Abstract
Background:
Hypertrophic scar formation is unpredictable and poorly understood, afflicting both the pediatric and adult populations. Treatment methods with conservative and invasive approaches have low rates of compliance and high rates of morbidity. The purpose of this study was to test a reproducible scar model and investigate a new technique of scar modification through the use of adipose- derived progenitor stromal cells (ASCs).
Methods:
Twenty thermal deep-partial thickness contact burns were created on the dorsum of three 8-week-old domestic swine and allowed to mature for 10 weeks. Scars were then injected with 2 cc saline, expanded autologous ASCs, or 2 cc fresh lipoaspirate and sampled at 2 week intervals up to 10 weeks postinjection. Volumetric analysis with a 3-D scanner, mechanical elasticity testing through negative pressure transduction, and standardized photography evaluation with Image J was performed. RNA sequencing was performed on scar tissue samples, cultured cells, and fresh lipoaspirate to determine relevant gene transcription regulation. Immunohistochemistry was used to verify expression level changes within the scars.
Results:
Volumetric analysis demonstrates a reduction in average scar thickness at 6 weeks when injected with ASCs (−1.6 cc3) and autologous fat (−1.95 cc3) relative to controls (−0.121 cc3; P < 0.05). A decrease in overall tissue compliance is observed with fat or ASC injection when compared with unburned skin at 8 weeks (35.99/37.94 versus 49.36 mm Hg × mm; P < 0.01). RNA sequencing demonstrates altered regulation of fibroblast gene expression and a decreased inflammatory profile when scars are injected with autologous fat/ASCs over controls.
Conclusion:
Early results suggest that autologous fat and/or ASCs may improve healing of hypertrophic scarring by altering the cellular and structural components during wound remodeling up to 20 weeks after injury. This may have beneficial applications in early treatment of large or cosmetically sensitive immature burn scars.
INTRODUCTION
Survival from thermally induced injuries has significantly improved in recent history, yet advances in aesthetic and functional outcomes inflicted by the resulting hypertrophic burn scar have not kept pace. Current modalities for the treatment of hypertrophic scars are not uniformly predictable, often relying on patient compliance and may potentially increase morbidity. The traditional surgical approach aimed at prevention of hypertrophic scarring is early excision and skin grafting of deep thermal injuries expected to require longer than 2 weeks to heal via secondary intention. Once a hypertrophic scar has developed, conservative treatment options include the use of pressure garments, massage, and silicone-based therapy, all of which are theorized to alter collagen deposition by influencing vascularity and oxygen tension.1–3 Invasive modalities consist of surgical excision and primary closure, adjacent tissue rearrangement, radiation, fractionated laser therapy, and intralesional injections of various medications.4–8
Recognition of the regenerative capabilities of adipose-derived stem cells (ASCs) in fat has lead to significant interest in their potential clinical use to modulate healing, given their sheer abundance in the donor sites, ease in harvest, and the density of pluripotent cells in fat compared with bone marrow.9 Clinically, improvement in skin quality was noted after lipofilling and recognizing its proangiogenic potential; Rigotti et al.10 and Serra-Renom et al.11 first demonstrated its effectiveness in the setting of radiodermatitis. The indications for lipofilling have since expanded to include scar contractures, acute burns, and healed burn scars.12,13
The effects of injected fat and ASCs on scar properties have been characterized using scar scales,13 histology,14 biomechanical tools,15 immunohistochemistry,14 and molecular analysis.15 Improvements in coloration, volume, pliability, and collagen arrangement have been demonstrated with both treatment modalities.13,14 On a molecular level, upregulation of vasculogenic markers and downregulation of fibrotic markers have also been documented using both techniques.16,17 However, comparative studies for fat and ACS demonstrating quantitative short- and long-term effects have not been reported and the superiority of 1 treatment modality has not been determined. Consequently, we developed a domestic porcine hypertrophic burn scar model18 to investigate the short-term effects of saline (injection trauma), injected fat, and ASCs on scar. Specifically, we sought to quantify the changes in the biomechanical properties, erythema and scar volume of hypertrophic burn scar following treatment and assess the histologic and protein expression changes following injection.
MATERIALS AND METHODS
Animal Study
The research was approved by the Institutional Animal Care and Use Committee at Cincinnati Children’s Hospital Medical Center (Protocol #1d12106). Three 8-week-old domestic swine (Sus scrofa domesticus) were purchased at around 35 kg weight and individually housed in the Animal Research Core at CCHMC. Cincinnati Children’s maintains a current Animal Welfare Assurance with the U.S. Department of Health and Human Services, Office of Laboratory Animal Welfare.
Porcine Surgical Technique
Using our established model,18 thermally induced, rectangular, deep-partial thickness contact burns were then created bilaterally approximately 5 cm from the dorsal midline. Each animal received 20 thermal wounds, 10 per side (Fig. 1). All eschars were allowed to auto-debride, and no intervention was employed to address chronic granulation tissue.
Fig. 1.
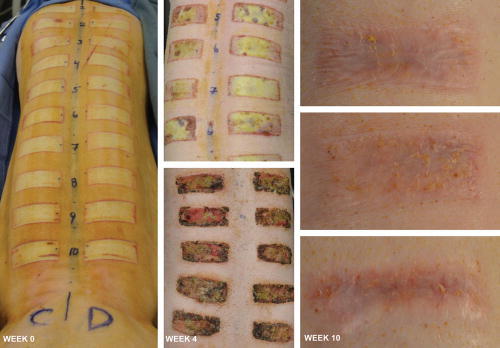
Deep-partial thickness contact burns created an eschar that persisted for 6 weeks with no intervention before resulting in raised, hyperemic scar tissue as seen by week 10.
Lipoaspirate Technique and Expansion of ASCs
Ten weeks following the initial thermal injury, 60 (n = 60) epithelialized burn scars were divided into 4 treatment groups. Eighteen scars (n = 18), 6 on each animal, were intradermally injected with: (1) 2 mL of normal saline; (2) 2 mL of resuspended ASCs or (3) 2 mL of autologous fat. Six (n = 6, 2 on each animal) scars were assigned as positive controls with no injection to maximize the number of treatment sites (Fig. 2). Scars were treated in a randomized controlled fashion along the dorsum of each animal to account for varying hypertrophic scar formation as evident in our pilot study.18
Fig. 2.

Scars were injected intradermally with 2 cc saline, expanded autologous ASCs, or 2 cc fresh lipoaspirate and sampled at 2-week intervals up to 10 weeks postinjection (20 weeks after original burn). Autologous fat cells were expanded to third passage with 2 × 106 cells resuspended in 2 cc normal saline.
To obtain autologous fat and ASCs, liposuction was performed on each animal’s ventral neck, yielding approximately 50–100 mL of lipoaspirate per animal. A superwet liposuction technique was utilized for fat harvest. The lipoaspirate fluid was separated from the adipose component. The adipose component of the aspirate was washed repeatedly with phosphate-buffered saline until the infranatant was free from blood. It was subsequently digested with collagenase I [0.1% in 1% bovine serum albumin in phosphate-buffered saline] for 30 minutes at 37°C with gentle shaking. Processed lipoaspirate (PLA) cells were pelleted by centrifugation and were resuspended in media consisting of Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) with 10% fetal bovine serum and 100X antibiotic/antimycotic, and then filtered through a sterile 100 μm nylon filter to remove debris before plating.
PLA cells were plated at a concentration of approximately 100,000 nucleated cells/cm2. All expanded cells were third passage cells. Lipoaspirate used in the immediate autologous fat injection treatment group was harvested using a closed system technique with the supernatant allowed to separate by gravity facilitating removal. Syringes will filled to 2 cc of volume without the supernatant. All treatment groups had injections of material by a Coleman micro-droplet technique. PLA cells were resuspended in 2 cc of normal saline before injection so that all treatment groups had 2 cc of total volume added to each scar.
Biomechanical and Image Analysis
Quantitative measurements were obtained from the hypertrophic scars by measuring biomechanical properties, calculating the degree of erythema using high-resolution digital photographs and assessing 3D surface topography. To measure 3D surface topography, the Artec 3-D scanner (Artec, Palo Alto, Calif.) was used to capture surface area and volume changes over time. The device was positioned approximately 48 inches from wound surface with multiple passes captured and averaged to generate a 3D model. The data were analyzed with 3dMDVultus software (3dMD, Atlanta, Ga.). Changes in wound volume were determined by subtracting the 3D surfaces for each scan from the scan taken before the first treatment. Measurements were obtained in 2-week intervals following treatment for 10 weeks for each scar.
Before analysis of the biomechanical data, we first accounted and corrected for the anatomic site variation in the dorsum of the pigs, confirming the expected differences between scars located more cranially and caudally. Biomechanical properties (Table 1) were measured at the center of each scar through 2 cycles at 10 mm Hg/s for 15 seconds with 5 seconds of relaxation between cycles using the BTC-2000 (SRLI Technologies, Nashville, Tenn.).19–21 Untreated hypertrophic burn scar skin served as the positive controls (2 per animal), and adjacent nonburned skin served as controls for the BTC-2000.
Table 1.
Biomechanical Data Definitions

RNA Sequencing
RNA sequencing was performed on all treatment groups and compared with normal skin. Additionally, 2 × 106 ASCs and 2 mL of fresh autologous adipose tissue harvested from 1 animal were sequenced. Four millimeter full-thickness punch biopsies were performed on all scars at weeks 1, 2, 6, and 10 postinjection. Samples frozen in RNase at −80° F had the RNA isolated and evaluated with the use of an RNeasy Mini-kit (Qiagen, Valencia, Calif.). RNA transcriptome gene analysis was performed and creation of a cDNA library to be compared with known genes and proteins involved in the inflammatory and wound healing cascade through the National Center for Biotechnology Information’s Entrez Gene Database (http://www.ncbi.nlm.nih.gov/gene).
Immunohistochemistry
A portion of the tissue biopsies was embedded in paraffin with immunohistochemistry performed on samples by the Cincinnati Children’s Hospital Medical Center Pathology Research Core. Myofibroblasts were stained using the antibody alpha smooth muscle actin (α-SMA), the Chromogen Substrate DAB, and the counterstain Mayer’s Hematoxylin. No antigen retrieval was required. Under light microscope 20× magnification, myofibroblast cell nuclei were manually counted on 5 separate fields within each slide and means recorded.
Statistical Analysis
The treatments were evaluated at weeks 4, 6, and 10 using linear mixed models procedures with time (week) as the repeated parameter with significance of P < 0.05 (SPSS, SPSS, Inc., Chicago, Ill.) with site, side, and pig number as covariates. Pairwise comparisons were made using least significant differences, and results were not corrected for multiple comparisons. Week 2 data were analyzed using general linear mixed models to determine initial differences since measurements were not made at baseline (week 0, the time at injection).
All data were expressed as averages and SD within each group. Student t tests were used for all other factor comparison with statistical significance accepted with a P value of < 0.05. Analysis was performed using Systat 13 (Systat Software, Inc., Chicago, Ill.).
RESULTS
There were significant differences for elasticity, elastic deformation, laxity, and ultimate deformation based on the anatomic site of the untreated hypertrophic scar (Fig. 3; Table 2). Caudal hypertrophic scars demonstrated less elasticity and elastic deformation compared with scars located more cranially. Table 2 reports the statistical results for each biomechanical characteristic for each week and treatments are coded as follows: 1 = burned untreated, 2 = saline, 3 = ACS, 4 = Fat, and 5 = normal skin (unburned).
Fig. 3.
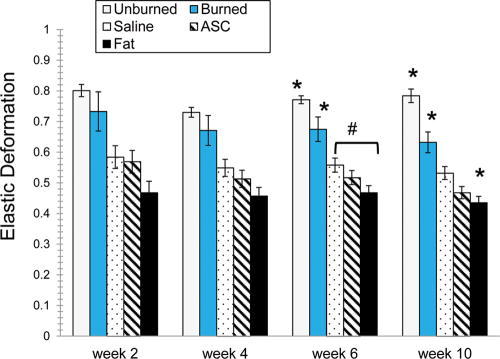
Differences in elasticity and elastic deformation varied based on anatomic site. Site 1 is the cranial end and site 10 is the caudal end on the swine’s dorsum. Site 3 was more elastic than site 7. For elastic deformation, site 9 had lower elastic deformation than all others except 10.
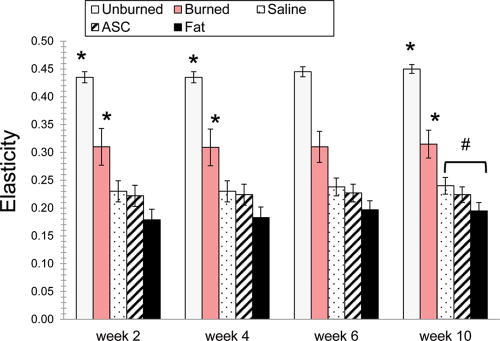
Table 2.
Biomechanical Data
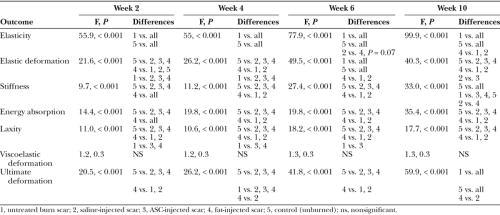
Elasticity and Elastic Deformation
At week 10, unburned skin (control) and treated burn scar demonstrated different elasticity from each other and all other treatments as indicated by * (F = 99.0; P < 0.001; Fig. 4). The saline-treated scars were more elastic than those treated with fat, as noted by #. Elastic deformation in unburned skin (control), untreated burned scar, and fat-treated burn scar graphs were each different from each other and all other treatments at week 10 (F = 40.3; P < 0.001). Scars injected with fat had significantly lower elastic deformation than saline and ASC treatments. At week 6, the fat and saline treatments were different.
Fig. 4.
All treatment groups demonstrated significantly decreased elasticity and elastic deformation compared with untreated burns through 10 weeks of sampling.
Effect of Treatment on Erythema
The surgery itself had an effect on burn scar erythema (excess erythema) as determined by comparing redness in high resolution images of scars before surgery and 2 weeks later. Excess erythema of untreated burn scars did not differ over course of observation. However, excess erythema was significantly higher for the saline and ASC injected groups (P < 0.05) and was higher for the fat treatment group (P = 0.07) 2 weeks after surgery.
At week 10, excess erythema was significantly higher for the ASC and fat injected groups versus untreated burn scar (F = 2.8; P = 0.04; Fig. 5). Similar trends were found at weeks 4 and 6 but did not reach statistical significance.
Fig. 5.
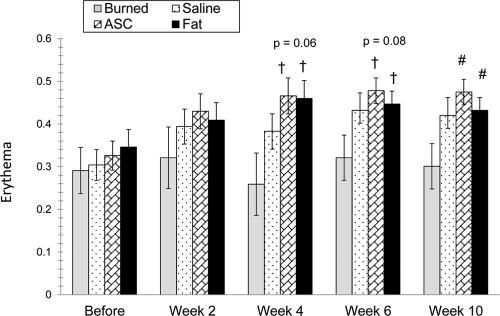
Erythema measured at week 2 demonstrates an increased amount of erythema in all treatment groups compared with burned groups alone (left). Erythema continued to be greater in injected samples up to 10 weeks (right).
Effects of Treatment on Topographical Volume
The greatest reduction in scar volume occurred from weeks 2 through 6 in scars that were injected with autologous fat and ASCs when compared with the control (−1.95 cm3 and −1.60 cm3 versus −0.12 cm3, respectively, P < 0.05; Fig. 6). Due to the rapid growth of the pigs, the scars could only be measured up to 6 weeks postinjection, prohibiting image overlay and software analysis.
Fig. 6.
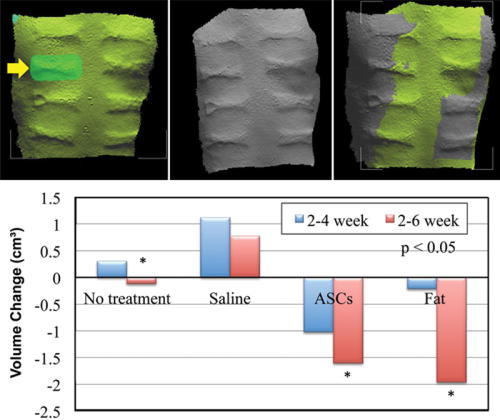
3-D volumetric analysis was performed on the scars between weeks 2 and 6. Scans were superimposed and thickness differences calculated between time points. ASCs and fat showed significant differences to no treatment with further reduction in volume of scars.
RNA Sequencing
One hundred forty-eight genes within our bioinformatics database, known to be involved with the wound healing cascade, fibroblast differentiation, and cellular signaling were analyzed. There were significant differences in gene expression levels in 99 of these known genes across treatment groups compared with untreated scar (P < 0.05; Fig. 7). The ASCs and autologous fat samples were also compared with the treatment groups. It is evident that the scars treated with autologous fat or ASC injection developed a heat signature similar to the isolated adipose or ASCs when comparing these 148 genes (Fig. 8).
Fig. 7.
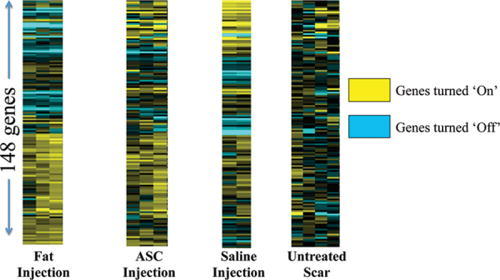
Heat map of different treatment groups. Each bar represents a different gene being expressed in a known database with clusters reflecting gene expression signatures indicative of how the added cell preparations modify the wound healing response.
Fig. 8.
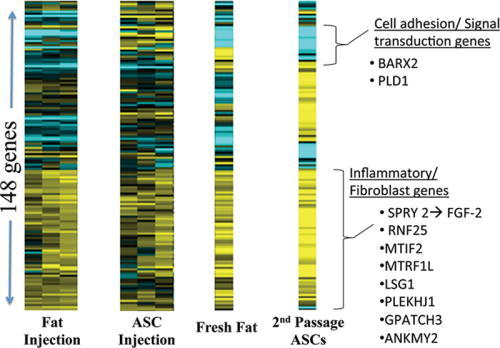
Scars injected with fat and/or ASC cells showed a stable gene expression signature most similar to cultured adipocytes, thus suggesting fat injection survivability at 10 weeks. There was also upregulation of genes involved in fibroblastic regulation.
One particular protein-encoding gene with significant differences in gene expression levels is the BARX2 gene. Known to be involved with myofibroblast migration and differentiation, the gene was upregulated in the untreated scar and saline injected scars up to 10 weeks postinjection (Fig. 9). There was more than a 2-fold decrease in expression levels of this gene when scars were injected with ASCs (0.69 ± 0.22) or autologous fat (0.79 ± .06) compared with untreated scar (1.76 ± 0.48) over 10 weeks (P < 0.03; Fig. 9). A second notable gene with upregulation in the autologous fat group and downregulation in the untreated group was the SPRY-2 gene, involved in fibroblast growth factor receptor ligand binding and activation. At 6 weeks, there was a 1.25 ± 0.45 normalized increase in expression at 6 weeks when fat injected and 1.05 ± 0.18 normalized decrease in expression in the nontreated scars at 6 weeks (P < 0.05).
Fig. 9.
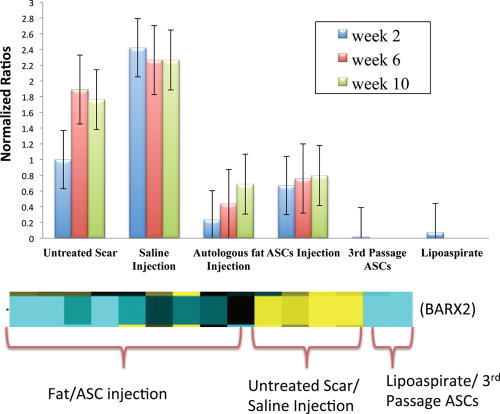
The normalized expression levels are upregulated 2–3 fold in untreated scars or saline-injected scars and a 2-fold decrease of BARX2 expression with fat or ASC injection. There is minimal BARX2 expression in ASCs or lipoaspirate alone.
Immunohistochemistry
To determine if downregulation of BARX2 after fat or ASC injection results in reduced myofibroblast presence, samples were stained with alpha-smooth muscle actin antibody (α-SMA antibody). Unburned skin showed a focus of SMA-positive endothelial cells activity at the dermal/epidermal junction (Fig. 10A). Vertical vessels were present, increased vascularity deep into the dermis, and a flattening of the papillary junction is evident at 6 weeks and 10 weeks in an untreated scar (Fig. 10B, C). Scars treated with autologous fat appear to have diminished vessel presence (Fig. 10D); however, myofibroblast cell counts did not differ significantly in the small sample size.
Fig. 10.
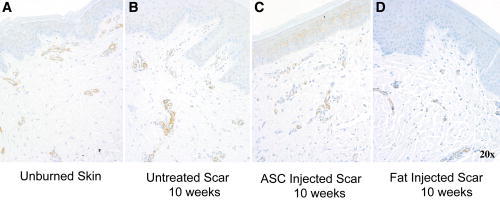
α-SMA antibody immunohistochemistry was performed with the myofibroblast staining brown. Unburned skin shows regularity of vessels at epidermal and dermal interface (A). Vertical vessels are again noted in the scars and amount of vascularity appears to be increased in untreated burns (B). Samples treated with autologous fat injections or ASCs at 10 weeks postinjection and 20 weeks postinjury appear to have decreased vessel penetration and presence (C, D).
DISCUSSION
Hypertrophic scars are described as overly projected, thickened, erythematous, indurated, fibrotic skin confined within the perimeter of the original tissue insult, the defining pathognomonic feature. Coupled with the appearance, common clinical symptoms may include pruritus, the sensation of tightness, decreased range of motion across joints and pain, ultimately causing significant morbidity.5,22 Both conservative and surgical treatment strategies have demonstrated variable success in improving these symptoms; however, the regenerative capacity of fat and its associated stem cells have shown promise as an alternative therapy. Multiple studies have documented the biomechanical improvements, histologic changes, and genetic expression of hypertrophic scar treated with fat and ASCs.23–26 Domestic swine were chosen due to the amount of tissue for analysis, similar skin characteristics to humans with regard to epidermal/dermal thickness, wound healing rates, and the propensity to develop hypertrophic scars following deep thermal injuries.27–31 The goal of this study was to quantify the changes in the biomechanical properties, color and volume following saline (injection trauma), fat and ASCs injection of hypertrophic burn scar using an established hypertrophic burn scar model in domestic swine.18 We hypothesized that the hypertrophic scars injected with fat and ASCs would improve over a 12-week period compared with untreated and saline-injected scar.
Unexpectedly, the biomechanical properties of hypertrophic scars injected with fat and ASCs did not improve over the 10 weeks following treatment. In addition, erythema also increased across all treatment groups but was statistically significant for the ASC and fat-injected scars compared with untreated hypertrophic scar. The scars injected with autologous fat and ASC, however, did show a statistically significant reduction in volume compared with the saline and control groups. Unfortunately, the rapid growth of the swine doubling of their weight over 20 weeks led to a significant elongation of the torso. Thus, our analysis of the scar volumes using image overlay after 6 weeks was not technically feasible.
The senior authors have observed clinically different results after human scars are injected with fat, whereby the scars become softer and more pliable after fat injection. We surmise that differences observed in this study are a result of increased tissue pressure resulting from injecting cells and fluid into a confined space. Scars are still in the collagen remodeling phase at 10 weeks postinjection and may have softened with further observation. To address the rapid growth of this animal model over time, we will use the Red Duroc or mini pig for further studies, which was previously cost-prohibitive in this pilot study. This will allow for longer observation times with less affect to scars by animal growth. The Red Duroc pig is also a more reliable animal model for consistent hypertrophic scar formation.32,33
Adipose-derived stem cells have regenerative capacity, are abundant, and are easy to harvest and isolate.34 Our results with RNA sequencing demonstrate that the addition of ASC or fresh lipoaspirate does have an effect on the wound healing of scars in this pig model. Interestingly, the heat signatures from samples injected with autologous fat or resuspended ASCs becomes more similar to autologous fat or ASCs alone when looking at genes involved with fibroblastic regulation. The results also suggest that even up to 10 weeks postinjection, there is a signature of gene regulation that implies the transplanted cells or downstream effects persist. This is evident again by the similar heat maps generated by the harvested fat and isolated ASCs to the autologously injected scars.
RNA sequencing measures gene expression levels or transcriptomes in a particular sample with variance leading to possible altered phenotypic expression. BARX2 is a homeobox transcription factor that is involved in myofibroblasts, cell adhesion, remodeling of actin myoskeleton, differentiation of smooth and skeletal muscle and is involved in the Wnt signaling pathway.35–38 Within hypertrophic scars, there is evidence of decreased myofibroblast apoptosis, which may contribute to the functional pathology as a result of excess contractile element production in the wound. Myofibroblasts have also been shown to produce increased deposition of extracellular matrices, and collagen I and III (Col1, Col3).23 BARX2 expression levels in scars decreased 2-fold 10 weeks following intradermal injection of ASCs or lipoaspirate. Confirmation with polymerase chain reaction of the 2-fold increase in expression levels of BARX2 is part of our ongoing studies. Although α-SMA staining appeared to demonstrate less myofibroblast penetration and localization around diminished vessel presence on light microscopy, significance could not be achieved likely due to low sample size. If the downregulation of BARX2 leads to a decrease in myofibroblast differentiation and migration, this would be expected to possibly alter overall tissue compliance and wound contracture with increased apoptosis of the proliferated fibroblastic cells in the hypertrophic scar.
Moreover, a significant study by Plikus et al.39 demonstrates evidence that myofibroblasts show plasticity and can transform to adipocytes through bone morphogenetic protein and ZFP423 pathways or when hair follicles and ASCs are present. Additionally, they showed that human keloid fibroblasts can be reprogrammed in to adipocytes when cultured with BMP2 or BMP4. Substantiating the effects of ASC treatment or influence on these embryonic pathways and mechanisms may translate to improved clinical outcomes and help determine relative time to treat in the wound healing process.
Further studies may yield relevant data on multiple other genes with significantly altered expression levels following ASC or autologous fat injection. Furthermore, SPRY-2 is known to be a down-regulator of fibroblast growth factor receptors, notably FGF-2, involved in myogenesis pathways and also cellular apoptosis.43,44 PLD1 encodes for phospholipases with cellular functions relating to inflammation, cell growth, signaling, and cellular death.45 A recent study The heatmap reveals a down regulatory effect when fat or ASCs are injected and increased expression on transcriptome levels in untreated or saline-treated scars. LSG1 is involved in cell viability. This study revealed 99 genes with significantly altered expression levels from lipoaspirate/ASC injection that may lead to changes in regulatory pathway involved in wound healing dynamics after thermal injury. Other areas of limitations to this study are many. The scars were still remodeling through the end of this study, and a longer sampling period would likely have a difference on overall tissue characteristics. As a pilot study, ongoing projects are aimed at longer observation times. It was technically challenging to place fat injections precisely into the dermis without some injected material traveling deep to dermis or out a puncture wound, so some test scars received varying amounts of treatment. Although 3 different animals were used, systemic or paracrine effects could not be accounted for as all treatment groups were performed on each animal. Further evaluation of fibrotic scar transformation will additionally focus on targeted pathways involving TGF-β/SMAD, fibroblasts, and keratinocytes. Lastly, the small amount of tissue gathered in the cone for negative pressure transduction measurements could have only represented the very surface of the scar and not overall compliance throughout. These concerns are all being addressed in current ongoing studies with the red Duroc pig model.
CONCLUSIONS
We created a reproducible, testable swine scar model in an effort to accelerate translational treatment approaches to achieve a softer, less erythematous, and flattened scar with less invasive techniques. ASC and fat injections appear to increase the rate at which erythema resolves and reduce the overall thickness of the scar in early wound healing. However, overall compliance of the tissue is decreased during wound remodeling. RNA sequencing shows persistent influence of injected fat cells, an increase in regulation of fibroblast gene expression, influence on inflammation, and demonstrates a gene expression profile in treatment groups similar to the cell of origin’s genes (ASCs).
Footnotes
Presented at the Plastic Surgery Meeting (ASPS) 2013 in San Diego, Calif. Award for Best Paper Presentation for the Research and Technology Session.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Weinstock-Zlotnick G, Torres-Gray D, Segal R. Effect of pressure garment work gloves on hand function in patients with hand burns: a pilot study. J Hand Ther. 2004;17:368. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2013;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217. [DOI] [PubMed] [Google Scholar]
- 4.Brewin MP, Lister TS. Prevention or treatment of hypertrophic burn scarring: a review of when and how to treat with the pulsed dye laser. Burns. 2014;40:797. [DOI] [PubMed] [Google Scholar]
- 5.Zurada JM, Kriegel D, Davis IC. Topical treatments for hypertrophic scars. J Am Acad Dermatol. 2006;55:1024. [DOI] [PubMed] [Google Scholar]
- 6.Walmsley GG, Maan ZN, Wong VW, et al. Scarless wound healing: chasing the holy grail. Plast Reconstr Surg. 2015;135:907. [DOI] [PubMed] [Google Scholar]
- 7.Khandelwal A, Yelvington M, Tang X, Brown S. Ablative fractional photothermolysis for the treatment of hypertrophic burn scars in adult and pediatric patients: a single surgeon’s experience. J Burn Care Res. 2014;35:455. [DOI] [PubMed] [Google Scholar]
- 8.Arno AI, Gauglitz GG, Barret JP, et al. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns. 2014;40:1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118:121S. [DOI] [PubMed] [Google Scholar]
- 10.Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409. [DOI] [PubMed] [Google Scholar]
- 11.Serra-Renom JM, Muñoz-Olmo JL, Serra-Mestre JM. Fat grafting in postmastectomy breast reconstruction with expanders and prostheses in patients who have received radiotherapy: formation of new subcutaneous tissue. Plast Reconstr Surg. 2010;125:12. [DOI] [PubMed] [Google Scholar]
- 12.Khouri RK, Smit JM, Cardoso E, et al. Percutaneous aponeurotomy and lipofilling: a regenerative alternative to flap reconstruction? Plast Reconstr Surg. 2013;132:1280. [DOI] [PubMed] [Google Scholar]
- 13.Klinger M, Caviggioli F, Klinger FM, et al. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610. [DOI] [PubMed] [Google Scholar]
- 14.Bruno A, Delli Santi G, Fasciani L, et al. Burn scar lipofilling: immunohistochemical and clinical outcomes. J Craniofac Surg. 2013;24:1806. [DOI] [PubMed] [Google Scholar]
- 15.Yun IS, Jeon YR, Lee WJ, et al. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012;38:1678. [DOI] [PubMed] [Google Scholar]
- 16.Sultan SM, Barr JS, Butala P, et al. Fat grafting accelerates revascularisation and decreases fibrosis following thermal injury. J Plast Reconstr Aesthet Surg. 2012;65:219. [DOI] [PubMed] [Google Scholar]
- 17.Sproat JE, Dalcin A, Weitauer N, et al. Hypertrophic sternal scars: silicone gel sheet versus Kenalog injection treatment. Plast Reconstr Surg. 1992;90:988. [PubMed] [Google Scholar]
- 18.Rapp SJ, Rumberg A, Visscher M, et al. Establishing a reproducible hypertrophic scar following thermal injury: a porcine model. Plast Reconstr Surg Glob Open. 2015;3:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis AM, Gerrand C, Griffin A, et al. Evaluation of clinical utility of BTC-2000 for measuring soft tissue fibrosis. Int J Radiat Oncol Biol Phys. 2004;60:286. [DOI] [PubMed] [Google Scholar]
- 20.Dobke MK, Dibernardo B, Thompson RC, et al. Assessment of biomechanical skin properties: is cellulitic skin different? Aesthet Surg J. 2002;22:260. [DOI] [PubMed] [Google Scholar]
- 21.Smalls LK, Randall Wickett R, Visscher MO. Effect of dermal thickness, tissue composition, and body site on skin biomechanical properties. Skin Res Technol. 2006;12:43. [DOI] [PubMed] [Google Scholar]
- 22.Atiyeh BS, El Khatib AM, Dibo SA. Pressure garment therapy (PGT) of burn scars: evidence-based efficacy. Ann Burns Fire Disasters. 2013;26:205. [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Zhang W, Gao J, et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi Z, Li-Na L, Qi Y, et al. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther. 2015;6:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mai TL, Allison N, Nathaniel PM, et al. Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing Tissue Eng Part A. 2013;19:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonathan R, Fabien B, Charlotte L, et al. Intradermal injection of human adipose-derived stem cells accelerates skin wound healing in nude mice. Stem Cell Res Ther. 2015;6:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan TP, Eaglstein WH, Davis SC, et al. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66. [DOI] [PubMed] [Google Scholar]
- 28.Seaton M, Hocking A, Gibran NS. Porcine models of cutaneous wound healing. ILAR J. 2015;56:127. [DOI] [PubMed] [Google Scholar]
- 29.Kim JY, Willard JJ, Supp DM, et al. Burn scar biomechanics after pressure garment therapy. Plast Reconstr Surg. 2015;136:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsaad SM, Ross EV, Smith WJ, et al. Analysis of depth of ablation, thermal damage, wound healing, and wound contraction with erbium YAG laser in a Yorkshire Pig Model. J Drugs Dermatol. 2015;14:1245. [PubMed] [Google Scholar]
- 31.Kim JS, Park S, Jeon BS, et al. Therapeutic effect of topical application of curcumin in treatment of radiation burns in a mini-pig model. J Vet Sci. 2016. Dec 30; 17(4): 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu KQ, Carrougher GJ, Gibran NS, et al. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair Regen. 2007;15:S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harunari N, Zhu KQ, Armendariz RT, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 2006;32:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22:313. [DOI] [PubMed] [Google Scholar]
- 35.Meech R, Gonzalez KN, Barro M, et al. Barx2 is expressed in satellite cells and is required for normal muscle growth and regeneration. Stem Cells. 2012;30:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang L, Hulin JA, Gromova A, et al. Barx2 and Pax7 have antagonistic functions in regulation of wnt signaling and satellite cell differentiation. Stem Cells. 2014;32:1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meech R, Gomez M, Woolley C, et al. The homeobox transcription factor Barx2 regulates plasticity of young primary myofibers. PLoS One. 2010;5:e11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makarenkova HP, Gonzalez KN, Kiosses WB, et al. Barx2 controls myoblast fusion and promotes MyoD-mediated activation of the smooth muscle alpha-actin gene. J Biol Chem. 2009;284:14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plikus MV, Guerrero-Juarez CF, Ito M, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science. 2017;355:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lito P, Mets BD, Appledorn DM, et al. Sprouty 2 regulates DNA damage-induced apoptosis in Ras-transformed human fibroblasts. J Biol Chem. 2009;284:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laziz I, Armand AS, Pariset C, et al. Sprouty gene expression is regulated by nerve and FGF6 during regeneration of mouse muscles. Growth Factors. 2007;25:151. [DOI] [PubMed] [Google Scholar]
- 42.Sethu S, Pushparaj PN, Melendez AJ. Phospholipase D1 mediates TNF alpha-induced inflammation in a murine model of TNF alpha-induced peritonitis. PLoS One. 2010;5:e10506. [DOI] [PMC free article] [PubMed] [Google Scholar]


