Supplemental Digital Content is available in the text.
Abstract
Background:
Postmastectomy breast reconstruction (PMBR) is an elective, preference-sensitive decision made during a stressful, time-pressured period after a cancer diagnosis. Shared decision making (SDM) can improve decision quality about preference-sensitive choices. Stakeholders’ perspectives on ways to support PMBR decision-making were explored.
Methods:
Forty semi-structured interviews with stakeholders (20 postmastectomy patients, 10 PMBR surgeons, 10 PMBR nurses) were conducted. Clinicians were recruited from diverse practices across the United States. Patients were recruited using purposive sampling with varying PMBR experiences, including no reconstruction. The interview guide was based on an implementation research framework. Themes were identified using grounded theory approach, based on frequency and emotive force conveyed.
Results:
Engagement in SDM was variable. Some patients wanted more information about PMBR from clinicians, particularly about risks. Some clinicians acknowledged highlighting benefits and downplaying risks. Many patients felt pressured to make a choice by their clinicians. Clinicians who successfully engaged patients through decisions often used outside resources to supplement conversations.
Conclusions:
Patient–clinician trust was critical to high-quality decisions, and many patients expressed decision regret when they were not engaged in PMBR discussions. Patients often perceived a race- or age-related bias in clinician information sharing. Interventions to support SDM may enhance decision quality and reduce decision regret about PMBR, ultimately improving patient-centered care for women with breast cancer.
INTRODUCTION
Breast cancer is the most common noncutaneous cancer among women in the United States.1 Universal coverage for postmastectomy breast reconstruction (PMBR) has led to a steady increase in its utilization among breast cancer patients.2 PMBR can improve quality of life, psychosocial and sexual well-being.3–5 However, it also comes with significant risks.6–10 As many as half of all PMBR patients may experience wound complications and up to one-quarter may experience complications severe enough to require additional surgeries.6,11
PMBR is an elective, preference-sensitive decision made during a stressful, time-pressured period after a cancer diagnosis. In order for patients to make high-quality decisions that are both informed and preference-concordant, accurate information about reconstruction procedures, their alternatives, and potential outcomes is critical.12,13 Unfortunately, 70% of patients considering PMBR may have knowledge deficits regarding its risks and benefits.14 Fewer than half of the patients report making high-quality PMBR decisions that are informed and consistent with patient preferences.13 Furthermore, there may be racial disparities in PMBR knowledge and receipt of PMBR surgery.15,16 Inadequate knowledge and choices inconsistent with preferences can lead to decisional regret, reduced quality of life, and secondary, costly procedures.17,18
To improve patient-centered care for women with breast cancer, additional research is needed on ways to support women’s preference-sensitive PMBR decisions. Shared decision making (SDM), a process of engaging patients in health decisions with multiple medically appropriate treatment options, can improve decision quality about preference-sensitive choices especially when facilitated by decision aids (DAs).19,20 As a first step toward developing a DA for PMBR, this study sought to explore stakeholders’ perspectives on ways to support PMBR decision-making through a series of semi-structured qualitative interviews.
METHODS
Theoretical Framework
Interview guides for patients and clinicians were developed based on an implementation research framework,21 as the ultimate goal of this work is to implement evidence-based approaches to SDM for PMBR. Stakeholders were engaged to identify existing facilitators and barriers to SDM for PMBR. Qualitative interviews were conducted for this phase of the project to capture in-depth experiences and perspectives of stakeholders, consistent with our implementation research framework. Qualitative research provides a foundation for understanding attitudes and beliefs about topics beyond what can be captured in quantitative surveys. It allowed stakeholders to openly discuss PMBR decisions from their own perspectives, rather than responding in ways driven by researchers. Results of this phase of the project will inform future work in a mixed-methods approach to study PMBR decisions.
Interview guides were revised after obtaining feedback from experts in the field of SDM and breast reconstruction, and pilot testing with volunteer participants. Questions explored knowledge about SDM and DAs, preferences for PMBR, and experience with patient–clinician discussions about PMBR decisions. Interview guides are displayed in supplemental online material (see pdf, Supplemental Digital Content 1, which displays a interview guide for patient and clinician stakeholders, http://links.lww.com/PRSGO/A609).
Study Population and Recruitment
Stakeholders included postmastectomy patients, plastic surgeons who perform PMBR, and perioperative nursing staff who care for surgically treated breast cancer patients. Interviewers had no prior relationship with their interviewees. This study was approved by the Washington University Institutional Review Board and Siteman Cancer Center’s Protocol Review Management Committee. Stakeholders completed informed consent. After informal conversations with surgeons, it was determined that incentives for surgeons would not impact the study; given budgetary limitations, only nurses and patients were remunerated for participation. Semistructured interviews, ranging from 17 to 58 minutes, were conducted between March and June 2017 by trained interviewers (J.H. and S.P.) supervised by an experienced qualitative researcher (M.P.). Interviews were audio recorded and transcribed verbatim. Recruitment continued until thematic saturation was reached, based on standard approaches to sampling strategies in qualitative research.22
Patients
Patients were purposively sampled from a database of those who had undergone mastectomy between January 1, 2016, and January 1, 2017, at 1 institution. Inclusion criteria for patients were female, ≥ 18 years of age, mastectomy for breast cancer ≤ 5 years before enrollment, no metastatic disease, could provide written consent in English, and completed surgery [as applicable: (1) formation of breast mound and symmetry procedure; (2) placement of nipple-areola reconstruction in cases of skin-sparing mastectomy; (3) patients 1 year from surgery if they did not receive postoperative chemotherapy, 18 months from surgery if they received postoperative chemotherapy, 2 years from surgery if they received radiation]. Some patients in the no reconstruction group had been referred to reconstructive surgeons before deciding not to have reconstruction; others only saw a breast surgeon without referral.
A chart review of 135 patients was conducted to determine agreement with enrollment criteria. Letters were sent to 81 participants to inform them of the study. We directly contacted 33 of those 81 patients based on their clinical characteristics, to achieve diversity in perspectives about reconstruction experiences. Of those, 20 agreed to participate, a 61% response rate of those contacted directly. African Americans were purposively overrecruited to explore preferences and perceived challenges when making PMBR decisions. Diversity in reconstructive experience (none, immediate/delayed, prosthetic/autologous reconstruction) was targeted (Table 1). All delayed reconstruction patients chose this option initially; none were failed prior reconstruction patients. Participants were recruited using mailed letters and phone calls, eligibility was confirmed by phone, and interviews were scheduled by phone or in person, according to patient preference.
Table 1.
Characteristics of Study Sample (n = 40)
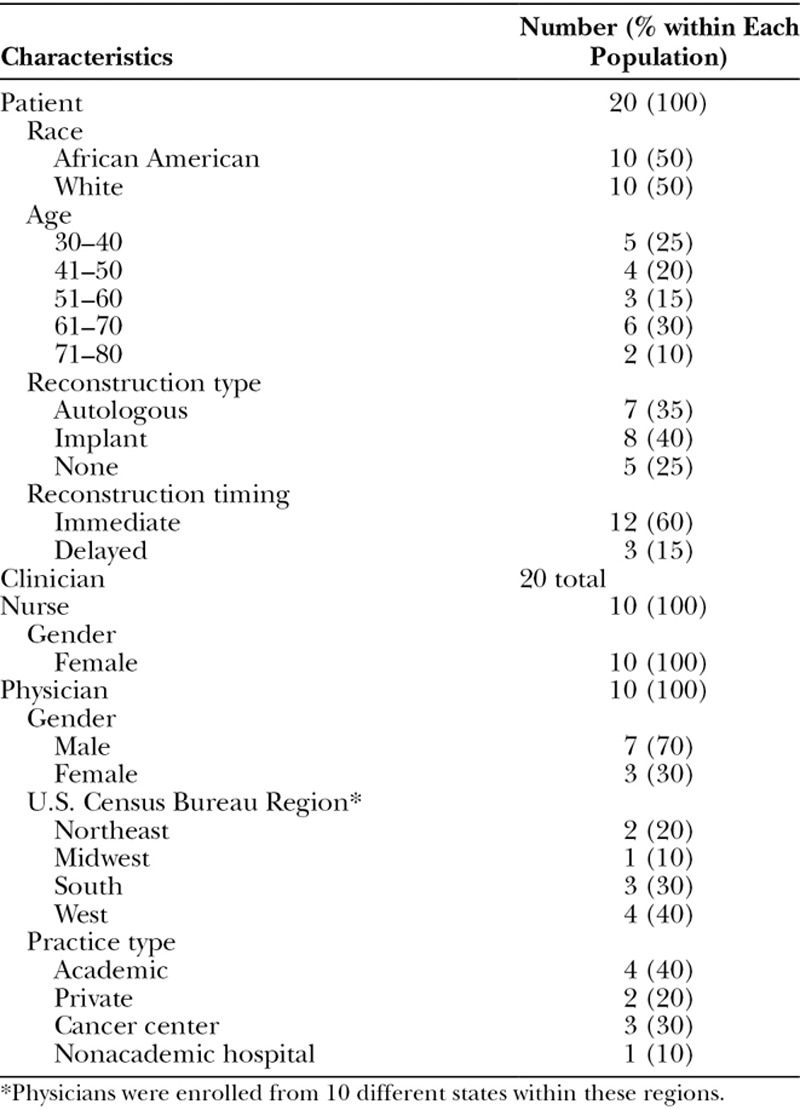
Clinicians
Plastic surgeons and nursing staff were enrolled using purposive and snowball sampling.23 Surgeons from various geographic areas in the United States and diverse practices (private/academic, rural/urban) were initially recruited via e-mail lists of national organizations. They could also refer us to others with expertise counseling patients about PMBR. Our goal was to recruit those across settings and geographic regions to provide a range of experiences. Nurses who treat breast reconstruction patients perioperatively or postoperatively were recruited via e-mail or in person from diverse areas of care at 1 academic institution. Inclusion criteria for clinicians included the following: performs PMBR or involved in perioperative care of surgically treated breast cancer patients and able to provide written consent in English. Screening and interviews were completed by phone.
Data Analysis
Nvivo 11 software (QSR International Pty., Ltd.) was used to code transcripts. Transcripts were reviewed, and preliminary codebooks were developed based on emerging themes and interview guides. Two team members coded 5 patient interviews each (J.H. and V.G.) and 5 clinician interviews each (S.P. and V.G.). The coding process was discussed, and the codebook was refined. Coders came to consensus on inconsistent codes. If consensus could not be reached, a third coder (M.P.) was consulted. Once coders obtained interrater reliability (kappa > 0.8; percent agreement > 97%), remaining transcripts were coded independently. Themes were identified using grounded theory approach,24 based on frequency of codes and emotive force conveyed.
RESULTS
Eighty-one potential patient participants were mailed letters; 33 of those mailed were also contacted by phone (15 answered). Nine enrolled from the mailed letters, and 11 who were reached by phone enrolled. Reasons for nonparticipation included transportation difficulties, not wanting to influence others by sharing experiences, and not wanting to relive experiences. Seventeen surgeons were contacted; 10 enrolled (58.8%). Fourteen nurses were contacted; 10 enrolled (71.4%). Clinicians cited time or scheduling constraints as reasons for nonparticipation.
A total of 40 interviews were conducted (20 patients, 20 clinicians). Table 1 describes sample characteristics. Table 2 outlines preexisting stakeholder knowledge of SDM and DAs.
Table 2.
Preexisting Stakeholder Knowledge of Concepts
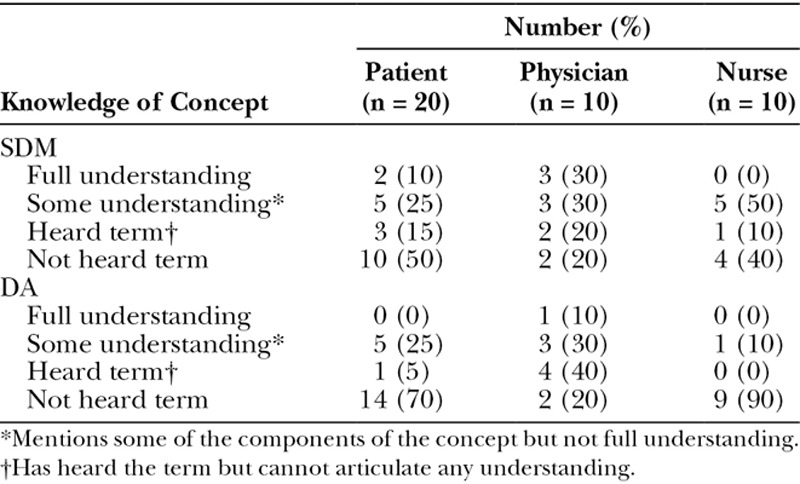
The main themes that emerged from the data are described below with illustrative quotes (P = patient, C = clinician).
Theme 1: Engagement in SDM is variable.
Stakeholders discussed widely varying levels of practice of, or participation in, SDM. Some described a very patient-centered process of engagement for breast reconstruction decisions.
“When I asked, ‘What would you suggest?’ He says, ‘I will give you all the support that you need, no matter what your decision is.’ That meant everything to me. He did not push me one way or the other. It was totally my decision how I wanted it done.” [P9, Delayed, autologous reconstruction]
“She said, ‘You can never ask too many questions.’ I said, ‘Okay, well let me ask then.’ I kept asking and talking...Whatever I feel comfortable with, she’s okay with...” [P18, Immediate, implant-based reconstruction]
“…you need to know where your patient wants to head… If you don’t listen to what they want, then they’re not gonna be happy in the end.” [C11]
However, many patients described feeling like they did not have a choice, even during this preference-sensitive decision.
“All the decisions are his [plastic surgeon]. I don’t think I have any decisions at this point. It’s whatever he wants to do, and now, he’s gonna do it.” [P4, Immediate, autologous and implant-based reconstruction]
“I do not feel like it was a shared decision. I felt like I was put out there. I was given a cookie cutter plan... It wasn’t about me.” [P12, Immediate, implant-based reconstruction]
“Nobody told me I had an option…There was never a lot of discussion on the options.” [P19, no reconstruction]
Some clinicians corroborated patients’ sentiments about clinician-dominated discussions.
“I don’t think the patients are really capable…of really understanding what they want. I think that it’s our job to guide them.” [C10]
“Not necessarily that it’s pushed on them, but…It’s expected that you go there [to have reconstructive surgery]… I don’t know if they [patients] always know that they have the option to just say, ‘I just want the mastectomy.’” [C12]
Theme 2: Stakeholders described many barriers to SDM, including limited information-sharing, clinician pressure, and clinician biases.
When SDM was not occurring, patients frequently wanted much more information about breast reconstruction, especially regarding the potential risks of complications.
“I was not aware of it [risk of implant-associated anaplastic large cell lymphoma]. We didn’t talk about that before those went in…the last thing you want from your cancer treatment is cancer.” [P16, Immediate, implant-based reconstruction]
“There was a lot I didn’t know. I didn’t research. I didn’t understand.” [P7, no reconstruction]
“Well, I thought it would just be, you know, just the recovering pain. I didn’t think about, hey, it’s gonna mess with other muscles in your body. It’s gonna cause you not to be able to raise your arm.” [P4, Immediate, autologous and implant-based reconstruction]
Many clinicians acknowledged highlighting benefits and downplaying risks.
“Yeah. I do address these things…[but] sometimes I brush over it a little bit.” [C1]
“Pain, complications…I don’t really tell them about that.” [C10]
Patients often felt pressure from their clinicians to choose 1 option or another.
“I said [to my doctor], ‘Well, can I think about it? Can I call you?’ He said, ‘No, you have to make a decision’… When he told me you just have to [decide]…I said, ‘Okay, let’s do it.’” [P18, Immediate, implant-based reconstruction]
“He [plastic surgeon] said…’You’ve got to make the right decision here. We need to do both sides…Please, let’s do both sides!’” [P1, Immediate, implant-based reconstruction]
Clinicians also referenced leading patients toward an option.
“At the end of the day, we have to be doctors. I don’t hesitate to point the patient in the right direction where they need to go.” [C10]
“…those women have so much on their mind…I think it’s easier for us to guide them to something, rather than them choosing.” [C12]
Some patients felt that clinicians were biased in their presentation of options because of the patient’s age, race, or socioeconomic status.
“I would’ve gladly talked with somebody…about it [reconstruction]…but that never happened. I guess they [clinicians] felt like a woman my age, you don’t matter… I was a poor, old, black lady, and it didn’t matter whether I had one breast….” [P19, no reconstruction]
“…he [plastic surgeon] said, ‘Well, you’re old anyhow, so what difference does it make?’ Isn’t that cruel? Those were the words.” [P1, Immediate, implant-based reconstruction]
“I don’t mean to sound—how can I say this professionally? I can’t find as many African American women doin’ it [reconstructive surgery].” [P7, no reconstruction]
Lack of SDM often led to decision regret.
“I regret that I didn’t have both of them removed at the time. Had I known what I know now…I would’ve had both breasts removed.” [P7, no reconstruction]
“If I’d known…I’d probably have taken the fat from somewhere else…” [P2, Immediate, autologous reconstruction]
“If anyone had actually explained to me about options, I possibly would have reconsidered a reconstruction.” [P19, no reconstruction]
“I did not research…had I did, I’d’a never made this decision…I woulda made so many better choices… if I knew…” [P4, Immediate, autologous and implant-based reconstruction]
Theme 3: SDM was particularly challenging when patients and clinicians disagreed about the best PMBR option for a patient. However, those who engaged in SDM during disagreements often ended up with more satisfied patients.
“Even being a [health professional], physicians intimidate me...I’m not one who’d speak up. I would probably switch doctors…but they wouldn’t let me [switch]. It made me angry.” [P17, Delayed, autologous reconstruction]
“I was just goin’ along, like, ‘Okay, okay.’ Then I didn’t get the results I wanted…now after what I’ve been through I would just say, ‘I would like to get a second opinion.’” [P21, Immediate, autologous and implant-based reconstruction]
“I said, ‘This is what I’ve been thinking about.’ She [the surgeon] was like, ‘Why?’…Again, don’t depend on them [clinicians] to tell you. You go do your research…” [P12, Immediate, implant-based reconstruction]
When challenges to SDM during times of disagreement were overcome, SDM was an effective technique to provide patient centered care and increase patient satisfaction.
“I was like, ‘No, we’re not doing that.’… he [plastic surgeon] was very understanding and open to it, and let me make the choice…He wasn’t mad or anything about it…I didn’t feel like he would hold it against me…He was very, very respectful of the response that I gave him…I think it was a really good decision. I’m very happy…” [P20, Delayed, autologous reconstruction]
“…she [surgeon] told me all about the whole process of having reconstructive surgery. I said no. No matter what she thought…she told me it was my decision...[when] I disagreed with anything she said or recommended—she said, ‘It’s up to you.’...I’m very happy with my decision.” [P15, no reconstruction]
“The bottom line is it’s your decision and if that’s what you choose to do that’s fine. Let’s just go over everything and make sure that you do know that I think this is the best option, but this [alternative] is what you chose, and that’s fine.” [C19]
Theme 4: Stakeholders described factors that facilitated SDM, including patient–clinician trust, time during and outside consultations, an engaged care team, and supplemental resources used outside of the clinic visit.
Patients who described trust in their clinicians felt decision-making was a shared experience.
“Like I said, she [surgeon] was open. I could sit there and talk to her just, not only as a doctor but as a friend. She always listened. She always looked at you when you talk. That was so very important to me.” [P22, no reconstruction]
“…the surgeon was so great about explaining it. I just felt comfortable. I was actually feeling blessed that I had a surgeon… I could talk to and not be intimidated by.” [P17, Delayed, autologous reconstruction]
Additionally, availability of time for consideration of options was described as an enabler to SDM by all stakeholders.
“Nothing was held back. Anything I asked was answered. I got plenty of time which was important to me…” [P17, Delayed, autologous reconstruction]
“…They [clinicians] make you feel like you’re the only one there in that office that day…They never rush you. You never felt like you were keeping them from someone else.” [P8, Immediate, implant-based reconstruction]
“…If you wanna discuss this again, you can call me, or you can come back, and we can go through it again. I think the most important thing is really just time and availability.” [C2]
Supplemental resources outside of the clinician/patient conversation and consultation were useful to increase engagement in SDM.
“It’s a long book [received from a friend], but it takes you step-by-step, telling you what the different kinds of breast cancer are, and what that means to you…it was the best tool that anybody could give me.” [P1, Immediate, implant-based reconstruction]
“I got online and Googled pictures, to see what that kind of reconstruction that I had looked like...” [P9, Delayed, autologous reconstruction]
“I came up with my decision…based off the information that I gathered off the internet, and just lookin’ at his [plastic surgeon’s] lil brochure.” [P4, Immediate, autologous and implant-based reconstruction]
“…we have a webinar to view…We send them an email link ahead of time…we have a…packet that we also give them in writing that goes over all the risks.” [C11]
“For [SDM] to really work, I feel like they have to be a well-informed patient. Whether that’s information that I’ve just provided, which can be overwhelming in one sitting, whether that’s information that they look up ahead of time…” [C16]
One caveat to the utility of supplemental resources was the credibility or applicability of the information accessed.
“I spent about five minutes on the internet. It scared me to death…If you want to call it a resource or a scare tactic, I’m not sure…” [P13, Immediate, implant-based reconstruction]
“…in the Googling and searching that I did online, I saw very little people—women of color that had had implants or reconstruction all together…Also, being larger… I didn’t quite know what that would translate like to a larger body.” [P10, Immediate, implant-based reconstruction]
“the patients…they take everything off of the Internet. It just causes so much more anxiety and so much pressure. I think that we need to actively push back against that with real conversations or descriptions from patients who’ve gone through the whole experience.” [C11]
DISCUSSION
Overall, clinician and patient stakeholders generally felt that SDM during PMBR choices was important. Some providers did an excellent job engaging patients in PMBR decisions and providing resources to support the decision process. However, this study suggests that SDM is variably implemented in practice, consistent with past research in other contexts.19,25,26 When SDM was not implemented, patients felt disappointed with the lack of information, particularly about potential PMBR risks. Clinicians acknowledged downplaying risks in some circumstances as well. Even in instances where knowledge was sufficient, power imbalance remained a barrier to participating in SDM.27 Patients often felt pressured to make choices, particularly when they felt that clinicians had age- or race-related biases regarding candidacy for PMBR. Some clinicians’ words were recalled by patients as being paternalistic, condescending, and dismissive. Many of these statements were quite alarming. Although there are situations where individual patient risk factors or treatment plans may limit available PMBR options, when these factors are not communicated clearly to patients, patients may feel less satisfied with their choices and PMBR outcomes.28 In fact, many patients expressed decision regret when they were not engaged in PMBR discussions. Clinicians’ communication style and approach to patient engagement clearly impacted patients’ recall of their PMBR experience.
Stakeholders described supplemental information sought outside of the consultation as useful for supporting patients’ choices. Concordant with expectancy theory,29 frequently referenced information that was desired included representative and realistic pictures specific to the type of reconstruction, race and build of the individual, highlighting scarring or lack thereof; and information from survivors about their experiences, specifically what to expect postoperatively. Representative photographs were particularly important for African American and older patients. Although previous studies have shown disparities in knowledge regarding and receipt of PMBR surgery by African Americans,15,16 this study is the first to our knowledge to describe how older and African American women may feel excluded from conversations and resources about PMBR. SDM and patient-centered communication across all demographics may help address previously identified disparities in PMBR knowledge and outcomes.
Patient–clinician trust was also critical in supporting patient choice and decreasing decision regret, particularly when patients and clinicians initially disagreed about PMBR. Patient stakeholders communicated a reluctance to disagree with clinicians, which may be attributed to fear that disagreement would not be socially acceptable.30 Clinician stakeholders noted disagreements could be “disappointing” or “frustrating.” When clinicians and patients successfully managed potential disagreements about PMBR choices through SDM, patients described feeling more confident in their choices and the patient–clinician relationship. An engaged care team, adequate time and availability, and supplemental information were all identified in this study as facilitators to SDM, and could be particularly useful tools during times of patient–clinician disagreement.
The results of this study should be viewed through a qualitative lens; although they provide in-depth perceptions that are both rich and meaningful, they do not necessarily reflect perceptions of all PMBR patients and clinicians. Additionally, patients who chose not to participate may have been different and more disadvantaged with their reconstruction experience. As a result, the findings here may not capture the more extreme cases of breast reconstruction decisions. Recruitment of only plastic and reconstructive surgeons was done to seek expertise in how to counsel women about reconstruction options. However, this may have limited perspectives since, in some settings, breast surgeons may do the initial counseling with patients about their reconstruction options. Although the surgeons were recruited nationally, patient and nurse stakeholders were recruited from a single academic institution.
Overall, these results suggest that interventions to support SDM may enhance decision quality and reduce decision regret about PMBR. Development of a DA that integrates patient-specific clinical features with patient preferences, and provides frequently sought information as identified by stakeholders, may facilitate a more standard approach to SDM for all patients, in a way that also respects patient diversity.31 Such interventions may help manage patient–clinician discussions and their relationship during this preference-sensitive choice, ultimately improving patient-centered care for women with breast cancer contemplating breast reconstruction.
ACKNOWLEDGMENTS
The authors acknowledge Margaret A. Olsen, PhD, and Clara N. Lee, MD, for their contribution to project conception and design.
Supplementary Material
Footnotes
Ms. Grabinski and Ms. Philpott contributed equally to this work.
To be presented at the Society for Medical Decision Making, 39th Annual Meeting, October 22, 2017, Pittsburgh, Pa.
Supported by the Siteman Cancer Center through a Siteman Investment Program Pre-R01 Award, funded by the Cancer Frontier Fund through the foundation for Barnes-Jewish Hospital and Siteman Cancer Center, to Drs. Myckatyn and Politi. Dr. Parikh is supported by a National Institutes of Health Institutional National Research Service Award (T32CA190194).
Ms. Grabinski was supported by a grant from the Siteman Cancer Center Leah Menshouse Springer Summer Opportunities Program. This study was approved by the Washington University Institutional Review Board (IRB# 201701045) and Siteman Cancer Center’s Protocol Review Management Committee.
Dr. Politi has a research contract (2017–2019) and previously (2016) received a speaker fee from Merck Sharpe & Dohme, both on topics unrelated to this article. Dr. Myckatyn is a consultant for and received investigator-initiated grant funding from Allergan Medical, Acelity, RTI Surgical, on topics unrelated to this article. The Article Processing Charge was paid for by The Siteman Investment Program Pre-R01 award.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.American Cancer Society, Cancer Statistics Center. 2017. Breast: at a glance; Available at https://cancerstatisticscenter.cancer.org/#/cancer-site/Breast. Accessed July 12, 2017. [Google Scholar]
- 2.Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150:9–16.. [DOI] [PubMed] [Google Scholar]
- 3.Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg. 2013;132:201e–209e.. [DOI] [PubMed] [Google Scholar]
- 4.Donovan K, Sanson-Fisher RW, Redman S. Measuring quality of life in cancer patients. J Clin Oncol. 1989;7:959–968.. [DOI] [PubMed] [Google Scholar]
- 5.Jagsi R, Li Y, Morrow M, et al. Patient-reported quality of life and satisfaction with cosmetic outcomes after breast conservation and mastectomy with and without reconstruction: results of a survey of breast cancer survivors. Ann Surg. 2015;261:1198–1206.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen MA, Nickel KB, Fox IK, et al. Incidence of surgical site infection following mastectomy with and without immediate reconstruction using private insurer claims data. Infect Control Hosp Epidemiol. 2015;36:907–914.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts A, Baxter N, Camacho X, et al. Once is rarely enough: a population-based study of reoperations after postmastectomy breast reconstruction. Ann Surg Oncol. 2015;22:3302–3307.. [DOI] [PubMed] [Google Scholar]
- 8.Olsen MA, Chu-Ongsakul S, Brandt KE, et al. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg. 2008;143:53–60; discussion 61.. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AA, Broderick KP, Belz J, et al. Uneventful versus successful reconstruction and outcome pathways in implant-based breast reconstruction with acellular dermal matrices. Plast Reconstr Surg. 2016;138:173e–183e.. [DOI] [PubMed] [Google Scholar]
- 10.Basta MN, Gerety PA, Serletti JM, et al. A systematic review and head-to-head meta-analysis of outcomes following direct-to-implant versus conventional two-stage implant reconstruction. Plast Reconstr Surg. 2015;136:1135–1144.. [DOI] [PubMed] [Google Scholar]
- 11.Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: a claims-based analysis. Ann Surg. 2016;263:219–227.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CN, Belkora J, Chang Y, et al. Are patients making high-quality decisions about breast reconstruction after mastectomy? [outcomes article]. Plast Reconstr Surg. 2011;127:18–26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CN, Deal AM, Huh R, et al. Quality of patient decisions about breast reconstruction after mastectomy. JAMA Surg. 2017;152:741–748.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CN, Ubel PA, Deal AM, et al. How informed is the decision about breast reconstruction after mastectomy?: A prospective, cross-sectional study. Ann Surg. 2016;264:1103–1109.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alderman AK, Hawley ST, Janz NK, et al. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population-based study. J Clin Oncol. 2009;27:5325–5330.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sisco M, Du H, Warner JP, et al. Have we expanded the equitable delivery of postmastectomy breast reconstruction in the new millennium? Evidence from the national cancer data base. J Am Coll Surg. 2012;215:658–666; discussion 666.. [DOI] [PubMed] [Google Scholar]
- 17.Sheehan J, Sherman KA, Lam T, et al. Association of information satisfaction, psychological distress and monitoring coping style with post-decision regret following breast reconstruction. Psychooncology. 2007;16:342–351.. [DOI] [PubMed] [Google Scholar]
- 18.Fischer JP, Fox JP, Nelson JA, et al. A longitudinal assessment of outcomes and healthcare resource utilization after immediate breast reconstruction—comparing implant- and autologous-based breast reconstruction. Ann Surg. 2015;262:692–699.. [DOI] [PubMed] [Google Scholar]
- 19.Zeuner R, Frosch DL, Kuzemchak MD, et al. Physicians’ perceptions of shared decision-making behaviours: a qualitative study demonstrating the continued chasm between aspirations and clinical practice. Health Expect. 2015;18:2465–2476.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barry MJ, Edgman-Levitan S. Shared decision making—pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781.. [DOI] [PubMed] [Google Scholar]
- 21.Proctor EK, Landsverk J, Aarons G, et al. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health. 2009;36:24–34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creswell JW. Qualitative Inquiry and Research Design: Choosing Among Five Traditions. 1998Thousand Oaks, CA: Sage Publications. [Google Scholar]
- 23.Marshall MN. Sampling for qualitative research. Fam Pract. 1996;13:522–525.. [DOI] [PubMed] [Google Scholar]
- 24.Corbin JM, Strauss AL, Strauss AL. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. 2008Third edition Los Angeles, Calif.: Sage Publications, Inc.. [Google Scholar]
- 25.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361–1367.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood). 2013;32:276–284.. [DOI] [PubMed] [Google Scholar]
- 27.Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94:291–309.. [DOI] [PubMed] [Google Scholar]
- 28.Ho AL, Klassen AF, Cano S, et al. Optimizing patient-centered care in breast reconstruction: the importance of preoperative information and patient-physician communication. Plast Reconstr Surg. 2013;132:212e–220e.. [DOI] [PubMed] [Google Scholar]
- 29.Vroom VH. Work and Motivation. 1964New York, N.Y.: J. Wiley. [Google Scholar]
- 30.Adams JR, Elwyn G, Légaré F, et al. Communicating with physicians about medical decisions: a reluctance to disagree. Arch Intern Med. 2012;172:1184–1186.. [DOI] [PubMed] [Google Scholar]
- 31.Thorne S, Oliffe JL, Stajduhar KI. Communicating shared decision-making: cancer patient perspectives. Patient Educ Couns. 2013;90:291–296.. [DOI] [PubMed] [Google Scholar]


