Abstract
OBJECTIVE
The objective of our study was to determine the clinical and MRI characteristics of clinically significant prostate cancer (PCA) (Gleason score ≥ 3 + 4) in men with Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) category 3 transition zone (TZ) lesions.
MATERIALS AND METHODS
From 2014 to 2016, 865 men underwent prostate MRI and MRI/ultrasound (US) fusion biopsy (FB). A subset of 90 FB-naïve men with 96 PI-RADSv2 category 3 TZ lesions was identified. Patients were imaged at 3 T using a body coil. Images were assigned a PI-RADSv2 category by an experienced radiologist. Using clinical data and imaging features, we performed univariate and multivariate analyses to identify predictors of clinically significant PCA.
RESULTS
The mean patient age was 66 years, and the mean prostate-specific antigen density (PSAD) was 0.13 ng/mL2. PCA was detected in 34 of 96 (35%) lesions, 14 of which (15%) harbored clinically significant PCA. In univariate analysis, DWI score, prostate volume, and PSAD were significant predictors (p < 0.05) of clinically significant PCA with a suggested significance for apparent diffusion coefficient (ADC) and prostate-specific antigen value (p < 0.10). On multivariate analysis, PSAD and lesion ADC were the most important covariates. The combination of both PSAD of 0.15 ng/mL2 or greater and an ADC value of less than 1000 mm2/s yielded an AUC of 0.91 for clinically significant PCA (p < 0.001). If FB had been restricted to these criteria, only 10 of 90 men would have undergone biopsy, resulting in diagnosis of clinically significant PCA in 60% with eight men (9%) misdiagnosed (false-negative).
CONCLUSION
The yield of FB in men with PI-RADSv2 category 3 TZ lesions for clinically significant PCA is 15% but significantly improves to 60% (AUC > 0.9) among men with PSAD of 0.15 ng/mL2 or greater and lesion ADC value of less than 1000 mm2/s.
Keywords: MRI, prostate, Prostate Imaging and Reporting System (PI-RADS), risk stratification, transition zone
The Prostate Imaging Reporting and Data System version 1 (PI-RADSv1) was released in 2012 [1], with the goal of standard-and reporting. Because of the rapid evolution of the field and some limitations of the initial iteration, PI-RADS version 2 (PI-RADSv2) was developed and released in December 2015 [2]. Only a few studies have attempted to validate PI-RADSv2 since its release [3–7]. Unlike BI-RADS on which PI-RADS was modeled, management recommendations are not included as part of PI-RADSv2.
Although it is generally accepted that MRI-targeted biopsy should be performed in lesions with an overall PI-RADSv2 category 4 or 5 [2, 8], the decision whether to pursue biopsy of a PI-RADSv2 category 3 lesion is not as clear given the lower yield for clinically significant prostate cancer (PCA) among these intermediate-risk lesions.
The objective of this study was to determine the clinical and MRI characteristics of clinically significant PCA (Gleason score ≥ 3 + 4) in PI-RADSv2 category 3 transition zone (TZ) lesions among men undergoing MRI/ultrasound (US) fusion biopsy (FB).
Materials and Methods
Study Population
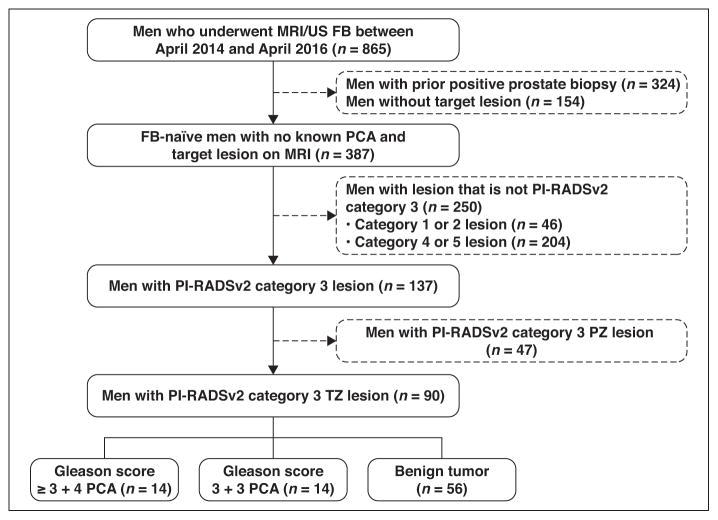
Between April 2014 and April 2016, a total of 3100 multiparametric MRI examinations were performed, yielding a total of 355 PI-RADSv2 category 3 TZ lesions. This institutional review board–approved HIPAA-compliant retrospective review concerns a subset of 865 consecutive men drawn from this larger experience who underwent multiparametric MRI followed by MRI/US FB (Artemis, Eigen), from which 90 men with 96 PI-RADSv2 category 3 TZ lesions were identified (Fig. 1) and analyzed. None of the subjects had undergone prior FB. Patients had serum prostate-specific antigen (PSA) measurement at the time of FB
Fig. 1.
Flowchart shows how final cohort of 90 men was derived along with biopsy outcomes in these men.
FB = fusion biopsy, PI-RADSv2 = Prostate Imaging Reporting and Data System version 2, PZ = peripheral zone, TZ = transition zone, PCA = prostate cancer, US = ultrasound.
MRI Technique
Multiparametric MRI examinations were performed on a 3-T platform (Magnetom, Trio, Verio, Skyra, or Prisma; Siemens Healthcare) with a pelvic phased-array coil. The complete MRI protocol has been published previously [9]. Briefly, the protocol included multiplanar T2-weighted imaging, DWI, and dynamic contrast-enhanced MRI (DCE-MRI) and was adherent with PI-RADSv2 technical recommendation criteria.
Image Analysis
Image analysis was performed by one of three fellowship-trained genitourinary radiologists, each of whom has interpreted more than 1000 prostate MRI examinations. Studies were read using software (DynaCAD for Prostate, version 3.0, Invivo). For studies performed after December 2015 (n = 12), lesions were prospectively assigned a PI-RADSv2 category. For studies performed before PI-RADSv2 was published (n = 78), images were retrospectively reviewed and assigned a PI-RADSv2 category by a fourth fellowship-trained genitourinary radiologist who has interpreted more than 500 prostate MRI examinations and was blinded to pathology and clinical data at the time of image analysis.
All quantitative imaging data were prospectively collected as follows: For determination of lesion apparent diffusion coefficient (ADC), an ROI was drawn on a single axial slice on the ADC map on which the tumor appeared the largest and most conspicuous [10]. The ROI encompassed the entirety of the lesion on the chosen slice. For determination of lesion perfusion metrics, a similar method was used, wherein an ROI was drawn on the single axial slice of the forward volume transfer constant (Ktrans) map on which the lesion appeared largest and most conspicuous. Prostate volume was calculated using manual contouring of multiparametric MRI examinations.
MRI/Ultrasound Fusion Biopsy
The reference standard in all cases was FB, which included systematic biopsy of 12 sites and additional targeted biopsy of each suspicious lesion identified on MRI, as described previously [11]. FB was performed by a single board-certified urologist with experience of approximately 1000 FBs at the onset of the study period [12]. One core of tissue was obtained approximately every 3 mm along the longest diameter of each target. A minimum of two core biopsy specimens was obtained per target. The median time between multiparametric MRI and FB was 28 days (range, 1–258 days). Biopsy specimens were interpreted by a board-certified genitourinary pathologist who specializes in PCA pathology.
Study Design
All lesions, by definition of the inclusion criteria, were PI-RADSv2 category 3 TZ lesions on T2-weighted imaging. Each PI-RADSv2 lesion was analyzed for the following features: location (apex, mid gland, base), DWI PI-RADSv2 score, ADC (calculated using b = 0 s/mm2), lesion size (longest diameter, as measured on axial T2-weighted imaging), DCE-MRI PI-RADSv2 assessment (positive or negative), and the following quantitative perfusion metrics: Ktrans, reverse reflux rate constant (kep), and initial area under the gadolinium concentration–time curve. The values obtained from the functional maps (ADC, Ktrans, kep, initial area under the gadolinium concentration–time curve) were measured as the mean of the lesion. Clinical features assessed included patient age, PSA value, prostate volume, and prostate-specific antigen density (PSAD). Clinically significant PCA was defined as a Gleason score of 3 + 4 or higher.
Statistical Analysis
Patient demographics and clinical data are reported using descriptive statistics. The chi-square and Spearman rank tests were used to evaluate the association between clinical and imaging features and increasing Gleason scores. Univariate logistic regression was used to evaluate the association between clinical and imaging features and the presence of clinically significant PCA, with p < 0.05 considered statistically significant. Multivariate logistic regression was then used to develop a predictive model using pertinent clinical and imaging covariates. Multiple models were tested with the efficacy of each logistic model estimated using the area under the ROC curve (AUC). The highest performing model is presented herein. Analysis of FB specimens was limited to the targeted biopsy cores, because the purpose was to determine biopsy outcomes of MRI-detected lesions. Statistical analysis was performed using statistical software (Stata, version 11, StataCorp).
Results
Patient Demographics and Clinical Features
A total of 865 men underwent FB between April 2014 and April 2016. From this group, a cohort of 90 men with 96 PI-RADSv2 category 3 TZ lesions was selected. Patient age, PSA value, prostate volume, and PSAD are presented in Table 1. Twenty of the 90 (22%) men had undergone at least one prior negative systematic prostate biopsy.
TABLE 1.
Patient Demographic Characteristics and Biopsy Results
| Characteristic or Result | Value |
|---|---|
|
| |
| Age (y) | |
| Mean | 66 |
| Range | 52–82 |
| PSA (ng/mL) | |
| Median | 5.55 |
| IQR | 4.3–7.9 |
| Prostate volume (cm3) | |
| Mean (SD) | 54.0 (22.8) |
| PSAD (ng/mL2) | |
| Mean (SD) | 0.13 (0.08) |
| Positive targeted biopsy results, no. (%)a | 34 (35) |
| Gleason score 3 + 3b | 20 (59) |
| Gleason score 3 + 4b | 10 (29) |
| Gleason score 3 + 5b | 1 (3) |
| Gleason score 4 + 3b | 3 (9) |
Note—PSA = prostate-specific antigen, IQR = interquartile range, PSAD = prostate-specific antigen density.
Denominator is total number of lesions (n = 96).
Denominator is total number of positive targeted biopsies (n = 34).
Biopsy Results
The mean number of cores per target was 3.7. PCA was detected in 34 of 96 lesions (35%), 14 (15%) of which represented clinically significant PCA based on targeted biopsy. In six of 90 (7%) men, the systematic biopsy results yielded a higher Gleason score than the targeted biopsy results. In all cases, the positive systematic cores were ipsilateral to the target, most often adjacent to it. Two of 90 men (2%) had clinically significant PCA detected only in systematic cores (Fig. 2).
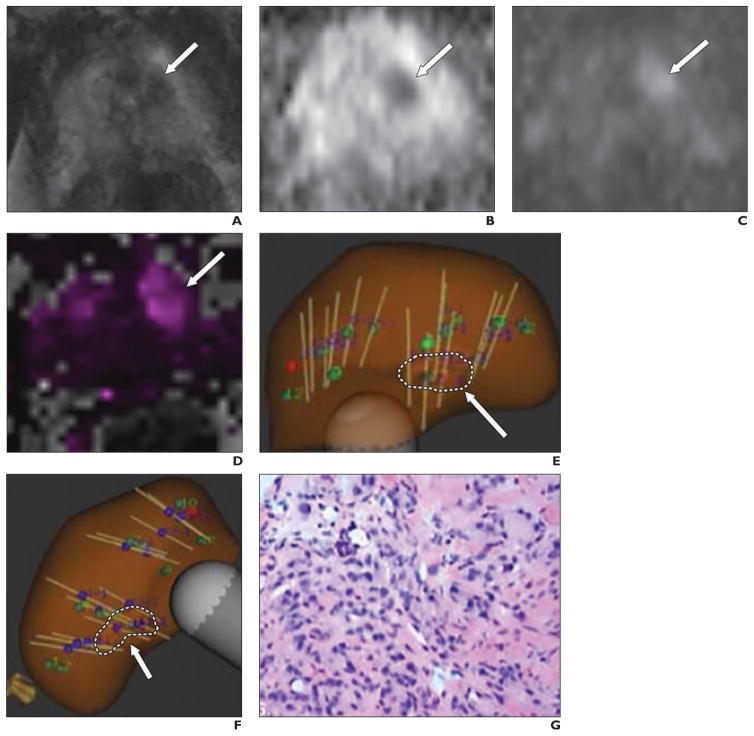
Fig. 2.
65-year-old man with prostate-specific antigen density of 0.15 ng/mL2 and 9-mm lesion in left anterior transition zone assessed as Prostate Imaging Reporting and Data System version 2 category 3.
A–D, Axial T2-weighted image (A), apparent diffusion coefficient (ADC) map (B), DW image (b = 1400 s/mm2) (C), and forward volume transfer constant (Ktrans) map (D) show lesion (arrows). Lesion ADC was 919 mm2/s.
E and F, Transverse (E) and longitudinal (F) images from MRI/ultrasound fusion biopsy show multiple biopsy cores traversing target (arrows, dashed lines).
G, Photomicrograph of pathologic specimen obtained at biopsy. Gleason score was 4 + 3.
Univariate Analysis
On univariate analysis, PSAD, prostate volume, and DWI score were significantly related to increasing Gleason score (p < 0.05), with PSA and ADC also suggestive of an association (p < 0.10), as shown in Table 2. The variables DWI score (p = 0.048), PSA (p = 0.030), prostate volume (p = 0.013), PSAD (p = 0.001), and ADC of less than 1000 mm2/s (p = 0.047) were significantly related to the presence of clinically significant PCA.
TABLE 2.
Clinical and Imaging Features Evaluated
| Univariate Analysis Variable | Clinically Significant Prostate Cancer (Gleason Score ≥ 3 + 4) | Prostate Cancer (Gleason Score = 6) | Benign | p |
|---|---|---|---|---|
|
| ||||
| Clinical features | ||||
| No. of lesions | 14 | 20 | 62 | |
| Age (y) | 0.649a | |||
| Mean (SD) | 65 (4.2) | 64 (8.7) | 64 (7.5) | |
| PSA (ng/mL) | 0.060a | |||
| Median (IQR) | 7.25 (5.3–10.9) | 5.5 (4.2–7.9) | 5.5 (3.8–7.5) | |
| Prostate volume (cm3) | 0.047a | |||
| Mean (SD) | 41 (11.4) | 63 (31.4) | 54 (22.9) | |
| PSAD (ng/mL2) | 0.002a | |||
| Mean (SD) | 0.29 (0.28) | 0.10 (0.05) | 0.11 (0.06) | |
| Imaging features | ||||
| Lesion diameter (mm) | 0.550a | |||
| Mean (SD) | 12.28 (4.1) | 12.15 (5.2) | 11.47 (4.1) | |
| DWI PI-RADSv2 score, no. (%) of lesionsb | 0.023a | |||
| 2 | 0 | 1 (5.0) | 1 (1.6) | |
| 3 | 6 (42.9) | 11 (55.0) | 45 (72.6) | |
| 4 | 8 (57.1) | 8 (40.0) | 15 (24.2) | |
| ADC (mm2/s) | ||||
| Mean (SD) | 982.4 (144) | 1017.5 (149) | 1058.2 (98) | 0.076a |
| DCE-MRI assessment, no. (%) of lesionsc | 0.377a | |||
| Positive | 9 (64.3) | 13 (65.0) | 45 (72.6) | |
| Negative | 5 (35.7) | 6 (30.0) | 15 (24.2) | |
| Mean Ktrans (SD) | 0.53 (0.42) | 0.37 (0.25) | 0.49 (0.32) | 0.394a |
| Mean kep (SD) | 1.77 (1.34) | 1.48 (0.90) | 1.71 (0.91) | 0.403a |
| Mean initial area under the gadolinium concentration–time curve (SD) | 9.54 (9.55) | 7.00 (4.3) | 9.01 (4.69) | 0.397a |
| Lesion location, no. (%) of lesions | 0.581d | |||
| Apex | 5 (35.7) | 5 (25.0) | 17 (27.4) | |
| Mid gland | 6 (42.9) | 13 (65.0) | 30 (48.4) | |
| Base | 3 (21.4) | 2 (10.0) | 15 (24.2) | |
Note—PSA = prostate-specific antigen, IQR = interquartile range, PSAD = prostate-specific antigen density, PI-RADSv2 = Prostate Imaging Reporting and Data System version 2, ADC = apparent diffusion coefficient, DCE-MRI = dynamic contrast-enhanced MRI.
Spearman rank test.
Denominator is 95 lesions.
Denominator is 93 lesions.
Chi-square test.
Multivariate Analysis
On multivariate analysis, PSAD and ADC were the most important covariates. The combination of both PSAD of 0.15 ng/mL2 or greater and lesion ADC of less than 1000 mm2/s yielded an AUC of 0.91 for correctly classifying men with clinically PCA (p < 0.001). If biopsy had been restricted to these criteria, only 10 of 90 men (11%) would have undergone FB, resulting in a clinically significant PCA detection rate of 60% with only eight of 90 (9%) false-negatives. Among the eight men who would have been misclassified, six had Gleason 3 + 4 tumors and two had Gleason 4 + 3 tumors. PSAD among the eight men who would have been misclassified (false-negatives) ranged from 0.07 to 1.19 ng/mL2 (median, 0.17 ng/mL2; interquartile range [IQR], 0.15–0.34 ng/mL2), and lesion ADC in these eight men ranged from 919 to 1206 mm2/s (median, 1110 mm2/s; IQR, 1015–1129 mm2/s).
Discussion
Data regarding the risk of clinically significant PCA associated with each PI-RADSv2 overall category remain limited. In a multireader reproducibility study of PI-RADSv2, Rosenkrantz et al. [6] reported an overall PCA detection rate of 15.4% among lesions with overall PI-RADSv2 category of less than 4, none of which was clinically significant PCA. Tan et al. [3] reported an overall PCA detection rate of 19.4% among PI-RADSv2 category 3 lesions, less than 10% of which constituted clinically significant PCA. These results reaffirm what other authors had previously reported for PI-RADSv1 in terms of biopsy yield for lesions with a category of 3. For example, Liddell et al. [8] evaluated 92 PI-RADSv1 category 3 lesions and reported an overall PCA detection rate of 7% and clinically significant PCA detection rate of 2%.
Given the low likelihood of clinically significant PCA in PI-RADSv2 category 3 lesions, especially lesions in the TZ, where interreader agreement and positive predictive value are lower [6, 13], we sought to identify ancillary clinical and imaging features that might improve risk stratification in this subset of patients. We observed that the combination of both PSAD of 0.15 ng/mL2 or greater and lesion ADC of less than 1000 mm2/s significantly increased the likelihood of clinically significant PCA in our cohort.
The PSAD threshold of 0.15 ng/mL2 was established by Epstein et al. [14] in 1994 and has been widely validated. Not surprisingly, in our cohort, men with a PSAD of 0.15 ng/mL2 or greater had a significantly higher likelihood of clinically significant PCA on FB.
The lesion ADC was an important predictor of clinically significant PCA on both univariate and multivariate analyses, in keeping with multiple prior studies that have documented an inverse relationship between lesion ADC and tumor grade [15–18]. Moreover, although PI-RADSv2 does not explicitly endorse a quantitative ADC assessment in the TZ, it does allow upgrading of a lesion with a category of 3 based on the presence of markedly restricted diffusion (coupled with size > 1.5 cm or invasive behavior). Our results suggest that restricted diffusion within a category 3 TZ lesion increases its likelihood of harboring clinically significant PCA irrespective of lesion size, given that the mean lesion size among category 3 lesions with clinically significant PCA was 12 mm in our cohort. Additionally, lesion diameter was not a significant predictor of clinically significant PCA on univariate analysis (p= 0.55).
DCE-MRI did not significantly contribute to the prediction of clinically significant PCA. Multiple prior studies have shown that there is little to no incremental benefit from the addition of DCE-MRI to T2-weighted imaging and DWI in the TZ [19, 20]. This is likely because benign prostatic hyperplasia nodules are markedly hypervascular and may show rapid enhancement and early washout, overlapping with the appearance of PCA.
Although systematic biopsy was performed concurrently with targeted biopsy in all patients, analysis was limited to the targeted biopsy results, because the primary aim of this work was to determine which MRI features predict clinically significant PCA. Although systematic biopsy yielded a higher Gleason score in a minority of patients (7%), the location of positive cores was ipsilateral to the MRI lesion in all cases, most often adjacent to it, suggesting that these cores, although systematic, may have reflected sampling of the MRI abnormality as well.
Our study has a few limitations. This study was a retrospective analysis of a relatively small group of patients, and image interpretation and tissue acquisition were performed by experts, so the results may not be generalizable to groups with less experience. Given that PI-RADSv2 categories were necessarily retrospectively assigned in most of the cohort, there is potential sampling bias; however, this bias is likely minimal given that the scoring system in place before the inception of PI-RADSv2 was very similar. All of the studies were performed at a single institution, so the ADC threshold reported herein, which included a b value of 0 s/mm2 in its calculation, may not be directly applicable to different scanners and platforms or calculations that do not include b values below 100 s/mm2. The clinically significant PCA detection rate of 15% among PI-RADSv2 category 3 TZ lesions in our cohort is slightly higher than that reported in previous publications [2, 3, 6] and could reflect enrichment of TZ lesions, given that they are more often occult at systematic biopsy and that nearly a quarter of our population had undergone at least one prior negative systematic biopsy. Last, although suggested by PI-RADSv2, the definition of clinically significant PCA that we used in this study (Gleason score ≥ 3 + 4) may not be universally agreed on because it does not account for tumor volume and is based on targeted biopsy rather than radical prostatectomy.
In conclusion, clinically significant PCA was detected in 15% of PI-RADSv2 category 3 TZ lesions. In our cohort, the combination of both PSAD of 0.15 ng/mL2 or greater and lesion ADC of less than 1000 mm2/s was significantly associated with clinically significant PCA. If biopsy had been restricted to these criteria, the number of men biopsied would have been decreased by 90%, yielding a clinically significant PCA detection rate of 60% with a false-negative rate of 9%.
Acknowledgments
This work was supported by the National Cancer Institute (R01CA158627) and the Integrated Diagnostics Program, Department of Radiological Sciences & Pathology, David Geffen School of Medicine at UCLA.
References
- 1.Barentsz JO, Richenberg J, Clements R, et al. European Society of Urogenital Radiology. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan N, Lin WC, Khoshnoodi P, et al. In-bore 3-T MR-guided transrectal targeted prostate biopsy: Prostate Imaging Reporting and Data System Version 2-based diagnostic performance for detection of prostate cancer. Radiology. 2016;283:130–139. doi: 10.1148/radiol.2016152827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargas HA, Hötker AM, Goldman DA, et al. Updated Prostate Imaging Reporting and Data System (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. 2016;26:1606–1612. doi: 10.1007/s00330-015-4015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auer T, Edlinger M, Bektic J, et al. Performance of PI-RADS version 1 versus version 2 regarding the relation with histopathological results. World J Urol. 2017;35:687–693. doi: 10.1007/s00345-016-1920-5. [DOI] [PubMed] [Google Scholar]
- 6.Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver reproducibility of the PI-RADS Version 2 lexicon: a multicenter study of six experienced prostate radiologists. Radiology. 2016;280:793–804. doi: 10.1148/radiol.2016152542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greer MD, Brown AM, Shih JH, et al. Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: a multireader study. J Magn Reson Imaging. 2017;45:579–585. doi: 10.1002/jmri.25372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liddell H, Jyoti R, Haxhimolla HZ. mp-MRI prostate characterised PIRADS 3 lesions are associated with a low risk of clinically significant prostate cancer: a retrospective review of 92 biopsied PIRADS 3 lesions. Curr Urol. 2015;8:96–100. doi: 10.1159/000365697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29:334–342. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta RT, Kauffman CR, Garcia-Reyes K, et al. Apparent diffusion coefficient values of the benign central zone of the prostate: comparison with low- and high-grade prostate cancer. AJR. 2015;205:331–336. doi: 10.2214/AJR.14.14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felker ER, Wu J, Natarajan S, et al. Serial magnetic resonance imaging in active surveillance of prostate cancer: incremental value. J Urol. 2016;195:1421–1427. doi: 10.1016/j.juro.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer. 2016;15:884–892. doi: 10.1002/cncr.29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller BG, Shih JH, Sankineni S, et al. Prostate cancer: interobserver agreement and accuracy with the revised Prostate Imaging Reporting and Data System at multiparametric MR imaging. Radiology. 2015;277:741–750. doi: 10.1148/radiol.2015142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 15.Glazer DI, Hassanzadeh E, Fedorov A, et al. Diffusion-weighted endorectal MR imaging at 3T for prostate cancer: correlation with tumor cell density and percentage Gleason pattern on whole mount pathology. Abdom Radiol (NY) 2017;42:918–925. doi: 10.1007/s00261-016-0942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo S, Kim SY, Cho JY, Kim SH. Preoperative evaluation of prostate cancer aggressiveness: using ADC and ADC ratio in determining Gleason score. AJR. 2016;207:114–120. doi: 10.2214/AJR.15.15894. [DOI] [PubMed] [Google Scholar]
- 17.Barbieri S, Brönnimann M, Boxler S, Vermathen P, Thoeny HC. Differentiation of prostate cancer lesions with high and with low Gleason score by diffusion-weighted MRI. Eur Radiol. 2017;27:1547–1555. doi: 10.1007/s00330-016-4449-5. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Reinikainen P, Vanhanen A, et al. Correlation between apparent diffusion coefficient value on diffusion-weighted MR imaging and Gleason score in prostate cancer. Diagn Interv Imaging. 2017;98:63–71. doi: 10.1016/j.diii.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Rosenkrantz AB, Kim S, Campbell N, Gaing B, Deng FM, Taneja SS. Transition zone prostate cancer: revisiting the role of multiparametric MRI at 3 T. AJR. 2015;204(web):W266–W272. doi: 10.2214/AJR.14.12955. [DOI] [PubMed] [Google Scholar]
- 20.Hoeks CM, Hambrock T, Yakar D, et al. Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology. 2013;266:207–217. doi: 10.1148/radiol.12120281. [DOI] [PubMed] [Google Scholar]