Abstract
Background
Recent studies shows that hyperbaric oxygen (HBO) therapy exerts some protective effects against neural injuries. The purpose of this study was to determine the neuroprotective effects of HBO following sciatic nerve transection (SNT).
Methods
Rats were randomly divided into five groups (n = 14 per group): Sham-operated (SH) group, SH + HBO group, SNT group, and SNT + pre- and SNT + post-HBO groups (100% oxygen at 2.0 atm absolute, 60 min/day for five consecutive days beginning on 1 day before and immediately after nerve transaction, respectively). Spinal cord segments of the sciatic nerve and related dorsal root ganglions (DRGs) were removed 4 weeks after nerve transection for biochemical assessment of malodialdehyde (MDA) levels in spinal cord, biochemical assessment of superoxide dismutase (SOD) and catalse (CAT) activities in spinal cord, immunohistochemistry of caspase-3, cyclooxigenase-2 (COX-2), S100beta (S100ß), and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) in spinal cord and DRG.
Results
The results revealed that MDA levels were significantly decreased in the SNT + pre-HBO group, while SOD and CAT activities were significantly increased in SNT + pre- and SNT + post-HBO treated rats. Attenuated caspase-3 and COX-2 expression, and TUNEL reaction could be significantly detected in the HBO-treated rats after nerve transection. Also, HBO significantly increased S100ß expression.
Conclusions
Based on these results, we can conclude that pre- and post-HBO therapy had neuroprotective effects against sciatic nerve transection-induced degeneration.
Keywords: Hyperbaric oxygen, Nerve transaction, Apoptosis, Inflammation
Background
After peripheral nerve transection, a proportion of sensory and motor neurons progressively die through apoptosis [1–3]. Therefore, it has been thought that the survival of the axotomized populations of neurons is the prime issue to restore the function of target organs [4]. Several mechanisms account for the apoptosis of neuronal cells after nerve transection, including excitotoxicity, alternations in electrical activity, oxidative stress, neurotrophic support deficit, neurotoxic inflammatory products, and alternation in cellular homeostasis [5–7]. Therefore, it has been postulated that the use of free radical scavengers [8], anti-inflammatory agents [9], and nerve growth factors [10] may offer some protection against neural apoptosis after peripheral nerve transection. Due to the complexity of the nerve cell destructive processes after nerve transection, a satisfactory therapeutic method has not been developed yet. So, finding an appropriate therapeutic method to reduce this process is an important issue in field of peripheral nerve regeneration.
Hyperbaric oxygen (HBO) therapy, treatment providing 100% oxygen at a pressure greater than that at sea level, can be considered as one of these methods. In this regard, studies documented that HBO have neuroprotective effects against traumatic brain and spinal cord injury [11, 12], ischemic brain and spinal cord injury [13, 14], neurodegenerative disorders [15, 16], oxidative damage in neuronal culture [17], and neuropathic pain [18]. Recently, HBO has been demonstrated to improve nerve regeneration [19, 20] following peripheral nerve injury. The beneficial effects can be attributed to some biological activities such as anti-oxidative [21, 22], anti-inflammatory [23, 24], and anti-apoptotic [25, 26] properties. Also, it was documented that HBO increased oxygen supply [27] and improved neural metabolism [28] after ischemia, along with promoting thrombolysis [29]. Despite the neuroprotective effects of HBO therapy against various experimental models of neural injury and disease, no studies have been conducted on the influence of HBO on neural apoptosis after nerve transection. Accordingly, we investigated the beneficial effects of HBO therapy on neuronal cell preservation after sciatic nerve transection.
Methods
Animals
A total of 70 adult male Sprague-Dawley rats were used in this study (laboratory animal research center, Sari, Iran). The animals were kept in the laboratory under constant conditions of light/dark cycle (12 h/12 h) and temperature (23 ± 1 °C). Experimental procedures and protocols used in this study were approved by ethical committee of Health Sciences, Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1396.2978).
Nerve transection and experimental design
Under anesthesia with intraperitoneal ketamine (60 mg/kg) plus xylazine (10 mg/kg), sciatic nerve was transected unilaterally (right side) at the level of the sacrotuberous ligament. Spontaneous regeneration of sciatic nerve was inhibited by excising a 3 mm segment of distal nerve stump, and reflection of the distal and proximal ends of the transected nerves [30]. The rats were placed in HBO chamber, the pressure was gradually raised to and maintained at 2.0 atm absolute (at a rate of 0.1 ATA/min), then allowed to breathe 100% oxygen for 60 min per day and decompressed to normal room pressure at a rate of 0.1ATA/min [31, 32]. All rats in the same group were kept in the chamber for 30 min to adapt it to the experimental conditions and then underwent treatment at the same time.
The animals were randomly allocated in five groups, each containing 14 rats: (Ι) Sham-operated (SH) group (underwent skin suture alone); (ΙΙ) SH + HBO group (underwent skin suture and received HBO); (III) sciatic nerve transection (SNT) group (underwent skin suture followed by sciatic nerve transection); (IV) SNT + pre-HBO group (HBO treatment beginning on 1 day before nerve transection and followed for 5 days); (V) SNT + Post-HBO group (HBO treatment for 5 days beginning on immediately after nerve transection and recovery from anesthesia).
Four weeks after nerve transection, each group of animals was divided into 2 subgroups: (A) in which rats (n = 7) were euthanized with an intraperitoneal injection of overdose of sodium pentobarbital, and spinal cord segments of the sciatic nerve removed from vertebral column for biochemical analysis, (B) in which rats (n = 7) were euthanized with an intraperitoneal injection of overdose of sodium pentobarbital, and spinal cord segments of the sciatic nerve and related dorsal root ganglions (ipsilateral L4 and L5 DRGs) removed for histopathological assessment and immunohistochemistry. The doses and treatment schedules were based on previous studies [29–31] and pilot experiments in our laboratory.
Biochemistry
Four weeks after nerve transection, the obtained spinal cord samples were immediately frozen and stored in a − 80 °C freezer for assays of tissue malondialdehyde (MDA) levels as a product of lipid peroxidation, and catalase (CAT) and superoxide dismutase (SOD) activities. Thiobarbituric acid reactive substances measurement was used to calculate of MDA level as micromoles per milligram of protein [33]. Catalase (CAT) enzyme activity was measured spectrophotometrically based on the reaction of the enzyme with methanol in the presence of hydrogen peroxide and expressed as unit per milligram of protein [34]. The estimation of superoxide dismutase (SOD) activity was based on the inhibition of superoxide radical reaction with pyrogallol which was determined spectrophotometrically by the absorbance at 420 nm and expressed as unit per milligram of protein [35].
Histopathology
Four weeks after nerve transection, the obtained spinal cord segments and related dorsal root ganglions were immediately fixed in 10% neutral buffered formalin. Five-micrometer serial transverse sections were taken from the paraffin-embedded blocks, deparaffinized, and then stained with Cresyl violet to assess the histopathological changes. All the histological assessments were done in a blinded fashion.
Immunohistochemistry
For immunohistochemistry, some sections were incubated with anti-caspase 3 rabbit polyclonal antibody (1:200 in PBS, v/v, Abcam), anti-COX 2 rabbit polyclonal antibody (1:200 in PBS, v/v, Abcam), and anti-S100ß rabbit polyclonal antibody (1:500 in PBS, v/v, Abcam) overnight at 4 °C. Sections were washed with PBS and incubated with secondary antibody conjugated with horseradish peroxidase (goat anti-rabbit IgG, Abcam) for 2 h. For quantitative analysis, immunohistochemical photographs (n = five photos from each five-micrometer serial transverse sections of ipsilateral spinal cord segments of the sciatic nerve and related dorsal root ganglions, the thickness of between sampled sections was 48 μm for spinal cord and 36 μm for DRG) from all rats in each experimental group were assessed by densitometry using ImageJ software. Data are expressed as a percentage of total tissue area.
TUNEL staining
TUNEL staining on some sections was performed using a TUNEL detection kit (Roche). Briefly, the sections incubated for 10 min with 3% H2O2 and for 15 min with proteinase-K, and then incubated with TUNEL reaction mixture for 60 min at 37 °C. In the following, the samples were incubated for 30 min with converter POD at 37 °C and demonstrated with DAB for 10 min. For quantitative analysis, immunohistochemical photographs (n = five photos from each five-micrometer serial transverse sections of ipsilateral spinal cord segments of the sciatic nerve and related dorsal root ganglions, the thickness of between sampled sections was 48 μm for spinal cord and 36 μm for DRG) from all rats in each experimental group were assessed by densitometry using ImageJ software. Data are expressed as a percentage of total tissue area.
Statistical analysis
Statistical analysis was performed with SPSS Version 15. Results were presented as mean values with standard deviations. Normality of the data determined by Kolmogrov-Smirnov (K-S) normality test. Also, analysis of variance (ANOVA) followed by Tukey′s multiple comparison tests were used to assess the results. A value of p < 0.05 was considered significant.
Results
Biochemical analysis
Biochemical analysis of the MDA levels, and SOD and CAT activities for all groups is shown in Table 1. Sciatic nerve transection in the SNT group produced a significant elevation (p < 0.01) in lipid peroxidation level compared to SH and SH + HBO groups. The MDA levels in the SNT + pre-HBO group were significantly lower than that those in the SNT group (p < 0.01). Treatment with HBO in the SNT + post-HBO group did not produced a significant decrease in MDA levels compared to SNT group (p > 0.05).
Table 1.
Effect of HBO on biochemical markers of rat spinal cord affected by sciatic nerve transection
| Experimental Groups | MDA μmol/mg-protein |
CAT unit/mg-protein |
SOD unit/mg-protein |
|---|---|---|---|
| SH | 60.67 ± 1.52 | 107 ± 26.87 | 10.33 ± 2.08 |
| SH + HBO | 60.33 ± 14.29 | 97 ± 8.48 | 9 ± 2.82 |
| SNT | 97.33 ± 1.52** | 15 ± 2.64*** | 1.83 ± 1.25* |
| SNT + pre-HBO | 65.67 ± 5.50## | 80 ± 17.06# | 9 ± 4.35# |
| SNT + post-HBO | 90.67 ± 15.89 | 75 ± 19.80# | 8.33 ± 1.52# |
Data are represented in Mean ± SD. ** p < 0.01 versus SH and SH + HBO; ## p < 0. 01 versus SNT; *** p < 0. 001 versus SH and SH + HBO; #p < 0. 05 versus SNT; * p < 0. 05 versus SH and SH + HBO by one-way ANOVA followed by Tukey′s post-hoc tests
Sciatic nerve transection in the SNT group produced a significant (p < 0.001) decrease in catalase (CAT) activity compared to SH and SH + HBO groups. The CAT activities in the SNT + pre- and SNT + post-HBO groups were significantly (p < 0.05) higher than that in the SNT group, while the differences between SNT + Pre- and SNT + post-HBO groups were not significant (p > 0.05).
Sciatic nerve transection in the SNT group produced a significant (p < 0.05) decrease in superoxide dismutase (SOD) activity compared to SH and SH + HBO groups. The SOD activities in the SNT + Pre- and SNT + Post-HBO groups were significantly (p < 0.05) higher than that in the SNT group, while the differences between SNT + Pre- and SNT + Post-HBO groups were not significant (p > 0.05).
Histopathologic assessment
Histological examination of the nerve-transected animals revealed cellular degeneration in sensory dorsal root ganglion neurons (Fig. 1a) and in spinal cord motoneurones (Fig. 1b). The changes include dissolution of Nissl bodies and displacement of the nucleus to the periphery, chromatolysis. Treatment with HBO in the SNT + Pre- and SNT + Post-HBO groups reduced the changes; so that normal microscopic appearance in some of neural cells was detected in dorsal root ganglion (Fig. 1c) and spinal cord (Fig. 1d). No detectable injury was shown in SH and SH + HBO groups.
Fig. 1.
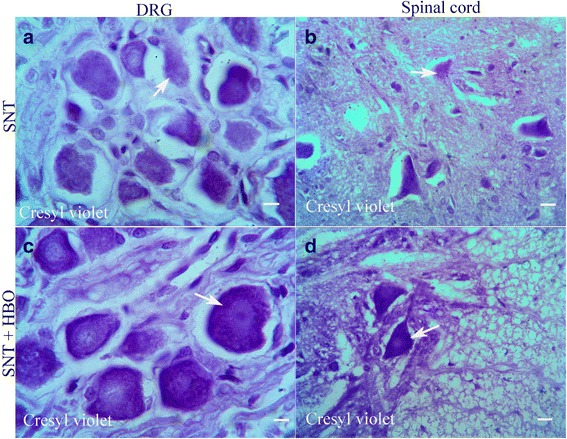
Light Photomicrographs of dorsal root ganglion (a) and spinal cord (b) horizontal section of SNT group show chromatolysis of the dorsal root ganglion neurons and anterior horn neurons of the spinal cord (arrows), while sections of dorsal root ganglion and spinal cord of SNT + HBO treated groups (c and d) show normal microscopic appearance in some of the sensory and motor neurons without chromatolysis (stained with cresyl violet; Scale bar = 100 μm)
Immunohistochemical assessment
Nerve transection in the SNT group increased the expression of caspase-3 in dorsal root ganglion (Fig. 2a) and spinal cord (Fig. 2b) compared to SH and SH + HBO groups. HBO treatment in the SNT + Pre- and SNT + Post-HBO groups reduced the degree of positive staining for caspase-3 in dorsal root ganglion (Fig. 2c) and spinal cord (Fig. 2d) compared to SNT group. Quantitative analysis of caspase-3 positive staining in the experimental groups is shown in Fig. 2e.
Fig. 2.
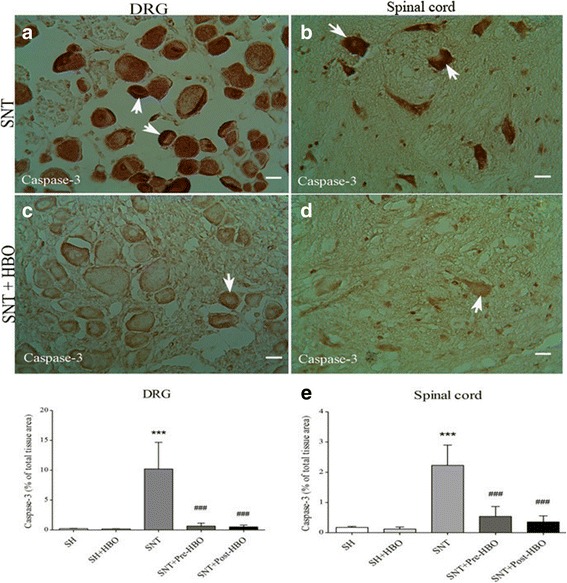
Light Photomicrographs show immunohistochemical staining of caspase-3 in dorsal root ganglion neurons and anterior horn neurons of the spinal cord in SNT (a and b) and SNT + HBO treated (c and d) groups, respectively. The positive staining of caspase-3 is presented by a brown color of cytoplasm (arrows) (Scale bar = 100 μm). The intensity of the immunohistochemical staining in sensory and motor neurons were decreased after HBO treatment. Densitometry analysis of immunohistochemical photomicrographs for caspase-3 was assessed (e). Data are expressed as a percentage of total tissue area. *** P < 0.001 versus SH and SH + HBO groups; ###P < 0.001 versus SNT group by one-way ANOVA followed by Tukey′s post-hoc tests
Nerve transection in the SNT group increased the expression of COX-2 in dorsal root ganglion (Fig. 3a) and spinal cord (Fig. 3b) compared to SH and SH + HBO groups. HBO treatment in the SNT + Pre- and SNT + Post-HBO groups reduced the degree of positive staining for COX-2 in dorsal root ganglion (Fig. 3c) and spinal cord (Fig. 3d) compared to SNT group. Quantitative analysis of COX-2 positive staining in the experimental groups is shown in Fig. 3e.
Fig. 3.
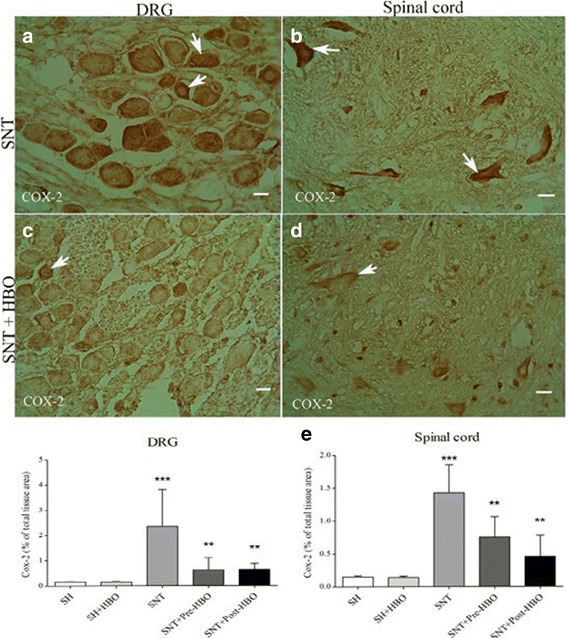
Light Photomicrographs show immunohistochemical staining of COX-2 in dorsal root ganglion neurons and anterior horn neurons of the spinal cord in SNT (a and b) and SNT + HBO treated (c and d) groups, respectively The positive staining of COX-2 is presented by a brown color of cytoplasm (arrows) (Scale bar = 100 μm). The intensity of the immunohistochemical staining in sensory and motor neurons were decreased after HBO treatment. Densitometry analysis of immunohistochemical photomicrographs for COX-2 was assessed (e). Data are expressed as a percentage of total tissue area. *** P < 0.001 versus SH and SH + HBO groups; ** P < 0.01 versus SNT group by one-way ANOVA followed by Tukey′s post-hoc tests
Nerve transection in the SNT group increased the expression of S100ß, a marker of Satellite and Schwann cells, in dorsal root ganglion (Fig. 4a) compared to SH and SH + HBO groups. HBO treatment in the SNT + Pre- and SNT + Post-HBO groups increased the degree of positive staining for S100ß in dorsal root ganglion (Fig. 4b) compared to SNT group. Quantitative analysis of S100ß positive staining in the experimental groups is shown in Fig. 4c.
Fig. 4.
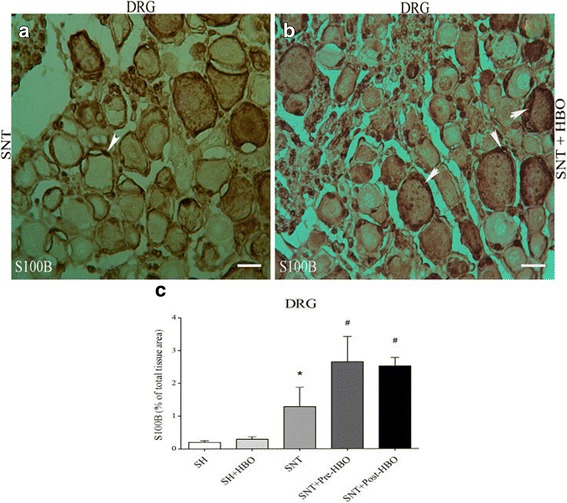
Light Photomicrographs show immunohistochemical staining of S100ß in dorsal root ganglion neurons of SNT (a) and SNT + HBO treated (b) groups, respectively. The positive staining of S100ß is presented by a brown color of cytoplasm (arrows) (Scale bar = 100 μm). The intensity of the immunohistochemical staining in sensory neurons were decreased after HBO treatment. Densitometry analysis of immunohistochemical photomicrographs for S100ß was assessed (c). Data are expressed as a percentage of total tissue area. * P < 0.05 versus SH and SH + HBO groups; #P < 0.05 versus SNT group by one-way ANOVA followed by Tuke’s post-hoc tests
TUNEL assessment
Almost no TUNEL-positive cells could be detected in SH and SH + HBO groups, whereas many cells were intensely stained in the dorsal root ganglion (Fig. 5a) and spinal cord (Fig. 5b) obtained from SNT group. In contrast, a small number of TUNEL-positive cells were detected in dorsal root ganglion (Fig. 5c) and spinal cord (Fig. 5d) obtained from HBO pre- and post-treated rats. Quantitative analysis of TUNEL- positive staining in the experimental groups is shown in Fig. 5e.
Fig. 5.
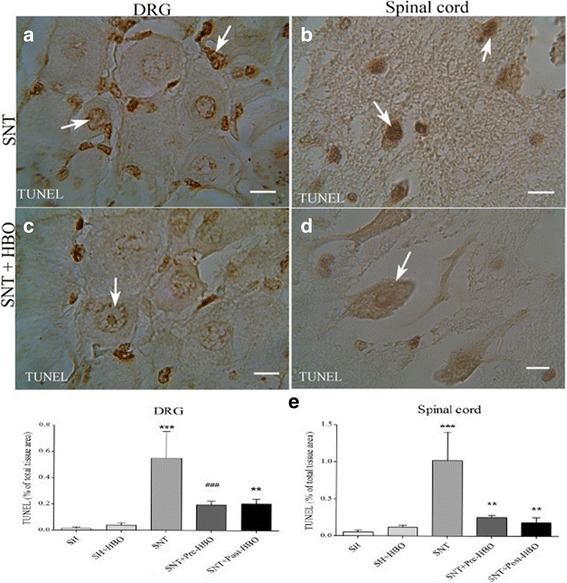
Light Photomicrographs show TUNEL-positive cells in dorsal root ganglion neurons and anterior horn neurons of the spinal cord in SNT (a and b) and SNT + HBO treated (c and d) groups, respectively. The positive staining of TUNEL is presented by a brown color of nucleus (arrows) (Scale bar = 100 μm). The intensity of the TUNEL staining in sensory and motor neurons were decreased after HBO treatment. Densitometry analysis of photomicrographs for TUNEL reaction was assessed (e). Data are expressed as a percentage of total tissue area. ***P < 0.001 versus SH and SH + HBO groups; **P < 0.01 versus SNT group; ###P < 0.001 versus SNT group by one-way ANOVA followed by Tukey’s post-hoc tests
Discussion
The current study indicated that hyperbaric oxygen therapy promotes neuron survival through attenuating apoptosis, inflammation, and lipid peroxidation, and also through improving antioxidant status after sciatic nerve transection.
Our immunohistochemical results showed that sciatic nerve transection considerably increased the expression of caspase-3 in sensory dorsal root ganglion neurons and in spinal cord motoneurones, which plays a critical role in apoptosis. On the contrary, our results showed that these up regulations significantly attenuated after HBO treatment, while the differences between pre- and post-treatment were not significant. To correlate neuronal cell apoptosis, we carried out TUNEL staining method. Retrograde neuronal apoptosis, which occurs in sensory dorsal root ganglion neurons and in spinal cord motoneurones, is one contributing factor of poor sensory recovery and reduced motor function after peripheral nerve transection [3, 36]. On the other hand, the survival of the neurons following injury is of great importance for the outcome of the axonal regeneration and target organ reinnervation. In this regard, it is well known that neuronal cell apoptosis after peripheral nerve transection is mediated through expression of apoptosis-related genes namely Bax, Bcl2, and caspase-3 [37, 38]. Several studies have shown that HBO prevents apoptosis in experimental neurological disorder models. In this regard, it was found that HBO therapy prevented apoptosis through opening of the mitochondrial ATP-sensitive potassium channels [39], decreasing of caspase-3 [40], and decreasing of phosphorelated-p38 mitogen-activated protein kinas [41] in ischemic brain. Also, it was documented that HBO therapy prevented apoptosis through decreasing of hypoxia-inducible factor-1α (HIF-1α) [42], adaptor molecule apoptosis-associated speck-like protein (ASC), and caspase-3 [43, 44] after spinal cord injury. Recently, it was found that hyperbaric oxygen therapy following chronic sciatic nerve constriction injury produced antinociceptive effects through different mechanisms such as downregulation of caspase-3 and inhibition of apoptosis [32].
Our immunohistochemical results showed that sciatic nerve transection considerably increased the expression of COX-2 in dorsal root ganglion and spinal cord. On the contrary, our results showed that these up regulations significantly attenuated after HBO treatment compared to non-treated rats, while the differences between pre- and post-treatment were not significant. Following peripheral nerve injury, various inflammatory mediators were upregulated in spinal cord and dorsal root ganglion [45, 46], which have been implicated in the axonal degerative process after injury. One of the inflammatory mediators that play an important role during these processes is cyclooxygenase-2 (COX-2) [47]. Several lines of evidence have shown that HBO treatment exerts neuroprotective effects via mechanisms such as inhibition of inflammation. In this regard, it was found previously that the antinociceptive effect of HBO treatment is associated somewhat with anti-inflammatory properties in a rat model of neuropathic pain [48]. HBO treatment decreased NF-kB, IL-1ß, and TNF-α levels after spinal cord injury [12]. Another study reported that HBO reduced COX-2 level in ischemic cerebral tissue [49].
In the present study, HBO treatment decreased malondialdehyde (MDA) levels as an index of lipid peroxidation in spinal cord of pre-treatment group compared to non-treated rats, while the difference in post-treatment group was not significant. Therefore, HBO pre-treatment was more effective than HBO post-treatment against lipid peroxidation. Also, HBO treatment increased catalase and superoxide dismutase activities as endogenous antioxidants in spinal cord of pre- and post-treatment groups compared to non-treated rats, while the differences between pre- and post-treatment were not significant. Free radical-induced lipid peroxidation is the primary pathway of peripheral nerve injury [50], which is elevated in the spinal cord after sciatic nerve transection [51]. Evidences also affirm that pheripheral nerve transection leads to pro-oxidative status in the spinal cord due to a decrease in antioxidant enzyme activities [52]. Studies revealed the alternation of enzymatic antioxidant activity after HBO exposure. Li et al. [21] reported that HBO induced tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzyme activity of catalase and superoxide dismutase. Also, it was found that HBO enhanced superoxide dismutase activity and reduced tissue damage after hypoxia-ischemia brain damage in neonatal rats [53].
Our immunohistochemical results showed that sciatic nerve transection increased the expression of S100ß in the dorsal root ganglion. Paradoxically, we found that HBO applied before or after SNT further increased S100ß expression significantly compared to SNT alone. Therefore, since it is well known that S100ß acts as an inhibitor of apoptosis and a stimulator of cell proliferation in neurodegenerative process [54], the increase in S100ß induced by SNT alone could be viewed as a compensatory mechanism of the body to deal with apoptosis after SNT (the present study) and other neural injuries [55, 56]. In this regard, previous studies have shown that S100ß expression upregulated in dorsal root ganglion after sciatic nerve transection [55]; so that treatment with S100ß decreased neural death after sciatic nerve transection [56]. Zhang et al. [57] found that HBO therapy after traumatic brain injury reduced neuronal loss and increased expression of S100 astrocyte marker in brain tissue.
Conclusions
The biochemical, histopathological, and immunohistochemical evidences demonstrated that pre- and post-HBO therapy had neuroprotective effects against sciatic nerve transection-induced degeneration. On the other hand, the results propose that HBO treatment is effective in protection of sensory and motor neurons against retrograde apoptosis and enhance neuronal survival time after peripheral nerve transection.
Acknowledgements
Not applicable.
Funding
This work was solely financed by Mazandaran University of Medical Sciences. No external funding was provided.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CAT
Catalse
- COX-2
Cyclooxigenase-2
- HBO
Hyperbaric oxygen
- MDA
Malodialdehyde
- S100ß
S100beta
- SH
Sham-operated
- SNT
Sciatic nerve transaction
- SOD
Superoxide dismutase
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Authors’ contributions
ZS carried out the animal experiments, histology, and immunohistochemistry analysis, interpreted results, and revised the manuscript for content. ARK designed and coordinated the study, acquired data, performed statistical analyses, interpreted results, and drafted the manuscript. HA performed biochemical analysis, interpreted results and revised the manuscript for content. ZZ interpreted results and revised the manuscript for content. KK interpreted results and revised the manuscript for content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Experimental procedures and protocols used in this study were approved by ethical committee of Health Sciences, Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1396.2978).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zahra Shams, Email: Zahrashams00@gmail.com.
Ali Reza khalatbary, Email: khalat90@yahoo.com.
Hassan Ahmadvand, Email: Hassan_a46@yahoo.com.
Zohreh Zare, Email: Zare1980@gmail.com.
Kosar Kian, Email: Kian48721@gmail.com.
References
- 1.Martin LJ, Kaiser A, Price AC. Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J Neurobiol. 1999;40:185–201. doi: 10.1002/(SICI)1097-4695(199908)40:2<185::AID-NEU5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Groves MJ, Christopherson T, Giometto B, et al. Axotomy-induced apoptosis in adult rat primary sensory neurons. J Neurocytol. 1997;26:615–624. doi: 10.1023/A:1018541726460. [DOI] [PubMed] [Google Scholar]
- 3.Tandrup T, Woolf CJ, Coggeshall RE. Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve. J Comp Neurol. 2000;422:172–180. doi: 10.1002/(SICI)1096-9861(20000626)422:2<172::AID-CNE2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Deumensm R, Bozkurt A, Meek MF, et al. Repairing injured peripheral nerves: bridging the gap. Prog Neurobiol. 2010;92:245–276. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Martin LJ. Motor neurons rapidly accumulate DNA single-strand breaks after in vitro exposure to nitric oxide and peroxynitrite and in vivo axotomy. J Comp Neurol. 2001;432:35–60. doi: 10.1002/cne.1087. [DOI] [PubMed] [Google Scholar]
- 7.Li HY, Ruan YW, Ren CR, et al. Mechanisms of secondary degeneration after partial optic nerve transection. Neural Regen Res. 2014;9:565–574. doi: 10.4103/1673-5374.130093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiarotto GB, Drummond L, Cavarretto G, et al. Neuroprotective effect of tempol (4 hydroxy-tempo) on neuronal death induced by sciatic nerve transection in neonatal rats. Brain Res Bull. 2014;106:1–8. doi: 10.1016/j.brainresbull.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Chen YS, Tseng FY, Tan CT, et al. Effects of methylprednisolone on nitric oxide formation and survival of facial motor neurons after axotomy. Brain Res. 2008;1197:23–31. doi: 10.1016/j.brainres.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira AL, Risling M, Negro A, et al. Apoptosis of spinal interneurons induced by sciatic nerve axotomy in the neonatal rat is counteracted by nerve growth factor and ciliary neurotrophic factor. J Comp Neurol. 2002;447:381–393. doi: 10.1002/cne.10248. [DOI] [PubMed] [Google Scholar]
- 11.Zhou BC, Liu LJ, Liu B. Neuroprotection of hyperbaric oxygen therapy in sub-acute traumatic brain injury: not by immediately improving cerebral oxygen saturation and oxygen partial pressure. Neural Regen Res. 2016;11:1445–1449. doi: 10.4103/1673-5374.175054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H, Xue L, Zhang X, et al. Hyperbaric oxygen therapy provides neuroprotection following spinal cord injury in a rat model. Int J Clin Exp Pathol. 2013;6:1337–1342. [PMC free article] [PubMed] [Google Scholar]
- 13.Chazalviel L, Blatteau JE, Vallée N, et al. Effects of normobaric versus hyperbaric oxygen on cell injury induced by oxygen and glucose deprivation in acute brain slices. Med Gas Res. 2016;6:169–173. doi: 10.4103/2045-9912.191364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Huang G, Zhang K, et al. Nrf2 activation in astrocytes contributes to spinal cord ischemic tolerance induced by hyperbaric oxygen preconditioning. J Neurotrauma. 2014;31:1343–1353. doi: 10.1089/neu.2013.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Li Y, Chen W, et al. Protective effect of hyperbaric oxygen on cognitive impairment induced by D-Galactose in mice. Neurochem Res. 2016;41:3032–3041. doi: 10.1007/s11064-016-2022-x. [DOI] [PubMed] [Google Scholar]
- 16.Pan X, Chen C, Huang J, et al. Neuroprotective effect of combined therapy with hyperbaric oxygen and madopar on 6-hydroxydopamine-induced Parkinson's disease in rats. Neurosci Lett. 2015;600:220–225. doi: 10.1016/j.neulet.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Li J, Zhang L, et al. Preconditioning with hyperbaric oxygen induces tolerance against oxidative injury via increased expression of heme oxygenase-1 in primary cultured spinal cord neurons. Life Sci. 2007;80:1087–1093. doi: 10.1016/j.lfs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Han G, Li L, Meng LX. Effects of hyperbaric oxygen on pain-related behaviors and nitric oxide synthase in a rat model of neuropathic pain. Pain Res Manag. 2013;18:137–141. doi: 10.1155/2013/147043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazario J, Kuffler DP. Hyperbaric oxygen therapy and promoting neurological recovery following nerve trauma. Undersea Hyperb Med. 2011;38:345–366. [PubMed] [Google Scholar]
- 20.Oroglu B, Turker T, Aktas S, et al. Effect of hyperbaric oxygen therapy on tense repair of the peripheral nerves. Undersea Hyperb Med. 2011;38:367–373. [PubMed] [Google Scholar]
- 21.Li J, Liu W, Ding S, et al. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–229. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Yang ZJ, Xie Y, Bosco GM, et al. Hyperbaric oxygenation alleviates MCAO-induced brain injury and reduces hydroxyl radical formation and glutamate release. Eur J Appl Physiol. 2010;108:513–522. doi: 10.1007/s00421-009-1229-9. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Tang J, Chen Q, et al. Hyperbaric oxygen preconditioning attenuates neuroinflammation after intracerebral hemorrhage in rats by regulating microglia characteristics. Brain Res. 2015;1627:21–30. doi: 10.1016/j.brainres.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Lin KC, Niu KC, Tsai KJ, et al. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J Trauma Acute Care Surg. 2012;72:650–659. doi: 10.1097/TA.0b013e31823c575f. [DOI] [PubMed] [Google Scholar]
- 25.Wee HY, Lim SW, Chio CC, et al. Hyperbaric oxygen effects on neuronal apoptosis associations in a traumatic brain injury rat model. J Surg Res. 2015;197:382–389. doi: 10.1016/j.jss.2015.04.052. [DOI] [PubMed] [Google Scholar]
- 26.Lu PG, Feng H, Yuan SJ, et al. Effect of preconditioning with hyperbaric oxygen on neural cell apoptosis after spinal cord injury in rats. J Neurosurg Sci. 2013;57:253–258. [PubMed] [Google Scholar]
- 27.Sunami K, Takeda Y, Hashimoto M, et al. Hyperbaric oxygen reduces infarct volume in rats by increasing oxygen supply to the ischemic periphery. Crit Care Med. 2000;28:2831–2836. doi: 10.1097/00003246-200008000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Calvert JW, Cahill J, Zhang JH. Hyperbaric oxygen and cerebral physiology. Neurol Res. 2007;29:132–141. doi: 10.1179/016164107X174156. [DOI] [PubMed] [Google Scholar]
- 29.Chazalviel L, Haelewyn B, Degoulet M, et al. Hyperbaric oxygen increases tissue-plasminogen activator-induced thrombolysis in vitro, and reduces ischemic brain damage and edema in rats subjected to thromboembolic brain ischemia. Med Gas Res. 2016;6:64–69. doi: 10.4103/2045-9912.184713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigneswara V, Berry M, Logan A, et al. Caspase-2 is upregulated after sciatic nerve transection and its inhibition protects dorsal root ganglion neurons from apoptosis after serum withdrawal. PLoS One. 2013;8:e57861. doi: 10.1371/journal.pone.0057861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan HC, Chin CS, Yang DY, et al. Human amniotic fluid mesenchymal stem cells in combination with hyperbaric oxygen augment peripheral nerve regeneration. Neurochem Res. 2009;34:1304–1316. doi: 10.1007/s11064-008-9910-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhao BS, Song XR, Hu PY, et al. Hyperbaric oxygen treatment at various stages following chronic constriction injury produces different antinociceptive effects via regulation of P2X4R expression and apoptosis. PLoS One. 2015;10:e0120122. doi: 10.1371/journal.pone.0120122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mihara S, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 34.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 35.Kuthan H, Haussmann HJ, Werringloer JA. Spectrophotometric assay for superoxide dismutase activities in crude tissue fractions. Biochem J. 1986;237:175–180. doi: 10.1042/bj2370175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira AL, Risling M, Deckner M, et al. Neonatal sciatic nerve transection induces TUNEL labeling of neurons in the rat spinal cord and DRG. Neuroreport. 1997;8:2837–2840. doi: 10.1097/00001756-199709080-00006. [DOI] [PubMed] [Google Scholar]
- 37.Gillardon F, Wickert H, Zimmermann M. Differential expression of bcl-2 and bax mRNA in axotomized dorsal root ganglia of young and adult rats. Eur J Neurosci. 1994;6:1641–1644. doi: 10.1111/j.1460-9568.1994.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 38.Saito H, Kanje M, Dahlin LB. Delayed nerve repair increases number of caspase 3 stained Schwann cells. Neurosci Lett. 2009;456:30–33. doi: 10.1016/j.neulet.2009.03.075. [DOI] [PubMed] [Google Scholar]
- 39.Lou M, Chen Y, Ding M, et al. Involvement of the mitochondrial ATP-sensitive potassium channel in the neuroprotective effect of hyperbaric oxygenation after cerebral ischemia. Brain Res Bull. 2006;69:109–116. doi: 10.1016/j.brainresbull.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Li JS, Zhang W, Kang ZM, et al. Hyperbaric oxygen preconditioning reduces ischemia-reperfusion injury by inhibition of apoptosis via mitochondrial pathway in rat brain. Neuroscience. 2009;159:1309–1315. doi: 10.1016/j.neuroscience.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Ostrowski RP, Graupner G, Titova E, et al. The hyperbaric oxygen preconditioning-induced brain protection is mediated by a reduction of early apoptosis after transient global cerebral ischemia. Neurobiol Dis. 2008;29:1–13. doi: 10.1016/j.nbd.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y, Liu XH, Qu SD, et al. Hyperbaric oxygen intervention on expression of hypoxia-inducible factor-1α and vascular endothelial growth factor in spinal cord injury models in rats. Chin Med J. 2013;126:3897–3903. [PubMed] [Google Scholar]
- 43.Long Y, Liang F, Gao C, et al. Hyperbaric oxygen therapy reduces apoptosis after spinal cord injury in rats. Int J Clin Exp Med. 2014;7:4073–4081. [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, Yang J, Li Z, et al. Hyperbaric oxygen treatment protects against spinal cord injury by inhibiting endoplasmic reticulum stress in rats. Spine. 2015;40:E1276–E1283. doi: 10.1097/BRS.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 45.Parente L, Perretti M. Advances in the pathophysiology of constitutive and inducible cyclooxygenases: two enzymes in the spotlight. Biochem Pharmacol. 2003;65:153–159. doi: 10.1016/S0006-2952(02)01422-3. [DOI] [PubMed] [Google Scholar]
- 46.Schäfers M, Svensson CI, Sommer C, et al. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broom DC, Samad TA, Kohno T, et al. Cyclooxygenase 2 expression in the spared nerve injury model of neuropathic pain. Neuroscience. 2004;124:891–900. doi: 10.1016/j.neuroscience.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Zhao BS, Meng LX, Ding YY, et al. Hyperbaric oxygen treatment produces an antinociceptive response phase and inhibits astrocyte activation and inflammatory response in a rat model of neuropathic pain. J Mol Neurosci. 2014;53:251–261. doi: 10.1007/s12031-013-0213-3. [DOI] [PubMed] [Google Scholar]
- 49.Cheng O, Ostrowski RP, Wu B, et al. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42:484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001;31:430–439. doi: 10.1016/S0891-5849(01)00597-4. [DOI] [PubMed] [Google Scholar]
- 51.Scheid T, Bosco LD, Guedes RP, et al. Sciatic nerve transection modulates oxidative parameters in spinal and supraspinal regions. Neurochem Res. 2013;38:935–942. doi: 10.1007/s11064-013-1000-9. [DOI] [PubMed] [Google Scholar]
- 52.Guedes RP, Bosco LD, Teixeira CM, et al. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res. 2006;31:603–609. doi: 10.1007/s11064-006-9058-2. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Chen YH, Lv HY, et al. Effect of hyperbaric oxygen on lipid peroxidation and visual development in neonatal rats with hypoxia-ischemia brain damage. Biomed Rep. 2016;5:136–140. doi: 10.3892/br.2016.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donato R, Sorci G, Riuzzi F, et al. S100B's double life: intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793:1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Huang YL, Ding M, Hansson HA. Dorsal root ganglion nerve cells transiently express increased immunoreactivity of the calcium-binding protein S-100beta after sciatic nerve transection. Brain Res. 1998;785:351–354. doi: 10.1016/S0006-8993(97)01425-X. [DOI] [PubMed] [Google Scholar]
- 56.Iwasaki Y, Shiojima T, Kinoshita M. S100 beta prevents the death of motor neurons in newborn rats after sciatic nerve section. J Neurol Sci. 1997;151:7–12. doi: 10.1016/S0022-510X(97)00073-7. [DOI] [PubMed] [Google Scholar]
- 57.Zhang XG, Jiang ZL, Wang GH, et al. Therapeutic efficacy of hyperbaric oxygen on traumatic brain injury in the rat and the underlying mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2012;28:42–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


