Abstract
Objective
To assess the trajectory of peri-operative brain growth in relationship to cardiac diagnosis and acquired brain injuries.
Methods
This is a cohort study of term neonates with hypoplastic left heart syndrome (HLHS) and transposition of the great arteries (TGA). Subjects underwent brain MRIs pre- and post-operatively to determine brain injury severity and total and regional brain volumes utilizing automated morphometry. Comparisons were made by cardiac lesion and injury status.
Results
79 subjects were included (49-TGA, 30- HLHS). HLHS subjects had more post-operative brain injury (55.6% vs. 30.4%, p= 0.03) and more severe brain injury (moderate to severe white matter injury (WMI), p= 0.01). Total and regional peri-operative brain growth was not different by brain injury status (either pre- or post-operative). However, subjects with moderate to severe WMI had a slower rate of brain growth in white and gray matter as compared to those with no injury. HLHS subjects had a slower rate of growth globally and in white and deep gray matter as compared to TGA (TBV- 12 cm3/week vs. 7 cm3; WM- 2.1 cm3/week vs. 0.6 cm3; deep GM- 1.5cm3/week vs. 0.7 cm3; p<0.001), after adjusting for gestational age at scan and the presence of brain injury. This difference remained after excluding subjects with moderate to severe WMI.
Conclusions
Neonates with HLHS have a slower rate of global and regional brain growth as compared to TGA, likely related to inherent physiologic differences post-operatively. These findings demonstrate the complex interplay between cardiac lesion, brain injury and brain growth.
Introduction
Advances in surgical techniques and peri-operative care have led to improved survival of newborns with critical congenital heart disease (CHD), such as hypoplastic left heart syndrome (HLHS) and transposition of the great arteries (TGA)1. Although there has been a decline in gross neurologic insults in these children, many experience behavioral, emotional, cognitive and motor impairments, suggesting widespread brain dysfunction2,3. Studies assessing neonates with TGA and HLHS as a single cohort have identified delayed brain development and a similar prevalence of pre-operative brain injury despite the fact that these lesions are anatomically and physiologically distinct from one another4–7. Interestingly, both lesions have evidence of altered fetal cerebral oxygen delivery and smaller total brain volumes as compared to the normal fetus, although the underlying mechanisms may differ in each lesion8–10.
Measurements of simple metrics of brain growth on MRI have identified smaller total and regional brain size at birth and infancy in CHD as compared to controls11,12; however the rate of brain growth was similar and did not differ by cardiac lesion11. Cerebral MRI volumetry is a quantitative measure of total and regional brain volume providing an accurate means to assess brain volume and growth trajectory13–15. Sophisticated automated volumetry techniques can model brain tissue characteristics during neonatal development and have identified decreased total and regional brain volumes in neonates with complex CHD prior to any corrective operation16. In particular, studies have shown associations between smaller total and regional brain volumes (white matter and cortical grey matter) with neurodevelopmental outcome in adolescents with CHD17, as well as association between brain volume at birth and neonatal neurobehavior18. These studies highlight the potential usefulness of this quantitative measure in identifying the patients at highest risk for neurodevelopmental impairment.
We sought to assess the trajectory of peri-operative brain growth in relationship to cardiac diagnosis and acquired brain injuries utilizing MR morphometry in a large sample of well characterized patients with standardized imaging time-points in the neonatal period. We hypothesize that patients with HLHS exhibit slower rates of brain growth due to a palliative, staged surgical approach with persistent cyanosis and potential for on-going postoperative brain injury. In contrast, newborns with TGA undergo definitive surgical correction with restoration of normal brain oxygen delivery, providing more favorable conditions for brain growth.
Methods
Between 2001–2014, newborns with critical CHD at the University of California San Francisco Benioff Children’s Hospital were consecutively invited to participate in a prospective protocol studying brain development and brain injury in CHD using magnetic resonance imaging (MRI). Brain imaging findings from earlier versions of this cohort were previously reported4,5. Patients who were born prior to 36 weeks gestation, had a suspected congenital infection, had clinical evidence of a congenital malformation or syndrome, and/or had a suspected or confirmed genetic or chromosomal anomaly were excluded. Once written informed consent was received, patients underwent brain MRI before and after cardiac surgery. The institutional committee on human research approved the study protocol.
Patients diagnosed as having d-TGA with or without a ventricular septal defect (VSD) or HLHS were included in this current study. HLHS was defined as the presence of 1 functioning right ventricle with varying degrees of severe left heart hypoplasia requiring a palliative surgical intervention for survival (i.e. Stage I operation) in the newborn period. All subjects had a Stage I (Norwood) operation with a RV-PA conduit (Sano) modification.
MRI Study
Preoperative MRI studies were performed as soon as the baby could be safely transported to the MRI scanner as determined by the clinical team. Postoperative studies were performed after completion of perioperative care and prior to discharge from the hospital. Imaging time points were separated by an average of 15 days in the entire cohort. Detailed MRI methods are listed in supplementary material. A neuroradiologist reviewed each MRI (A.J.B.). Brain injury was characterized as stroke, white matter injury (WMI), intraventricular hemorrhage (IVH), and/or global hypoxic ischemic injury as previously described5,6. Post-operative brain injuries are limited to newly acquired lesions not evident on the pre-operative scan. WMI was classified as mild (1–3 foci each < 2mm), moderate (>3 foci or any foci > 2mm), or severe (>5% of white matter volume)5. Intraventricular hemorrhage was characterized as grade I, II, III, or IV using the system of Papile et al19. In addition, brain injury severity (BIS) was determined for each patient as previously described20. The BIS was assigned as follows: 0 indicates normal (no injury); 1, minimal injury (minimal WMI and IVH grade I or II); 2, stroke (all stroke); and 3, moderate to severe injury (moderate and severe WMI, IVH grade III or global hypoxic-ischemic injury).
Morphometry
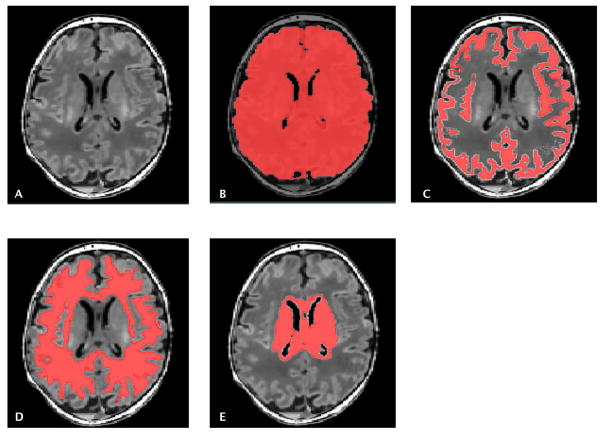
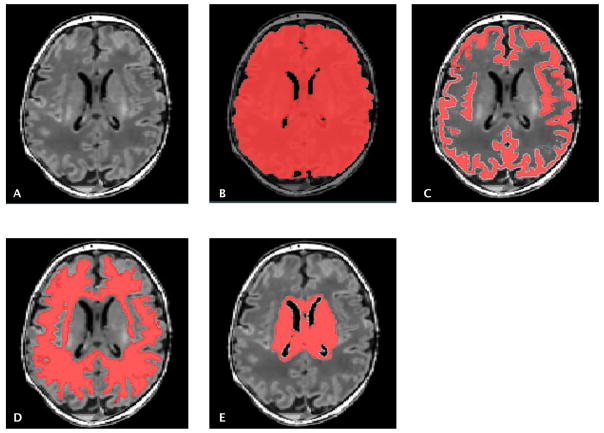
Using an automated approach, segmentation of the gray matter (GM), white matter (WM) and deep gray matter structure (combining the thalamus, the basal ganglia and the periventricular germinal zone) was performed, as previously described21 (Figure 1). Detailed methods are listed in supplemental material.
Figure 1.
T1 weighted MRI image (A). Automatic segmentation of brain structures to measure total brain (B), gray matter (C), white matter (D) and deep gray matter volumes (E).
Clinical Variables
Clinical data were prospectively collected from the medical records by a team of trained neonatal research nurses and reviewed by a pediatric intensivist (P.M.) blinded to all neuroimaging findings.
Statistical Analysis
Demographic characteristics, descriptors of brain injury and clinical variables were compared between patients with HLHS and d-TGA using standard descriptive statistics. To analyze brain structural volumes, we used general linear models that included gestational age (GA) at scan, type of cardiac lesion (TGA or HLHS), BIS and sex as dependent variables while TBV, GM, WM, or deep GM volumes were set as the independent variables. As most subjects were scanned twice (one before and one after the surgery), mixed-effect linear models were used to take into account multiple measurements per subject. To assess whether pre-operative or post-operative brain injury influenced brain volumes, all subjects were first dichotomized into those with a cumulative BIS score > 0 and those with a BIS score = 0 based on the maximum value between pre-op and post-op BIS scores. In a separate analysis, an interaction term was included as BIS x GA at scan into the linear model in order to assess differences in brain growth rate depending on the presence of brain injury.
Finally, to assess difference by cardiac lesion, a linear model that contained a group term of cardiac lesion type (TGA or HLHS) and GA at scan as the main effect term was performed, including an interaction term (cardiac lesion x GA at scan). Adjustments were made for the presence of brain injury. The Bonferroni adjustment for multiple comparisons was used to obtain corrected p-values, which after adjusting for 8 comparisons was p= 0.006.
Results
A total of 79 infants were included in this study, 49 with d-TGA and 30 with HLHS. There were a total of 79 pre-operative brain MRI’s and 73 post-operative brain MRI’s. The post-operative MRI was not analyzed in 5 subjects due to motion degradation. One subject did not have a post-operative MRI due to pacemaker placement. Demographics and clinical variables are listed in Tables 1 and 2. Both groups were similar in pre-operative clinical characteristics including birth weight and birth head circumference. Post-operatively, HLHS subjects had more frequent cardiac arrest events and a longer length of hospital stay as compared to TGA subjects. In addition, the post-operative MRI was performed on average 6.5 days later in the HLHS group (DOL MRI: median 24 days, IQR: 20–30) as compared to the TGA group (DOL MRI median 17.5 days, IQR: 15–25, p= 0.001). The average time between scans was 15 days (IQR 10–21) in the entire cohort, 12.5 days in TGA (IQR 9–17) and 19 days in HLHS (IQR 15–25). Weight at the time of the second MRI was similar in both groups (TGA: 3492.9g, 95% CI: 3364.6–3621.4; HLHS: 3308.5g, 95% CI: 3183.1–3433.8); p= 0.06) with a similar increase in weight from birth to the time of the second MRI in both groups (TGA: 96.1 grams; HLHS: 96.6 grams) (Table 1).
Table 1.
Demographics by Cardiac Lesion
| TGA (n= 49) | HLHS (n= 30) | p-value | |
|---|---|---|---|
| EGA Delivery, wk (mean, 95% CI) | 39.2 (38.8–39.6) | 38.9 (38.4–39.3) | 0.35 |
| Birth weight, g (mean, 95% CI) | 3396.8 (3228.0–3565.6) | 3211.9 (3030.6–3393.3) | 0.16 |
| Male, N(%) | 39 (79.6%) | 16 (53.3%) | 0.02 |
| Birth Head Circumference, cm (mean, 95% CI) | 34.0 (33.7–34.3) | 34.1 (33.6–34.6) | 0.78 |
| Weight at MR2, g (mean, 95% CI) | 3492.9 (3364.6–3621.4) | 3308.5 (3183.1–3433.8) | 0.06 |
TGA= d- transposition of the great arteries; HLHS= hypoplastic left heart syndrome; EGA= estimated gestational age; CI= confidence interval
Table 2.
Pre- and post-operative Clinical Variables by cardiac lesion
| TGA (n= 49) | HLHS (n= 30) | p-value | |
|---|---|---|---|
| Balloon atrial septostomy N (%) | 30 (61.2%) | 1 (3.3%) | < 0.001 |
| Lowest pre-op O2 sat Mean (95%CI) | 56.9 (52.0–61.7) | 80.1 (76.2–84.0) | < 0.001 |
| Pre-op pH (1st ABG) Mean (95% CI) | 7.27 (7.23–7.31) | 7.29 (7.24–7.35) | 0.43 |
| Pre-op Base excess (1st ABG) Mean (95% CI) | −6.2 (−8.5 to −3.9) | −4.1 (−6.7 to −1.4) | 0.23 |
| Pre-op Cardiac Arrest N (%) | 1 (2.0%) | 2 (6.7%) | 0.55 |
| Day of life operation Median (IQR) | 8 (5.5–11) | 8 (6–11) | 0.99 |
| Day of life MRI 1 Median (IQR) | 5 (3–6) | 5 (3–6) | 0.96 |
| Post-op Cardiac Arrest N (%) | 0 | 5 (16.7%) | 0.006 |
| Post-op ECLS N (%) | 2 (4.1%) | 4 (13.3%) | 0.19 |
| Post-op duration of mechanical ventilation Median (IQR) | 5 (3–7) | 8 (6–10) | 0.0002 |
| Day of life MRI2 Median (IQR) | 17.5 (15–25) | 24 (20–30) | 0.001 |
| Hospital LOS Mean (95% CI) | 23.8 (19.2–28.3) | 40 (30.0–50.0) | 0.001 |
TGA= d-transposition of the great arteries; HLHS= hypoplastic left heart syndrome; ABG= arterial blood gas; IQR= inter-quartile range; ECLS= extra-corporeal life support; LOS= length of stay
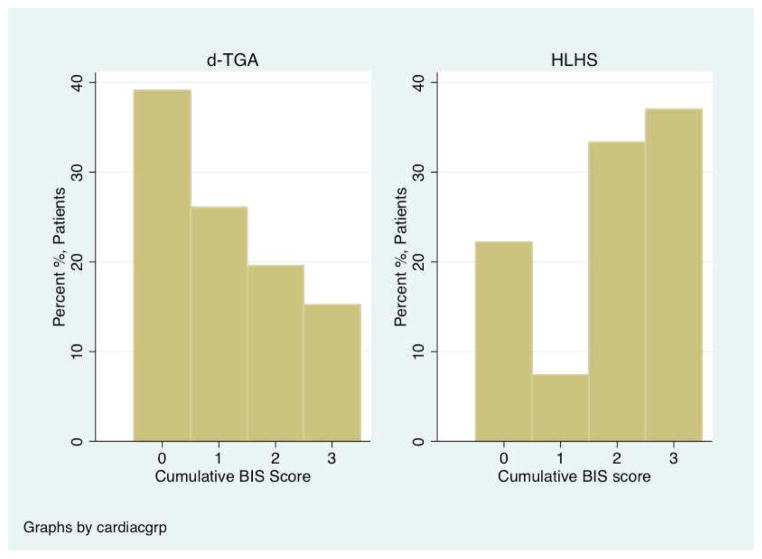
The frequency of pre-operative brain injury was highest in HLHS, but not statistically significant (TGA= 14.3%, HLHS= 30%, p= 0.09). In contrast, HLHS subjects had a higher prevalence of new post-operative injury (TGA= 30.4%, HLHS= 55.6%, p= 0.03), mostly in the form of stroke (Table 3). The cumulative BIS score, which represents the highest score on either MRI, is represented in Figure 2. HLHS subjects had higher cumulative BIS scores than TGA subjects (BIS = 2 and 3) (p= 0.01).
Table 3.
Pre- and post-operative brain injury by cardiac lesion
| TGA (n= 49) | HLHS (n= 30) | P-value | |
|---|---|---|---|
| Pre-operative | |||
| Any Injury | 17/49 (34.7%) | 13/30 (43.3%) | 0.44 |
| WMI | 7/49 (14.3%) | 9/30 (30.0%) | 0.09 |
| Stroke | 12/49 (24.5%) | 6/30 (20.0%) | 0.64 |
| New Post-operative* | |||
| Any Injury | 14/46 (30.4%) | 15/27 (55.6%) | 0.03 |
| WMI | 13/46 (28.3%) | 9/27 (33.3%) | 0.65 |
| Stroke | 1/46 (2.2%) | 7/27 (25.9%) | 0.003 |
WMI= white matter injury; TGA= transposition of the great arteries; HLHS= hypoplastic left heart syndrome
Post-op MRI missing in 3 subjects with TGA and 3 subjects with HLHS due to death of subject
Figure 2.
Distribution of the cumulative Brain Injury Severity (BIS) score by cardiac lesion. The cumulative BIS score is the worst score when combining the pre- and post-operative MRI. A test for trends demonstrates less severe cumulative BIS scores in the TGA group (p = 0.01).
Morphometry
Total and regional brain volumes increased significantly with increasing age in the entire cohort, even when accounting for the presence of brain injury, sex and the type of cardiac lesion (p < 0.0001 for all regions).
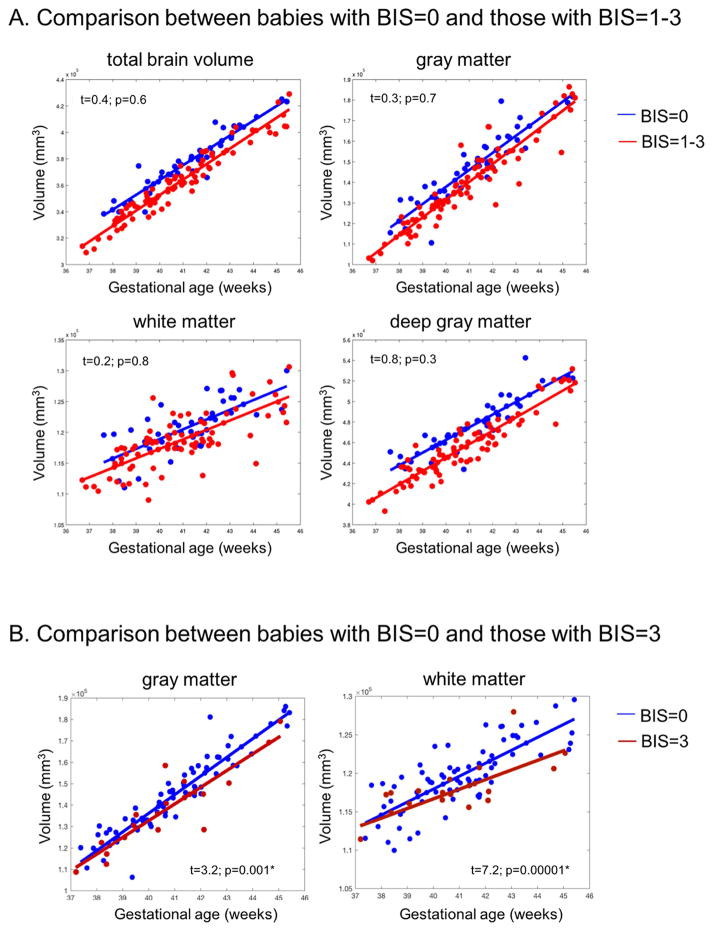
Subjects were dichotomized by cumulative BIS score (BIS =0 vs. BIS>0). Overall, mean global and regional brain volumes (all pre- and post-operative data points combined) were higher in subjects without brain injury as compared to those with brain injury, except in WM (TBV: 385 cm3 v. 359 cm3, p< 0.0001; GM: 147 cm3 vs. 139 cm3, p<0.0001; Deep GM: 48 cm3 vs. 46 cm3, p< 0.004; WM: 121 cm3 vs. 116 cm3, p=0.007). Although pre-operative brain injury did not predict pre-operative brain volumes, it was associated with significantly lower GM volumes on the post-operative MRI (147 cm3 vs. 157 cm3, p = 0.0001). Similarly, post-operative brain injury was associated with lower GM volumes on the post-operative MRI (149 cm3 vs. 159 cm3, p = 0.0001). In contrast, pre-operative brain volumes were not associated with new post-operative brain injury (p =0.2). Despite the difference in brain volumes at single points in time, the rate of global and regional brain growth did not correlate with the presence of brain injury noted on the pre- or post-operative MRI (Rate of growth in those with no brain injury (cumulative BIS=0) vs. those with brain injury (cumulative BIS>0)- TBV: 12.3 cm3 vs. 12.4 cm3, p>0.3; GM: 8.15 cm3 vs. 8.14 cm3, p>0.5; WM: 5.52 cm3 vs. 5.46 cm3, p>0.4; Deep GM: 1.21 cm3 vs. 1.18 cm3, p>0.3) (Figure 3A, Supplementary Figure 1A). However, when only evaluating patients with the most severe form of brain injury (moderate to severe WMI, cumulative BIS= 3), the rate of growth in WM and GM was significantly slower as compared to those with no brain injury (GM: 6.7 cm3/week vs. 8.1 cm3/week, p= 0.001; WM: 1.2 cm3/week vs. 1.8 cm3/week, p < 0.001) (Figure 3B, supplementary Figure 1B).
Figure 3.
A) Brain global/regional volume changes in the presence of brain injury (red line- cumulative BIS > 0) and without brain injury (blue line- cumulative BIS = 0). The x-axis represents the gestational age at the time of MRI and the y-axis represents the volume in mm3. The plot includes pre- and post-operative brain MRI measures for each subject with a best-fitted line. Plots with paired observations for each subject are included in supplementary Figure 1. Overall, mean global and regional (gray matter and deep gray matter) brain volumes were lower in subjects with brain injury as compared to those without brain injury. However, the rate of global and regional volume change over time was not associated with brain injury (all p-values > 0.1). B) Brain regional volume changes in gray and white matter in the presence of no brain injury (blue line- cumulative BIS= 0) and in those with moderate to severe white matter injury (dark red line- cumulative BIS= 3). Those with moderate to severe WMI have significantly slower growth in gray matter and white matter as compared to those without injury (p= 0.001 and p< 0.0001 respectively).
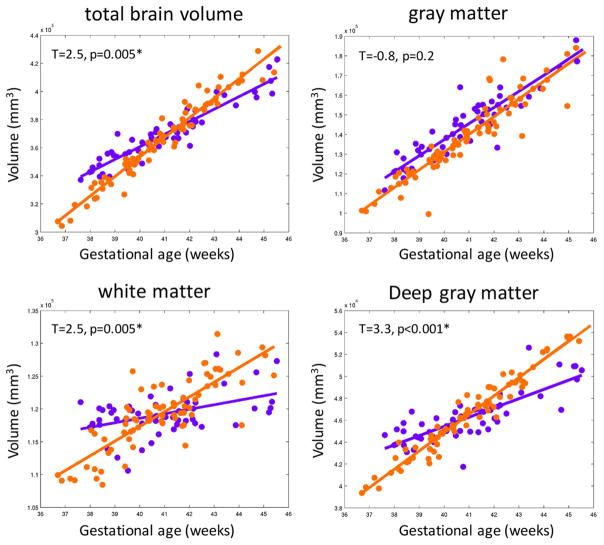
When assessing differences by cardiac lesion, there was no difference in overall mean total and regional measures of brain volume (all pre- and post-operative data points combined and when assessing each time point separately) in subjects with TGA compared to HLHS (TBV: 375 cm3 vs. 372 cm3, p >0.2; GM: 142 cm3 vs. 145 cm3, p>0.1; WM: 120 cm3 vs. 117 cm3, p=0.1; Deep GM: 47 cm3 vs. 46 cm3, p>0.1). However, D-TGA subjects had a faster rate of increase in global and regional brain growth as compared to HLHS subjects, even when adjusting for gestational age at scan and the presence of any injury (TBV- 12 cm3/week vs. 7 cm3/week; WM volume- 2.1 cm3/week vs. 0.6 cm3/week; deep GM volume-1.5 cm3/week vs. 0.7 cm3/week; p<0.001; Figure 4, supplementary Figure 2). GM did not show a significant difference in growth by cardiac lesion (p=0.2). Even when excluding subjects with moderate to severe WMI (BIS =3), those with D-TGA had a faster rate of increase in global and regional brain growth as compared to HLHS subjects (p < 0.001 for TBV, deep GM and WM).
Figure 4.
The rate of change in global and regional brain volumes by cardiac lesion after adjusting for the presence of brain injury. The x-axis represents the gestational age at the time of MRI and the y-axis represents the volume in mm3. The plots include pre- and post-operative brain MRI measures for each subject with a best-fitted line. Plots with paired observations for each subject are included in supplementary Figure 2. Subjects with HLHS (purple line) have a slower rate of growth in total brain volume, white matter and deep gray matter as compared to those with d-TGA (orange line). *Significant after Bonferroni correction, p<0.006.
Discussion
Our results demonstrate better perioperative brain growth in patients with d-TGA as compared to HLHS. Specifically, there was a 40% increase in the rate of brain growth in WM, GM and TBV in d-TGA subjects in this short period from preoperative to postoperative imaging. Interestingly, although subjects with brain injury had overall smaller brain volumes at individual points in time as compared to those without injury, the strongest predictor of brain growth trajectory was cardiac lesion. This is the first report, to our knowledge, directly comparing the peri-operative patterns of brain growth between two infant groups with common, yet physiologically distinct critical congenital cardiac defects utilizing advanced MR morphometry.
In the fetal time period, both d-TGA and HLHS fetuses have evidence of delayed brain development (TBV and metabolic brain development)9, although the underlying mechanisms likely differ by cardiac physiology and anatomy. In particular, based on fetal lamb models, those with HLHS have abnormalities in both perfusion (due to retrograde flow from the ductus arteriosus) and oxygenation of cerebral blood flow. In contrast, those with d-TGA are more likely to have abnormalities in oxygenation of cerebral blood flow10. Ultimately, both lesions result in decreased cerebral oxygen delivery and consumption8. Recently Rudolph suggested that decreased substrate delivery to the brain such as glucose may be the primary cause for the abnormalities in brain development22. Most studies find no differences by cardiac lesion in the degree of fetal or neonatal pre-operative brain development4,7. Some evidence suggests that within a particular group, specific anatomic features such as aortic atresia in HLHS leads to the greatest degree of delayed microstructural brain development23. Our study did not demonstrate significant differences pre-operatively in measures of brain volumes when comparing HLHS to d-TGA. This demonstrates that although these lesions are physiologically different, brain growth is similar during fetal and up to the pre-operative time period.
After birth and prior to their neonatal operation, patients with both d-TGA and HLHS are at risk for brain injury in the form of WMI and stroke5,24,25. Indeed, both groups have a similar risk of brain injury, likely secondary to shared risk factors such as hypoxia, length of time to surgery, embolism and preoperative cardiac arrest26–29. New, post-operative brain injury is common and tends to be more prevalent in subjects with single ventricle physiology5. Similarly, we found that post-operative brain injury was more common in the HLHS group with a higher number of small focal strokes, consistent with embolism. As opposed to TGA patients, HLHS patients after a Norwood procedure are vulnerable to embolic injury, which may be modifiable based on post-operative management and hospital length of stay. The relationship between brain development and injury is complex and dependent on methods used to measure brain development. Several studies agree that brain immaturity, measured utilizing semi-quantitative methods, is a risk factor for pre-operative brain injury20,28,30. However, our measures of total and regional brain volumes pre-operatively did not seem to predict the presence of new post-operative brain injury. The impact of brain injury on measures of ongoing brain development is less clear. For example, our group has shown that pre-operative brain injury predicts delayed post-operative metabolic and microstructural brain development20. We found that pre-operative brain injury is associated with smaller post-operative gray matter volumes and that overall brain volumes were smaller in those with injury as compared to those without injury. However, we found that rate of brain growth did not differ by the presence of brain injury globally or regionally. Thus, although brain injury is associated with brain volume loss, there appears to be minimal influence on continued brain growth at least in the peri-operative time period. On the most severe end of the spectrum, subjects with moderate to severe WMI exhibit a slower rate of growth in white and gray matter. We believe this result is driven by the complex interplay between cardiac lesion, brain injury and brain growth. In our cohort, a larger percentage of subjects with HLHS had moderate to severe WMI, suggesting that cardiac lesion is the initial risk factor that influences both injury and brain growth. In fact, when subjects with moderate to severe WMI were removed from the analysis, those with d-TGA continued to have an even faster rate of global and regional brain growth as compared to HLHS.
Studies analyzing pyramidal tract maturation in newborns with CHD have demonstrated less rapid changes in fractional anisotropy over the peri-operative time period in subjects with pre-operative brain injury31, similar to what is seen in premature infants with brain injury32. This suggests that the effects of brain injury on brain development may differ at macroscopic and microscopic scales. The techniques utilized to measure these different aspects of brain development are unique and offer different perspectives on brain maturity. More sensitive measures of brain growth in regions corresponding to the location of acquired focal lesions may lead to a deeper understanding of the longer term effects of milder forms of brain injury on brain growth.
The relationship between brain volumes in particular and injury is inherently complicated. Brain injury may lead to swelling and increased volume acutely, followed by tissue loss, potential repair and finally, in the immature brain, variable subsequent growth. Brain growth is one component of several factors that may impact long term neurologic outcomes in patients with complex CHD. The experience with preterm infants has demonstrated the close interplay between injury and continued brain growth and their collective influence on outcomes. Specifically, studies in premature infants have demonstrated long term effects of WMI or periventricular leukomalacia (PVL) on brain growth and function33. Although PVL is localized to the white matter, it can lead to disruption of cortical activity 34 and gray matter hypoplasia 35, with important consequences for developing cortical circuits36–39. Neuropathologic studies in non-survivors of critical CHD have shown abnormalities in the thalamus with thalamic neuronal loss and gliosis40, regions critical for working memory and attention, a common deficit noted in survivors of critical CHD. Our data demonstrates that moderate to severe WMI does appear to slow the rate of brain growth in white and gray matter. Although this is likely largely driven by cardiac lesion, the impact of injury on brain growth and long term neurodevelopmental outcomes warrants further study.
Our findings demonstrate a clear link between cardiac sub-group and ongoing peri-operative brain growth. Given the high burden of acquired injury and delayed brain development early in life, the potential for repair and optimal brain growth is critical. Although HLHS and TGA patients appear to share similar fetal and pre-operative risk factors, they differ significantly post-operatively. D-TGA subjects undergo a corrective operation (arterial switch operation) restoring normal cardiovascular physiology, while those with HLHS undergo a series of palliative operations never achieving normal circulation, with ongoing hypoxia and risk for diminished systemic perfusion. Patients with single ventricle physiology rely upon circulation in series that may result in diminished systemic perfusion with compromised overall cerebral blood flow. There are several other differences between these groups including operative strategies (low or full flow bypass only vs. circulatory arrest or regional cerebral perfusion) and post-operative management (length of stay, duration of mechanical ventilation) that influence our findings. Finally, somatic growth likely plays a role in brain development, particularly for those with single ventricle physiology. However, in the short time period evaluated in our study, both groups had a similar rate of weight gain in the peri-operative time period. In line with the cardiac physiology influences on brain development in CHD, we recently reported a relationship between the benefits of prenatal diagnosis and a healthier pre-operative state with perioperative brain development41.
Ibuki et al demonstrated normalization of total and frontal brain volumes at three years of age in d-TGA subjects but not in single ventricle patients42. Their data suggested an association between hypoxia (SpO2) and measures of neurodevelopmental outcome (psychomotor development index on the Bayley’s scale of infant development-II) in the single ventricle group. Their study was limited due to a small sample size, considerable variability and long time intervals between imaging studies. In our study, the larger sample size, standardized imaging timepoints in the neonatal peri-operative period, and the short interval between MRI studies strengthens our conclusions and suggests that the differences in brain growth by cardiac lesion begin soon after the neonatal operation.
Our findings suggest improved peri-operative brain growth in TGA subjects, however; evidence suggests that these patients continue to have structural and functional neurologic abnormalities later in life. TGA adolescents have altered grey matter volume and thickness43 as well as diminished white matter microstructure17,44,45 as compared to healthy controls. Neurodevelopmental assessments have revealed ongoing impairments in executive skills, visual-spatial skills and the need for remedial services46, suggesting that abnormal fetal and pre-operative physiology and acquired brain injury may have lasting effects. Indeed, patients with HLHS demonstrate the greatest deficits and neurodevelopmental morbidity2,47–49.
Our findings are limited by not knowing the exact timing of acquired brain injury despite two imaging time points in the peri-operative time period. Although we are invoking developmental etiologies (lack of oxygen and substrate delivery) as the primary influence on neonatal brain growth, the impact of injury as a destructive etiology on brain growth requires a larger sample size and more imaging timepoints, particularly in the transitional period after birth.
In conclusion, brain growth in the peri-operative time period is influenced more by cardiac sub-group than by brain injury. HLHS subjects have a slower rate of peri-operative brain growth globally and regionally in WM and deep GM. Further studies are needed to determine the predictive abilities of this measure of brain development on long term neurodevelopmental outcomes, with a goal of identifying specific impairments as targets for early intervention.
Supplementary Material
Central Picture.
Legend: T1 weighted MRI image (A). Automatic segmentation of brain structures to measure total brain (B), gray matter (C), white matter (D) and deep gray matter volumes (E).
Central Message.
Neonates with hypoplastic left heart syndrome have a slower rate of peri-operative brain growth than d- transposition of the great arteries. Brain injury has less influence on neonatal brain growth.
Perspective Statement.
Brain injury and delayed brain development are common in neonates with hypoplastic left heart syndrome (HLHS) and d-Transposition of the great arteries (TGA). Despite similarities in brain health pre-operatively, neonates with HLHS have a slower rate of on-going brain growth in the peri-operative time period, a reflection of the post-operative physiologic differences between these two cardiac lesions.
Acknowledgments
Funding Sources: This work was supported by grants K23 NS099422, R01 NS40117, R01NS063876, R01EB009756, R01HD07274, P01 NS082330 and P50 NS35902 from the National Institutes of Health; grant MOP93780 from the Canadian Institutes of Health Research; grant 5-M01-RR-01271 from the National Center for Research Resources; grants 5-FY05-1231 and 6-FY2009-303 from the March of Dimes Foundation; grant 0365018Y from the American Heart Association; and grant 2002/3E from the Larry L. Hillblom Foundation. Dr. Miller is the Bloorview Children’s Hospital Chair in Pediatric Neuroscience.
Glossary of Abbreviations
- CHD
congenital heart disease
- HLHS
hypoplastic left heart syndrome
- TGA
d-transposition of the great arteries
- MRI
magnetic resonance imaging
- WMI
white matter injury
- IVH
intraventricular hemorrhage
- BIS
brain injury severity
- GM
gray matter
- WM
white matter
- TBV
total brain volume
- GA
gestational age
- DOL
day of life
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose
IRB Approval: This study was approved by the UCSF IRB study number 10-03749, original approval date October 2001.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karamlou T, Diggs BS, Ungerleider RM, Welke KF. Evolution of treatment options and outcomes for hypoplastic left heart syndrome over an 18-year period. J Thorac Cardiovasc Surg. 2010;139(1):119–26. doi: 10.1016/j.jtcvs.2009.04.061. discussion126–7. [DOI] [PubMed] [Google Scholar]
- 2.Newburger JW, Sleeper LA, Bellinger DC, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125(17):2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellinger DC, Newburger JW, Wypij D, Kuban KCK, duPlesssis AJ, Rappaport LA. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19(1):86–97. doi: 10.1017/S1047951108003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 5.McQuillen PS, Barkovich AJ, Hamrick SEG, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38(2 Suppl):736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 6.Block AJ, McQuillen PS, Chau V, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2010;140(3):550–557. doi: 10.1016/j.jtcvs.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137(3):529–36. doi: 10.1016/j.jtcvs.2008.10.025. –discussion536–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Macgowan CK, Sled JG, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131(15):1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121(1):26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph A. Congenital Diseases of the Heart. John Wiley & Sons; 2011. [Google Scholar]
- 11.Ortinau C, Inder T, Lambeth J, Wallendorf M, Finucane K, Beca J. Congenital heart disease affects cerebral size but not brain growth. Pediatric Cardiology. 2012;33(7):1138–1146. doi: 10.1007/s00246-012-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortinau C, Beca J, Lambeth J, et al. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J Thorac Cardiovasc Surg. 2012;143(6):1264–1270. doi: 10.1016/j.jtcvs.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gholipour A, Estroff JA, Barnewolt CE, Connolly SA, Warfield SK. Fetal brain volumetry through MRI volumetric reconstruction and segmentation. Int J Comput Assist Radiol Surg. 2011;6(3):329–339. doi: 10.1007/s11548-010-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzawa J, Matsui M, Konishi T, et al. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb Cortex. 2001;11(4):335–342. doi: 10.1093/cercor/11.4.335. [DOI] [PubMed] [Google Scholar]
- 15.Weisenfeld NI, Warfield SK. Automatic segmentation of newborn brain MRI. Neuroimage. 2009;47(2):564–572. doi: 10.1016/j.neuroimage.2009.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhein von M, Buchmann A, Hagmann C, et al. Severe Congenital Heart Defects Are Associated with Global Reduction of Neonatal Brain Volumes. The Journal of Pediatrics. 2015;167(6):1259–1263. e1. doi: 10.1016/j.jpeds.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Rhein von M, Buchmann A, Hagmann C, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137(Pt 1):268–276. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- 18.Owen M, Shevell M, Donofrio M, et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. The Journal of Pediatrics. 2014;164(5):1121–1127. e1121. doi: 10.1016/j.jpeds.2013.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. The Journal of Pediatrics. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 20.Dimitropoulos A, McQuillen PS, Sethi V, et al. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81(3):241–248. doi: 10.1212/WNL.0b013e31829bfdcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H, Lepage C, Maheshwary R, et al. NEOCIVET: Towards accurate morphometry of neonatal gyrification and clinical applications in preterm newborns. Neuroimage. 2016;138:28–42. doi: 10.1016/j.neuroimage.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudolph AM. Impaired cerebral development in fetuses with congenital cardiovascular malformations: Is it the result of inadequate glucose supply? Pediatr Res. 2016;80(2):172–177. doi: 10.1038/pr.2016.65. [DOI] [PubMed] [Google Scholar]
- 23.Sethi V, Tabbutt S, Dimitropoulos A, et al. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res. 2013;73(5):661–667. doi: 10.1038/pr.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licht DJ, Wang J, Silvestre DW, et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg. 2004;128(6):841–849. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12 Suppl 1):I109–I114. [PubMed] [Google Scholar]
- 26.McQuillen PS, Hamrick SEG, Perez MJ, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113(2):280–285. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 27.Petit CJ, Rome JJ, Wernovsky G, et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119(5):709–716. doi: 10.1161/CIRCULATIONAHA.107.760819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goff DA, Shera DM, Tang S, et al. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. J Thorac Cardiovasc Surg. 2013 Jul; doi: 10.1016/j.jtcvs.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch JM, Buckley EM, Schwab PJ, et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2014 Jun; doi: 10.1016/j.jtcvs.2014.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139(3):543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partridge SC, Vigneron DB, Charlton NN, et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59(4):640–651. doi: 10.1002/ana.20772. [DOI] [PubMed] [Google Scholar]
- 32.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32(9):496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Back SA, Miller SP. Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Ann Neurol. 2014;75(4):469–486. doi: 10.1002/ana.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McQuillen PS, Ferriero DM. Perinatal subplate neuron injury: implications for cortical development and plasticity. Brain Pathol. 2005;15(3):250–260. doi: 10.1111/j.1750-3639.2005.tb00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inder TE, Huppi PS, Warfield S, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46(5):755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 37.Volpe JJ. Encephalopathy of congenital heart disease- destructive and developmental effects intertwined. The Journal of Pediatrics. 2014;164(5):962–965. doi: 10.1016/j.jpeds.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Duerden EG, Guo T, Dodbiba L, et al. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann Neurol. 2016;79(4):548–559. doi: 10.1002/ana.24601. [DOI] [PubMed] [Google Scholar]
- 39.Tam EWY, Miller SP, Studholme C, et al. Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. The Journal of Pediatrics. 2011;158(3):366–371. doi: 10.1016/j.jpeds.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinney HC, Panigrahy A, Newburger JW, Jonas RA, Sleeper LA. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 2005;110(6):563–578. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- 41.Peyvandi S, De Santiago V, Chakkarapani E, et al. Association of Prenatal Diagnosis of Critical Congenital Heart Disease With Postnatal Brain Development and the Risk of Brain Injury. JAMA Pediatr. 2016 Feb; doi: 10.1001/jamapediatrics.2015.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibuki K, Watanabe K, Yoshimura N, et al. The improvement of hypoxia correlates with neuroanatomic and developmental outcomes: comparison of midterm outcomes in infants with transposition of the great arteries or single-ventricle physiology. J Thorac Cardiovasc Surg. 2012;143(5):1077–1085. doi: 10.1016/j.jtcvs.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 43.Watson CG, Asaro LA, Wypij D, Robertson RL, Newburger JW, Rivkin MJ. Altered Gray Matter in Adolescents with d-Transposition of the Great Arteries. The Journal of Pediatrics. 2016;169:36–43. e1. doi: 10.1016/j.jpeds.2015.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivkin MJ, Watson CG, Scoppettuolo LA, et al. Adolescents with D-transposition of the great arteries repaired in early infancy demonstrate reduced white matter microstructure associated with clinical risk factors. J Thorac Cardiovasc Surg. 2013;146(3):543–9. e1. doi: 10.1016/j.jtcvs.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rollins CK, Watson CG, Asaro LA, et al. White matter microstructure and cognition in adolescents with congenital heart disease. The Journal of Pediatrics. 2014;165(5):936–44. e1–2. doi: 10.1016/j.jpeds.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124(12):1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marelli A, Miller SP, Marino BS, Jefferson AL, Newburger JW. Brain in Congenital Heart Disease Across the Lifespan: The Cumulative Burden of Injury. Circulation. 2016;133(20):1951–1962. doi: 10.1161/CIRCULATIONAHA.115.019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16(Suppl 1(S1)):92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 49.Wernovsky G. Outcomes regarding the central nervous system in children with complex congenital cardiac malformations. Cardiol Young. 2005;15(Suppl 1):132–133. doi: 10.1017/s1047951105001162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.