Summary:
Craniofacial defects often result in aesthetic and functional deficits, which affect the patient’s psyche and wellbeing. Patient-specific implants remain the optimal solution, but their use is limited or impractical due to their high costs. This article describes a fast and cost-efficient workflow of in-house manufactured patient-specific implants for craniofacial reconstruction and cranioplasty. As a proof of concept, we present a case of reconstruction of a craniofacial defect with involvement of the supraorbital rim. The following hybrid manufacturing process combines additive manufacturing with silicone molding and an intraoperative, manual fabrication process. A computer-aided design template is 3D printed from thermoplastics by a fused deposition modeling 3D printer and then silicone molded manually. After sterilization of the patient-specific mold, it is used intraoperatively to produce an implant from polymethylmethacrylate. Due to the combination of these 2 straightforward processes, the procedure can be kept very simple, and no advanced equipment is needed, resulting in minimal financial expenses. The whole fabrication of the mold is performed within approximately 2 hours depending on the template’s size and volume. This reliable technique is easy to adopt and suitable for every health facility, especially those with limited financial resources in less privileged countries, enabling many more patients to profit from patient-specific treatment.
INTRODUCTION
An anatomically correct reconstruction of the highly complex shape of the craniofacial skeleton is challenging, especially when certain anatomical landmarks are lost. Often extensive experience and precise 3D conceptual ability are needed. Computer-aided design (CAD)/computer-aided manufacturing (CAM) has been continuously improved over the past few decades and has found its way into daily clinical work. The possibilities of CAD/CAM supported anatomical reconstruction are important because a haptic 3D model provides the surgeon and the patient with an additional understanding of the individual’s specific anatomy. In the case presented here, the patient had previously suffered severe trauma to the head, resulting in resorption of the fractured bony parts and retraction of the surrounding tissue. This led to rhythmic pulsation of the overlying scar. Many techniques for reconstruction of craniofacial bony defects have already been described.1–5 Only a few offer a solution when bone is missing due to osseous resorption or comminuted fractures. Some achieve a satisfactory fit and contour by taking impressions of the defect over the skin. Producing implants by traditional methods is economical and leads to good results; however, with CAD/CAM, more accurate results may be achieved.6 Because prices for CAD/CAM have been continuously decreasing over the years, it is now widely available. The workflow presented here does not require any production outsourcing so that patient-specific reconstruction can be performed on the spot, on the same day, and at minimal costs.
In times of economic restructuring of the health care system and decreasing subsidies for medical treatment, cost-efficient therapies are favored. The subsidies often do not cover the expenses for patient-specific implants from a manufacturer or accessibility is limited, which is the case in many less privileged countries. Considering these circumstances, the technique presented here offers the possibility for many more patients to profit from patient-specific treatment.
MATERIALS AND METHODS
A medical file format Digital Imaging and Communications in Medicine is created from a spiral CT scan of the head (SOMATOM Definition Flash, Siemens Healthcare GmbH, Erlangen, Germany) and a virtual 3D rendering is obtained using 3D reconstruction software (MIMICS, version 19.0, Materialise Inc., Leuven, Belgium). A spiral CT scan in polytrauma patients usually has adequate quality at a 1-mm slice thickness in the skull region. However, in this case, because of secondary reconstruction, a new CT scan was obtained with a slice thickness of 2 mm, which is sufficient. The contralateral intact skull is superimposed onto the bony defect by a digital subtraction mirror imaging process. The 2 images are merged, and the final implant shape is cropped. The segmentation allows removal of redundant parts. A design optimization software (3-matic, version 11.0, Materialise Inc., Leuven, Belgium) permits modifications at the mesh level. Both software programs are certified for clinical use. A Standard Tessellation Language file is created and transferred to a standard commercial 3D printer [MakerBot Replicator Desktop 3D Printer (5th Generation), MakerBot Industries, Brooklyn, N.Y.) to print the implant template in polylactic acid (PLA) thermoplastics. For better visualization and planning, an anatomical model is created (Fig. 1). To save printing time, depending on the schedule and time-pressure, a reduction of nonrequired parts in the model is recommended.
Fig. 1.
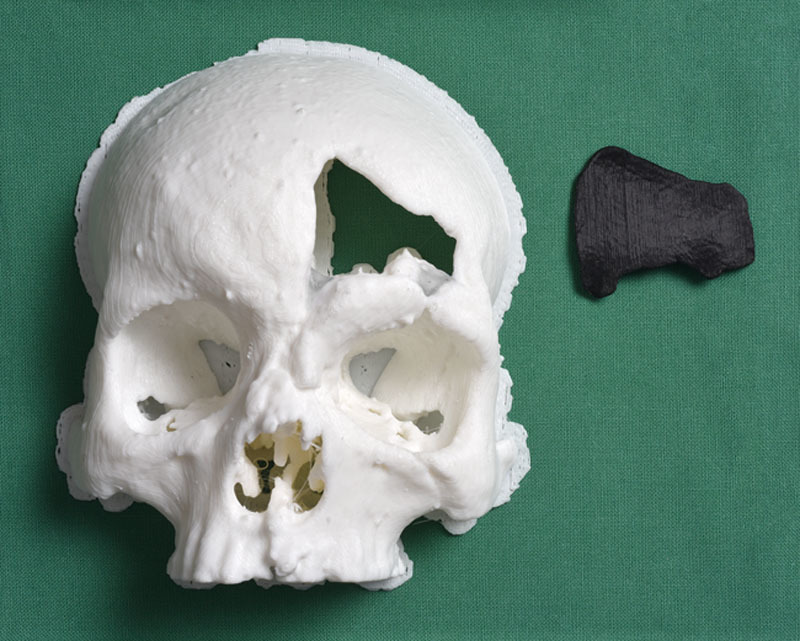
Model of the forehead (white) and implant template (black).
The molding is performed with an addition-type silicone (PRESIDENT soft putty, Coltène/Whaledent AG, Altstätten, Switzerland). A thin layer of separating liquid (GI-MASK universal separator for silicones, Coltène/Whaledent AG, Altstätten, Switzerland) prevents adherence. Small retention spikes are created to ensure accuracy of fit (Fig. 2).
Fig. 2.

Silicone mold and implant template.
Afterward, the template is not needed anymore and can be disposed of. The sterilization of the mold is typically carried out in an autoclave. The form stability of this silicone after sterilization is certified. This hybrid manufacturing process originates from the fact that PLA cannot resist steam sterilization and is not certified for implantation. Finally, the silicone mold is used intraoperatively in a compression molding technique. Although surgical exploration of the defect is underway, the polymethylmethacrylate (PMMA), for example, bone cement (PALACOS R+G, Heraeus Kulzer GmbH, Hanau, Germany) is prepared by mixing the polymer powder with the liquid monomer according to the manufacturer’s instructions. It is then poured into the mold and pressed into form. The molding process can be performed by a resident or perioperative nurse during surgical exploration. To ensure constant compression, small metal wires are applied (Fig. 3).
Fig. 3.
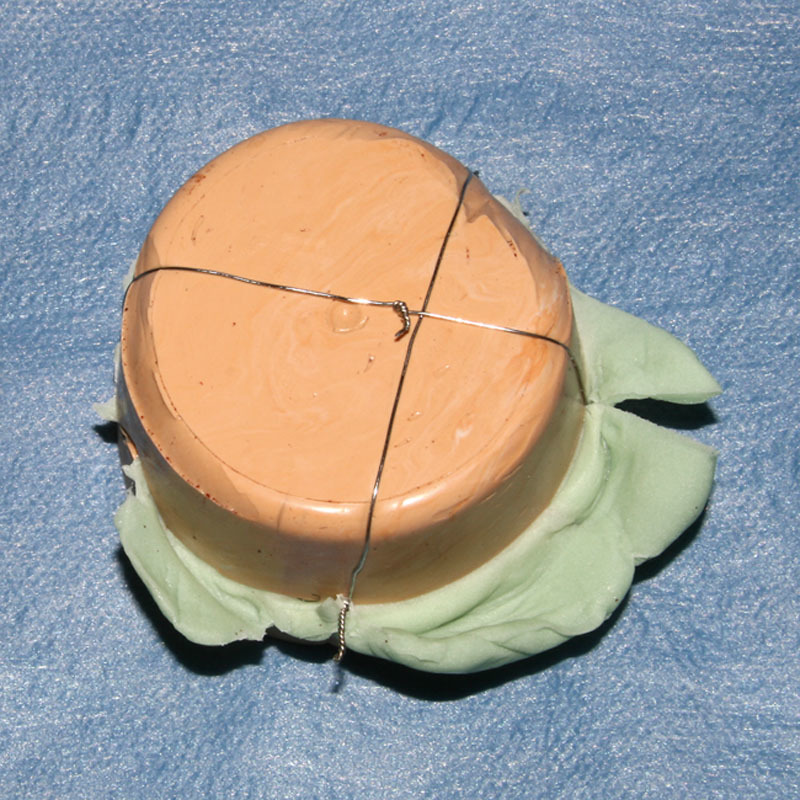
Compression molding technique.
After a short exothermic reaction, the implant is hardened out. Now the metal wires and the silicone mold are removed and the implant is slightly refined (Fig. 4).
Fig. 4.

Patient-specific implant made from PALACOS R+G.
After completing exploration of the defect, the implant can be immediately applied and fixed to the bone with titanium mini-plates and screws. A postoperative radiological alignment check is recommended to confirm correct anatomical reconstruction.
The above-mentioned case was the first one of a series of reconstructions and demonstrates the feasibility of this technique. Even large-size calvarial defects with a complex geometrical shape have since been successfully reconstructed by cranioplasty (Fig. 5).
Fig. 5.
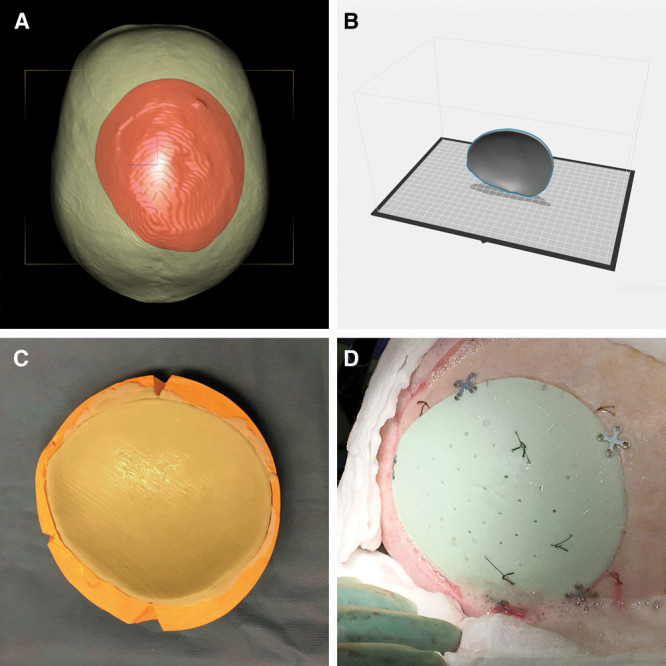
Preoperative planning (A), implant design (B), compression molding technique (C), intraoperative result (D).
Another case demonstrates the superior fit and contour of a preoperatively planned, patient-specific implant compared with a manually shaped one (Fig. 6).
Fig. 6.
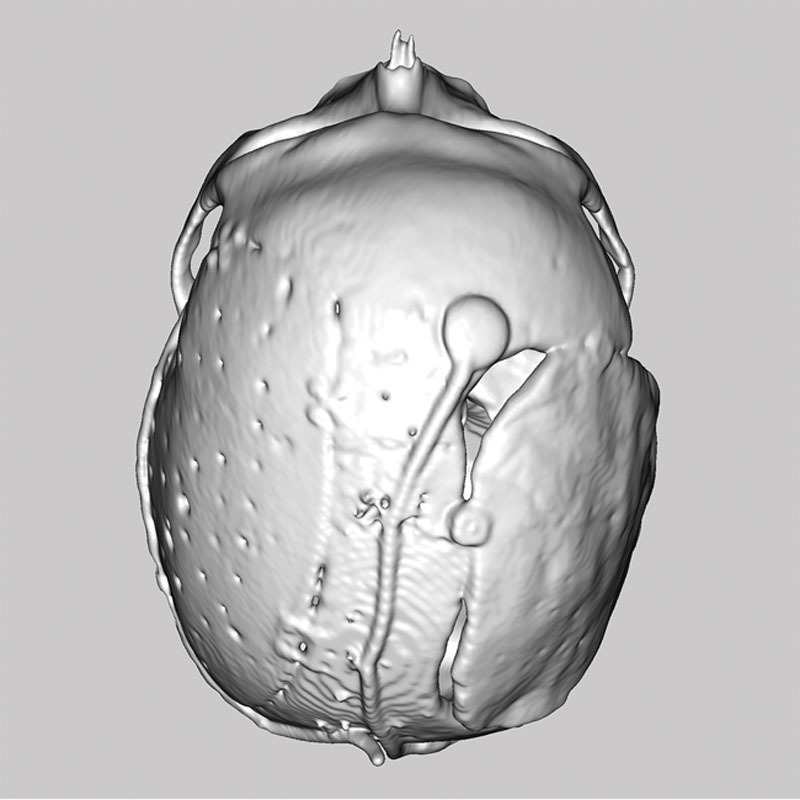
Preoperatively planned, patient-specific implant (left side) and manually shaped implant (right side).
This postoperative radiological alignment check shows a precise reconstruction of the left calvarial side by application of a preoperatively planned, patient-specific implant. The manually shaped implant on the right side has insufficient closure and is inferior to the reconstruction of the opposite side.
DISCUSSION
There are various materials for craniofacial reconstruction, for example, autografts and metal or nonmetal allografts.7 Choosing the appropriate material for a specific defect remains difficult and should always be done with caution. Harvesting autografts like split calvarial grafts is convenient, has a long tradition, low donor-site morbidity but is time consuming.8,9 Occasionally, bone graft resorption can result in a contour deformity and could lead to a secondary operation.10 In contrast, reconstruction with metal allografts is simple and has the lowest rate of graft infection but is unsuitable for complex defects.11 To achieve an adequate contour of large-size defects in cranioplasty, an additional application of cement over the titanium mesh may be needed.12 The use of nonmetal allografts like PMMA has been a gold standard among neurosurgeons for many years. Acrylate polymers were already being used in the 1940s during World War II.13
In the literature, the risk of local thermic and toxic lesion of the surrounding tissues as well as fatal systemic allergic reactions have been described regarding the application of acrylic bone cement directly onto the dura mater.14,15 It has been shown in animal studies that increasing heating to 50°C for 1 minute leads to bone tissue injury.16 To avoid these risks, the PMMA is generally removed before fully cured and reinserted manually after cooling off. However, this can lead to a loss of shape resulting in an insufficient fit, as shown in Figure 6. Extracorporeal fabrication by a compression molding technique as in this workflow does not carry such risks. The intraoperative fabrication of the implant additionally allows minor alterations to be made to accommodate poorly visible or completely invisible bony margins identified during exploration, which were not detected radiologically. Possible disadvantages of the compression molding technique might become apparent when it comes to reconstructing large-size defects. At a certain critical size, the difficulty of constant compression of the mold increases and may result in poor-quality implants. However, large-size calvarial defects in cranioplasty have been successfully reconstructed with the above-mentioned technique.
Over the years, advanced medical imaging has become of significant importance in surgery. It facilitates a better understanding of the patient’s specific anatomy, preoperative planning, creation of cutting guides, 3D printed prosthetics and has educational purposes.17,18 Preoperative planning and designing of the implant leads to a decrease in surgical time as no time is wasted on shaping and anatomical reconstruction is achieved more easily. The reconstructed defect in this case had a complex 3D geometry with involvement of the supraorbital rim, which can be challenging when shaping by hand. Manually shaping an implant takes considerably more time in the operating room compared with this method, whereby the preoperative planning time is longer. In cranial vault reconstruction with 3D models, a reduction in the amount of blood transfusion and operation time can be achieved.19 Furthermore, operation time for cranioplasty can be considerably reduced by working with prefabricated implants.20 Consequently, this technique reduces the duration of general anesthesia, which results in a decrease of costs and also leads to less comorbidity.
The CAD/CAM option that employs easy to use and widely accessible utilities without the need to outsource production has an optimal benefit-cost ratio. The required medical grade materials can be found in most of the noteworthy laboratories associated with departments for Plastic and Reconstructive Surgery or the dental laboratories of departments for Oral and Maxillofacial Surgery. The basic costs for printing the 3D model and template are estimated at about 20 USD, the medical grade silicone is around 40 USD, the PMMA (40 g) is around 120 USD, and miscellaneous expenses like metal wires, separating liquid for silicones, autoclave sterilization sum up to 50 USD. Not included are the costs of 1–2 hours working time as well as the acquisition costs for hardware and software licensing fees. The preoperative printing time takes around 1 hour but can take up to 5 hours for palm-size templates. In the coming decades, 3D printers will improve further and the printing time will continue to decline.
The acquisition costs for standard commercial 3D printers can vary between 1,000 and 3,000 USD and PLA filaments cost around 20–60 USD/kg. The software MIMICS, version 19.0 and 3-matic, version 11.0 were used because they were already implemented at our clinic. The annual license fees for these software options are estimated at a couple of thousand USD, whereby the digital subtraction mirror imaging process is just a minor feature and is also offered by a wide range of cheaper software options. For example, the certified medical software DDS-Pro (Digital Dental Service Ltd, London, United Kingdom) is able to supply all the required features at a price of about 300 USD for a life-long license. Overall, the total cost of manufacturing this patient-specific implant is estimated at around 250 USD.
In reconstructive surgery, there are numerous techniques that make the molding process redundant but the majority of them are expensive. For instance, a simple plain titanium mesh has a price in the lower 4-digit USD range. A patient-specific polyetheretherketone implant for craniofacial reconstruction is in the upper 4-digit USD range depending on its size and has a production time of about 2 weeks.21 Most of the patient-specific polyetheretherketone implants are currently milled and not printed.
CAD/CAM techniques have great potential to improve various surgical procedures in the coming years as steady research on this topic is done, specifically, upcoming research on 3D bioprinting could give rise to new techniques for 3D manufacturing of biological implants in the future. However, any newly developed implants will certainly remain more expensive and harder to obtain for a long time.
CONCLUSIONS
The technique involves a comprehensive workflow to fabricate inexpensive patient-specific implants without outsourcing any production steps. Overall, a considerable reduction in operation time and an accurate reconstruction of the original skull contour can be achieved. This could enable every health facility, even those with limited financial resources, possibly in remote locations where access to manufacturers is limited, to achieve precise craniofacial reconstruction with inexpensive patient-specific implants.
Footnotes
Drs. Jaquiéry and Thieringer contributed equally to this work.
Presented at the 31st Annual Conference of the Swiss Society of Oral and Maxillo-Facial Surgery (SSOMFS) 2016, Solothurn, Switzerland.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Asimacopoulos TJ, Papadakis N, Mark VH. A new method of cranioplasty. Technical note. J Neurosurg. 1977;47:790–792.. [DOI] [PubMed] [Google Scholar]
- 2.Fathi AR, Marbacher S, Lukes A. Cost-effective patient-specific intraoperative molded cranioplasty. J Craniofac Surg. 2008;19:777–781.. [DOI] [PubMed] [Google Scholar]
- 3.Sorour M, Caton WL, 3rd, Couldwell WT. Technique for methyl methacrylate cranioplasty to optimize cosmetic outcome. Acta Neurochir (Wien). 2014;156:207–209.. [DOI] [PubMed] [Google Scholar]
- 4.Stieglitz LH, Gerber N, Schmid T, et al. Intraoperative fabrication of patient-specific moulded implants for skull reconstruction: single-centre experience of 28 cases. Acta Neurochir (Wien). 2014;156:793–803.. [DOI] [PubMed] [Google Scholar]
- 5.Peltola MJ, Vallittu PK, Vuorinen V, et al. Novel composite implant in craniofacial bone reconstruction. Eur Arch Otorhinolaryngol. 2012;269:623–628.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar NG, Rangarajan H, Shourie P. Cranioplasty of hemispherical defects using high impact methylmethacrylic plate. J Craniofac Surg. 2015;26:1882–1886.. [DOI] [PubMed] [Google Scholar]
- 7.Aydin S, Kucukyuruk B, Abuzayed B, et al. Cranioplasty: review of materials and techniques. J Neurosci Rural Pract. 2011;2:162–167.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers GF, Greene AK. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg. 2012;23:323–327.. [DOI] [PubMed] [Google Scholar]
- 9.Tessier P, Kawamoto H, Posnick J, et al. Taking calvarial grafts, either split in situ or splitting of the parietal bone flap ex vivo—tools and techniques: V. A 9650-case experience in craniofacial and maxillofacial surgery. Plast Reconstr Surg. 2005;116:54S–71S; discussion 92S-94S.. [DOI] [PubMed] [Google Scholar]
- 10.Moreira-Gonzalez A, Jackson IT, Miyawaki T, et al. Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg. 2003;14:144–153.. [DOI] [PubMed] [Google Scholar]
- 11.Matsuno A, Tanaka H, Iwamuro H, et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien). 2006;148:535–540; discussion 540.. [DOI] [PubMed] [Google Scholar]
- 12.Kumar NG, Sudeep S, Balwan R. Cranioplasty of hemispherical defects using calcium phosphate cements along with titanium mesh: our experience. J Maxillofac Oral Surg. 2015;14:920–924.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Small JM, Graham MP. Acrylic resin for the closure of skull defects. Br J Surg. 1945;33:106–113.. [DOI] [PubMed] [Google Scholar]
- 14.Golz T, Graham CR, Busch LC, et al. Temperature elevation during simulated polymethylmethacrylate (PMMA) cranioplasty in a cadaver model. J Clin Neurosci. 2010;17:617–622.. [DOI] [PubMed] [Google Scholar]
- 15.Meel BL. Fatal systemic allergic reaction following acrylic cranioplasty: a case report. J Clin Forensic Med. 2004;11:205–207.. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson RA, Albrektsson T, Magnusson B. Assessment of bone viability after heat trauma. A histological, histochemical and vital microscopic study in the rabbit. Scand J Plast Reconstr Surg. 1984;18:261–268.. [DOI] [PubMed] [Google Scholar]
- 17.Gerstle TL, Ibrahim AM, Kim PS, et al. A plastic surgery application in evolution: three-dimensional printing. Plast Reconstr Surg. 2014;133:446–451.. [DOI] [PubMed] [Google Scholar]
- 18.Kamali P, Dean D, Skoracki R, et al. The current role of three-dimensional printing in plastic surgery. Plast Reconstr Surg. 2016;137:1045–1055.. [DOI] [PubMed] [Google Scholar]
- 19.Imai K, Tsujiguchi K, Toda C, et al. Reduction of operating time and blood transfusion for craniosynostosis by simulated surgery using three-dimensional solid models. Neurol Med Chir (Tokyo). 1999;39:423–426; discussion 427.. [DOI] [PubMed] [Google Scholar]
- 20.Kumar NG, Sreenivas M, Gowda S. Cranioplasty of large cranial defects with porous polyethylene implants. J Craniofac Surg. 2016;27:e333–e335.. [DOI] [PubMed] [Google Scholar]
- 21.Manrique OJ, Lalezarzadeh F, Dayan E, et al. Craniofacial reconstruction using patient-specific implants polyether ether ketone with computer-assisted planning. J Craniofac Surg. 2015;26:663–666.. [DOI] [PubMed] [Google Scholar]


