BACKGROUND:
Perioperative fentanyl has been reported to induce hyperalgesia and increase postoperative pain. In this study, we tried to investigate behavioral hyperalgesia, the expression of proinflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), and the activation of microglia in the spinal cord and dorsal root ganglion (DRG) in a rat model of surgical plantar incision with or without perioperative fentanyl.
METHODS:
Four groups of rats (n = 32 for each group) were subcutaneously injected with fentanyl at 60 μg/kg or normal saline for 4 times with 15-minute intervals. Plantar incisions were made to rats in 2 groups after the second drug injection. Mechanical and thermal nociceptive thresholds were assessed by the tail pressure test and paw withdrawal test on the day before, at 1, 2, 3, 4 hours, and on the days 1–7 after drug injection. The lumbar spinal cord, bilateral DRG, and cerebrospinal fluid of 4 rats in each group were collected to measure IL-1β, IL-6, and TNF-α on the day before, at the fourth hour, and on the days 1, 3, 5, and 7 after drug injection. The lumbar spinal cord and bilateral DRG were removed to detect the ionized calcium-binding adapter molecule 1 on the day before and on the days 1 and 7 after drug injection.
RESULTS:
Rats injected with normal saline only demonstrated no significant mechanical or thermal hyperalgesia or any increases of IL-1β, IL-6, and TNF-α in the spinal cord or DRG. However, injection of fentanyl induced analgesia within as early as 4 hours and a significant delayed tail mechanical and bilateral plantar thermal hyperalgesia after injections lasting for 2 days, while surgical plantar incision induced a significant mechanical and thermal hyperalgesia lasting for 1–4 days. The combination of fentanyl and incision further aggravated the hyperalgesia and prolonged the duration of hyperalgesia. The fentanyl or surgical incision upregulated the expression of IL-1β, IL-6, and TNF-α in the spinal cord and bilateral DRG for more than 7 days and increase of ionized calcium-binding adapter molecule 1 in the spinal cord. The combination of fentanyl and incision resulted in higher increase of IL-1β, IL-6, and TNF-α in the spinal cord and bilateral DRG.
CONCLUSIONS:
The surgical plantar incision with or without perioperative fentanyl induced significant mechanical and thermal hyperalgesia, an increased expression of IL-1β, IL-6, TNF-α in the spinal cord and DRG, and activation of microglia in the spinal cord.
KEY POINTS.
Question: Did perioperative fentanyl induced any behavioral or molecular changes in spinal cord, dorsal root ganglia (DRG), and cerebrospinal fluid in rats?
Findings: An increased expression of proinflammatory cytokines including interleukin-1β, interleukin-6, and tumor necrosis factor-α in the spinal cord and bilateral DRG, and activation of microglia in spinal cord in a rat model of plantar incision with or without perioperative fentanyl.
Meaning: The findings suggest that increased expression of proinflammatory cytokines including in the spinal cord and bilateral DRG, and activation of microglia in the spinal cord, may contribute to the development and maintenance of mechanical and thermal hyperalgesia induced by fentanyl and/or incision, which provide a new insight into the treatment of opioid-induced hyperalgesia or postsurgical pain.
Repeated and/or prolonged administration of opioids has been clearly demonstrated to induce abnormal pain or hyperalgesia paradoxically in human and animal models.1 A series of studies have also indicated that perioperative opioids are associated with a significant increase in acute pain after surgery, opioid-induced hyperalgesia (OIH), and prolongation of postoperative pain.2–4
Recent systematic reviews suggested that perioperative OIH was mainly associated with the use of a high dose of remifentanil, but the correlation was less clear for other opioids, such as fentanyl and sufentanil, because of limited data.2,3 The authors recognized that cumulative dose, duration administration, and modality of withdrawal may be possible determinant factors of remifentanil-induced hyperalgesia.2,5 Although the mechanism that underlies remifentanil-induced hyperalgesia has not been completely understood, much evidence suggests that the activation of glutamatergic system or N-methyl-d-aspartate receptors in the central nervous system plays an important role.2,6 In addition, recent evidence indicates that the activation of spinal glia and immune system is also essential for the development of OIH.4,7,8
Remifentanil is a short-acting opioid widely administered during surgery due to its characteristics of rapid onset, predictable rapid recovery profile, and dosing reliability. Meanwhile, long-acting opioids such as fentanyl are also often used during surgery. In animal models, fentanyl was clearly demonstrated to induce hyperalgesia and increase postoperative pain.9,10 Interestingly, relatively recent human trials demonstrated a higher dose of fentanyl-induced hyperalgesia from 4.5 to 6.5 hours in healthy volunteers.11 However, to the best of our knowledge, few studies have investigated the interaction of surgery and perioperative fentanyl on hyperalgesia, although some studies indicated that remifentanil or fentanyl could aggravate surgical incision–induced hyperalgesia in mice or rats.12,13 Heinl et al14 demonstrated that pronociceptive mechanisms of OIH by remifentanil, fentanyl, and morphine were differential because of their different pharmacological characteristics. Therefore, further study needs to further explore the reason for perioperative fentanyl-induced hyperalgesia. Given that the activation of spinal glia and upregulation of proinflammatory cytokines are critical in the development of OIH,4,7,8 the present study aimed to investigate hyperalgesia interaction, the changes of spinal and dorsal root ganglion (DRG) immune system, and proinflammatory cytokines in a rat model of surgical incision with or without perioperative fentanyl.
METHODS
Animals
This study obtained the approval of the Institutional Lab oratory Animal Care and Use Committee of the Sun Yat-sen University and was performed according to the guidelines of the Physiological Society of China regarding the care of experimental animals. All experiments were performed on adult male Sprague Dawley rats (200–250 g, purchased from Sun Yat-sen University). The animals were housed in 4 per cage under a 12-hour light/dark cycle, 25°C ± 2°C in a specific pathogen-free environment. Food and water were available at any time. All rats were marked with picric solution and randomly assigned into 4 groups (n = 32 rats), including normal saline (NS), fentanyl (F), incisional (I), and fentanyl plus incisional (FI).
Medication and Measurement Protocol
Four groups of Sprague Dawley rats, as described above, received subcutaneous injection of fentanyl (60 µg/kg) or NS in a volume of 1.2 mL/kg for 4 times with 15-minute intervals, referred to previous study.10 Behavioral tests, as described below, were performed daily for 11 days (D−4 to D−1, D1–D7) and at hours 1, 2, 3, and 4 (H1, H2, H3, H4) after fentanyl or saline injection on the treatment day. The 3-day behavioral tests before treatment (D−4 to D−2) were performed for rats to be habitual to equipment and environment. The values on day 1 before treatment were recorded as baseline values. All behavioral tests were performed by a single investigator blinded to the treatment groups. Each test (between 9:00 and 14:00) was repeated 3 times with 3-minute intervals to get an average as the final value. The lumbar spinal cord (L3–L6), both sides of DRG (L3–L6), and cerebrospinal fluid (CSF) were collected on D−1, H4, D1, D3, D5, and D7 to examine the expression of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). The lumbar spinal cord (L4) and both sides of DRG (L4) were removed after being perfused with 4% paraformaldehyde on D−1, D1, and D7 for immunofluorescence assay (Figure 1).
Figure 1.
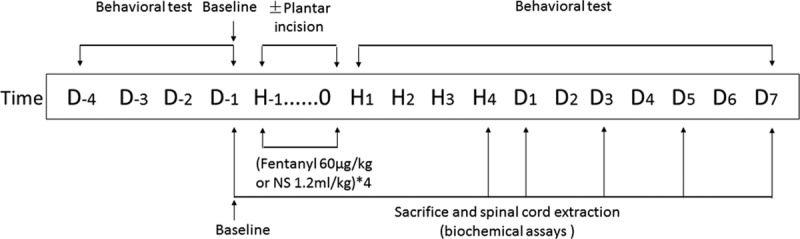
Medication and measurement protocol. Four groups of Sprague Dawley rats received subcutaneous injection of fentanyl (60 µg/kg) or normal saline (1.2 mL/kg) 4 times with 15-minute intervals. Rats that received surgery underwent surgical incision in the plantar of the left hind paw followed by the second fentanyl injection. Behavioral tests were performed daily for 11 d (D−4 to D−1, D1–D7) and at 1, 2, 3, and 4 h (H1, H2, H3, H4) after the treatment. The thermal and mechanical thresholds on D-1 were recorded as baseline. The lumbar spinal cord and both ipsilateral and contralateral dorsal root ganglion to surgical sites were collected on D-1, H4, D1, D3, D5, and D7 to examine the expression of interleukin-1β, interleukin-6, and tumor necrosis factor-α. NS indicates normal saline.
Surgery
Rats in groups I and FI were anesthetized with 1.5%–2% isoflurane (Jiupai company, Hebei, China) delivered via a nose cone. After skin degeneration, a 1-cm incision was made through the skin, fascia, and muscle of the plantar aspect of the left hind paw. The incision was started at 0.5 cm from the proximal edge of the heel and extended to the toes, as described previously.15 A 4-0 silk was used to suture the skin after the wound was pressed with a cotton swab to stop bleeding. All incisions were made after the second injection.
Behavior Tests
Tail Pressure Test.
Tail pressure test was performed as described previously.16,17 An electronic device (ZH-YLS-3E, Anhui Zhenghua Company, China) was used to evaluate mechanical nociceptive thresholds (MNTs). The rat was fixed in a cylindrical fixing device and a marker was made at 10 cm from the tail tip. The marked part of the tail was put on the platform where a plastic wedge above provided the pressure on the surface of the tail. The pressure was stopped and the value was recorded if the rat flicked the tail, screamed, or struggled. A cutoff was set at 600g to avoid tissue damage.
Thermal Test.
The thermal test was performed as described previously.18 Briefly, a Plantar Analgesia Meter (IITC Life Science Inc, Woodland Hills, CA) was used to evaluate thermal nociception. The rat was put in a plexiglass box on a glass plate. A beam of light passed through the glass to the sole near to toes ipsilateral and contralateral to surgical site. The intensity of the beam was set at 30W and the diameter was 5 mm. The paw withdrawal latency (PWL) was recorded as the length of time between the onset of the light beam and paw withdrawal. The 20-second cutoff time was set to avoid tissue damage.
Enzyme-Linked Immunosorbent Assay
CSF and the lumbosacral enlargement of the spinal cord (L3–L6) and both sides of DRG (L3–L6) were collected and all DRGs were divided into left and right halves. Each tissue sample was homogenized and centrifuged with icy phosphate-buffered saline (PBS; weight/volume ratio: 1 mg/10 μL), and the supernatants were removed for immediate use. The amounts of IL-1β, TNF-α, and IL-6 were assayed using commercially available rat-specific ELISA kits (Yikesai, Shanghai, China) in accordance with the manufacturer’s instruction. The optical density (value) of each well was determined using a microplate reader set to 450 nm within 10 minutes.
Because the amount of CSF was insufficient for detection of the 3 cytokines, we finally detected IL-1β and TNF-α involved in pain.19
Immunofluorescence
Increased microglial activity is evidenced by a profound shift in morphology and upregulation of ionized calcium-binding adapter molecule 1 (Iba1) that can be easily visualized using immunofluorescence.20 Perfused spinal cord (L4) and bilateral DRG (L4) were sectioned into 5 μm coronal sections. Three sections were sectioned per 1 tissue sample. Briefly, sections were rinsed extensively in PBS for 3 times followed by a 10-minute fixation in 4% paraformaldehyde. The tissue was then incubated in a primary antibody solution (rabbit anti-Iba 1 1:1000; wako 019-19741) in PBS for 24 hours at 4°C. After being rinsed with PBS, the tissue was incubated for 1 hour in a secondary antibody (biotinylated immunoglobulin G goat antirabbit 1:600), rinsed with PBS, and then incubated for 1 hour. After being rinsed in PBS, the tissue was then incubated in 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (Beyotime, Shanghai, China) for 10 minutes. The intensity of fluorescence was analyzed using Image J (National Institutes of Health, Bethesda, MD) analysis software.
Statistical Analysis
All data were presented as mean ± standard deviation. SPSS for Windows 16.0 (SPSS Inc, Chicago, IL) was used for statistical analysis. Changes in continuous variables over time were analyzed with repeated-measures analysis of variance, followed by the Tukey’s test. Difference among groups at each time point and difference among multiple time points in each group were analyzed with analysis of variance and the Tukey’s test. The paired-samples t test was used to assess paired comparisons values (incised ipsilateral site versus contralateral site). The criterion for statistical significance was P < .05. Data points in each group at each time point outside the scope of mean ± 3 standard deviation were considered to be apparent outliers and were excluded before statistical analysis.
RESULTS
Fentanyl and/or Incision Induced Mechanical and Thermal Hyperalgesia in Rats
The changes in MNTs induced by fentanyl and/or incision are shown in Figure 2A. No statistically significant difference of MNTs was observed among the 4 groups on D−1 (1-way analysis of variance; P > .05) and for days (D0–D7) after NS injections in the control (NS) group (1-way analysis of variance; P > .05). A significant mechanical antinociceptive effect, as evidenced by a significant increase of the MNTs in tail pressure test, was observed at hours 1, 2, and 3 in group F and group FI after subcutaneous injections of fentanyl. On the contrary, a significant mechanical hyperalgesia, as evidenced by a significant decrease of the MNTs in tail pressure test, was observed on days 1–3 after fentanyl injections. The decrease of MNTs in group FI sustained for 3 days (D1–D3), while that in group F sustained for 2 days (D1 and D2). Furthermore, the values of MNTs in group F on days 1 and 2, and those in group FI on days 1–3, were significantly lower than those in the control group (P < .05 by the Tukey’s test). These results indicated that the mechanical hyperalgesia in group FI was longer than that in group F. Additionally, the values of MNTs were significantly lower in group FI than those in group F on D3 (P < .05 by the Tukey’s test). In brief, the above results suggested that mechanical hyperalgesia induced by perioperative fentanyl and surgery was more severe than that induced by fentanyl only. Similarly, the decrease of MNTs in group FI, which sustained for 3 days (D1–D3), was longer than that in group I, which sustained for 1 day (D1). The values of MNTs were significantly lower in group FI than those in group I on days 2–4 (P < .05 by the Tukey’s test). These results indicated that perioperative fentanyl aggravated postoperative mechanical hyperalgesia induced by surgery.
Figure 2.
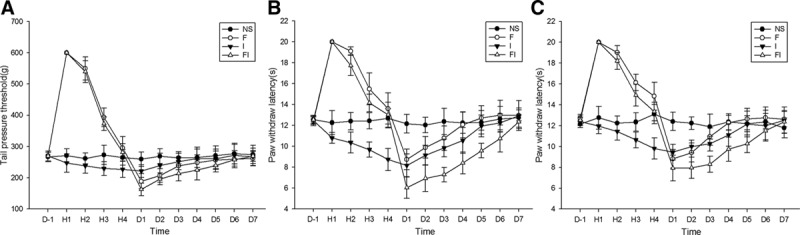
The mechanical and thermal hyperalgesia induced by fentanyl with or without surgical incision in rats. Sprague Dawley rats received subcutaneous fentanyl (60 μg/kg × 4) or normal saline (NS) and/or plantar incision (group NS = the control group; group F = rats received fentanyl only; group I = rats received NS and plantar incision; group FI = rats received fentanyl and plantar incision). Tail pressure threshold (g) (A) and paw withdraw latency (s) of surgical paws (B) and contralateral paws (C) on the day before (D−1), at 1, 2, 3, 4 h and on the days 1, 2, 3, 4, 5, 6, 7 after drug injections (D1–D7) are shown as mean ± standard deviation (n = 8).
The changes in thermal nociceptive latencies ipsilateral to surgical site (shown as PWLs) induced by fentanyl and/or incision are shown in Figure 2B. Similar to tail pressure tests, a significant decrease of the PWLs in paw withdraw test was observed on days 1–5 after fentanyl injections. The thermal hyperalgesia in group FI lasting for 5 days (D1–D5) was longer than that in group F lasting for 2 days (D1 and D2). Further, the values of PWLs in group FI were significantly lower than those in group F on days 2–6 (P < .05 by the Tukey’s test). These results suggested that ipsilateral thermal hyperalgesia induced by perioperative fentanyl and surgery was more severe than that induced by fentanyl only. In group I, the PWLs significantly decreased at third and fourth hours and on D1–D4 after surgery and saline injection. The values of PWLs in group FI were significantly lower than those in group I on days 1–3 (P < .05 by the Tukey’s test). These results indicated that perioperative fentanyl aggravated postoperative ipsilateral thermal hyperalgesia induced by surgery.
Interestingly, we found that the extent and duration of PWL decrease in contralateral sites (Figure 2C) were similar to those in ipsilateral sites (Figure 2B) in groups F, I, and FI. However, the thermal hyperalgesia demonstrated in contralateral sites lasting for 4 days (D0–D3) was shorter than that in ipsilateral sites lasting for 5 days (D0–D4) in group I. Again, values of PWLs in ipsilateral sites were significantly lower than those in contralateral sites on days 1 and 2 in group I and on days 1 and 3 in group FI after surgery (P < .05 by paired-samples t test). These results indicated that the thermal hyperalgesia in contralateral sites was weaker than in ipsilateral sites in groups I and FI.
Fentanyl and/or Incision Induced the Increase of Proinflammatory Cytokines in Spinal Cord and Bilateral DRG
The expressions of IL-1β (Figure 3), IL-6 (Figure 4), and TNF-α (Figure 5) in spinal cord and bilateral DRG were determined on the day before, at hour 4, and on days 1, 3, 5, and 7 after fentanyl or saline injections. The levels of IL-1β, IL-6, and TNF-α in the spinal cord and bilateral DRG in the control (NS) group and the levels of IL-1β and TNF-α in CSF in 4 groups of NS, F, I, and FI did not change following time course. However, the levels of IL-1β significantly increased in spinal cord on days 1 and 3 in groups F and I, on days 1, 3, 5, and 7 in groups FI compared to baseline, and on days 1, 3, 5, and 7 in groups F, I, and FI, compared to the control group (P < .05 by the Tukey’s test). It significantly increased in both sides of DRG in groups F, I, and FI on days 1, 3, 5, and 7 after fentanyl or saline injections, compared to the baseline and the control group (P < .05 by the Tukey’s test).
Figure 3.
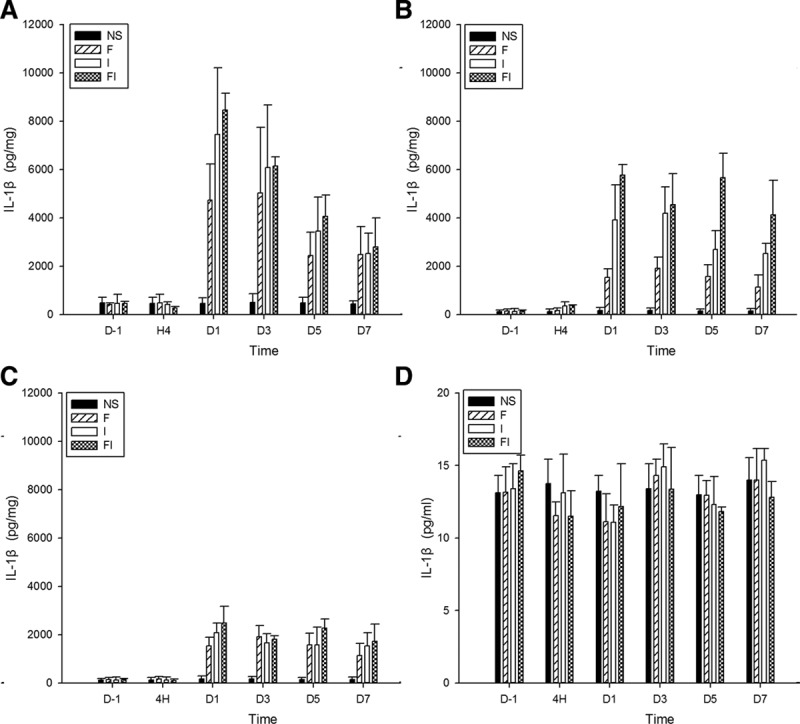
The expression of interleukin-1β (IL-1β) in the spinal cord, bilateral dorsal root ganglion (DRG), and cerebrospinal fluid (CSF) after treatment of fentanyl and/or surgical incision. Sprague Dawley rats received subcutaneous fentanyl (60 μg/kg × 4) or normal saline (NS) and/or plantar incision (group NS = the control group; group F = rats received fentanyl only; group I = rats received NS and plantar incision; group FI = rats received fentanyl and plantar incision). Lumbar spinal cord (A), ipsilateral DRG (B), contralateral DRG (C) to surgical sites and CSF (D) of 4 rats were collected in each group. The expression of IL-1β (pg/mg for tissue, pg/mL for CSF) on the day before (D−1), at 4 h (H4) and on the days 1, 3, 5, 7 after drug injections (D1, D3, D5, and D7) were detected by enzyme-linked immunosorbent assay. All data are shown as mean ± standard deviation (n = 4).
Figure 4.
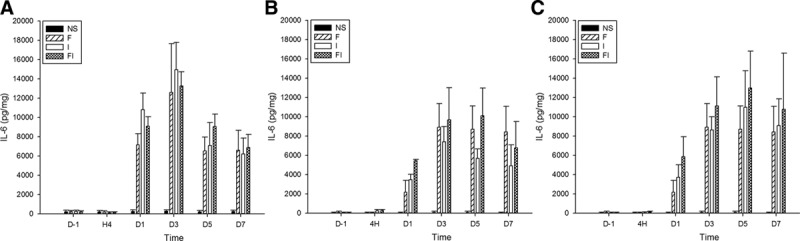
The expression of interleukin-6 (IL-6) in the spinal cord and bilateral dorsal root ganglion (DRG) after treatment of fentanyl and/or surgical incision. Sprague Dawley rats received subcutaneous fentanyl (60 μg/kg × 4) or normal saline (NS) and/or plantar incision (group NS = the control group; group F = rats received fentanyl only; group I = rats received NS and plantar incision, group FI = rats received fentanyl and plantar incision). Lumbar spinal cord (A), ipsilateral DRG (B), and contralateral DRG (C) to surgical sites of 4 rats were collected in each group. The expression of IL-6 (pg/mg) on the day before (D−1), at 4 h (H4), and on the days 1, 3, 5, 7 after drug injections (D1, D3, D5, and D7) were detected by enzyme-linked immunosorbent assay. All data are shown as mean ± standard deviation (n = 4).
Figure 5.
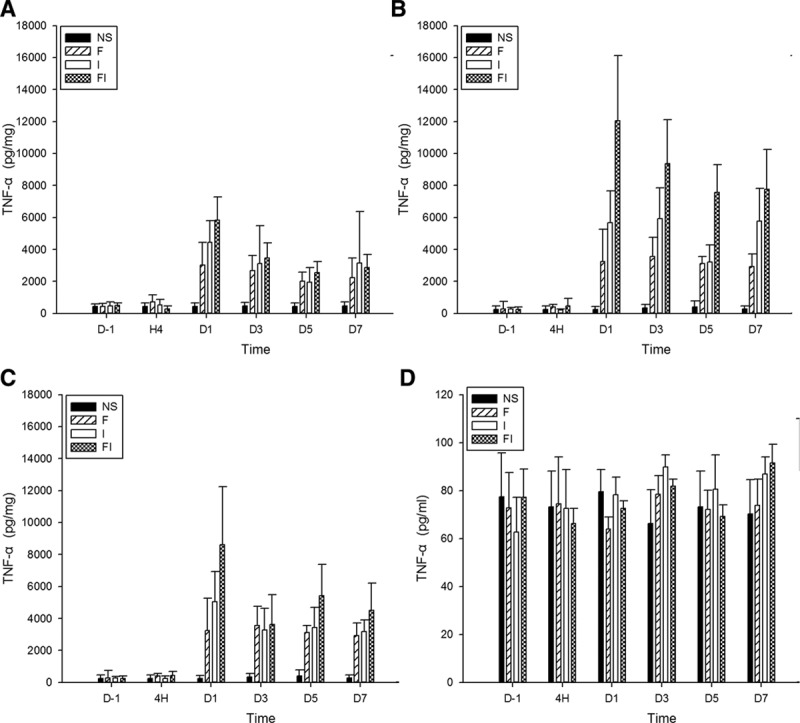
The expression of tumor necrosis factor (TNF-α) in the spinal cord, bilateral dorsal root ganglion (DRG), and cerebrospinal fluid (CSF) after treatment of fentanyl and/or surgical incision. Sprague Dawley rats received subcutaneous fentanyl (60 μg/kg × 4) or normal saline (NS) and/or plantar incision (group NS = the control group; group F = rats received fentanyl only; group I = rats received NS and plantar incision, group FI = rats received fentanyl and plantar incision). Lumbar spinal cord (A), ipsilateral DRG (B), contralateral DRG (C), and CSF (D) to surgical sites of 4 rats were collected in each group. The expression of TNF-α (pg/mg for tissue, pg/mL for CSF) on the day before (D−1), at 4 h (H4) and on the days 1, 3, 5, 7 after drug injections (D1, D3, D5, and D7) were detected by enzyme-linked immunosorbent assay. All data are shown as mean ± standard deviation (n = 4).
Furthermore, the levels of IL-1β in group FI were significantly higher than those in group F on day 1 after fentanyl injection in spinal cord, higher than those in group F on days 1, 3, 5, and 7, higher than those in group I on days 1 and 5 after fentanyl injection in ipsilateral DRG, and higher than those in group F on day 1 after fentanyl injection in contralateral DRG (P < .05 by the Tukey’s test). These results indicated that intraoperative fentanyl and surgery induced a higher increase of IL-1β in spinal cord and bilateral DRG than fentanyl only, and induced higher increases of IL-1β in ipsilateral DRG than surgery only.
Additionally, the levels of IL-1β in ipsilateral DRG were significantly higher than those in contralateral DRG on day 3 in group I, on days 1, 3, 5, and 7 in group FI (P < .05 by paired-samples t test). These results indicated that surgery only or surgery and intraoperative fentanyl induced a higher increase of IL-1β in ipsilateral DRG than in contralateral DRG.
Similarly, the levels of IL-6 significantly increased in spinal cord in groups F, I, and FI on days 1, 3, 5, and 7 and after fentanyl or saline injections, compared to the baseline and the control group (P < .05 by the Tukey’s test). It significantly increased on days 3, 5, and 7 in groups F and I and on days 1, 3, 5, and 7 in group FI in ipsilateral DRG, significantly increased on days 3, 5, and 7 in groups F, I, and FI in contralateral DRG compared to baseline (P < .05 by the Tukey’s test). The levels of IL-6 were significantly higher on days 1, 3, 5, and 7 in groups F and FI and on days 1, 3, and 5 in group I in bilateral DRG compared to control group (P < .05 by the Tukey’s test).
Furthermore, the levels of IL-6 in group FI were significantly higher than those in group F at hour 4 and on day 1, and higher than those in group I on day 1, after fentanyl injection in ipsilateral DRG (P < .05 by the Tukey’s test). The levels of IL-6 in group FI were significantly higher than those in group F on day 1 after fentanyl injection in contralateral DRG (P < .05 by the Tukey’s test). These results indicated that intraoperative fentanyl and surgery induced a higher increase of IL-6 in bilateral DRG than fentanyl only and induced a higher increase of IL-6 in ipsilateral DRG than surgery only.
Similarly, the levels of TNF-α significantly increased in spinal cord on day 7 in groups F and I, on days 1 and 7 in group FI, increased in bilateral DRG on days 5 and 7 in group F, increased in ipsilateral DRG on days 1, 3, and 7 in group I and on days 1, 3, 5, and 7 in group FI, increased in contralateral DRG on days 1 and 7 in group I and on days 1, 5, and 7 in group FI after fentanyl or saline injections, compared to the baseline (P < .05 by the Tukey’s test). The levels of TNF-α were significantly higher in spinal cord on days 1 and 5 in groups F and I, and on days 1, 3, and 5 in group FI, higher in both sides of DRG on days 3, 5, and 7 in groups F, and on days 1, 3, 5, and 7 in groups I and FI after fentanyl or saline injections, compared to the control group (P < .05 by the Tukey’s test).
Furthermore, the levels of TNF-α in group FI were significantly higher than those in group F on day 1 after fentanyl injection in spinal cord, higher than those in group F on days 3, 5, and 7, and higher than those in group I on days 1 and 5 after fentanyl injection in ipsilateral DRG, and higher than those in groups F on day 1 after fentanyl injection in contralateral DRG (P < .05 by the Tukey’s test). These results indicated that intraoperative fentanyl and surgery induced a higher increase of TNF-α than fentanyl only in spinal cord and bilateral DRG, and induced a higher increase of TNF-α in ipsilateral DRG than surgery only.
Additionally, the levels of TNF-α in ipsilateral DRG were significantly higher than those in contralateral DRG on day 3 in group FI (P < .05 by paired-samples t test), indicating that intraoperative fentanyl and surgery induced a higher increase of TNF-α in ipsilateral DRG than in contralateral DRG.
Fentanyl and/or Incision Induced the Activation of Microglia in the Spinal Cord
Microglia activity, as evidenced by Iba1 immunoreactivity, was significantly increased in animals that received fentanyl injections and/or plantar incision in the spinal cord (Figure 6A). The intensity of Iba1 in the 2 halves of the spinal cord was increased on day 1 in groups F and I, and on days 1 and 7 in group FI (P < .05 by the Tukey’s test). There was no significant difference between the each half of the spinal cord (P > .05 by the paired-samples t test). No significant differences among groups and time points were noted in Iba1 immunoreactivity in both sides of DRG (Figure 6B, C) (P > .05 by the Tukey’s test).
Figure 6.
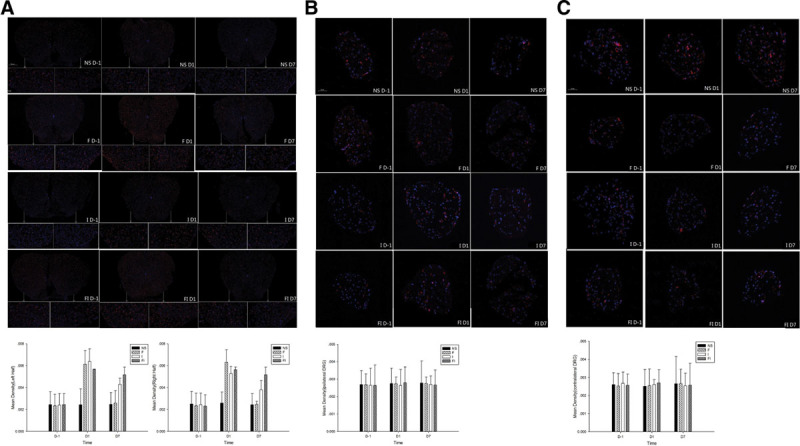
The expression of ionized calcium-binding adapter molecule 1 (Iba1) in the spinal cord, bilateral dorsal root ganglion (DRG) after treatment of fentanyl and/or surgical incision. Sprague Dawley rats received subcutaneous fentanyl (60 μg/kg × 4) or normal saline (NS) and/or plantar incision (group NS = the control group; group F = rats received fentanyl only; group I = rats received NS and plantar incision; group FI = rats received fentanyl and plantar incision). Lumbar spinal cord (A), ipsilateral DRG (B), and contralateral DRG (C) to surgical sites of 4 rats were collected in each group. Three sections were sectioned per 1 tissue sample. The expression of Iba 1 on the day before (D−1) and on the days 1 and 7 after drug injections were detected by immunofluorescence. The panels where the arrow points are the same parts enlarged. All sections costained for Iba 1(red) and DAPI (blue) are shown in the figure. All data are shown as mean ± standard deviation (n = 12).
DISCUSSION
The main finding of this study is that surgery and/or fentanyl administration could induce mechanical and thermal nociceptive hyperalgesia and an increase of proinflammatory cytokines including IL-1β, IL-6, and TNF-α in the spinal cord and DRG ipsilateral and contralateral to the surgical site in rats.
The plantar incision usually induces postoperative mechanical allodynia and thermal hyperalgesia in rats.12,21,22 Previous studies also suggested that high doses of fentanyl induced a significant delayed nociceptive hyperalgesia.10,12,23 Our study was consistent with these studies.10,12,21–23 Further, our study indicated that the extent and duration of hyperalgesia induced by fentanyl and surgery were more severe than that of fentanyl or surgery only, which is also consistent with the previous studies.12 In the present study, we not only assessed the mechanical hyperalgesia using the tail pressure test but also investigated the thermal hyperalgesia in both ipsilateral and contralateral sites to surgical incision. However, in other studies, the authors assessed the hyperalgesia only in the surgical sites.12,13 Astonishingly, the mechanical hyperalgesia by the tail pressure test and thermal hyperalgesia in contralateral sites by the plantar test apparatus were significant and obvious, almost similar to the results in ipsilateral sites. Certainly, the thermal hyperalgesia in contralateral sites was weaker than that in ipsilateral sites. These results suggest that central sensitization may largely be involved in the development of delayed hyperalgesia induced by surgery and/or perioperative fentanyl, considering the tail pressure test which reflects spinal reflex involving both sites of the spinal cord17,24 and the significant contralateral hyperalgesia.
In the present study, we also found that IL-1β, IL-6, and TNF-α significantly increased in the spinal cord and bilateral DRG, following fentanyl with or without surgical incision. It has been reported that chronic administration of morphine could activate spinal glia and upregulate TNF-α, IL-1β, and IL-6 in the spinal cord; L5 spinal nerve nerve-injury further enhanced this phenomenon8 and that perioperative infusion of remifentanil could induce activation of spinal glia and increase of spinal IL-6 and TNF-α.4 However, to the best of our knowledge, the present study reveals for the first time that acute or short-term fentanyl with or without surgery results in the increase of proinflammatory cytokines including IL-1β, IL-6, and TNF-α in both the spinal cord and bilateral DRG. Interestingly, it was found that a higher expression of IL-1β, IL-6, and TNF-α in spinal cord and bilateral DRG was induced in rats receiving both surgery and fentanyl than in those that received surgery or fentanyl only, and that the increase of IL-1β and TNF-α in ipsilateral DRG were higher than those in contralateral DRG in rats receiving surgery (Figures 3–5). These molecular changes highly corresponded to the behavioral manifestation, which indicated an association of increased cytokines in the spinal cord and DRG and hyperalgesia induced by fentanyl and/or incision, and that not only the central but peripheral mechanism has also contributed to the effect at some extent.
The mechanism of spinal and/or DRG proinflammatory cytokines involving fentanyl- and/or surgical incision–induced hyperalgesia is unclear. In the present study, we observed the increase of microglia activation (Figure 6A) in the spinal cord after fentanyl with or without surgical incision, consistent with the previous studies indicating that opiate could prime glia for activation of toll like receptor 4/Nuclear factor-κB signaling pathway and then enhanced production of proinflammatory cytokines,7,25 which may be important for the development and maintenance of hyperalgesia.4,25,26 However, it was unclear what were the possible reasons that caused the increase of cytokines in DRG involving fentanyl and/or surgical incision as we did not find any evidences of microglia activation in bilateral DRG on days 1 and 7 after drug injection. Previous studies indicated an early increase of glial fibrillary acidic protein in satellite glial cell (indicating activation of astroglia) in DRG after acute morphine injection and in a rat model of chronic constriction injury to sciatic nerve which subsequently contributed to the production of a number of proinflammatory cytokines.27,28 Combined with the evidence that there were no differences of microglia activation between both sides of spinal cord in rats receiving surgery with or without fentanyl while the level of IL-1β and TNF-α in the ipsilateral DRG was higher than that in the contralateral DRG, it suggested that other mechanisms, such as astrocyte, neuron, and vascular endothelial cells in DRG, may also contribute to the release or production of proinflammatory cytokines.29 Furthermore, proinflammatory cytokines have been confirmed to enhance neuronal activity and the abnormal activation of N-methyl-d-aspartate receptors in the spinal dorsal horn, which may provoke hyperalgesia.30,31 Recent evidence also indicates that the upregulated expression of inflammatory cytokines synthesized by DRG neurons and associated glial in the spinal cord can produce changes in the excitability of nociceptive sensory neurons.31,32 All evidence described above, thus, points to a strong association between an increase of spinal and/or DRG proinflammatory cytokines and hyperalgesia induced by fentanyl and/or surgical incision.
Another interesting finding is that proinflammatory cytokines in the contralateral DRG increased in rats that received surgery only. It is easy to understand that the proinflammatory cytokines may increase in the contralateral DRG, besides in the spinal cord and ipsilateral DRG, in rats that received fentanyl with or without surgery. However, it is unclear why surgery only can induce an increase of proinflammatory cytokines in the contralateral DRG. In the present study, we found that microglia activation similarly increased in both sides of spinal cord in rats that received surgeries only (Figure 6A), which may subsequently induce an increase of proinflammatory cytokines. However, we did not find any changes of IL-1 β or TNF-α in CSF following any interventions including fentanyl injection and/or surgery. Thus, we supposed that proinflammatory cytokines that increased in contralateral DRG might be the connection between spinal microglia and DRG by cellular junction or other unclear mechanism rather than proinflammatory cytokines diffused into CSF.
In conclusion, our study found an increased expression of proinflammatory cytokines including IL-1β, IL-6, and TNF-α in the spinal cord and bilateral DRG, and the activation of microglia in spinal cord in a rat model of plantar incision with or without perioperative fentanyl, which may contribute to the development and maintenance of mechanical and thermal hyperalgesia.
DISCLOSURES
Name: Lu Chang, MD.
Contribution: This author helped design the study, perform the experiments, collect the data, analyze the data, and write the manuscript.
Name: Fang Ye, MD.
Contribution: This author helped design the study, perform the experiments, collect the data, analyze the data, and write the manuscript.
Name: Quehua Luo, MD.
Contribution: This author helped perform the experiments and collect the data.
Name: Yuanxiang Tao, MD, PhD.
Contribution: This author helped write the manuscript.
Name: Haihua Shu, MD, PhD.
Contribution: This author helped design the study, analyze the data, and write the manuscript.
This manuscript was handled by: Jianren Mao, MD, PhD.
Footnotes
Funding: This study was supported by National Natural Science Foundation of China (Project No. 81571071) and Guangdong Provincial Natural Science Foundation of China (Project No. 2014A030313203).
The authors declare no conflicts of interest.
L. Chang and F. Ye contributed equally to this study and share first authorship.
Reprints will not be available from the authors.
REFERENCES
- 1.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–161.. [PubMed] [Google Scholar]
- 2.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014;112:991–1004.. [DOI] [PubMed] [Google Scholar]
- 3.Angst MS. Intraoperative use of remifentanil for TIVA: postoperative pain, acute tolerance, and opioid-induced hyperalgesia. J Cardiothorac Vasc Anesth. 2015;29Suppl 1S16–S22.. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Zhang W, Liu Y, Liu X, Ma Z, Gu X. Intrathecal injection of JWH015 attenuates remifentanil-induced postoperative hyperalgesia by inhibiting activation of spinal glia in a rat model. Anesth Analg. 2014;118:841–853.. [DOI] [PubMed] [Google Scholar]
- 5.Cabañero D, Puig MM. Immediate and delayed remifentanil-induced hypersensitivity. Anesth Analg. 2012;115:977–978.. [DOI] [PubMed] [Google Scholar]
- 6.Song YK, Lee C, Seo DH, Park SN, Moon SY, Park CH. Interaction between postoperative shivering and hyperalgesia caused by high-dose remifentanil. Korean J Anesthesiol. 2014;66:44–51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waxman AR, Arout C, Caldwell M, Dahan A, Kest B. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci Lett. 2009;462:68–72.. [DOI] [PubMed] [Google Scholar]
- 10.Célèrier E, Rivat C, Jun Y, et al. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–472.. [DOI] [PubMed] [Google Scholar]
- 11.Mauermann E, Filitz J, Dolder P, Rentsch KM, Bandschapp O, Ruppen W. Does fentanyl lead to opioid-induced hyperalgesia in healthy volunteers?: a double-blind, randomized, crossover trial. Anesthesiology. 2016;124:453–463.. [DOI] [PubMed] [Google Scholar]
- 12.Rivat C, Vera-Portocarrero LP, Ibrahim MM, et al. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur J Neurosci. 2009;29:727–737.. [DOI] [PubMed] [Google Scholar]
- 13.Célérier E, González JR, Maldonado R, Cabañero D, Puig MM. Opioid-induced hyperalgesia in a murine model of postoperative pain: role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology. 2006;104:546–555.. [DOI] [PubMed] [Google Scholar]
- 14.Heinl C, Drdla-Schutting R, Xanthos DN, Sandkühler J. Distinct mechanisms underlying pronociceptive effects of opioids. J Neurosci. 2011;31:16748–16756.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–872.. [DOI] [PubMed] [Google Scholar]
- 16.Shu H, Hayashida M, Arita H, et al. Pentazocine-induced antinociception is mediated mainly by μ-opioid receptors and compromised by κ-opioid receptors in mice. J Pharmacol Exp Ther. 2011;338:579–587.. [DOI] [PubMed] [Google Scholar]
- 17.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–419.. [PubMed] [Google Scholar]
- 18.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88.. [DOI] [PubMed] [Google Scholar]
- 19.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50.. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki Y, Ohsawa K, Kanazawa H, Kohsaka S, Imai Y. Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochem Biophys Res Commun. 2001;286:292–297.. [DOI] [PubMed] [Google Scholar]
- 21.Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America. 2005;23:1–20.. [DOI] [PubMed] [Google Scholar]
- 22.Yang T, Yang P, Jiang LM, Zhou RY. Activation of spinal NF-КB mediates pain behavior induced by plantar incision. Int J Clin Exp Med. 2015;8:9149–9155.. [PMC free article] [PubMed] [Google Scholar]
- 23.Van Elstraete AC, Sitbon P, Benhamou D, Mazoit JX. The median effective dose of ketamine and gabapentin in opioid-induced hyperalgesia in rats: an isobolographic analysis of their interaction. Anesth Analg. 2011;113:634–640.. [DOI] [PubMed] [Google Scholar]
- 24.Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304.. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo W, Wang H, Watanabe M, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mika J, Rojewska E, Makuch W, Przewlocka B. Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience. 2010;165:1420–1428.. [DOI] [PubMed] [Google Scholar]
- 28.Berta T, Liu T, Liu YC, Xu ZZ, Ji RR. Acute morphine activates satellite glial cells and up-regulates IL-1β in dorsal root ganglia in mice via matrix metalloprotease-9. Mol Pain. 2012;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol. 2013;716:106–119.. [DOI] [PubMed] [Google Scholar]
- 30.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462.. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;194:417–49.. [DOI] [PMC free article] [PubMed] [Google Scholar]


