Abstract
Objective:
Salt reduction is one of the most cost-effective interventions for the prevention of noncommunicable diseases, but there are no studies evaluating the effectiveness of national strategies in low or lower middle income countries. This study aimed to examine the effect of an 18-month nation-wide salt reduction strategy in Samoa.
Methods:
Two nationally representative cross-sectional surveys of adults aged 18–64 years, measuring 24-h urinary salt excretion and salt-related knowledge, attitudes and behaviours, were conducted before (2013) and after (2015) the intervention.
Results:
There were 234 participants at baseline (response rate 47%) and 479 at 18 months (response rate 61%). There was no change in mean population salt intake between 2013 (7.31 g/day) and 2015 (7.50 g/day) (0.19, 95% confidence interval −0.50 to 0.88; P = 0.588). There were significant changes in the proportion of the population who always or often add discretionary salt when eating (−16.2%, P = 0.002), the proportion who understood the adverse effects of salt (+9.0%, P = 0.049) and the proportion using one or more methods to control their salt intake (+20.9%, P < 0.001). A total of 73% reported that they had heard or seen the salt reduction messages.
Conclusion:
With widespread awareness of the salt reduction message and some improvements in salt-related knowledge and behaviours following the intervention, Samoa is now well positioned to implement much-needed structural initiatives or policies to reduce salt in the food supply.
Keywords: cardiovascular diseases, developing countries, hypertension, nutrition, public health, salt, sodium
BACKGROUND
Cardiovascular diseases (CVDs) are the leading cause of deaths worldwide, and are responsible for 17.5 million deaths each year, or 31% of all deaths [1]. About 75% of these deaths occur in low-income and middle-income countries (LMICs) [2]. Raised blood pressure (BP) is the single leading risk factor for death and disability worldwide, and in low-income and some middle-income regions, the prevalence is rising or constant at a high level [3,4]. Excess salt intake is a major contributor to raised BP, which in turn increases the risk of CVD [5]. In 2010, excess salt intake was estimated to cause one in every 10 CVD deaths worldwide [6]. Systematic reviews and meta-analyses of randomized trials repeatedly demonstrate that reducing salt intake can lower BP [7,8]. Some observational studies [9–11] have suggested increased vascular risks with salt reduction to a low level but the totality of evidence suggests that salt reduction is beneficial for health [7,8,12–14]. As such, salt reduction is amongst the top five priority actions for addressing the noncommunicable disease (NCD) crisis [15] and the WHO has recommended it as a ‘best buy’ strategy – an action that has high impact, is cost-effective, affordable and feasible to implement on a large-scale in any resource setting [2].
Although deaths from CVD have been declining in high-income countries, there have been rapid increases in LMICs; many of these deaths are premature [16]. This results in a substantial economic burden to a country's healthcare system and economy [17]. Despite the potential public health benefits of salt reduction, there has been limited uptake of interventions and implementation research to understand how best to achieve a sustained reduction in salt intake in LMICs [18,19]. Implementation research can help in understanding what interventions are available, effective and best suited for different contexts [20]. In 2014, only 25% of LMICs (34 of 138) had a national salt reduction strategy, compared with 52% of high income countries [18]. Fourteen of the 34 LMICs with salt reduction programmes are in the Western Pacific Region and the majority (10) of these are in Pacific Island countries and territories where the WHO has been actively supporting salt reduction efforts [21].
Samoa is a lower middle income country where NCDs are responsible for 70% of deaths and CVDs account for 37% of all deaths [22]. Approximately, 40% of the adult population have raised BP and 54% are obese [22]. Worsening diets and increasing reliance on processed foods are likely to be a major contributor [17,23,24]. The Government of Samoa and the Ministry of Health (MOH) recognized salt reduction as a priority action as part of their National Food and Nutrition Policy and strategy for the Prevention of NCDs [25,26]. The Samoan Government was also one of the first countries to integrate the assessment of salt intake into its WHO STEPS survey as there was no previous population-level data on salt consumption [27]. In 2013, a nation-wide multifaceted salt reduction project was implemented. In addition to measuring and lowering salt intake in Samoa, the project aimed to understand the issues related to implementation so as to bridge the gap between efficacious interventions validated in controlled settings and their effective implementation in the real world. The objective of this study was to assess the effect of the salt reduction strategy on population salt intake and salt-related knowledge, attitudes and behaviours (KABs).
METHODS
Study design and participant selection for surveys
The MASIMA (Monitoring and Action on Salt in Samoa) project used two nationally representative cross-sectional surveys conducted before (2013) and after (2015) the intervention to evaluate the impact of a population-based strategy to reduce salt intake. It was part of a broader research project about the effectiveness of salt reduction in the Pacific Islands [28]. Participants were urban and rural residents aged 18–64 years, who were not pregnant or lactating at the time of the surveys. Households were stratified into four regions by the Samoa Bureau of Statistics – Apia Urban Area, North West Upolu, Rest of Upolu and Savaii – based on the 2011 Population and Housing Census for Samoa. The same stratified multistage cluster sampling method, using probability sampling proportional to size to select enumeration areas, was used at baseline and at 18 months (of intervention) to select samples representative of the target population. The baseline survey was part of a broader WHO STEPS survey, and every fifth STEPS-selected individual was invited to participate in the salt subsurvey. More information about the baseline survey has been reported elsewhere [29]. Whereas at 18 months, a standalone salt survey was carried out using different survey methods. A total of 94 enumeration areas were selected, comprising 32 clusters from urban areas and 62 from rural areas. Five households were randomly selected from each urban enumeration area and 10 households were randomly selected from each rural enumeration area. Then, for each selected household, one individual who met the criteria was randomly selected using the lottery simple random sampling without replacement technique [30]. All participants provided written consent and the study was approved by the University of Sydney Human Research Ethics Committee (no. 15359), the WHO and MOH, Samoa Health Research Committee.
Multifaceted salt reduction intervention
The multifaceted salt reduction intervention, adapted from the WHO's framework for creating an enabling environment for salt reduction [31], was implemented from March 2014 to September 2015. The intervention targeted the whole country and had three main goals as described in Table 1. The strategy was developed and delivered by the MOH, Government of Samoa to ensure it was culturally appropriate. The George Institute for Global Health and WHO provided ongoing technical support and a 2-day training workshop on the implementation of a salt reduction strategy. Two MOH implementation officers were recruited through the project to lead the salt reduction activities (Table 1). The implementation staff were supported by other MOH teams (nutrition and legal), other Government Ministries (Ministry of Education, Sports and Culture; Ministry of Women, Community and Social Development; Ministry of Commerce, Industry and Labour – MCIL and Ministry of Foreign Affairs and Trade), religious groups, schools and the Samoa Parliamentary Advocacy Groups for Healthy Living where relevant.
TABLE 1.
Monitoring and action on salt in Samoa (MASIMA) salt reduction interventions in Samoa
| Goals | Strategies | Actions | Deliverers |
| Influence policy and food environment to reduce salt consumption | 1. Advocate for the Food Act to include:a. Labelling of sodium content on packaged foodsb. Mandatory salt targets for processed foods2. Engage the food industrya. Monitor salt content in foods for meetings3. Incorporate salt targets into the School Nutrition Standards | • Shop surveys and food composition analysis to determine salt content in foods• Consultation on Food Act with food industry• Proposals for incorporating PICs regional salt targets for foods into the Food Act• Proposals to incorporate salt targets in the School Nutrition Standards and monitor compliance• Food regulations as part of the Food Act were approved by director general and sent to cabinet (November 2016) | MOH (nutrition and legal team), WHO, SPAGHL, nutrition team, MCIL Codex Committee, MFAT and MESC |
| Mobilize the community to take action to reduce salt | 4. Engage and mobilize community leaders to raise awareness and share tips to reduce salt intake including:a. Government ministriesb. Church leaders and groupsc. Village mayorsd. Schools and tertiary institutionse. Health workers and community health outreach programmesf. Food industry (restaurants, foods distributors/producers) | • Awareness and engagement talks about salt reduction with female mayors of Savaii & Upolu, other ministries’ community programmes, church groups, international rugby sevens tournament and schools• Dissemination of salt reduction resources (stickers, posters, leaflets and DVDs) to government ministries, NGOs, schools, GP clinics, health centres and hospitals (particularly to people with raised blood pressure)• Educate restaurants and food industry about salt• Presentations about salt reduction project at annual health sector forum (Nov 2014) and health promotion seminar for church conference (July 2015) | MOH, MWCSD and health partners |
| Increase awareness through media and advocacy campaigns | 5. Disseminate salt reduction materials (leaflets, posters, bookmarks, stickers, salt reduction DVD) to community about the adverse health effects of excess salt intake, commonly eaten foods that are high in salt and should be avoided (e.g. processed foods) and tips to reduce the use of salt (use lemon, herbs and spices)6. Raise awareness through TV, radio, newspaper articles, billboards and Facebook and Salt Awareness Week activities | • Community awareness and distribution of resources (leaflets, posters, factsheets) during USO bike ride awareness programme (August 2014) and healthy lifestyle week (November 2014)• Salt reduction billboard display• Television and radio advertisements for World Salt Awareness Week (WSAW, March 2014 and 2015)• 2015 WSAW: mass media campaign, distribution of resources, Slash the salt community concert | MOH |
DVDs, digital video disc; GP, general practice; MCIL, Ministry of Commerce, Industry and Labour; MESC, Ministry of Education, Sports and Culture; MFAT, Ministry of Foreign Affairs and Trade; MOH, Ministry of Health; MWCSD, Ministry of Women, Community and Social Development; PIC, Pacific Island countries and territories; SPAGHL, Samoa Parliamentary Advocacy Groups for healthy living.
Sample size calculation
Based on the number of participants at baseline (before exclusions for incomplete demographic information, n = 293), we estimated that a sample of 500 adults at 18 months would provide a power of 80% with alpha = 0.05, to detect a minimum difference of 0.7 g/day (10%) in salt intake, assuming a SD of 3.5. Based on the anticipated response rate of 65%, 780 participants were sampled and invited to participate in the survey at 18 months.
Data collection
At baseline, data were collected by trained researchers as part of the 2013 WHO STEPS survey. The salt monitoring training was undertaken over one and a half days as part of the broader WHO STEPS training programme and included instructions on collecting 24-h urine samples and administering the salt KAB questionnaire. In 2015, other researchers were trained over four and a half days to follow similar data collection protocols. Training included random selection of individuals from the household, obtaining consent, administering the demographic and KAB questionnaire, physical measurements (height, weight and BP), instructions on how to collect 24-h urine samples and a pilot survey.
Randomly selected individuals were invited to take part in the survey and were asked to provide written consent following an explanation of the purpose and procedures of the salt survey. At baseline, as part of the WHO STEPS survey, participants attended fixed sites [church halls, village fales (common Samoan houses or structures for holding meetings)] for data collection, whereas in the survey at 18 months, data collection took place at the participants’ home. Researchers administered the demographic and salt-related KAB questionnaire that was based on the WHO STEPS Instrument [27]. Data on age (years), sex (male or female), education (highest level of education completed), employment (main work status over the past year), area (rural or urban as defined by the Samoa Bureau of Statistics), history of hypertension (as previously diagnosed by a doctor or health worker) and use of BP lowering medication (in the last 2 weeks) were collected. The KAB questionnaire included two questions testing the participant's knowledge about the adverse effects of salt and the recommended intake, two questions related to their attitude towards salt reduction and perceived level of consumption and four questions about their salt intake behaviour including discretionary salt use at the table or during cooking. Researchers also undertook measurements of height, weight and BP according to the WHO STEPS protocol [27]. Participants’ weight was measured to the nearest 0.1 kg using digital Omron scales and height was measured to the nearest 0.1 cm using Seca portable stadiometers. BP was measured while seated by using a validated automatic BP monitor with an appropriately sized cuff. Three BP measurements were taken and the average of the last two measurements was used for the analysis. Interviewers were instructed to provide participants with a referral form to the health centre and a leaflet about salt reduction if their measured SBP was more than 140 mmHg or DBP was more than 90 mmHg.
One 24-h urine sample was collected from each participant for the measurement of 24-h urinary sodium, potassium and creatinine excretion. Research staff of both surveys were trained to instruct participants to collect 24-h urine using the same protocol with the exception that at baseline, participants were asked to start urine collection at least 1 day after fasting blood glucose and cholesterol measurements were collected for the STEPS survey. On first contact, researchers provided the urine containers and advised participants to start urine collection after discarding the first urine void the next morning, recording start and finish times on the containers. Researchers picked up the urine samples the day after collection and transported them to the laboratory in which the urine volumes were measured. Aliquots from the 24-h samples were then stored in a refrigerator until they were analysed for sodium, potassium and creatinine concentrations. The biochemists who performed the urinary electrolyte measurements were not involved with the implementation of the interventions. A 24-h urine volume of less than 500 ml or a 24-h urinary creatinine excretion of less than 4 or more than 25 mmol in women and less than 6 or more than 30 mmol in men was considered inaccurate and thus excluded from analyses [11].
Outcome measures
The primary outcome was the difference in mean population salt intake as measured by 24-h urine collection in separate samples in 2013 (pre-intervention) and 2015 (postintervention). Secondary outcomes included the difference in population KABs related to salt intake.
To capture intervention reach, the question ‘Have you heard about or seen any promotion regarding the Slash the Salt campaign?’ was added to the questionnaire post-intervention. If participants answered yes, they were asked how. The potential sources of exposure that could be selected included stickers, radio, television, community events, information leaflet/poster, billboards and friends and family. Mean salt intake was analysed based on the number of different exposures to the salt reduction campaign that participants had heard or seen.
Statistical analysis
All analyses were weighted to reflect the multistage cluster sampling design and the Samoa population distribution based on the 2011 Census [32]. As weighting by the probability of selection (sampling weight) and age, sex and area distribution (population weight) did not address the balance of other demographic characteristics, raking (sample balancing) by the five demographic variables (age, sex, area, education and employment) was undertaken to match the two samples to the Census population [33]. Thus, more exclusions were undertaken to only include participants with complete data on these five demographic variables in the main analysis.
A survey-adjusted linear regression (accounting for strata and cluster effects, with raking weights and finite population correction factor) was used to determine the difference in salt intake and potassium intake between baseline and 18 months. A crude analysis was performed and any variable with indications of association with salt intake and potassium intake (P < 0.20) were included in the adjusted models. The covariates tested were age, sex, area (urban and rural), education, employment, height, weight and urinary creatinine excretion. The change in the proportions of the population above 5 g/day of salt (the WHO recommended amount) and 10 g/day were also calculated. Similarly, survey-adjusted logistic regression was used to measure change in salt knowledge, attitudes and behaviour between baseline and 18 months. Covariates included in the model include age, sex, area, education, employment, height, weight and history of hypertension.
Various sensitivity analyses were conducted to examine the robustness of the conclusions of the primary analysis. Other methods were used to adjust for potential inaccurate 24-h urine collections. We explored the change in the sodium-to-creatinine ratio (Na : Cr) and sodium-to-potassium ratio (Na : K) between baseline and post-intervention to account for any variation in urine completeness [34]. Subgroup analyses were also undertaken to determine the change in salt intake by different urinary creatinine excretion and urine volume categories, which are indicators of the completeness of 24-h urine collection.
Statistical analyses were conducted by using STATA IC version 13.0 for Windows (StataCorp LP, College Station, Texas, USA) and SAS 9.3 (SAS Institute, Cary, North Carolina, USA) for the analyses. Results are reported as mean, SD, standard error (SE) and 95% confidence interval as appropriate. All analyses were two-sided, and P less than 0.05 was considered significant.
RESULTS
Sample characteristics
There were 500 randomly selected individuals at baseline of whom 71.2% consented and provided urine samples. After exclusion of persons with suspected inaccurate urine collection based on pre-established criteria (63) and incomplete demographic information (59), 234 participants were eligible for analysis at baseline (Fig. 1). At 18 months, 780 participants were randomly selected of whom 82.3% consented and provided urine samples. After exclusion of inaccurate urine collection (161) and incomplete demographic information (2), 479 participants were eligible for analysis.
FIGURE 1.
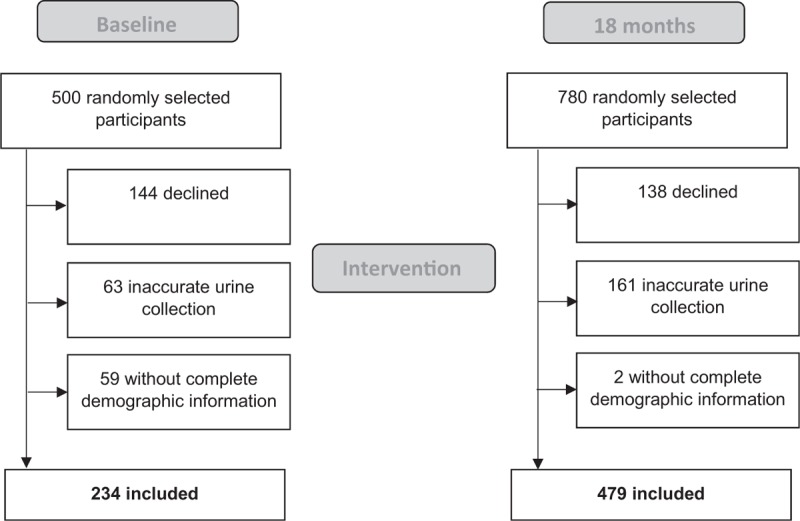
Flow chart of participant recruitment and exclusion of participants.
Table 2 shows the demographic characteristics of participants at baseline and at 18 months unweighted, weighted by age, sex and area distribution and raked to match the Samoa adult population. Prior to weighting, the two samples at baseline and 18 months were significantly different in terms of age, area, education and employment (P < 0.011 for each). After weighting by age, sex and area there were still significant demographic differences in the distribution of education and employment (P < 0.05 for each) between the two samples.
TABLE 2.
Characteristics of participants at baseline and 18 months
| Unweighted | Weighted (by age, sex and area) | Rakeda | ||||||||
| Variable | Baseline, n = 293 | 18 months, n = 481 | P value | Baseline, n = 293 | 18 months, n = 481 | P value | Baseline, n = 234 | 18 months, n = 479 | P value | 2011 Samoa census data |
| Age [years (mean, SE)] | 37.3 (0.8) | 40.0 (0.6) | 0.011 | 36.1 (1.1) | 37.4 (0.7) | 0.341 | 36.5 (1.4) | 36.7 (0.8) | 0.923 | 36.2 |
| Female (%) | 58.4 | 53.0 | 0.148 | 48.0 | 48.0 | 1.000 | 48.0 | 48.0 | 0.996 | 48.0 |
| Rural (%) | 91.1 | 81.3 | 0.010 | 79.3 | 79.3 | 1.000 | 79.4 | 79.4 | 0.997 | 79.3 |
| Education (%) | ||||||||||
| Completed primary school or less | 21.3 | 39.7 | <0.001 | 16.7 | 38.3 | <0.001 | 21.2 | 21.2 | 1.000 | 21.1 |
| Completed secondary school | 63.2 | 44.7 | 64.6 | 44.2 | 60.9 | 60.9 | 61.1 | |||
| Completed tertiary school | 15.5 | 15.6 | 18.7 | 17.4 | 17.9 | 17.9 | 17.8 | |||
| Employment | ||||||||||
| Employed | 20.7 | 36.3 | 0.001 | 25.7 | 37.1 | 0.050 | 45.8 | 45.8 | 1.000 | 45.9 |
| Unemployed | 6.3 | 4.4 | 4.4 | 4.6 | 2.7 | 2.7 | 2.7 | |||
| Non-economically active | 73.0 | 59.3 | 69.9 | 58.3 | 51.5 | 51.5 | 51.4 | |||
| Height [cm (mean, SE)] | 166.6 (0.5) | 166.8 (0.4) | 0.849 | 167.7 (0.7) | 167.2 (0.5) | 0.552 | 168.1 (0.8) | 167.4 (0.5) | 0.414 | – |
| Weight [kg (mean, SE)] | 90.8 (1.2) | 93.2 (1.0) | 0.113 | 90.4 (1.4) | 92.7 (1.4) | 0.226 | 92.2 (1.8) | 91.9 (1.3) | 0.900 | – |
| BMI [kg/m2 (mean, SE)] | 32.8 (0.4) | 33.7 (0.4) | 0.105 | 32.2 (0.4) | 33.2 (0.5) | 0.110 | 32.7 (0.6) | 33.0 (0.5) | 0.694 | – |
| SBP [mmHg (mean, SE)] | 126.2 (1.2) | 131.6 (0.9) | <0.001 | 126.0 (1.6) | 130.3 (1.1) | 0.024 | 126.4 (1.7) | 129.4 (1.1) | 0.127 | – |
| DBP [mmHg (mean, SE)] | 76.4 (0.7) | 85.2 (0.6) | <0.001 | 75.4 (1.0) | 83.9 (0.8) | <0.001 | 76.3 (1.2) | 83.8 (0.9) | <0.001 | – |
| Diagnosed with hypertension by doctor or healthcare worker (%) | 5.8 | 31.8 | <0.001 | 5.1 | 28.8 | <0.001 | 8.0 | 28.1 | <0.001 | – |
| Urinary volume [ml (mean, SE)] | 1272.7 (37.4) | 1408.5 (29.2) | 0.006 | 1243.6 (43.8) | 1423.7 (32.9) | 0.002 | 1254.3 (56.1) | 1419.1 (34.8) | 0.016 | – |
| Creatinine [mmol (mean, SE)] | 12.1 (0.3) | 13.7 (0.2) | <0.001 | 12.7 (0.4) | 14.0 (0.3) | 0.005 | 13.0 (0.5) | 14.0 (0.3) | 0.070 | – |
SE, standard error.
aNo missing data were imputed; only respondents with complete data on age, sex, area, education and employment were included.
Following raking (sample balancing) by all demographic variables, there were some significant differences between the samples at baseline and 18 months. At 18 months, participants had higher mean urinary volume (P = 0.016), higher mean DBP (P < 0.001) and more reported being diagnosed with hypertension by a doctor or healthcare worker (P < 0.001) (Table 2). There were no significant differences in average population height, weight, BMI and SBP between participants at baseline and 18 months (Table 2).
Change in population salt intake
There was no significant difference in mean salt intake (P = 0.588) as measured by 24-h urinary excretion after raking the samples and adjusting for potential confounders (Table 3). The adjusted mean population 24-h urinary salt excretion for Samoan adults aged 18–64 years was 7.31 g/day (SE 0.30) at baseline and 7.50 g/day (SE 0.19) at 18 months. There was also no significant difference in the proportion of the population meeting the WHO 5 g/day target (P = 0.672) and those exceeding 10 g/day of salt (P = 0.555) pre-intervention and post-intervention.
TABLE 3.
Adjusted change in salt intake and potassium intake between baseline and 18 months
| Variable | Baseline | 18 months | Difference (95% CI) | P value |
| Salt intake [g/day (mean, SE)]a | 7.31 (0.3) | 7.50 (0.2) | 0.19 (−0.50 to 0.88) | 0.588 |
| Salt intake above the 5 g/day WHO target (%)a | 70.6 | 72.6 | 2.0 (−7.3 to 11.3) | 0.672 |
| Salt intake above 10 g/day (%)a | 20.7 | 23.3 | 2.6 (−6.0 to 11.3) | 0.555 |
| Potassium intake [mmol (mean, SE)]b | 55.30 (1.5) | 60.61 (2.3) | 5.30 (−0.21 to 10.82) | 0.059 |
CI, confidence interval; SE, standard error.
aAdjusted for age, sex, height, weight and urine creatinine.
bAdjusted for age, sex area height, weight, education and urine creatinine.
Subgroup analyses demonstrated that the intervention had no statistically significant differential effect between men and women (P for interaction = 0.317) or the urban and rural area (P for interaction = 0.281). There was no evidence of association between SBP or DBP with salt intake at baseline, 18 months or combined in an adjusted regression with age, sex, area, education, employment, height, weight and potassium (P > 0.138 for each) (Supplementary file 1). Adjusted population urinary potassium excretion was 55.30 mmol/day (SE 1.52) at baseline and 60.61 mmol/day (SE 2.28) at 18 months, however, the difference was not statistically significant (P = 0.059).
Table 4 shows the results from sensitivity analyses. There was no change in adjusted Na : Cr ratio (P = 0.989) and a non-significant change of −0.27 in the Na : K ratio (P = 0.104) (Table 4). The analysis of change in salt intake based on different groups of urinary volume and creatinine representing different levels of urine collection completeness (group 1: <25th percentile, group 2: 25–75th percentile, group 3: >75th percentile) also showed no significant difference in salt intake between baseline and 18 months in each category (P > 0.179 for each urine volume group and P > 0.651) (Table 5).
TABLE 4.
Effect of using different methods to adjust for potential inaccurate 24-h urine collection on salt intake estimates – sensitivity analyses of change in adjusted salt intake: adjusted sodium to creatinine and sodium to potassium ratios at baseline and 18 months
| Baseline | 18 months | Difference (95% CI) | P value | |
| Sodium: creatinine ratio (mmol/mmol, SE)a | 10.01 (0.5) | 10.00 (0.3) | −0.01 (−1.07 to 1.06) | 0.989 |
| Sodium: potassium ratio (mmol/mmol, SE)b | 2.68 (0.1) | 2.40 (0.1) | 0.27 (−0.60 to 0.06) | 0.104 |
CI, confidence interval; SE, standard error.
aAdjusted for sex, height, weight and employment.
bAdjusted for age, sex, area, weight and education.
TABLE 5.
Effect of using different methods to adjust for potential inaccurate 24-h urine collection on salt intake estimates – sensitivity analyses of change in adjusted salt intake by categories of urine volume or urinary creatinine at baseline and 18 months
| Adjusted salt intake (g/day, SE)a | ||||
| Baseline | 18 months | Difference (95% CI) | P value | |
| By urine volume categories | ||||
| Urine volume <900 ml (<25th percentile) | 5.25 (0.3) | 5.48 (0.3) | 0.22 (−0.50 to 0.94) | 0.543 |
| Urine volume between 900 and 1700 ml (25–75th percentile) | 7.90 (0.6) | 7.64 (0.3) | −0.25 (−1.52 to 1.02) | 0.694 |
| Urine volume >1700 ml (>75th percentile) | 8.54 (0.6) | 9.58 (0.4) | 1.04 (−0.48 to 2.55) | 0.179 |
| By urinary creatinine categories | ||||
| Urinary creatinine <9.6 mmol (<25th percentile) | 5.54 (0.4) | 5.70 (0.4) | 0.16 (−0.81 to 1.12) | 0.749 |
| Urinary creatinine between 9.6 and 16.7 mmol (25–75th percentile) | 7.13 (0.4) | 7.36 (0.3) | 0.23 (−0.77 to 1.23) | 0.651 |
| Urinary creatinine >16.7 mmol (>75th percentile) | 9.52 (0.9) | 9.64 (0.4) | 0.12 (−1.63 to 1.87) | 0.892 |
SE, standard error.
aAdjusted for age, sex, height and weight.
Change in salt-related knowledge, attitudes and behaviours
Table 6 shows the changes in knowledge, attitude and behaviours towards salt reduction between baseline and 18 months when adjusted for covariates. There was a significant increase in the proportion of participants who understood that high salt consumption could cause serious health problems (from 81 to 90%, P = 0.049) and several improvements in self-reported behaviours related to salt intake. These included a decrease in the proportion of participants who always or often add salt to food when eating (from 50 to 33%, P = 0.002) and who always or often ate processed foods (from 60 to 49%, P = 0.020) and an increase in the proportion who reported using one or more methods to control their salt intake (from 73 to 93%, P < 0.001), particularly through the use of spices rather than salt in cooking (from 48 to 76%, P < 0.001). However, there was no change in the proportion of participants who knew the recommended daily salt intake was less than 5 g (22 vs. 20%, P = 0.638) or in the proportion of participants who thought that they consumed too much salt and that lowering salt was important in their diet (P > 0.183 for each). There were reductions in the proportion of the population who reported controlling their salt intake through either checking the sodium on packaged food labels (from 43 to 29%, P = 0.015) or avoiding eating out (from 62 to 40%, P < 0.001) (Fig. 2).
TABLE 6.
Changes in salt-related knowledge, attitude and behaviours before and after the intervention
| Salt-related knowledge, attitudes and behaviour | Baselinea | 18 monthsa | Difference (95% CI) | P value |
| Knowledge | ||||
| Agreed that salt could cause health problems (%) | 80.6 | 89.6 | 9.0 (−0.6 to 18.7) | 0.049 |
| Correctly identified the recommended amount of salt: <5 g (%) | 22.2 | 20.2 | −2.0 (−10.4 to 6.4) | 0.638 |
| Attitude | ||||
| Perceived salt consumption: far too much (%) | 23.2 | 17.7 | −5.5 (−13.7 to 2.7) | 0.183 |
| Claimed that lowering salt is very or somewhat important (%) | 92.1 | 93.0 | 0.9 (−4.3 to 6.0) | 0.740 |
| Behaviour | ||||
| Always/often add salt to food (%) | 49.6 | 33.4 | −16.2 (−26.2 to −6.3) | 0.002 |
| Always/often add salt in cooking (%) | 57.8 | 66.2 | 8.4 (−2.3 to 19.1) | 0.123 |
| Always/often eat processed foods (%) | 60.4 | 49.4 | −11.0 (−20.1 to −2.0) | 0.020 |
| Performed any method to control salt intake (%) | 72.5 | 93.4 | 20.9 (13.1–28.7) | <0.001 |
| Avoid consumption of processed foods (%) | 60.2 | 68.1 | 8.0 (−2.4 to 18.3) | 0.130 |
| Look at sodium labels (%) | 42.9 | 29.0 | −13.9 (−25.5 to −2.4) | 0.017 |
| Do not add salt at the table (%) | 45.5 | 47.9 | 2.4 (−8.9 to 13.7) | 0.677 |
| Buy low sodium alternatives (%) | 54.0 | 56.9 | 3.0 (−8.7 to 14.6) | 0.620 |
| Do not add salt when cooking (%) | 48.5 | 45.1 | −3.4 (−15.1 to 8.4) | 0.576 |
| Use spices other than salt in cooking (%) | 48.2 | 76.4 | 28.2 (17.2–39.2) | <0.001 |
| Avoid eating out (%) | 61.7 | 39.9 | −21.8 (−33.0 to −10.6) | <0.001 |
CI, confidence interval.
aAdjusted for age, sex, rurality, education, employment, height, weight and history of hypertension.
FIGURE 2.
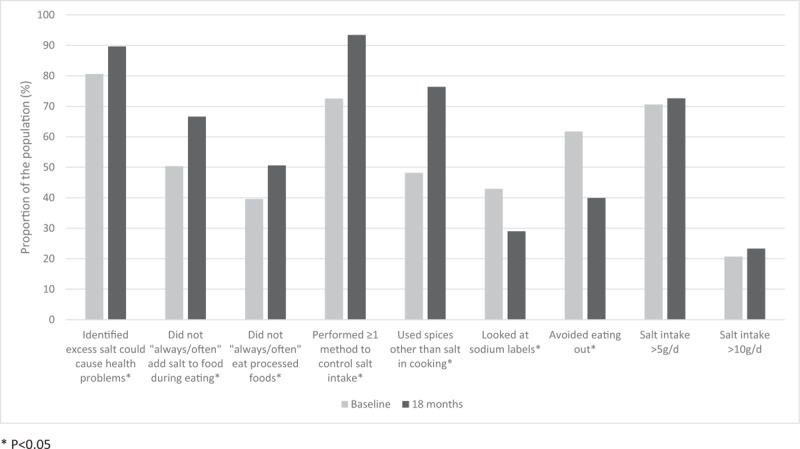
Change in salt intake and reported salt-related knowledge and behaviours between baseline and 18 months in the Samoan adult population.
The measure of the campaign's reach showed that 73% of participants were exposed to salt reduction messages from one or more sources. After adjusting for potential covariates, those who heard or saw the salt reduction message from four or more of the potential seven sources (more than half) of the campaign had a significantly lower mean adjusted salt intake of 5.71 g/day compared with those who did not recall any exposure to the salt reduction campaign (7.68 g/day, P = 0.001) (Table 7).
TABLE 7.
Adjusted salt intake at 18 months based on number of exposures to the salt reduction campaign
| Number of sources of exposure to the campaign | Mean salt intakea (g/day) | 95% Confidence interval | P value |
| 0 (no exposure) | 7.68 | 7.02–8.34 | Ref |
| 1–3 sources (1–50%) | 7.80 | 7.30–8.30 | 0.775 |
| 4 or more sources (>50%) | 5.71 | 4.79–6.63 | 0.001 |
aAdjusted for age, sex, height, weight and creatinine.
DISCUSSION
The current study found no change in average population salt intake after an 18-month nation-wide salt reduction strategy in Samoa. There were, however, reductions in reported discretionary salt use when eating, improvements in some salt-lowering behaviours and increased knowledge about the adverse health effects of excess salt intake. The intervention also achieved widespread reach, with almost three of four adults recalling that they had heard or seen the salt reduction campaign. This suggests that the foundation for further interventions and improvements is now in place and increased efforts to improve the food supply and environment would likely benefit the Samoan population, given the high and rising burden from hypertension and dietary risks [22,35].
There are several possible explanations for why the study did not find any change in population salt intake after the 18-month intervention. First, the surveys undertaken at baseline and 18 months were independent and used methods that resulted in recruited samples with substantially different demographics and clinical characteristics. Some of these differences are likely to be associated with behaviours related to salt intake and may have confounded the measure of outcomes. Despite efforts to ensure that random, nationally representative samples were chosen at each time point, methodological differences related to the baseline survey being part of the STEPS survey may have affected the final sample and the measurement of salt intake. This is reflected in significant differences in age, area (rural vs. urban), education and employment between the two samples prior to weighting or raking. Although raking was conducted in an attempt to adjust for differences in demographic factors and adjustments were made to account for potential confounders in the regression model, it was likely to be not effective and there was residual confounding. This is indicated by the significantly higher average DBP, higher proportion of people diagnosed with hypertension and higher average urine volume at 18 months compared with baseline even after adjustments. Non-identical data collection procedures at baseline and 18 months, such as the measurement of BP in different environments (e.g. in fixed sites with proper set up at baseline compared with participants’ home at 18 months) may have also contributed to the differences observed in BP. It is likely that the significant increase in mean DBP was an anomaly and these methodological issues may explain the lack of association between SBP or DBP and salt intake found in our study. This highlights the challenges of implementing consistent sampling and survey methods at different times, despite rigorous training for researchers conducting the surveys. To observe trends in salt intake over time, it will be crucial that the next WHO STEPS survey in Samoa, scheduled to take place in 2018, replicates the 2013 survey protocols.
The second potential explanation for a null effect is related to limited intervention dose and duration. Previous successful national programs in the United Kingdom, Finland and Japan were implemented over at least 5 years [18,36,37]. Although the 18-month intervention made important progress in terms of influencing policy in Samoa, there was not enough time for the changes to take effect. For example, although a Food Act has been passed and maximum salt limits for foods will be included, the regulation is yet to be implemented and enforced. Therefore, it is unlikely that any changes in the sodium content of foods have occurred to date. This means that this study only assesses the effects of the awareness campaigns and community engagement initiatives – strategies which aim to change individuals’ behaviours. These behaviour-change interventions have been important, particularly as there was no previous public education about salt reduction and salt added by the consumer during cooking or eating is still a major source of salt in the Samoan diet. Although they have likely led to some of the improvements in salt-related knowledge and behaviours, previous reviews suggest these interventions alone are unlikely to have a substantial impact on population salt intake [19,38]. Complementary structural interventions that create a healthier food supply and environments that support lower salt intake such as reformulation to reduce salt content in foods, fiscal, trade or healthy food procurement policies are needed to ensure a substantial and sustained impact [18,19]. The intensity of the behaviour-change interventions may also not be enough to influence the population's usual salt consumption, as the mean number of sources of exposure to the campaign was 1.37. This is consistent with a recent review, which suggested programmes that utilize several delivery modes to reach the wider public in conjunction with in-depth education delivered to specific groups were more effective in improving salt reduction behaviours [38]. In addition, there were some evidence to suggest behaviour-change interventions based on theoretical frameworks such as Communication for Behaviour Impact or the PRECEDE–PROCEED were effective in reducing salt intake [38]. Future implementation research should consider the intervention dose and structural initiatives that are timely and feasible, particularly in lower resource countries.
A third explanation is that a secular upward trend in salt consumption overwhelmed any intervention effect and that salt intake levels in Samoa were already relatively low compared with other countries [39,40]. The study did not include a control population so the impact of the secular trends could not be captured. Samoa, like many Pacific Island countries, is still in a stage of nutrition transition from traditional foods such as starchy root vegetables, coconut, fish and fruits to a diet of refined cereals, meats, fats, canned and processed foods that are higher in salt [23,41–44]. Previous studies in other Pacific Island countries have found such dietary changes towards a westernized diet have been linked with increasing salt intake [43,45–48]. The proportion of the population that reported avoiding eating out decreased significantly. This is a potential indicator of changing diets and urbanization which is further supported by data from the MCIL in Samoa, which showed that the number of restaurants had increased from 160 in 2013 to 210 in 2015, a 31% increase over the duration of the study, mostly in the urban region (Supplementary file 2). This could also be one of the contributors to the rising trend in BP amongst Pacific Island countries [4]. This suggests that continued efforts to reduce salt intake and improve nutrition through structural interventions and policies are even more crucial in LMICs like Samoa that are undergoing a nutrition transition [23]. These structural interventions should also consider the additional complexities that these countries may face such as heavy reliance on imported foods, food insecurity and undernutrition [43].
It is likely that a combination of these explanations resulted in a null effect in population salt intake following the intervention in Samoa. These reasons for a null effect are common amongst community-based prevention programs and have been identified in a review of 32 health promotion programmes [49]. Furthermore, a recent Cochrane review found that in 10 countries with national salt reduction strategies, five had a significant decrease in salt intake but three (Austria, Netherlands, and United States) had no change and two (Canada and Switzerland) had an increase in salt intake [19]. Similarly, some suggested reasons for no reduction in salt intake include a lack of structural interventions and short intervention duration. These studies, nevertheless, provide important findings about the feasibility of implementing and evaluating large-scale salt reduction strategies and building our understanding of the challenges faced in the real world.
An important limitation of the study is the uncontrolled pre-intervention and post-intervention study design. This meant the study was unable to account for other factors that could potentially affect salt intake and causal inferences cannot really be made. However, it was not feasible to implement a national strategy comprising of national policies and communication activities as part of a controlled trial. The incorporation of the baseline salt intake measurement in the STEPS survey caused methodological differences in sampling and survey procedures; however, it ensured greater efficiency of resources, regular surveillance and international comparability. The next STEPS survey in 2018 will provide more comparable sampling and survey methods. Another challenge was the complexity and burden associated with the use of 24-h urine collection to measure salt intake in low-resource settings. This is reflected in the high number of urine samples that were excluded based on a pre-established criteria for suspected inaccurate collections. This also made it difficult to achieve a large enough sample (and therefore, statistical power) to detect a modest change in salt intake. Despite the use of a pre-established criteria to exclude suspected incomplete urines, there was still a significantly higher mean urine volume of 165 ml at 18 months compared with baseline and a borderline significant increase in mean urinary creatinine of 1 mmol (P = 0.07), which suggests there may be more under-collection at baseline, which can affect the comparability of the measurements. This highlights the complexities of collecting 24-h urine particularly in lower resource settings and the need to develop innovative methods of measuring salt intake that are less burdensome, complex and costly. This would also allow programmes to give greater focus, resource and funds to the implementation of interventions.
Although there were some limitations, the strengths of the study are that it is nationally representative, the gold standard method for measuring salt intake was used, in-depth analyses were undertaken to adjust for known confounders, multiple indicators were measured and the data collection tools and processes used were part of the WHO STEPS resources, so the findings are internationally comparable. Our study adds important and transferrable findings about the real-world implementation and evaluation of large-scale salt reduction interventions in lower resource settings.
In conclusion, the high and increasing burden of diet-related illness and hypertension in Samoa and similar Pacific Island countries requires urgent action [4,35]. Although the MASIMA salt reduction strategy did not change population salt intake, there is now widespread awareness of the salt reduction message, increased knowledge of the adverse health effects and improvements in salt-related behaviour. This places Samoa in a good position to implement much-needed structural interventions and policies that will lower salt in the food supply and environment. Accelerated efforts and regular monitoring particularly through the STEPS survey are required long-term to achieve the WHO recommended salt target of less than 5 g/day and minimize diet-related diseases. Future research in innovative methods of measuring salt intake that are less burdensome, complex and costly are also vital in allowing lower resource countries to give greater focus to the implementation of interventions.
ACKNOWLEDGEMENTS
The authors wish to thank the Samoan Ministry of Health, the Samoa Bureau of Statistics, the National Health Services Laboratory, the WHO staff in Samoa, in Western Pacific Regional Office and in Geneva, the Ministry of Women, Community and Social Development, the NCD Committee, the STEPS and salt survey teams and all of the survey participants for their support and interest in the study. The authors also wish to thank Kris Rogers at the George Institute for Global Health for his continued advice and support with the data analysis.
The Project is funded by the National Health and Medical Research Council of Australia under the Global Alliance for Chronic Disease (GACD) Hypertension Program (no. 1040178). K.T. is supported by a National Health and Medical Research Council of Australia postgraduate scholarship (no. 1115169) and VicHealth for work on salt reduction. B.N. is supported by a National Health and Medical Research Council of Australia Principal Research Fellowship (APP1106947). He works within a NHMRC Centre for Research Excellence (APP1117300) and holds an NHMRC Program Grant (APP1052555). M.M. is supported by a NHMRC Centre for Research Excellence in Obesity Policy and Food Systems (no. 1041020). J.W. is supported by a National Health and Medical Research Council/National Heart Foundation Career Development Fellowship (no. 1082924) on International strategies to reduce salt. J.W. has funding from WHO, VicHealth and the National Health and Medical Research Council of Australia for research on salt reduction.
Conflicts of interest
J.W. is Director of the WHO Collaborating Centre on Population Salt Reduction with a remit to support countries to implement and evaluate salt reduction programmes in line with the WHO target for all countries to reduce population salt intake by 30% by 2025. All other authors declare that they have no conflicts of interest related to this study.
Supplementary Material
Supplementary Material
Reviewers’ Summary Evaluations
Reviewer 1
The manuscript entitled "Effects of MASIMA: a nationwide strategy to reduce salt intake in Samoa Short title: Salt reduction strategy in Samoa" by K. Trieu et al. is an interesting study dealing with salt intake reduction in Samoa's population. The results are quite surprising.
Reviewer 2
Trieu et al report results of two nationally representative cross-sectional surveys aiming examining the benefit on salt intake of a 18-month nationwide salt reduction strategy in Samoa. They report no effect on urinary sodium excretion as a gold standard evaluation of salt intake despite some improvements in salt-related knowledge and behaviors following the intervention. Although with mainly negative results, the manuscript is interesting and well written but deserves some criticisms.
Footnotes
Abbreviations: KAB, knowledge, attitudes and behaviour; LMIC, low and middle income country; MASIMA, monitoring and action on salt in Samoa; MCIL, Ministry of Commerce, Industry and Labour; MESC, Ministry of Education, Sports and Culture; MFAT, Ministry of Foreign Affairs and Trade; MOH, Ministry of Health; MWCSD, Ministry of Women, Community and Social Development; PICs, Pacific Island countries and territories; SPAGHL, Samoa Parliamentary Advocacy Groups for Healthy Living
REFERENCES
- 1.The World Health Organization. Global status report on noncommunicable diseases 2014. Geneva, Switzerland: The World Health Organization; 2014. [Google Scholar]
- 2.Alwan A. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: The World Health Organization; 2011. [Google Scholar]
- 3.Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386:2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017; 389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 h urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 1988; 297:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014; 371:624–634. [DOI] [PubMed] [Google Scholar]
- 7.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013; 346:f1325. [DOI] [PubMed] [Google Scholar]
- 8.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013; 346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 2014; 371:601–611. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371:612–623. [DOI] [PubMed] [Google Scholar]
- 11.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011; 305:1777–1785. [DOI] [PubMed] [Google Scholar]
- 12.He F, Appel L, Cappuccio F, de Wardener H, MacGregor G. Does reducing salt intake increase cardiovascular mortality? Kidney Int 2011; 80:696–698. [DOI] [PubMed] [Google Scholar]
- 13.Poggio R, Gutierrez L, Matta MG, Elorriaga N, Irazola V, Rubinstein A. Daily sodium consumption and CVD mortality in the general population: systematic review and meta-analysis of prospective studies. Public Health Nutr 2014; 18:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster J, Waqanivalu T, Arcand J, Trieu K, Cappuccio FP, Appel LJ, et al. Understanding the science that supports population-wide salt reduction programs. J Clin Hypertens (Greenwich) 2017; 19:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the noncommunicable disease crisis. Lancet 2011; 377:1438–1447. [DOI] [PubMed] [Google Scholar]
- 16.The World Health Organization. Projections of mortality and causes of death 2015 and 2030. Geneva, Switzerland: The World Health Organization; 2015. [Google Scholar]
- 17.Anderson I. The economic costs of noncommunicable diseases in the Pacific Islands: a rapid stocktake of the situation in Samoa, Tonga and Vanuatu. Washington, DC: The World Bank; 2012. [Google Scholar]
- 18.Trieu K, Neal B, Hawkes C, Dunford E, Campbell N, Rodriguez-Fernandez R, et al. Salt reduction initiatives around the world – a systematic review of progress towards the global target. PLoS One 2015; 10:e0130247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaren L, Sumar N, Barberio AM, Trieu K, Lorenzetti DL, Tarasuk V, et al. Population-level interventions in government jurisdictions for dietary sodium reduction. Cochrane Database Syst Rev 2016; 9:CD010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridde V. Need for more and better implementation science in global health. BMJ Glob Health 2016; 1:e000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christoforou A, Snowdon W, Laesango N, Vatucawaqa S, Lamar D, Alam L, et al. Progress on salt reduction in the pacific islands: from strategies to action. Heart Lung Circ 2015; 24:503–509. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Samoa. NCD country profiles. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 23.Thow AM, Heywood P, Schultz J, Quested C, Jan S, Colagiuri S. Trade and the nutrition transition: strengthening policy for health in the Pacific. Ecol Food Nutr 2011; 50:18–42. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization Regional Office for the Western Pacific. Diet, the food supply and obesity in the Pacific. Geneva, Switzerland: The World Health Organization; 2003. [Google Scholar]
- 25.Ministry of Health Samoa. National noncommunicable disease policy 2010–2015: preventing chronic conditions – working towards a healthy life for all Samoans. Apia, Samoa: Government of Samoa; 2010. [Google Scholar]
- 26.Government of Samoa Ministry of Health. National food and nutrition policy 2013. Apia, Samoa: Government of Samoa; 2013. [Google Scholar]
- 27.World Health Organization.. WHO STEPS Instrument (Core and Expanded). The WHO STEPwise approach to noncommunicable disease risk factor surveillance (STEPS). Geneva, Switzerland: The World Health Organization; 2016. [Google Scholar]
- 28.Webster J, Snowdon W, Moodie M, Viali S, Schultz J, Bell C, et al. Cost-effectiveness of reducing salt intake in the Pacific Islands: protocol for a before and after intervention study. BMC Public Health 2014; 14:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webster J, Su’a SAF, Ieremia M, Bompoint S, Johnson C, Faeamani G, et al. Salt intakes, knowledge, and behavior in Samoa: monitoring salt-consumption patterns through the World Health Organization's surveillance of noncommunicable disease risk factors (STEPS). J Clin Hypertens (Greenwich) 2016; 18:884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy P, Lemeshow S. Sampling of populations: methods and applications. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- 31.Webster JL, Dunford EK, Hawkes C, Neal BC. Salt reduction initiatives around the world. J Hypertens 2011; 29:1043–1050. [DOI] [PubMed] [Google Scholar]
- 32.Samoa Bureau of Statistics.. Population and housing census 2011. Samoa: Samoa Bureau of Statistics; 2012. [Google Scholar]
- 33.Izrael D, Hoaglin D, Battaglia M. A SAS macro for balancing a weighted sample. Proceedings of the twenty-fifth annual SAS users group international conference 2000. p. 258. [Google Scholar]
- 34.Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 2014; 129:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute for Health Metrics and Evaluation. GBD compare. Seattle: IHME, University of Washington; 2015. [Google Scholar]
- 36.He FJ, Brinsden HC, MacGregor GA. Salt reduction in the United Kingdom: a successful experiment in public health. J Hum Hypertens 2014; 28:345–352. [DOI] [PubMed] [Google Scholar]
- 37.He FJ, MacGregor GA. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis 2010; 52:363–382. [DOI] [PubMed] [Google Scholar]
- 38.Trieu K, McMahon E, Santos JA, Bauman A, Jolly KA, Bolam B, et al. Review of behaviour change interventions to reduce population salt intake. Int J Behav Nutr Phys Act 2017; 14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol 2009; 38:791–813. [DOI] [PubMed] [Google Scholar]
- 40.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013; 3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes RG. Diet, food supply and obesity in the Pacific. Manila: World Health Organization Regional Office for the Western Pacific; 2003. [Google Scholar]
- 42.Snowdon W, Raj A, Reeve E, Guerrero R, Fesaitu J, Cateine K, et al. Processed foods available in the Pacific Islands. Global Health 2013; 9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seiden A, Hawley NL, Schulz D, Raifman S, McGarvey ST. Long-term trends in food availability, food prices, and obesity in Samoa. Am J Hum Biol 2012; 24:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiBello JR, McGarvey ST, Kraft P, Goldberg R, Campos H, Quested C, et al. Dietary patterns are associated with metabolic syndrome in adult Samoans. J Nutr 2009; 139:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodge AM, Dowse GK, Zimmet PZ, Collins VR. Prevalence and secular trends in obesity in pacific and Indian Ocean island populations. Obes Res 1995; 3:77s–88s. [DOI] [PubMed] [Google Scholar]
- 46.Galanis DJ, McGarvey ST, Quested C, Sio B, Afele-Fa’amuli SA. Dietary intake of modernizing Samoans: implications for risk of cardiovascular disease. J Am Diet Assoc 1999; 99:184–190. [DOI] [PubMed] [Google Scholar]
- 47.Cassels S. Overweight in the Pacific: links between foreign dependence, global food trade, and obesity in the Federated States of Micronesia. Global Health 2006; 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans M, Sinclair RC, Fusimalohi C, Liava’a V. Globalization, diet, and health: an example from Tonga. Bull World Health Org 2001; 79:856–862. [PMC free article] [PubMed] [Google Scholar]
- 49.Merzel C, D’Afflitti J. Reconsidering community-based health promotion: promise, performance, and potential. Am J Public Health 2003; 93:557–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


