Abstract
IMPORTANCE
The long-term clinical effects of wartime traumatic brain injuries (TBIs), most of which are mild, remain incompletely described. Current medical disability cost estimates from world conflicts continually surpass projections. Additional information regarding long-term functional trajectory is needed to reduce this extensive public health burden.
OBJECTIVES
To examine 5-year clinical outcomes leveraging existing clinical data collected at 1 year after injury in the same patients and to identify early risk factors for long-term disability.
DESIGN, SETTING, AND PARTICIPANTS
This prospective, longitudinal study enrolled active-duty US military after concussive blast injury (n = 50) in the acute to subacute stage and combat-deployed control individuals (n = 44) in Afghanistan or after medical evacuation to Germany from November 1, 2008, through July 1, 2013. One- and 5-year clinical evaluations were completed in the United States. All concussive blast injuries met the Department of Defense definition of mild, uncomplicated TBI. In-person clinical evaluations included standardized evaluations for neurobehavior, neuropsychological performance, and mental health burden that were essentially identical to the evaluations completed at 1-year follow-up. Data were analyzed from October 1 through November 30, 2016.
MAIN OUTCOMES AND MEASURES
Changes in the in-person standardized evaluations for neurobehavior, neuropsychological performance, and mental health burden from the 1- to 5-year follow-up. Predictive modeling was used to identify early risk factors for long-term disability.
RESULTS
Among the 94 participants (87 men [93%] and 7 women [7%]; mean [SD] age, 34 [8] years), global disability, satisfaction with life, neurobehavioral symptom severity, psychiatric symptom severity, and sleep impairment were significantly worse in patients with concussive blast TBI compared with combat-deployed controls, whereas performance on cognitive measures was no different between groups at the 5-year evaluation. Logistic regression on the dichotomized Extended Glasgow Outcome Scale (GOS-E) at 5 years as a measure of overall disability identified brain injury diagnosis, preinjury intelligence, motor strength, verbal fluency, and neurobehavioral symptom severity at 1 year as risk factors for a poor outcome at 5 years, with an area under the curve of 0.92 indicating excellent prediction strength. Thirty-six of 50 patients with concussive blast TBI (72%) had a decline in the GOS-E from the 1- to 5-year evaluations, in contrast with only 5 of 44 combat-deployed controls (11%). Worsening of symptoms in concussive blast TBI was also observed on measures of posttraumatic stress disorder and depression. Service members with concussive blast TBI experienced evolution, not resolution, of symptoms from the 1- to 5-year outcomes.
CONCLUSIONS AND RELEVANCE
Considerable decline was observed in military service members with concussive blast TBI when comparing 1- and 5-year clinical outcomes. These results advocate for new treatment strategies to combat the long-term and extremely costly effect of these wartime injuries.
Traumatic brain injury (TBI) affects roughly 3.5 million individuals annually in the United States,1 and approximately 75% of TBIs are attributable to mild or concussive events.2 An estimated 20% of the deployed US military suffered a head injury in the wars in Iraq and Afghanistan3; 83.3% endured a mild, uncomplicated TBI or concussion.4–6 Compared with civilians, service members with TBI have a high rate of comorbid mental health conditions. Previous studies7 found that 89% of veterans diagnosed with TBI also had a psychiatric diagnosis, with the median cost of health care in these comorbid cases being 4 times that of veterans with psychiatric diagnosis without TBI. Although civilian studies8 have reported poor outcomes on the Extended Glasgow Outcome Scale (GOS-E) in 22% to 33% of patients with mild TBI, reports of mild TBI sustained in combat have identified 62% to 96% with poor outcomes on the same measure.9–12
The long-term clinical effects of wartime injuries remain incompletely described.13,14 Previous studies15–18 have been based largely on self-report and screening tools to define TBI, rather than direct clinical assessments in cohorts identified at the time of injury and prospectively studied. Although much effort has been expended to better understand this type of concussive TBI, many studies in active-duty US military and veterans16,19–26 have been restricted to cross-sectional evaluations, often involving retrospective medical record review19–21 or self-report16,18,22–24,26,27 and considering only later stages of injury.25,28
In contrast, our group was provided the unique opportunity to examine patients in the acute9,29 to subacute10–12,30 stage after combat-related concussive TBI with prospective follow-up to 1-year9–12,30 and now 5-year evaluations. This longitudinal observation has allowed for a greater appreciation of functional postinjury trajectory and the effect on long-term outcome. The present study objective was to examine 5-year clinical outcomes leveraged with existing early clinical data in the same patients. Our goal was to identify predictors of poor outcome in service members diagnosed with concussive blast TBI. Given the extraordinary effect of these wartime injuries on service members, their families, communities, and the health care delivery system, we have an imperative to determine factors that will aid in identifying those at risk to better target treatments and therapies, providing proactive resolutions to this potentially high-cost and lifelong health burden.
Methods
Participants were originally enrolled in 1 of 4 previous cohorts.9–12,29,30 The present study is the 5-year evaluation in a continued prospective, observational, longitudinal research study. We report the 5-year clinical outcomes compared with 1-year findings in the following participant groups: patients with concussive blast TBI and combat-deployed control individuals. This study was approved by the institutional review boards of the University of Washington, Seattle, and the US Army Medical Research Materiel Command and was performed in accordance with the approved protocol. All par ticipants provided additional written informed consent for the 5-year evaluation; no surrogate consent was allowed (eMethods in the Supplement).
Selection and enrollment for the 5-year evaluation focused on individuals who had been enrolled at the time of injury, completed the 1-year evaluation, and were at a minimum of 4 years past the original enrollment. Inclusion criteria are reported elsewhere.9,10,30 In brief, participants were service members deployed to combat from November 1, 2008, through July 1, 2013, for whom original enrollment was completed directly in Afghanistan9,29 or after medical evacuation to Landstuhl Regional Medical Center in Landstuhl, Germany.10–12,30 Diagnosis of head injury was determined by trained medical personnel working in the TBI clinics in Germany. For the concussive blast TBI group, all available clinical histories indicated blast exposure plus another mechanism of head injury, such as a fall, motor vehicle crash, or being struck by a blunt object. All patients with concussive blast TBI were evacuated to Germany, and their cases met the Department of Defense definition of mild, uncomplicated TBI. None had a prior TBI or psychiatric diagnosis in their medical records. All combat-deployed controls were found in clinical evaluation to be free of TBI signs and symptoms, and we reviewed their medical records to confirm no history of TBI diagnosis, psychiatric diagnosis, or blast exposure. Combat-deployed controls were enrolled directly in Afghanistan or after evacuation to Germany for noncombat diagnoses such as gastrointestinal tract issues or dermatitis. Subgroup analysis by evacuation status in the control group identified no differences in any of the clinical outcome measures,10 and the data were combined for this study.
Clinical Assessments
In-person clinical evaluations at the University of Washington included a structured neurobehavioral interview, neuropsychological battery consisting of 10 cognitive tests, and structured psychiatric evaluation identical to the 1-year follow-up with additional self-administered questionnaires. Evaluations lasted approximately 5 hours, including 1 hour of standardized neurologic examination and 2 hours each for cognitive testing and psychiatric evaluation. Participants took all medications as prescribed by their clinical health care professionals. All tests were performed from 8 AM to 5 PM in private, quiet, well-lighted rooms. All examiners were blinded to other clinical information, although during the interviews, participant group often became clear in given endorsements of prior events. All examiners were psychometrists who underwent standardized training for administration.
Overall global disability was assessed using the GOS-E.31,32 Poor outcome was defined as a GOS-E score of 6 or less, indicating moderate to severe disability. Additional information on the GOS-E and for neuropsychological battery details can be found in the eMethods in the Supplement.
The neurologic assessment included a structured interview designed for patients with TBI (Neurobehavioral Rating Scale–Revised33), 2 headache interviews capturing frequency and intensity (Migraine Disability Assessment34 and the 6-item Headache Impact Test35), the Neurological Outcome Scale for Traumatic Brain Injury36–38 designed to assess focal neurologic deficits associated with TBI, and a TBI history intake interview modified from the Brain Injury Screening Questionnaire39 to confirm life history of head injury exposure and identify new head injuries sustained since the last evaluation. Participants completed the Quality of Life After Brain Injury40,41 questionnaire capturing life satisfaction.
The psychiatric evaluation included structured interviews and self-administered questionnaires. The Clinician-Administered PTSD (Posttraumatic Stress Disorder) Scale for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (CAPS)42 and Montgomery-Asberg Depression Rating Scale (MADRS)43 for depression were administered as structured interviews before the participant completed the PTSD Checklist–Military Version,44 Beck Depression Inventory,45 anxiety module of the Brief Symptom Inventory,46 Insomnia Severity Index,47 and Michigan Alcoholism Screening Test.48 The CAPS was scored using the rules from Blake et al.49
Statistical Analysis
Data were analyzed from October 1 through November 30, 2016. Group differences in patient characteristics were assessed using the 2-sided Fisher exact and Mann-Whitney U tests as appropriate. Group differences in the 5-year outcome were evaluated using standard linear and logistic regression with adjustment for imbalances in patient characteristics. Extension of the 5-year outcome analyses to adjust for the corresponding 1-year outcome was performed using linear and logistic mixed-effects regression modeling, with random intercepts and slopes for each participant and an unstructured correlation matrix. Risk factors for a poor 5-year outcome were determined using multivariate logistic regression modeling based on TBI diagnosis, demographic characteristics, and 1-year clinical outcome data using the Akaike information criterion50 to determine the optimal combination of variables. The number of events per variable for the best Akaike information criterion model was 8, which although lower than the minimum recommended number (10–15), is likely valid given the conditions of this particular analysis.51 The area under the receiver operating characteristic curve was calculated to assess model predictive accuracy.
Results
In total, 94 service members completed the 5-year evaluation, including 50 patients with concussive blast TBI and 44 combat-deployed controls (87 men [93%] and 7 women [7%]; mean [SD] age, 34 [8] years) (Table). General demographic details of participants, such as the numbers of officers vs enlisted personnel and race/ethnicity, did not significantly differ across groups. Combat-deployed controls were slightly older (mean [SD] age, 35 [8] vs 32 [8] years; P = .02, 2-sided Mann-Whitney U test), included more women (6 [14%] vs 1 [2%]; P = .048, Fisher exact test), had a higher educational level (mean [SD], 15.0 [2.5] vs 13.0 [1.5] years; P = .001, 2-sided Mann-Whitney U test), and included fewer members of the US Army (30 [68%] vs 45 [90%]; P = .01, Fisher exact test), compared with patients with concussive blast TBI. All results were adjusted by age, educational level, sex, rank, branch of service, and race/ethnicity. All cross-sectional associations between measures were adjusted for these factors by fitting linear (continuous) and logistic (binary) regression models, and adjusted P values are reported. When comparing the number of service members in each group who separated from the service within the first 5 years, we found that 15 combat-deployed controls (34%) and 37 patients with concussive blast TBI (74%) had left the military (P < .001, Fisher exact test). Although all 44 combat-deployed controls maintained employment, only 36 patients with concussive blast TBI (72%) were employed (P < .001, Fisher exact test).
Table.
Participant Characteristics at 5-Year Follow-up
| Characteristic | Study Groupa | P Value | |
|---|---|---|---|
| Combat-Deployed Controls (n = 44) |
Concussive Blast TBI (n = 50) |
||
| Age, mean (SD), y | 35(8) | 32 (8) | .02b |
| Educational level, mean (SD), y | 15.0 (2.5) | 13.0 (1.5) | .001b |
| Sex | |||
| Male | 38 (86) | 49 (98) | .048c |
| Female | 6 (14) | 1 (2) | |
| Race/ethnicity | |||
| White | 30 (68) | 36 (72) | .82c,d |
| African American | 10 (23) | 6 (12) | |
| Hispanic/Latino | 4(9) | 6 (12) | |
| Asian | 0 | 2 (4) | |
| Branch of service | |||
| US Army | 30 (68) | 45 (90) | .01c,e |
| US Air Force | 8 (18) | 0 | |
| US Marine Corps | 3 (7) | 5 (10) | |
| US Navy | 3 (7) | 0 | |
| Military rank | |||
| Enlisted | 39 (89) | 48 (96) | .25c |
| Officer | 5 (11) | 2 (4) | |
| Service separation | 15 (34) | 37 (74) | <.001c |
| Employment | 44 (100) | 36 (72) | <.001c |
Abbreviation: TBI, traumatic brain injury.
Data are presented as number (percentage) of participants unless otherwise indicated.
Calculated using the Mann-Whitney U test.
Calculated using the Fisher exact test.
Indicates white vs other.
Indicates Army vs other.
Global Outcome
Patients with concussive blast TBI fared worse on global outcome measures as evidenced by the GOS-E and the Quality of Life After Brain Injury questionnaire at the 5-year follow-up (both adjusted P < .001) (Figure 1). Good outcome for the GOS-E was defined as a score of 7 or 8, with 6 or lower considered to be a poor outcome. Five combat-deployed controls (11%) met this criterion, whereas 36 patients with concussive blast TBI (72%) were found to also have poor outcomes (Figure 1A). Comparing 1-year with 5-year GOS-E scores in the same participant, 5 combat-deployed controls (11%) had experienced decline by their 5-year evaluation. In contrast, 36 patients who experienced concussive blast TBI (72%) declined into or further in the moderate to severe disability range. A small subset in each cohort (10 patients with concussive blast TBI [20%] and 15 combat-deployed controls [34%]) improved, defined as a 1-year GOS-E score of 6 or less and a 5-year GOS-E score of 7 or 8. Many participants reported a significant effect on quality of life as observed in Figure 1B; 6 combat-deployed controls (14%) and 23 patients with concussive blast TBI (46%) scored in the bottom 3 categories of no to moderate life satisfaction (adjusted P < .001).
Figure 1. Overall Disability and Quality of Life at 5-Year Follow-up.
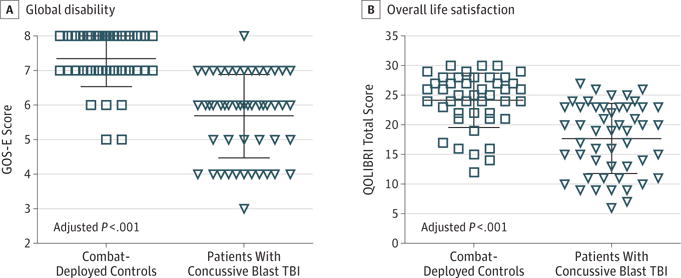
A, Global disability was assessed using the Extended Glasgow Outcome Scale (GOS-E; scores range from 3–8, with 7 or 8 categorized as a good outcome and ≤6 categorized as a poor outcome). Five controls (11%) and 36 patients with concussive blast traumatic brain injury (TBI) (72%) had a poor outcome. B, Overall life satisfaction was assessed using the Quality of Life After Traumatic Brain Injury (QOLIBRI; range, 6–30, with higher scores indicating greater life satisfaction). Each data marker represents an individual; horizontal black lines, mean (SD).
Neurobehavioral Evaluation
The patients with concussive blast TBI had significantly elevated levels of neurobehavioral symptoms (unadjusted mean [SD], 5 [4]) compared with combat-deployed controls at their 5-year evaluation (unadjusted mean [SD], 14 [7]; adjusted P < .001) (Figure 2A). This difference was not driven by focal neurologic deficits, evidenced by the Neurological Outcomes Scale for Traumatic Brain Injury (unadjusted mean [SD], 1.1 [1.4] for controls vs 1.6 [1.5] for the TBI group; adjusted P = .53) (Figure 2B). Headache impairment was worse in the concussive blast TBI group (adjusted P < .001) (Figure 2C). With use of the clinical cutoff of 11 for Migraine Disability Assessment scores and 50 for Headache Impact Test scores, moderate to severe headache impairment was observed in 10 combat-deployed controls (23%) and 31 patients with concussive blast TBI (62%) on the Migraine Disability Assessment and in 19 combat-deployed controls (43%) and 43 patients with concussive blast TBI (86%) on the Headache Impact Test. No participant reported a TBI diagnosis since the 1-year evaluation on the history of head injury in the intake interview. Seven combat-deployed controls (16%) and 27 patients with concussive blast TBI (54%) endorsed events suggestive of concussion, although few sought medical attention. These events were primarily ground-level falls and hits to the head during training exercises for combat-deployed controls and fights and motor vehicle crashes for patients with concussive blast TBI.
Figure 2. Neurobehavioral Symptoms, Neurologic Deficits, and Headache Impairment at 5-Year Follow-up.
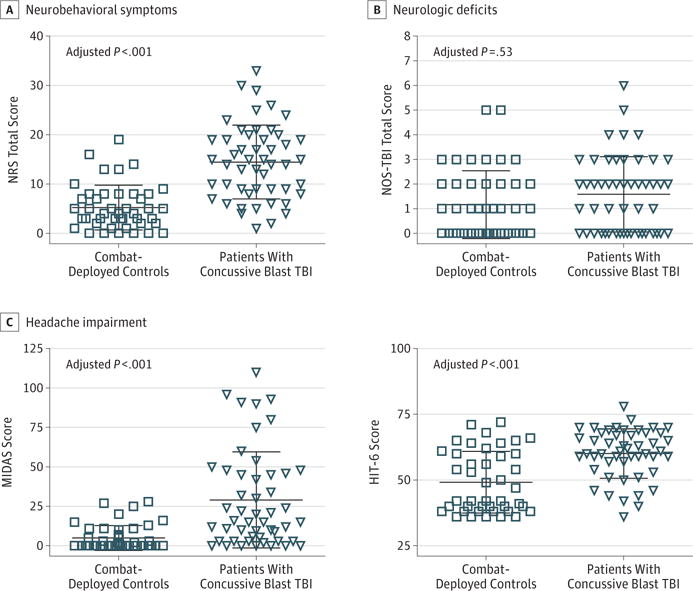
A, Overall neurobehavioral symptom severity was assessed using the Neurobehavioral Rating Scale–Revised (NRS; scores range from 0–33, with higher scores indicating more symptoms; maximum score, 87). B, Focal neurologic deficits were assessed using the Neurological Outcome Scale for Traumatic Brain Injury (NOS-TBI; scores range from 0–6, with higher scores indicating more deficits; maximum score, 58). C, Headache impairment was assessed using the Migraine Disability Scale (MIDAS; scores range from 0–110, with higher scores indicating more headache disabiltiy; maximum score, 270) and the 6-item Headache Impact Test (HIT-6; scores range from 36–78, with higher scores indicating more headache impairment; maximum score, 78). TBI indicates traumatic brain injury. Each data marker represents an individual; horizontal black lines, mean (SD).
Neuropsychological Performance
Cognitive performance was tested across 10 domains of function (eTable 1 in the Supplement). Compared with combat-deployed controls, patients with concussive blast TBI had comparable neuropsychological function across all domains at 5-year follow-up, with slight differences in visuospatial learning (total trials correct, 50.98 [9.74] vs 47.44 [11.34]; adjusted P = .05), fine motor speed and coordination (mean [SD] Grooved Pegboard score, 67.49 [8.77] vs 72.29 [10.89] seconds; adjusted P = .07), mental flexibility (mean [SD] Trails B test time, 56.37 [15.65] vs 63.16 [24.02] seconds; adjusted P = .05), and verbal fluency (Controlled Oral Word Association mean [SD] total score, 46.11 [10.67] vs 40.66 [9.62]; adjusted P = .04). None of the differences remained statistically significant after correction for multiple comparisons (ie, P < .002).
Psychiatric Evaluation
Mental health symptoms in multiple domains were significantly elevated in patients with concussive blast TBI compared with combat-deployed controls at the 5-year evaluation. Symptoms of posttraumatic stress measured by structured interview (CAPS) and self-report (PTSD Checklist–Military Version) were significantly more severe in patients with concussive blast TBI (adjusted P < .001 for both) (Figure 3A). Severity of depressive symptoms measured by structured interview (MADRS) and self-report (Beck Depression Inventory) was similarly disparate across groups (adjusted P = .001 for both) (Figure 3B). Using the clinical cutoffs of 65 for CAPS and 20 for MADRS, we found that 4 combat-deployed controls (9%) met the criteria for moderate to severe PTSD and 6 (14%) for moderate to severe depression. In contrast, 24 patients with concussive blast TBI (48%) had moderate to severe PTSD and 20 (40%) had moderate to severe depression. When comparing the 1- and 5-year PTSD data in the same participant, 9 combat-deployed controls (21%) and 19 patients with concussive blast TBI (38%) scored more than 10 points worse on the CAPS at their second evaluation. Both groups had roughly 20% with substantial exacerbation in depression defined as greater than a 10-point increase on the MADRS during this period (8 combat-deployed controls [18%] and 10 patients with concussive blast TBI [20%]).
Figure 3. Psychiatric Symptom Severity at 5-Year Follow-up.
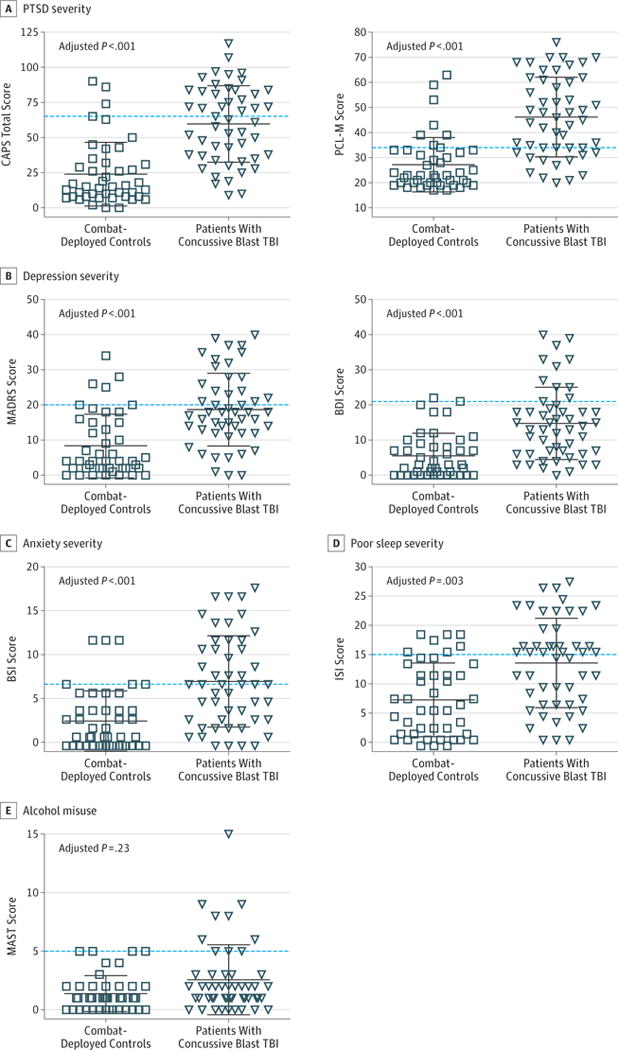
A, Posttraumatic stress disorder (PTSD) severity was assessed using the Clinician-Administered PTSD Scale for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (CAPS; scores range from 0–117, with higher scores indicating greater PTSD severity; maximum score, 136) and the self-administered PTSD Checklist–Military Version (PCL-M; scores range from 17–76, with higher scores indicating greater PTSD severity; maximum score, 85). B, Depression severity was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS; scores range from 0–40, with higher scores indicating greater depression severity; maximum score, 60) and the self-administered Beck Depression Inventory (BDI; scores range from 0–40, with higher scores indicating greater depression severity; maximum score, 63). C, Anxiety symptom severity was assessed using the anxiety module of the Brief Symptom Inventory (BSI; scores range from 0–18, with higher scores indicating greater anxiety severity; maximum score, 24). D, Severity of poor sleep assessed using the Insomnia Severity Index (ISI; scores range from 0–28, with higher scores indicating worse sleep impairment; maximum score, 28). E, Alcohol misuse was assessed using the Michigan Alcoholism Screening Test (MAST; scores range from 0–15, with higher scores indicating greater alcohol impairment; maximum score, 22). TBI indicates traumatic brain injury. Each data marker represents an individual; horizontal black lines, mean (SD); and dashed lines, threshold for moderate to severe symptoms for each evaluation.
The groups also significantly differed on measures of anxiety (adjusted P = .001) and sleep impairment (adjusted P = .003), whereas no significant difference was observed in alcohol misuse (adjusted P = .23) (Figure 3C–E). Using the clinical cutoffs of 7 for the anxiety module of the Brief Symptom Inventory and 15 for the Insomnia Severity Index (sleep impairment measure), 6 combat-deployed controls (14%) and 27 patients with concussive blast TBI (54%) had moderate to severe anxiety symptoms, and 9 combat-deployed controls (20%) and 28 patients with concussive blast TBI (56%) had moderate to severe sleep impairment. Of interest, between the 1- and 5-year study evaluations, 18 combat-deployed controls (41%) and 40 patients with concussive blast TBI (80%) endorsed seeking assistance from a licensed mental health care professional, defined as a psychologist, psychiatrist, therapist, social worker, or other licensed, credentialed mental health care professional. Only 9 combat-deployed controls (20%) and 9 patients with concussive blast TBI (18%) reported that the mental health programs helped.
Predictors of Poor Outcome
We used the 1-year clinical outcome data to determine what demographic, neurologic, neuropsychological, and psychiatric factors, including interim head impact exposures best predicted 5-year poor global outcome, defined as a GOS-E score of 6 or less (moderate to severe disability). Of the 94 participants, 84 had complete 1-year clinical outcome data that were used for analysis. Logistic regression using dichotomized GOS-E of 7 to 8 (good outcome) and 6 or less (poor outcome) identified the best-fit model by Akaike information criterion to include TBI diagnosis, neurobehavioral symptom severity (a 29-domain measure of neurologic, cognitive, and mental health function), performance on a 7.5-m (25-ft) walk (motor strength, balance, and coordination), performance on the Wechsler Test of Adult Reading (preinjury intelligence measure),52 and performance on Controlled Oral Word Association (verbal fluency)53 (Figure 4 and eTable 2 in the Supplement), with an area under the curve of 0.92. In the small subset showing improvement, the best-fit model included control status, younger age, and lower depression severity on the MADRS, with an area under the curve of 0.88.
Figure 4. One-Year Predictors of 5-Year Global Outcomes.
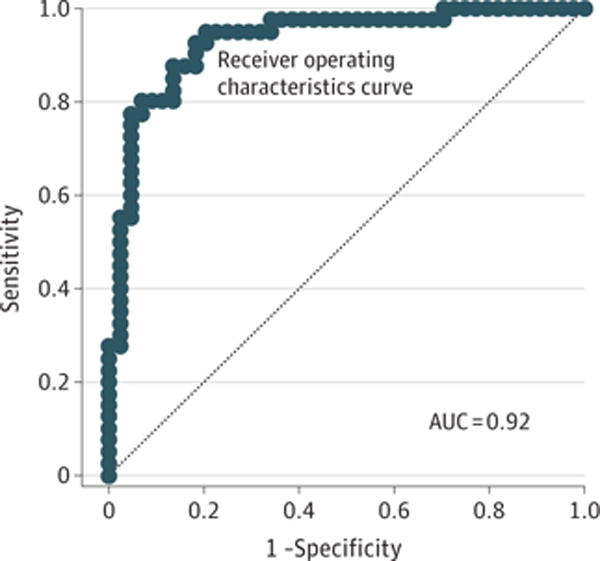
Receiver operating characteristics curve for the best-fit logistic regression model of 1-year clinical predictors of 5-year overall outcome disability defined by the dichotomized Extended Glasgow Outcome Scale, with scores of 7 or 8 categorized as good outcome and 6 or below categorized as disabled. The best model by Akaike information criterion contained the diagnoses of traumatic brain injury, neurobehavioral symptom severity (a 29-domain measure of neurological, cognitive, and mental health function), performance on the 7.5-m (25-ft) walk (a measure of motor strength, balance, and coordination), performance on the Wechsler Test of Adult Reading (a measure of preinjury intelligence), and performance on the Controlled Oral Word Association test (a measure of verbal fluency). AUC indicates area under the curve; dashed line, line of unity.
Discussion
These findings indicate that service members who sustained blast concussion in combat have worse early long-term outcomes compared with combat-deployed controls. The rate of disability observed in concussive blast TBI is much higher than in otherwise comparable civilian studies, even in mild TBI with polytrauma, and speaks to a higher percentage of persistent postinjury symptoms in service personnel.54–61 A substantial number of patients with concussive blast TBI continued to experience decline from the 1- to 5-year evaluations. The historic mantra in medicine regarding general stability at 6 months after injury appears to be challenged in this population, with progression of postconcussive symptoms well after this time frame.
Although cognitive performance was not substantially different between patients with concussive blast TBI and combat-deployed controls, considerable disparities in psychiatric symptom severity were observed. Specifically, symptoms of PTSD, depression, and anxiety were significantly elevated in participants with concussive blast TBI, and a substantial number of these individuals were found to have worsening impair ment compared with their 1-year assessment. Chronic sleep impairment was also significantly worse in patients with concussive blast TBI. We find the combination of sustained, elevated psychiatric symptoms and sleep impairment concerning, given the growing literature on the long-term health implications of these 2 conditions. Chronic sleep impairment has been reported to affect metabolic and cardiovascular health as well as overall disease-associated mortality.62 Although our study identified impairment sustained during the course of this first 5-year period after injury, given the young age of these service members (most in their 30s), questions arise regarding how these individuals will progress with natural aging and what health complications they will face in the future.
For this reason, we examined predictors of the 5-year outcome leveraging early clinical data collected in the same participant to target modifiable risk factors to reduce the long-term effect of these exposures. By logistic regression, we identified TBI diagnosis, preinjury intelligence, motor strength, verbal fluency, and neurobehavioral symptom severity at 1 year as risk factors for 5-year poor outcomes, with an area under the curve of 0.92 indicating excellent prediction strength. Among these, neurobehavioral symptoms may be a particularly important modifiable target for early intervention in service members with concussion.63
Strengths and Limitations
Strengths of this study include the use of a prospective, observational, longitudinal cohort design; enrollment of all combat-deployed, active-duty US military in the acute9,29 to subacute11,12,30 phase after injury; repeated assessment by identical clinical measures at the 1- and 5-year evaluations; and blinded clinical evaluations completed by trained personnel at each point. Limitations include a modest sample size by group, lack of comprehensive preinjury and acute postinjury clinical data for comparison with later outcomes, heterogeneous treatment across centers and in the United States after injury, and possible unmeasured covariates that may influence results.
Conclusions
Together these findings indicate progression of symptom severity beyond 1 year after injury. Many service members with concussive blast TBI experience evolution rather than resolution of symptoms from the 1- to 5-year outcomes. Even a small percentage of combat-deployed controls appeared to experience worsening over time. In both groups, this finding appears to be driven more by psychiatric symptoms than by cognitive deficits. Efforts are under way to replicate these findings in a larger cohort and to extend evaluation to later years.
These results speak to the need for new approaches to co-morbid mental health treatment and long-term care, given that most of the patients in our study (80%) sought help but a much smaller number (19%) found sustained resolution. Targeting psychological health domains identified herein and by others21,64–69 might provide the highest potential for a long-lasting effect of treatment. This process may aid in reducing the public health burden and extensive cost facing our service members and our nation in the decades to come. Peak disability payout for veterans of world conflicts has been reported to incur decades after the conflict is over. World War I (1917–1918) disability cost reportedly peaked in 1969, World War II (1941–1945) disability cost reportedly peaked in 1980, and Vietnam (1959–1975) disability cost was still increasing in 2011.70 With the Global War on Terrorism (2001–2014) recently ending and already exceeding cost projections, the true effect will likely not be felt for years to come.71 We believe that by being informed from longitudinal studies such as this one, the medical community can be proactive in combatting the potentially negative and extremely costly effect of these wartime injuries.
Supplementary Material
Key Points.
Questions
What early clinical variables are best associated with long-term outcome after concussive blast injury, and do patients experience symptom resolution?
Findings
In this longitudinal case-control study of military service members, brain injury diagnosis, preinjury intelligence, motor strength, verbal fluency, and neurobehavioral symptom severity at 1 year were most associated with 5-year global outcome. Thirty-six of 50 patients with blast concussion experienced a decline from the 1- to 5-year evaluations.
Meaning
Considerable decline was observed in service members with concussive blast injury, supporting the need for new treatment strategies to combat the long-term and extremely costly effects of these wartime injuries.
Acknowledgments
Funding/Support: This study was supported by Department of Defense grant W81XWH-13-2-0095 through the Chronic Effects of Neurotrauma Consortium and by grant 1R01NS091618-01 from the National Institute of Neurological and Communication Disorders and Stroke, National Institutes of Health.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Mac Donald had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mac Donald, Dikmen, Temkin.
Acquisition, analysis, or interpretation of data: Mac Donald, Barber, Jordan, Johnson, Fann, Temkin.
Drafting of the manuscript: Mac Donald.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Mac Donald, Barber, Johnson, Temkin.
Obtained funding: Mac Donald, Temkin.
Administrative, technical, or material support: Mac Donald, Jordan, Johnson, Fann.
Study supervision: Mac Donald, Fann.
Conflict of Interest Disclosures: None reported.
Disclaimer: Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the US government, Department of Defense, or the US Department of Veterans Affairs, and no official endorsement should be inferred.
Additional Contributions: We thank the service members, their families, commanding officers, and clinicians for making this study possible. Paul Helmer, MS, Brie Sullivan, BS, Andrea Winters, BS, Brian Douay, BS, Steve Ottinger, BS, Colleen Tuffy, BS, Mellissa Tong, BS, and Trista Protzeler, BS, of the psychometric team at the University of Washington, Seattle, assisted with this study. They were compensated for their contributions to the study.
References
- 1.Coronado VG, McGuire LC, Sarmiento K, et al. Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J Safety Res. 2012;43(4):299–307. doi: 10.1016/j.jsr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) NCfIPaC. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 3.Taniellian T, Jaycox L. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008. [Google Scholar]
- 4.DVBIC. DoD Numbers for Traumatic Brain Injury, Worldwide—Totals, 2000–2012 (Q1-Q3) 2013 http://www.dvbic.org/sites/default/files/uploads/dod-tbi-worldwide-2000-2013-Q3-as-of-05%20Nov-2013.pdf. Accessed December 10, 2013.
- 5.Casscells S. In: Traumatic brain injury: definition and reporting. Aso D, editor. Washington, DC: Department of Defense; 2007. [Google Scholar]
- 6.Defense and Veterans Brain Injury Center. DoD Worldwide TBI Numbers (2000–2016, Q1–Q3) 2016 http://dvbic.dcoe.mil/files/tbi-numbers/DoD-TBI-Worldwide-Totals_2016_Q3_Nov-10-2016_v1.0_508_2016-12-27.pdf. Accessed January 14, 2017.
- 7.Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50(4):342–346. doi: 10.1097/MLR.0b013e318245a558. [DOI] [PubMed] [Google Scholar]
- 8.McMahon P, Hricik A, Yue JK, et al. TRACK-TBI Investigators Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J Neurotrauma. 2014;31(1):26–33. doi: 10.1089/neu.2013.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mac Donald CL, Adam OR, Johnson AM, et al. Acute post-traumatic stress symptoms and age predict outcome in military blast concussion. Brain. 2015;138(Pt 5):1314–1326. doi: 10.1093/brain/awv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mac Donald CL, Johnson AM, Wierzechowski L, et al. Outcome trends after US military concussive traumatic brain injury [published online June 27, 2016] J Neurotrauma. 2016 doi: 10.1089/neu.2016.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mac Donald CL, Johnson AM, Wierzechowski L, et al. Prospectively assessed clinical outcomes in concussive blast vs nonblast traumatic brain injury among evacuated US military personnel. JAMA Neurol. 2014;71(8):994–1002. doi: 10.1001/jamaneurol.2014.1114. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald CL, Johnson AM, Nelson EC, et al. Functional status after blast-plus-impact complex concussive traumatic brain injury in evacuated United States military personnel. J Neurotrauma. 2014;31(10):889–898. doi: 10.1089/neu.2013.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman JC, Diaz-Arrastia R. Military traumatic brain injury: a review. Alzheimers Dement. 2014;10(3 suppl):S97–S104. doi: 10.1016/j.jalz.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Boyle E, Cancelliere C, Hartvigsen J, Carroll LJ, Holm LW, Cassidy JD. Systematic review of prognosis after mild traumatic brain injury in the military: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95(3 suppl):S230–S237. doi: 10.1016/j.apmr.2013.08.297. [DOI] [PubMed] [Google Scholar]
- 15.Vanderploeg RD, Belanger HG, Horner RD, et al. Health outcomes associated with military deployment: mild traumatic brain injury, blast, trauma, and combat associations in the Florida National Guard. Arch Phys Med Rehabil. 2012;93(11):1887–1895. doi: 10.1016/j.apmr.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 17.Lippa SM, Pastorek NJ, Benge JF, Thornton GM. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J Int Neuropsychol Soc. 2010;16(5):856–866. doi: 10.1017/S1355617710000743. [DOI] [PubMed] [Google Scholar]
- 18.Polusny MA, Kehle SM, Nelson NW, Erbes CR, Arbisi PA, Thuras P. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to Iraq. Arch Gen Psychiatry. 2011;68(1):79–89. doi: 10.1001/archgenpsychiatry.2010.172. [DOI] [PubMed] [Google Scholar]
- 19.Eskridge SL, Macera CA, Galarneau MR, et al. Influence of combat blast-related mild traumatic brain injury acute symptoms on mental health and service discharge outcomes. J Neurotrauma. 2013;30(16):1391–1397. doi: 10.1089/neu.2012.2537. [DOI] [PubMed] [Google Scholar]
- 20.Galarneau MR, Woodruff SI, Dye JL, Mohrle CR, Wade AL. Traumatic brain injury during Operation Iraqi Freedom: findings from the United States Navy–Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108(5):950–957. doi: 10.3171/JNS/2008/108/5/0950. [DOI] [PubMed] [Google Scholar]
- 21.Kontos AP, Kotwal RS, Elbin RJ, et al. Residual effects of combat-related mild traumatic brain injury. J Neurotrauma. 2013;30(8):680–686. doi: 10.1089/neu.2012.2506. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy JE, Cullen MA, Amador RR, Huey JC, Leal FO. Symptoms in military service members after blast mTBI with and without associated injuries. NeuroRehabilitation. 2010;26(3):191–197. doi: 10.3233/NRE-2010-0555. [DOI] [PubMed] [Google Scholar]
- 23.Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol. 2008;167(12):1446–1452. doi: 10.1093/aje/kwn068. [DOI] [PubMed] [Google Scholar]
- 24.Wilk JE, Herrell RK, Wynn GH, Riviere LA, Hoge CW. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom Med. 2012;74(3):249–257. doi: 10.1097/PSY.0b013e318244c604. [DOI] [PubMed] [Google Scholar]
- 25.Verfaellie M, Lafleche G, Spiro A, III, Tun C, Bousquet K. Chronic postconcussion symptoms and functional outcomes in OEF/OIF veterans with self-report of blast exposure. J Int Neuropsychol Soc. 2013;19(1):1–10. doi: 10.1017/S1355617712000902. [DOI] [PubMed] [Google Scholar]
- 26.Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24(1):14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 27.Reid MW, Miller KJ, Lange RT, et al. A multisite study of the relationships between blast exposures and symptom reporting in a post-deployment active duty military population with mild traumatic brain injury. J Neurotrauma. 2014;31(23):1899–1906. doi: 10.1089/neu.2014.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer BL, Parsons M, Durgerian S, et al. Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J Neurotrauma. 2014;31(2):169–179. doi: 10.1089/neu.2013.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adam O, Mac Donald CL, Rivet D, et al. Clinical and imaging assessment of acute combat mild traumatic brain injury in Afghanistan. Neurology. 2015;85(3):219–227. doi: 10.1212/WNL.0000000000001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mac Donald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in US military personnel. N Engl J Med. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 32.Pettigrew LE, Wilson JT, Teasdale GM. Reliability of ratings on the Glasgow Outcome Scales from in-person and telephone structured interviews. J Head Trauma Rehabil. 2003;18(3):252–258. doi: 10.1097/00001199-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Levin HS, High WM, Goethe KE, et al. The Neurobehavioural Rating Scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry. 1987;50(2):183–193. doi: 10.1136/jnnp.50.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart WF, Lipton RB, Whyte J, et al. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology. 1999;53(5):988–994. doi: 10.1212/wnl.53.5.988. [DOI] [PubMed] [Google Scholar]
- 35.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12(8):963–974. doi: 10.1023/a:1026119331193. [DOI] [PubMed] [Google Scholar]
- 36.McCauley SR, Wilde EA, Kelly TM, et al. The Neurological Outcome Scale for Traumatic Brain Injury (NOS-TBI), II: reliability and convergent validity. J Neurotrauma. 2010;27(6):991–997. doi: 10.1089/neu.2009.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilde EA, McCauley SR, Kelly TM, et al. The Neurological Outcome Scale for Traumatic Brain Injury (NOS-TBI), I: construct validity. J Neurotrauma. 2010;27(6):983–989. doi: 10.1089/neu.2009.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilde EA, McCauley SR, Kelly TM, et al. Feasibility of the Neurological Outcome Scale for Traumatic Brain Injury (NOS-TBI) in adults [published correction in J Neurotrauma. 2010;27(7):1355] J Neurotrauma. 2010;27(6):975–981. doi: 10.1089/neu.2009.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dams-O’Connor K, Cantor JB, Brown M, Dijkers MP, Spielman LA, Gordon WA. Screening for traumatic brain injury: findings and public health implications. J Head Trauma Rehabil. 2014;29(6):479–489. doi: 10.1097/HTR.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Truelle JL, Koskinen S, Hawthorne G, et al. Qolibri Task Force Quality of life after traumatic brain injury: the clinical use of the QOLIBRI, a novel disease-specific instrument. Brain Inj. 2010;24(11):1272–1291. doi: 10.3109/02699052.2010.506865. [DOI] [PubMed] [Google Scholar]
- 41.von Steinbuechel N, Wilson L, Gibbons H, et al. QOLIBRI overall scale: a brief index of health-related quality of life after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(11):1041–1047. doi: 10.1136/jnnp-2012-302361. [DOI] [PubMed] [Google Scholar]
- 42.Weathers FW, Keane TM, Davidson JR. Clinician-Administered PTSD Scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 43.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 44.Yeager DE, Magruder KM, Knapp RG, Nicholas JS, Frueh BC. Performance characteristics of the Posttraumatic Stress Disorder Checklist and SPAN in Veterans Affairs primary care settings. Gen Hosp Psychiatry. 2007;29(4):294–301. doi: 10.1016/j.genhosppsych.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Homaifar BY, Brenner LA, Gutierrez PM, et al. Sensitivity and specificity of the Beck Depression Inventory-II in persons with traumatic brain injury. Arch Phys Med Rehabil. 2009;90(4):652–656. doi: 10.1016/j.apmr.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 47.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 48.Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127(12):1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 49.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 50.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Second International Symposium on Information Theory; 1973; Budapest, Hungary. pp. 267–281. [Google Scholar]
- 51.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler D. Wechsler Test of Adult Reading (WTAR) Manual. New York, NY: Psychological Corp; 2001. [Google Scholar]
- 53.Benton A, Amsher K. Multilingual Aphasia Examination. 3rd. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- 54.Sigurdardottir S, Andelic N, Roe C, Schanke AK. Cognitive recovery and predictors of functional outcome 1 year after traumatic brain injury. J Int Neuropsychol Soc. 2009;15(5):740–750. doi: 10.1017/S1355617709990452. [DOI] [PubMed] [Google Scholar]
- 55.Benedictus MR, Spikman JM, van der Naalt J. Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Arch Phys Med Rehabil. 2010;91(9):1436–1441. doi: 10.1016/j.apmr.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 56.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995;45(7):1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 57.Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ. 2000;320(7250):1631–1635. doi: 10.1136/bmj.320.7250.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosenthal AC, Livingston DH, Lavery RF, et al. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J Trauma. 2004;56(5):1042–1048. doi: 10.1097/01.ta.0000127767.83267.33. [DOI] [PubMed] [Google Scholar]
- 59.Lannsjö M, Raininko R, Bustamante M, von Seth C, Borg J. Brain pathology after mild traumatic brain injury: an exploratory study by repeated magnetic resonance examination. J Rehabil Med. 2013;45(8):721–728. doi: 10.2340/16501977-1169. [DOI] [PubMed] [Google Scholar]
- 60.Jacobs B, Beems T, Stulemeijer M, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma. 2010;27(4):655–668. doi: 10.1089/neu.2009.1059. [DOI] [PubMed] [Google Scholar]
- 61.Stulemeijer M, van der Werf SP, Jacobs B, et al. Impact of additional extracranial injuries on outcome after mild traumatic brain injury. J Neurotrauma. 2006;23(10):1561–1569. doi: 10.1089/neu.2006.23.1561. [DOI] [PubMed] [Google Scholar]
- 62.Research IoMUCoSMa. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 63.Bell KR, Fann JR, Brockway JA, et al. Telephone problem solving for service members with mild traumatic brain injury: a randomized, clinical trial. J Neurotrauma. 2017;34(2):313–321. doi: 10.1089/neu.2016.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stein MB, Ursano RJ, Campbell-Sills L, et al. Prognostic indicators of persistent post-concussive symptoms after deployment-related mild traumatic brain injury: a prospective longitudinal study in US Army soldiers. J Neurotrauma. 2016;33(23):2125–2132. doi: 10.1089/neu.2015.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA. 2010;303(19):1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haagsma JA, Scholten AC, Andriessen TM, Vos PE, Van Beeck EF, Polinder S. Impact of depression and post-traumatic stress disorder on functional outcome and health-related quality of life of patients with mild traumatic brain injury. J Neurotrauma. 2015;32(11):853–862. doi: 10.1089/neu.2013.3283. [DOI] [PubMed] [Google Scholar]
- 67.Vuletic S, Bell KR, Jain S, et al. CONTACT Investigators Telephone problem-solving treatment improves sleep quality in service members with combat-related mild traumatic brain injury: results from a randomized clinical trial. J Head Trauma Rehabil. 2016;31(2):147–157. doi: 10.1097/HTR.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 68.Storzbach D, O’Neil ME, Roost SM, et al. Comparing the neuropsychological test performance of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans with and without blast exposure, mild traumatic brain injury, and posttraumatic stress symptoms. J Int Neuropsychol Soc. 2015;21(5):353–363. doi: 10.1017/S1355617715000326. [DOI] [PubMed] [Google Scholar]
- 69.Walker WC, Franke LM, McDonald SD, Sima AP, Keyser-Marcus L. Prevalence of mental health conditions after military blast exposure, their co-occurrence, and their relation to mild traumatic brain injury. Brain Inj. 2015;29(13–14):1581–1588. doi: 10.3109/02699052.2015.1075151. [DOI] [PubMed] [Google Scholar]
- 70.Bilmes L, Stiglitz J. The long-term costs of conflict: the case of the Iraq War. In: Braddon D, Hartley K, editors. Elgar Handbook on the Economics of Conflict. Cheltenham, England: Edward Elgar Publishers; 2011. [Google Scholar]
- 71.Bilmes L. Soldiers Returning From Iraq and Afghanistan: The Long-term Costs of Providing Veterans Medical Care and Disability Benefits. Boston, MA: Harvard University, John F. Kennedy School of Government; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


