Abstract
Objective:
Soft-tissue sarcomas are most frequently located deep within myofascial compartments. Superficial soft-tissue sarcomas (S-STS) are relatively less common and may be managed differently than deep sarcomas because generous resection margins are often possible without sacrificing critical structures. We sought to investigate the frequency and types of soft-tissue reconstructive procedures that are required following excision of S-STS.
Methods:
We reviewed 457 consecutively treated patients with S-STS with a minimum 2-year follow-up from our prospectively maintained database between 1989 and 2009.
Results:
Mean follow-up was 10.5 years (range, 2–23). Four hundred twenty-one tumors (91%) were excised with negative margins, 38 (8.3%) had microscopically positive margins, and three (0.7%) had grossly positive margins. One patient required an amputation. In 271 (58%) patients, the wounds were closed primarily. In comparison, 93 patients (20%) required a rotation flap, 70 (15%) required a split-thickness skin graft, and 23 (5%) underwent a free tissue transfer (ie, advanced reconstructive procedure). The overall complication rate was 12%, although 43% of patients undergoing free tissue transfer developed complications (P = 0.04). An unplanned excision before referral to our center was a risk factor for local recurrence (P = 0.03) when residual tumor was recovered in the reexcision specimen pathologically.
Conclusions:
Although concern about the morbidity associated with a free tissue transfer (ie, advanced reconstructive procedure) may potentially limit the adequacy of resection in some patients with S-STS, the results of this study showed that the majority of patients had complete excisions with negative margins and primary closure. Obtaining a negative margin when excising a known or suspected S-STS rarely requires an advanced reconstructive procedure and almost never results in loss of limb.
INTRODUCTION
Soft-tissue sarcomas are mesenchymal tumors that occur most commonly in the extremities, but with a cumulative incidence of less than 1% per year.1 Although most extremity and truncal soft-tissue sarcomas are located deep within myofascial compartments, superficial soft-tissue sarcomas (S-STS) are relatively less common and differ from deep STS in several important ways.2–7 S-STS are frequently smaller3,4 than deep tumors, and as a result are associated with lower rates of distant metastasis and higher rates of disease-free survival (DFS).4,8 Resection of S-STS rarely involves critical structures, such as major nerves and arteries, which are typically involved with deeper tumors. However, due to their small size and superficial location, these lesions may be not be recognized as malignant on initial presentation and are frequently treated by marginal or intralesional resection before referral to a dedicated sarcoma center, a scenario often referred to as an “unplanned excision.”9,10 In these cases, resection margins are frequently positive necessitating further treatment.
Using the largest series of consecutively treated patients with S-STS to date, this study aims to investigate the frequency and types of soft-tissue reconstructive procedures that are required following definitive resection of S-STS. In this study, we also investigate relevant oncologic outcomes.
METHODS AND MATERIALS
Patients and Treatment
Between January 1986 and December 2009, 1,295 consecutive patients with soft-tissue sarcoma of the trunk and extremities were enrolled in the prospectively maintained database. Among these patients, 457 (35%) underwent surgical resection for a histologically proven S-STS of the extremities or trunk. We defined S-STS as a malignant mesenchymal neoplasm located exclusively superficial to the fascia of the underlying muscular compartment. This assessment was based on magnetic resonance imaging as well as clinical assessment at the time of surgery. For instance, a patient assessed as having an S-STS preoperatively but found to have fixation to or transgression through the underlying fascia was not classified as having an S-STS. Patients with Kaposi’s sarcoma and atypical fibroxanthoma were excluded. Malignant ulceration was defined as visible ulceration or fungation of the tumor through the skin.
The surgical plan for each patient was made with priority given to performing a wide excision of the tumor (or tumor bed in the case of patients undergoing revision surgery). For patients in whom free tissue transfer was expected (ie, advanced reconstructive procedure requiring a multidisciplinary surgical team), the plastic surgery team was available to perform immediate reconstruction. For patients in whom reconstruction was expected to be performed by the orthopedic oncologist (eg, basic rotation flaps, skin grafts), the plastic surgery team was not directly involved in the case. In all patients, the soft-tissue reconstruction was performed during the same operation as the resection.
Data Collection and Statistical Analysis
A retrospective patient review of a prospectively collected database was performed following Research Ethics Board approval. Data collected included patient age and gender, tumor size and location, tumor grade, treatment before referral to the sarcoma center, local recurrence following prior treatment elsewhere, utilization of adjuvant therapy, margins at definitive surgical resection (ie, closest margin, closest radial margin), and disease status at diagnosis and final follow-up. The deep margin for all patients in this study was fascia. The goal for margin of resection was 2 cm, though margins were considered negative if they demonstrated no signs of microscopically positive margins at the time of resection. The type of soft-tissue reconstructive procedure required after definitive resection was categorized as primary wound closure, skin graft alone, local rotation flap +/- skin graft, and free tissue transfer (ie, advanced reconstructive procedure) +/- skin graft. For patients who had undergone prior “unplanned excision” before referral, we examined whether they had positive resection margins and whether there was residual tumor recovered in the reexcision specimen pathologically. Once patients had been seen by a specialty sarcoma center, we examined whether surgical margins were positive or negative and noted local recurrence, overall survival (OS), and overall DFS. Finally, we determined which reconstructive surgery, if any, patients required after completing their definitive surgical excision.
Patient, disease, and treatment-related variables for patients with different types of soft-tissue reconstruction were compared using Fisher’s exact test or the chi-square test. Survival curves were calculated using the Kaplan-Meier method and survival differences were compared by the log-rank test. To investigate for differences between reconstructive type and disease variables (eg, tumor size), the log rank test was applied. Logistic regression models were also used to evaluate the effect of patient and tumor characteristics on local recurrence and OS outcomes in the form of hazard ratios; these ratios are factors that indicate the multiplicative factor of comparative risk between 2 groups.
RESULTS
Patients and Disease Characteristics
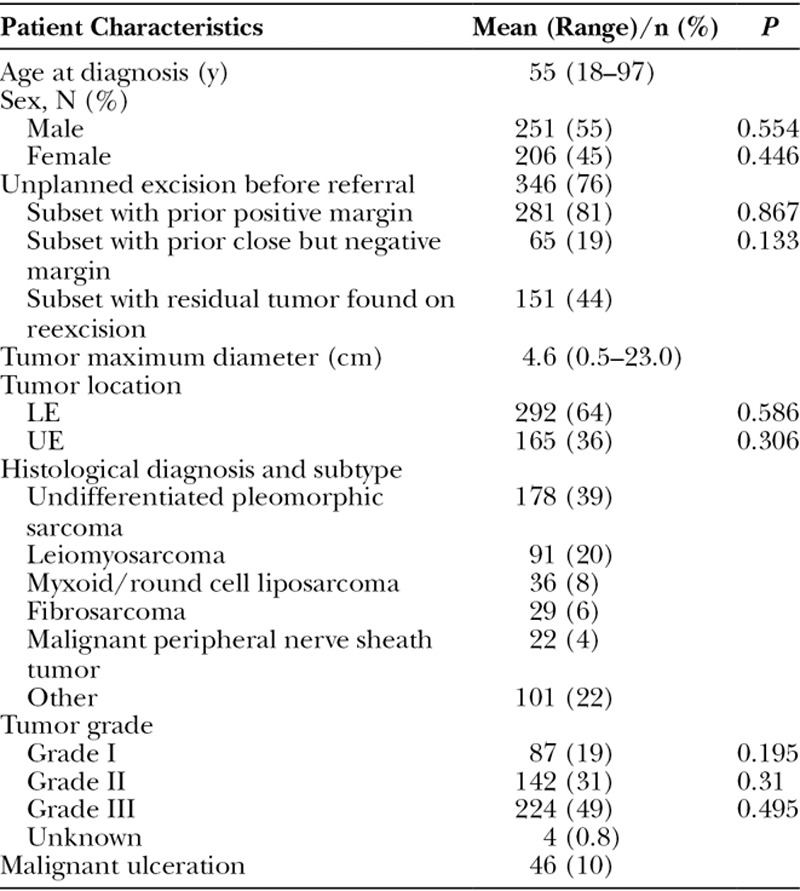
A total of 457 patients were identified in our database that had STS-E with a mean follow-up of 10.5 years (range, 2–23 years). Baseline patient and tumor features are presented in Table 1. Mean patient age was 55 years (range, 18–97 years). Three hundred forty-six patients (76%) had undergone an unplanned excision before referral to our institution; of these, resection margins were positive in 281 patients (81%). Two hundred ninety-two tumors (64%) were located on the lower extremity and 165 tumors (36%) were located on the upper extremity. Mean tumor size was 4.6 cm (range, 0.5–23 cm). The most common histologies were undifferentiated pleomorphic sarcoma (n = 178; 39%), leiomyosarcoma (n = 91; 20%), and myxoid liposarcoma (n = 36; 8%; Table 1). There were 87 grade I tumors (19%), 142 grade II tumors (31%), and 224 grade III tumors (49%). In 4 tumors, a grade was not assigned. Malignant ulceration was present at the time of surgery in 46 tumors (10%). There were no patients with known metastatic disease at the time of surgery. There were no statistically significant differences in patient and tumor characteristics.
Table 1.
Baseline Patient and Tumor Characteristics
Treatment Modalities and Reconstructive Techniques
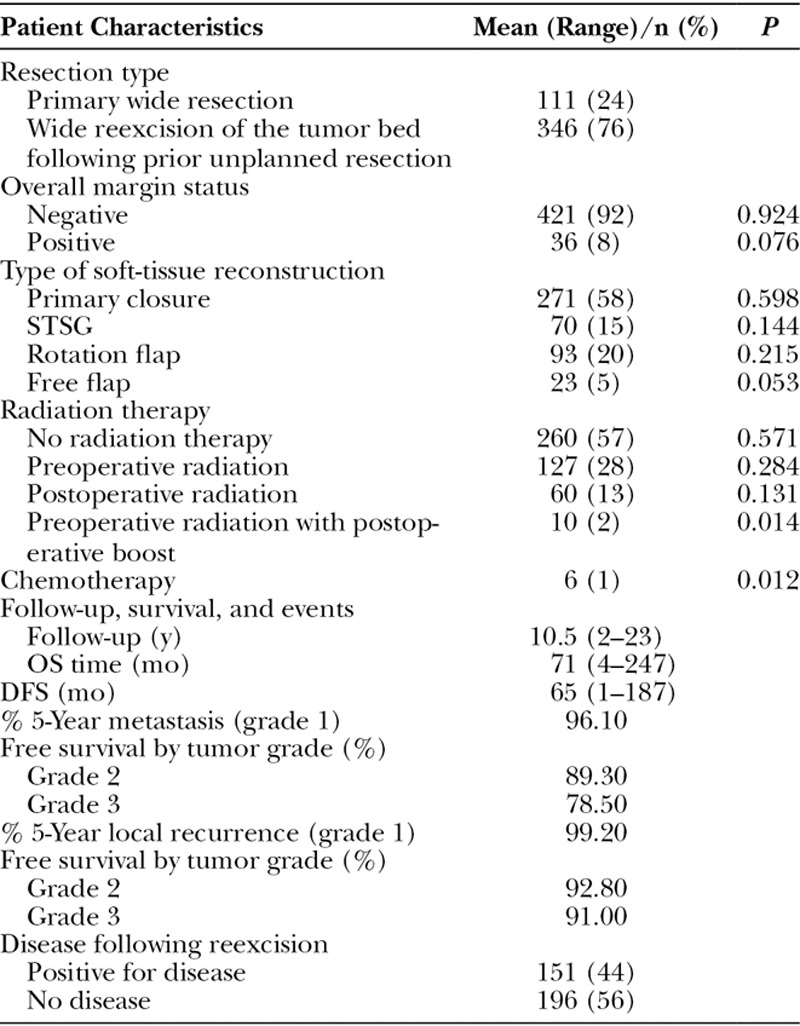
Definitive resection margins were negative in 421 patients (92%) and positive in 36 patients (8%; Table 2). The majority of patients (n = 346; 76%) underwent wide reexcision at our center following a prior unplanned resection performed elsewhere. One hundred ninety-seven patients (43%) were treated with adjuvant or neoadjuvant radiation therapy after referral to our institution, whereas only 6 patients (1%) received adjuvant or neoadjuvant chemotherapy. Two hundred seventy-one patients (58%) underwent wide resection and primary wound closure. Ninety-three patients (20%) underwent wide resection and closure with a rotation flap and split-thickness skin graft (STSG). Seventy patients (15%) underwent wide resection and coverage with an STSG alone. Twenty-three patients (5%) required a free tissue transfer for coverage of the resected site.
Table 2.
Baseline Treatment Characteristics
Complications and Outcomes
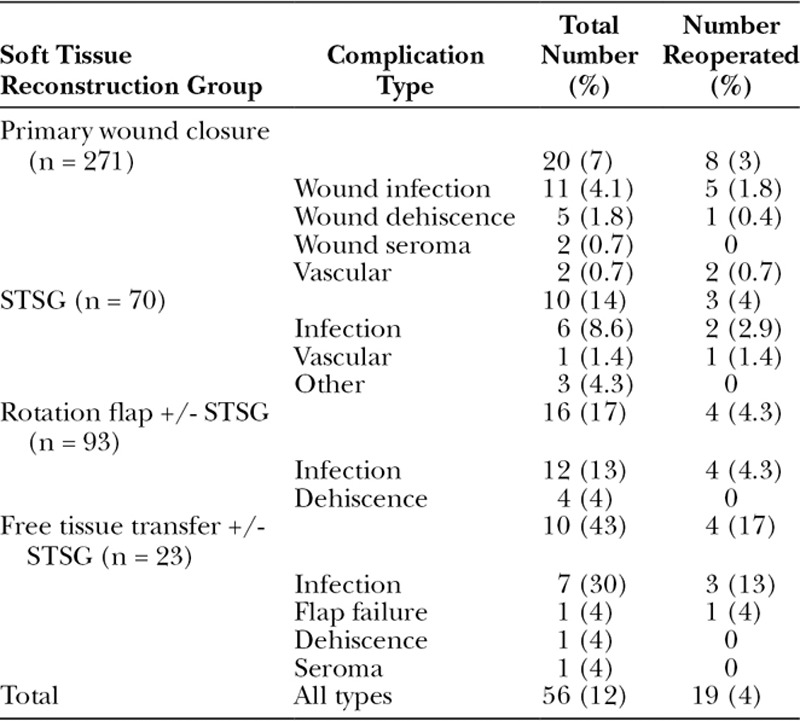
Postoperative complications by reconstruction group are listed in Table 3. Fifty-six patients (12%) had a complication directly related to surgery, and 19 (34%) of them required reoperation. Patients undergoing free tissue transfer were the most likely to have a complication (10/23; 43%; P = 0.04). In comparison, complications occurred in 16 of 93 patients (17%) treated with a rotation flap and 10 of 70 (14%) who had an STSG alone. Only 20 of 271 patients (8%) treated with primary wound closure developed a complication. However, this group included 2 patients with significant complications: One patient underwent resection of a posterior leg S-STS and, following primary closure, developed compartment syndrome. She was treated with fasciotomies and delayed wound closure. Another patient had microscopically positive margins after resection of a distal leg S-STS and developed a wound infection. She elected to have a transtibial amputation rather than another attempt at limb-preserving resection (which likely would have required a free tissue transfer) or radiation therapy.
Table 3.
Postoperative Complications Stratified by Reconstruction Group
Mean OS was 71 months with a mean DFS of 65 months (Table 2). Five-year local recurrence-free survival rates were greater than 90% for tumors of every grade (Fig. 1A; P = 0.058). Five-year metastasis-free survival was 96.1%, 89.3%, and 78.5% for grades 1, 2, and 3, respectively (Fig. 1B; P < 0.001).
Fig. 1.
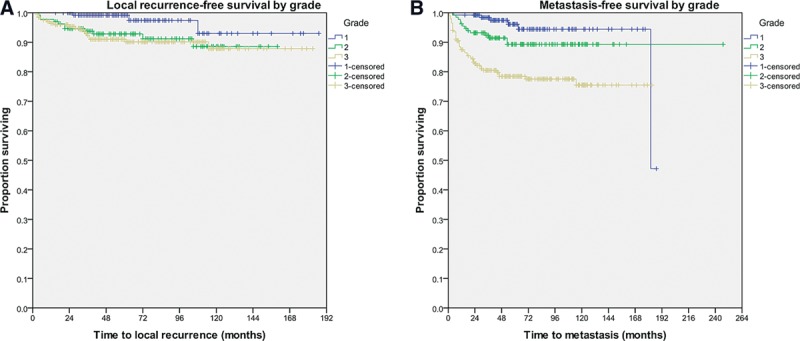
Kaplan-Meier curves illustrating 5-year local recurrence-free survival (A) and 5-year metastasis-free survival according to grade (B). A, With respect to local recurrence-free survival, patients with grades 1, 2, and 3 tumors had a survival rate of 99.2%, 92.8%, and 91%, respectively (P = 0.058). B, With respect to metastasis-free survival, patients with grades 1, 2, and 3 tumors had a survival rate of 96.1%, 89.3%, and 78.5%, respectively (P < 0.001).
Patients who underwent an unplanned excision before referral had an increased risk of local recurrence but only when residual tumor was recovered pathologically from reexcision specimen (P = 0.009; hazard ratio, 3.1). This event occurred in 151 of 346 patients (44%) and was associated with a hazard ratio for local recurrence of 5.9 when compared with those patients in whom tumor was not recovered upon reexcision. These patients were not more likely to require an advanced reconstructive procedure or to have a complication.
DISCUSSION
S-STS are underrepresented in the literature despite having significantly different biology and clinical outcomes from their deep equivalents. An oft-cited reason for inadequate margins obtained in the treatment of S-STS of the extremities is the desire to avoid the need for soft-tissue reconstructive procedures.10 However, although wide excision of S-STS may be viewed as undesirable from a reconstructive and cosmetic perspective, multiple studies have shown that obtaining an adequate resection margin is a critical for local control and an independent predictor of disease-free and metastasis-free survival.4–6,11 No data exist to describe what types of reconstructive procedures are typically required or their frequency following excision of S-STS of the extremities. Therefore, we performed a dedicated analysis of the largest series of consecutively treated patients with S-STS of the extremities to determine the utilization and complications associated with soft-tissue reconstructive procedures.
We demonstrate that limb preservation can almost always be accomplished when treating patients with S-STS. Only 1 patient in this series of 457 patients required an amputation, despite an overall complication rate of 12%. This paucity of amputations underlies an important difference between superficial and deep STS. Wound complications including infection and dehiscence following treatment of patients for deep STS often result in compromise of skeletal, neurologic, or vascular structures that are critical for limb preservation. In comparison, patients with S-STS may often require reoperation but rarely result in loss of the limb following similar complications.
At our institution, orthopedic oncologists perform STSGs and many rotation flaps. A separate plastic microsurgical reconstructive team performs more complex rotation flaps and all free tissue transfers. In the present study, only 5% of patients required a free tissue transfer. The majority of patients (58%) required only primary wound closure, and another 35% required closures that could be performed by an orthopedic oncologist. This was not due to less aggressive resection techniques, as the surgical resection margins were negative in 92% of the patients in this series.
In general, the complication rate increased with the complexity of the reconstructive procedure required. Free tissue transfer was associated with the highest rate of complications (43%) and reoperation (13%), but there were no failures of limb salvage in this treatment group. There was free anterolateral thigh flap to the dorsal forearm that failed due to thrombosis. This flap was partially debrided and the resulting wound healed by secondary intention. Although the lowest rate of complications was, not surprisingly, associated with primary wound closure, this group of patients also encountered the 2 most severe complications—1 patient required emergent fasciotomies for compartment syndrome and another patient with contaminated margins developed a severe infection that ultimately required an amputation. Two patients initially treated with primary closure required conversion to skin grafts after wound dehiscence and open wound management. These were the only 2 patients who required conversion from one reconstructive type to another.
Recently, there has been increasing attention focused on the effect of an unplanned sarcoma excision before referral for definitive treatment.10,12–15 Unplanned excisions are often the result of an understandable failure to identify a superficial mass as a sarcoma. The majority of patients in this study (n = 346; 76%) were referred to our center secondary to unplanned surgical excisions performed elsewhere, and most of them also had positive surgical margins (281; 81%), whereas a smaller group (65; 19%) had negative but very close (eg, 1–2 mm) margins. In this series, having had a prior unplanned excision did not predict the need for an advanced reconstructive procedure. It also did not generally affect oncologic outcomes although those patients in whom tumor was identified pathologically in the reexcision specimen were at higher risk to develop a local recurrence (P = 0.005). We would consider offering adjuvant radiation therapy to such patients depending on the status of their final resection margins. However, the use of radiation therapy may significantly impact reconstructive decision making and is well known to affect wound healing.16,17
The demographic characteristics of patients with S-STS in this series have several differences compared with other reports of extremity S-STS. In the present series, 68% of tumors were smaller than 5 cm in maximum diameter. Although the relative prevalence of small (ie, less than 5 cm) tumors in this series may have influenced the low rate of advanced reconstructive procedures, it should be noted that other series of S-STS had an even greater proportion of small tumors. Cany et al.5 reported that 92% of S-STS in their series of 105 patients were smaller than 5 cm, and Salas et al.7 reported that 76% of S-STS in their series of 367 patients were under 5 cm.
Using the largest reported series of consecutively treated patients with S-STS, we demonstrate that completely resected S-STS rarely require advanced reconstructive procedures. The importance of proper surgical planning and management for patients with S-STS, with an emphasis on wide negative margin excision, cannot be understated. Concerns about the need for using free soft-tissue transfers (ie, advanced reconstructive procedures) should not be a factor in limiting surgical margins or otherwise compromising a complete excision. Although advanced reconstructive techniques, when required, carry a high rate of complications, this does not compromise the likelihood of limb salvage. The biggest dilemma in dealing with S-STS remains the high proportion of patients who continue to be treated initially in the community as benign lesions and are only referred to specialty sarcoma centers following incomplete resections. This will only be improved through development and dissemination of management guidelines to facilitate early referral of a greater proportion of patients with superficial masses who actually have S-STS.18
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. World Health Organization Classification of tumours of soft tissue and bone. 20134th Edition Lyon, France: IARC Press. [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, et al. In: American Joint Committee on Cancer Staging Manual. 2010:New York, N.Y.: Springer; 291. [Google Scholar]
- 3.Brooks AD, Heslin MJ, Leung DH, et al. Superficial extremity soft tissue sarcoma: an analysis of prognostic factors. Ann Surg Oncol. 1998;5:41–47.. [DOI] [PubMed] [Google Scholar]
- 4.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689.. [DOI] [PubMed] [Google Scholar]
- 5.Cany L, Stoeckle E, Coindre JM, et al. Prognostic factors in superficial adult soft tissue sarcomas: analysis of a series of 105 patients. J Surg Oncol. 1999;71:4–9.. [DOI] [PubMed] [Google Scholar]
- 6.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14:869–877.. [DOI] [PubMed] [Google Scholar]
- 7.Salas S, Stoeckle E, Collin F, et al. Superficial soft tissue sarcomas (S-STS): a study of 367 patients from the French Sarcoma Group (FSG) database. Eur J Cancer. 2009;45:2091–2102.. [DOI] [PubMed] [Google Scholar]
- 8.Ravaud A, Bui NB, Coindre JM, et al. Prognostic variables for the selection of patients with operable soft tissue sarcomas to be considered in adjuvant chemotherapy trials. Br J Cancer. 1992;66:961–969.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel HJ, Brown O, Lopez-Ben R, et al. Unplanned surgical excision of extremity soft tissue sarcomas: patient profile and referral patterns. J Surg Orthop Adv. 2009;18:93–98.. [PubMed] [Google Scholar]
- 10.Noria S, Davis A, Kandel R, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am. 1996;78:650–655.. [DOI] [PubMed] [Google Scholar]
- 11.Pisters PW, Pollock RE, Lewis VO, et al. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg. 2007;246:675–681; discussion 681.. [DOI] [PubMed] [Google Scholar]
- 12.Umer HM, Umer M, Qadir I, et al. Impact of unplanned excision on prognosis of patients with extremity soft tissue sarcoma. Sarcoma. 2013;2013:498604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alamanda VK, Delisca GO, Mathis SL, et al. The financial burden of reexcising incompletely excised soft tissue sarcomas: a cost analysis. Ann Surg Oncol. 2013;20:2808–2814.. [DOI] [PubMed] [Google Scholar]
- 14.Kang S, Han I, Lee SA, et al. Unplanned excision of soft tissue sarcoma: the impact of the referring hospital. Surg Oncol. 2013;22:e17–e22.. [DOI] [PubMed] [Google Scholar]
- 15.Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1997;66:81–87.. [DOI] [PubMed] [Google Scholar]
- 16.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241.. [DOI] [PubMed] [Google Scholar]
- 17.Townley WA, Mah E, O’Neill AC, et al. Reconstruction of sarcoma defects following pre-operative radiation: free tissue transfer is safe and reliable. J Plast Reconstr Aesthet Surg. 2013;66:1575–1579.. [DOI] [PubMed] [Google Scholar]
- 18.Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


