Abstract
Brain activity during wakefulness is associated with high metabolic rates that are believed to support information processing and memory encoding. In spite of loss of consciousness, sleep still carries a substantial energy cost. Experimental evidence supports a cerebral metabolic shift taking place during sleep that suppresses aerobic glycolysis, a hallmark of environment-oriented waking behavior and synaptic plasticity. Recent studies reveal that glial astrocytes respond to the reduction of wake-promoting neuromodulators by regulating volume, composition and glymphatic drainage of interstitial fluid. These events are accompanied by changes in neuronal discharge patterns, astrocyte-neuron interactions, synaptic transactions and underlying metabolic features. Internally-generated neuronal activity and network homeostasis are proposed to account for the high sleep-related energy demand.
Introduction
Sleep is a universal and evolutionarily conserved behavior shared by species in the animal kingdom regardless of the great diversity of their ecological constraints. Although we do not yet have a fundamental understanding of why animals need to sleep, it is generally accepted that sleep allows the brain to perform critical operations that are largely incompatible with wakefulness [1]. The brain is a constant energy sink, accounting for up to one fifth of total body metabolism. Most of this energy utilization is due to information processing by neuronal-glial networks in the cortical grey matter [2]. The latter is necessary to implement appropriate behavioral responses to the afferent stimuli that are constantly barraging sense organs during wakefulness. Sleep interrupts the connection with the external world, but not the high cerebral metabolic demand. First, brain energy expenditure in non rapid eye movement (NREM) sleep only decreases to ~85% of the waking value, which is higher than the minimal amount of energy required to sustain consciousness [3**]. Second, rapid eye movement (REM) sleep is as expensive as wakefulness and the suspension of thermoregulation during REM sleep is paradoxically associated with increases in brain metabolic heat production and temperature [4]. Third, neither torpor/hibernation (for instance, in mammals undergoing daily hypothermia) [5] nor several anesthetic states [6] can completely redeem sleep need and recovery, in spite of the loss of consciousness and the accompanying decrease of energy use. Sleep must be related to some essential functions that are adaptive to the organism in the face of their relatively high energy requirements. Here we discuss how the glymphatic and ionostatic functions of the brain contribute to shape the metabolic correlates of sleep and associated neuronal network homeostasis.
Oxidative shift in brain energy metabolism during state transitions
Cerebral energy production is reliant on uptake and metabolism of circulating glucose as well as oxygen diffusing from bloodstream supporting the near-complete oxidation of the sugar. The oxygen-glucose utilization stoichiometry (oxygen-glucose index, OGI) is about 5.1–5.4 in quiet waking conditions (note that 6 mol of oxygen are required to fully oxidize 1 mol of glucose). The excess carbohydrates are processed through aerobic glycolysis, so termed because the pyruvate generated from glucose is reduced to lactate instead of being oxidized within the tricarboxylic acid cycle, regardless of adequate oxygenation (i.e. independent of oxygen availability) [7]. Aerobic glycolysis produces only 6% the amount of ATP generated by oxidative phosphorylation, yet it is substantially upregulated during active waking resulting in elevated production of lactate. Advances in amperometric substrate-specific biosensors technology have allowed to monitor on a second-by-second time resolution the extracellular concentration of several important metabolites in the brain of freely-behaving rodents. Together with microdialysis and biochemical assays, these studies show that during NREM sleep cerebral glucose increases [8–10,11*] and lactate decreases [8,11*,12–16] relative to quiet waking. Glucose and lactate levels are reported to be somewhat similar in REM sleep and waking [9,17], although during engagement in complex tasks glucose drops [17,18] and lactate rises [13,17] further than during quiet waking. The extracellular glutamate concentration is lower during NREM sleep compared with wakefulness and REM sleep, supporting a decrease in glutamatergic neurotransmission [11*,19,20]. Finally, the transition from wakefulness to sleep comes along with a transient surge in ATP levels in wake-active brain regions [21] that likely reflects the initial decrease in ATP degradation as sleep supervenes [22].
The above-mentioned changes in brain metabolite levels confirm and extend previous reports obtained in human subjects (but also in other mammals) that measured alterations in cerebral metabolic rates in different states relative to resting conditions. Specifically, active waking (e.g., sensory stimulation) is accompanied by much higher increases in metabolic rate of glucose than oxygen (with OGI decreasing to 4–5) [23–26]. Opposite, sleep is characterized by much higher decreases in metabolic rate of glucose than oxygen (with OGI approaching or exceeding 6; OGI>6 implies oxidation of substrates other than glucose, e.g., fatty acids) [27–34]. To summarize, state transitions are associated with changes in brain energy metabolism whose magnitude is governed by oxygen consumption and is thus relatively modest (about 15%). However, on top of these absolute changes there is a state-dependent metabolic shift affecting the degree of aerobic glycolysis, with sleep being more oxidative than wakefulness [35] (Figure 1). For comparison, body metabolic rates fall at least by 25% from quiet wakefulness to sleep, and by much more when physical activity is taken into account. Interestingly, the physiological changes taking place during sleep (e.g., decrease in muscle activity and thermogenesis) come with a slight reduction in respiratory quotient, indicating a transition to lower carbohydrate/fat oxidation ratio at the whole body level.
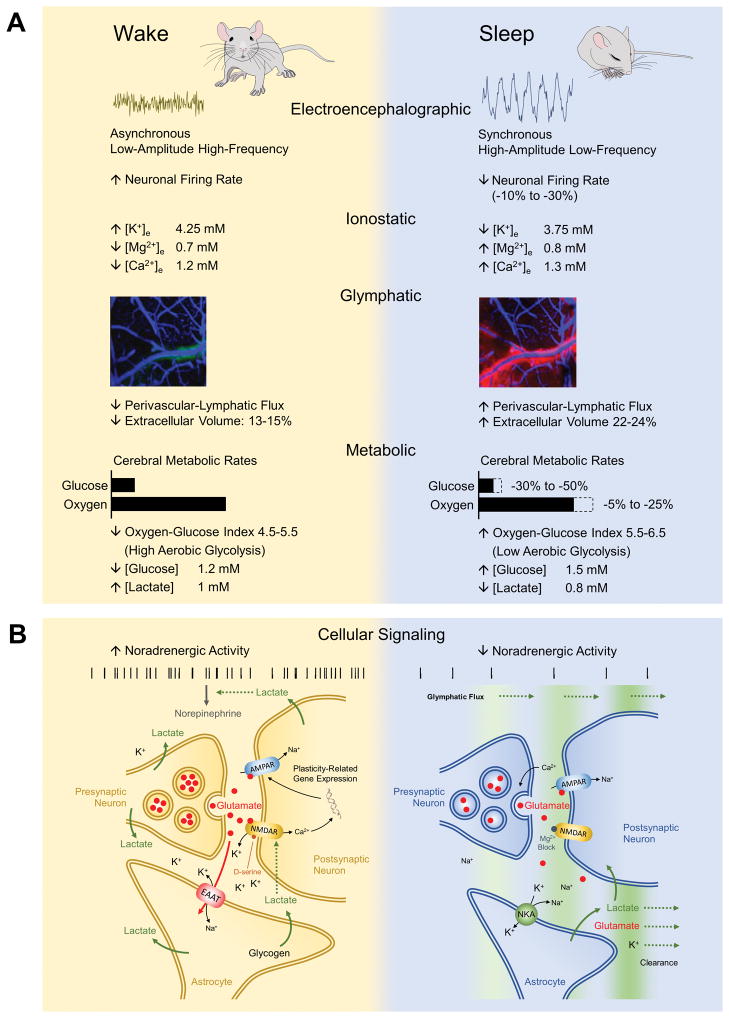
Figure 1. Physiological correlates of brain state changes across the sleep-wake cycle.
(A) Electroencephalographic, ionostatic, glymphatic and metabolic features of wakefulness and sleep states. Sleep is characterized by the appearance of synchronous slow wave activity underlying a reduction in neuronal firing rate and reshaped firing patterns. Neuronal excitability is suppressed through alterations in interstial fluid ion composition as well as glymphatic clearance of neuroactive compounds. These events are accompanied by a metabolic shifts from elevated aerobic glycolysis during wakefulness to more oxidative metabolism during sleep. Approximate values of main cerebral ions and metabolites are shown. (B) Schematics of state-dependent astrocyte-neuron interactions. Noradrenergic tone drives state transitions and maintains brain state by acting at different targets, including ionostatic and glymphatic control systems. During wakefulness, neuronal and astrocytic metabolism is characterized by high rates of aerobic glycolysis, glycogen degradation and lactate production in a NE-dependent manner. Lactate in turn potentiates NE release by noradrenergic terminals and it is involved in NMDAR-mediated synaptic plasticity mechanisms and expression of plasticity-related genes. During sleep, suppression of noradrenergic activity enhances glymphatic clearance of lactate and possibly also glutamate and K+. Clearance is facilitated by increased extracellular space volume (i.e. reduced intracellular volume) and decreased synaptic coverage by astrocytic processes. K+ is also sequestrated by astrocytic NKA, which contributes to the decreased extracellular K+ and associated neuronal excitability underlying sleep. In this state, increased levels of extracellular Ca2+ and Mg2+ enhances release probability of vesicles (possibly with reduced quantal content) while blocking NMDAR activation, thereby changing synaptic plasticity rules. EAAT, excitatory amino acid transporter; NMDAR, N-methyl-D-aspartate receptor; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; NKA, Na+/K+-activated adenosine trisphosphatase.
Coupling between aerobic glycolysis and plasticity by norepinephrine
The benefits for adopting the inefficient metabolic strategy of aerobic glycolysis during active waking behavior are presently unknown, but aerobic glycolysis is abolished by noradrenergic blockade, suggesting that it is related to the processing of sensory information [reviewed by 7]. Indeed, responsiveness to and discrimination between meaningful and meaningless environmental stimuli hinges on the activation of wake-promoting systems. During NREM sleep, firing of cholinergic and noradrenergic neurons is dramatically suppressed and forebrain acetylcholine (Ach) and norepinephrine (NE) levels are reduced (during REM sleep cholinergic activity is reinstated while noradrenergic neurons cease firing altogether). As a result, in the sleeping state the brain no longer process information in a task-relevant manner [e.g., 36] and memory encoding (i.e. formation) is set aside [37].
Aerobic glycolysis is developmentally regulated and correlates with expression of genes involved in synaptic growth and plasticity [38]. In the adult human brain, aerobic glycolysis is elevated in sites exhibiting intense plasticity genes expression and sustained activity like the medial prefrontal cortex, an area extensively implicated in learning and memory [39]. A recent human study reported high levels of aerobic glycolysis in task-relevant neocortical regions that strongly correlated with behavioral adaptation and functional connectivity [40**]. In rodent hippocampus and amygdala, increased glucose utilization and degradation of astrocytic glycogen contribute to the increase in extracellular lactate concentration required for memory processing [41–43]. Lactate potentiates neuronal N-methyl-D-aspartate (NMDA) receptor signaling (necessary for long-term potentiation) and triggers the expression of genes involved in synaptic plasticity [44,45]. The induction of plasticity-related genes depends on the activity of the noradrenergic system and thus can only occur during wakefulness [46]. In turn, lactate can regulate NE availability by stimulating its release from locus coeruleus axonal varicosities [47]. Notably, sleep stimulates the clearance of brain lactate through the glymphatic system [48*], a process dependent on reduced noradrenergic activity and mediated by astrocytic aquaporin-4 (AQP4) water channels [49]. Cerebral perivascular-lymphatic drainage is an important route for lactate dispersal [50] and its suppression during wakefulness may thus contribute to maintain brain lactate levels, noradrenergic tone and synaptic plasticity [48*,51]. It should be noted that changes in neuromodulatory tone drive brain state changes and influence plasticity-related processes in all brain cell types, including astrocytes, neurons, oligodendrocytes and microglia [reviewed by 52].
State-dependent astrocyte-neuron functional and metabolic interactions
The transition from wakefulness to sleep is accompanied by a marked expansion of extracellular space [49,53] as well as rapid and sustained decrease in extracellular K+ and increase in extracellular Ca2+ and Mg2+ [54**]. These changes in interstitial fluid volume and ionic composition are reversed during the transition from sleep to wakefulness and are largely dependent on neuromodulators, while they survive suppression of neuronal firing [49,54**]. As wake-promoting neuromodulators generally decrease membrane K+ conductance, these findings suggest the involvement of Na+/K+-activated adenosine trisphosphatase (NKA) activity. During sleep-wake cycle, NE has been reported to stimulate neuronal and inhibit astrocytic NKA [55], advancing the possibility that sleep promotes extracellular K+ removal and volume increase by a transient disinhibition of the osmogenic astrocytic NKA. Both neurons and astrocytes swell upon elevated extracellular K+ or oxygen/glucose deprivation, however neurons are remarkably more osmo-resistant than AQP4-expressing astrocytes [56,57]. Interestingly, NE has repeatedly been found to alter astrocytic morphology in vitro and in situ, a mechanism seemingly associated with increments of distal processes volume and surface area [53 and references therein]. Arousal upregulates genes related to extracellular matrix and cytoskeleton involved in the elongation of peripheral astrocytic processes, bringing them closer to the synaptic cleft during wakefulness compared with sleep [58*]. Alternatively or in addition, alike for lactate the enhancement of glymphatic system may contribute to K+ clearance during sleep (Figure 1).
The combination of interstitial fluid ionic changes brought about by sleep onset and progression might affect the balance between presynaptic and postsynaptic activation. Low extracellular K+ is associated with reduced synaptic failures and high extracellular Ca2+ is known to enhance transmitter release, whereas high extracellular Mg2+ increases blockade of NMDA receptors [54**,59]. The decrease in insterstitial K+ concentration is consistent with widespread neuronal hyperpolarization and consequent reduction in neuronal firing rates. Compared with quiet waking, average neuronal firing rates do indeed slightly decrease during NREM sleep, increase during active waking and do not appreciably change during REM sleep [60,61*]. Declines in neuronal firing rates (e.g., periodic neuronal silence during NREM slow-wave activity) are intrinsically associated with reduced synaptic failures in the majority of central synapses [62]. In addition, decreased Ach and NE levels during sleep might suppress astrocytic release (or astrocyte-mediated neuronal release) of ATP/adenosine and D-serine, thereby abrogating the effects of adenosine in inhibiting presynaptic glutamate release and potentiating postsynaptic responses as well as the effect of D-serine in acting as co-agonist at NMDA receptors [63]. Reduced synaptic coverage by astrocytes during sleep also entails decreased glutamate reuptake and consequent increase in glutamate dwell time and spillover, with profound consequences on neuronal synchronization and synaptic plasticity rules [58*]. Together, these observations indicate that sleep is seemingly characterized by an increase in release probability (i.e. decrease in synaptic failures). Synaptic failures enhance information transmission efficiency [2] and are necessary for basic neuronal computations, such as sensory adaptation, gain control and direction selectivity [64], something that is manifestly important during wakefulness.
Fundamental differences in the balance between presynaptic and postsynaptic activation can be well responsible for the changes in OGI observed during the sleep-wake cycle. The machinery for axonal vesicle transport and presynaptic vesicle recyling largely depends on glycolytic energy [65–68], consistent with low mitochondrial density in presynaptic terminals [69]. Action potential waveform and transmitter release can be modulated by astrocytes [70] and are influenced by glycolytic energy provision [71] as well as axonal mitochondrial trafficking [72]. Uptake of extracellular K+ (most of which exits neurons via NMDA receptors) and glutamate by astrocytes causes and perhaps requires upregulation of glycogenolysis and glycolysis [73–75]. Dendritic spine remodeling during long term potentiation is to a large extent dependent on astrocytic glycogenolysis [76]. High rates of Ca2+ entry (e.g., through postsynaptic NMDA receptors) above the threshold for mitochondrial calcium uniporter stimulate tricarboxylic acid cycle dehydrogenases but at the same time favor lactate production by impairing malate-aspartate NADH shuttle [7,77]. The ability of glycolysis and oxidative phosphorylation to sustain different aspects of neurotransmission has been hitherto difficult to determine, but it likely depends on neuronal presynaptic and postsynaptic transactions [78]. At least in part, the aforementioned events are directly or indirectly the result of astrocytic response to neuromodulators, consistent with the idea that these cells can drive state transitions [79].
Network homeostasis as an energy-consuming facet of sleep
Sleep is an adaptive behavior that increases fitness and behavioral performance, as evidenced by the dramatic adverse effects of sleep deprivation, including reduced alertness and ability to acquire and store information [1]. Adaptation to the environment, a crucial factor impacting on the probability of survival, entails the capacity to modify behavior to mutating conditions. Refinement of neuronal circuits occur in an experience-dependent manner, whereby synapses constantly undergo Hebbian forms of synaptic plasticity like long-term depression and potentiation. If unopposed such mechanism may result in severe widening of neuronal firing rate distribution [80], so that distinct neuronal populations either fire together or are silent, even in the presence of synaptic rescaling. Against this destabilizing force, homeostatic plasticity mechanisms are proposed to maintain the stability of average neuronal activity by adjusting (i.e. either upscaling or downscaling) synaptic strengths [81].
Sleep and wakefulness have recently been found to produce distinct plasticity effects on neuronal networks. In particular, wakefulness is associated with homeostatic changes targeting average neuronal firing set-points [82*] whereas sleep is associated with homeostatic changes targeting neuronal firing distribution [61*]. It seems that sleep brings about a spike rate homogenization effect, that is increasing the activity of slow-firing neurons and decreasing the activity of fast-firing neurons. To this end, the drifting levels of NE and other subcortical neuromodulators occurring during sleep have been suggested to shift plasticity rules between depression and potentiation [61*]. These findings can be interpreted in keeping with the concept of predictive coding, in a nutshell being the idea that the brain contains a representation of the reality used during wakefulness for unconscious inference [83]. The role of NE would be that of inducing the processing of the sole residual mismatch between external information and the inner model of the world, something that dramatically improves energy efficiency. Accordingly, NE decreases the influence of the internal representation to the afferent sensory input, suggesting that it prevents the interpretation of sensory information based only on previous learning [84]. The representation of reality is altered during wakefulness in the presence of environmentally-oriented sensory-motor cortical activity and requires NE, lactate and astrocytic participation in synaptic plasticity [51]. As illustrated above, cessation of noradrenergic firing during sleep substantially modifies astrocyte-neuron interactions. The resulting internally-generated neuronal activity may enable environment-independent changes to internal representations, which would otherwise become progressively resistant to, and worsen the efficiency of, reorienting behavioral responses.
Conclusions
The varying degree of aerobic glycolysis between sleep and wakefulness likely reflects the altered astrocytic participation in neuronal activity and the ensuing changes in synaptic plasticity brought about by differences in neuromodulatory tone. During sleep, selective homeostatic changes are obtained by exposing synapses to neuronal discharge patterns that are energy demanding. Overall, both in terms of energy metabolism and neuronal activity, the immediate sleep “savings” are quantitatively minor as they might support processes necessary to improve and/or restore the ability to learn and remember during subsequent wake periods. The exact biological mechanisms underlying the homeostatic control of cortical synapses are largely unknown, but astrocytes are undoubtely involved in the remodeling of neuronal activity and synaptic plasticity occurring in response to wake-promoting neuromodulators [85,86].
Highlights.
Cerebral aerobic glycolysis and lactate levels are elevated during wakefulness
Sleep is metabolically expensive and increases oxygen-glucose utilization stoichiometry
Noradrenergic tone targets brain ionostatic, glymphatic and metabolic functions
Astrocytes affect state-specific presynaptic/postsynaptic neuronal activity and plasticity
Acknowledgments
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 701635. The content is solely the responsibility of the authors and does not necessarily represent the official views of the European Union.
Footnotes
Conflict of interest statement
Nothing declared.
References
- 1.Vyazovskiy VV. Sleep, recovery, and metaregulation: explaining the benefits of sleep. Nat Sci Sleep. 2015;7:171–184. doi: 10.2147/NSS.S54036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- **3.Stender J, Mortensen KN, Thibaut A, Darkner S, Laureys S, Gjedde A, Kupers R. The Minimal Energetic Requirement of Sustained Awareness after Brain Injury. Curr Biol. 2016;26:1494–1499. doi: 10.1016/j.cub.2016.04.024. In vivo human [18F]-fluorodeoxyglucose positron-emission tomography study of patients in minimally conscious state and unresponsive wakefulness syndrome as well as healthy subjects. The specific amount of glucose utilization required to sustain conscious awareness was identified as 42% of the healthy control value. This result reveals that the energy expended by the brain during wakefulness is due to processes (e.g., information processing) that operate beyond the function of supporting consciousness. [DOI] [PubMed] [Google Scholar]
- 4.Wehr TA. A brain-warming function for REM sleep. Neuroscience & Biobehavioral Reviews. 1992;16:379–397. doi: 10.1016/s0149-7634(05)80208-8. [DOI] [PubMed] [Google Scholar]
- 5.Vyazovskiy VV, Palchykova S, Achermann P, Tobler I, Deboer T. Different Effects of Sleep Deprivation and Torpor on EEG Slow-Wave Characteristics in Djungarian Hamsters. Cereb Cortex. 2017:1–12. doi: 10.1093/cercor/bhx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pick J, Chen Y, Moore JT, Sun Y, Wyner AJ, Friedman EB, Kelz MB. Rapid eye movement sleep debt accrues in mice exposed to volatile anesthetics. Anesthesiology. 2011;115:702–712. doi: 10.1097/ALN.0b013e31822ddd72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dienel GA, Cruz NF. Aerobic glycolysis during brain activation: adrenergic regulation and influence of norepinephrine on astrocytic metabolism. J Neurochem. 2016;138:14–52. doi: 10.1111/jnc.13630. [DOI] [PubMed] [Google Scholar]
- 8.Van den Noort S, Brine K. Effect of sleep on brain labile phosphates and metabolic rate. Am J Physiol. 1970;218:1434–1439. doi: 10.1152/ajplegacy.1970.218.5.1434. [DOI] [PubMed] [Google Scholar]
- 9.Netchiporouk L, Shram N, Salvert D, Cespuglio R. Brain extracellular glucose assessed by voltammetry throughout the rat sleep-wake cycle. Eur J Neurosci. 2001;13:1429–1434. doi: 10.1046/j.0953-816x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 10.Dash MB, Bellesi M, Tononi G, Cirelli C. Sleep/wake dependent changes in cortical glucose concentrations. J Neurochem. 2013;124:79–89. doi: 10.1111/jnc.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Naylor E, Aillon DV, Barrett BS, Wilson GS, Johnson DA, Johnson DA, Harmon HP, Gabbert S, Petillo PA. Lactate as a biomarker for sleep. Sleep. 2012;35:1209–1222. doi: 10.5665/sleep.2072. In vivo measurements of brain glucose, lactate and glutamate during the sleep-wake cycle in freely-behaving mice. An unprecedented sensitivity and temporal resolution was obtained by using substrate-specific amperometric biosensors in conjunction with electroencephalograph and electromyograph monitoring. Based on the excellent correlation between lactate and brain state, the study identified the monocarboxylate as an ideal biomarker of sleep. Notably, glutamate changes lagged behind those of lactate, while glucose only transiently reflected state transitions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clegern WC, Moore ME, Schmidt MA, Wisor J. Simultaneous electroencephalography, real-time measurement of lactate concentration and optogenetic manipulation of neuronal activity in the rodent cerebral cortex. J Vis Exp. 2012:e4328. doi: 10.3791/4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shram N, Netchiporouk L, Cespuglio R. Lactate in the brain of the freely moving rat: voltammetric monitoring of the changes related to the sleep-wake states. Eur J Neurosci. 2002;16:461–466. doi: 10.1046/j.1460-9568.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- 14.Rempe MJ, Wisor JP. Cerebral lactate dynamics across sleep/wake cycles. Front Comput Neurosci. 2014;8:174. doi: 10.3389/fncom.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dash MB, Tononi G, Cirelli C. Extracellular levels of lactate, but not oxygen, reflect sleep homeostasis in the rat cerebral cortex. Sleep. 2012;35:909–919. doi: 10.5665/sleep.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter D, Dawson RM. Brain metabolism in emotional excitement and in sleep. Am J Physiol. 1948;154:73–79. doi: 10.1152/ajplegacy.1948.154.1.73. [DOI] [PubMed] [Google Scholar]
- 17.Cespuglio R, Netchiporouk L, Shram N. Glucose and Lactate Monitoring Across the Rat Sleep–Wake Cycle. In: Marinesco S, Dale N, editors. Microelectrode Biosensors. Humana Press; 2013. pp. 241–256. [Google Scholar]
- 18.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naylor E, Aillon DV, Gabbert S, Harmon H, Johnson DA, Wilson GS, Petillo PA. Simultaneous real-time measurement of EEG/EMG and L-glutamate in mice: A biosensor study of neuronal activity during sleep. J Electroanal Chem (Lausanne) 2011;656:106–113. doi: 10.1016/j.jelechem.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong-Riley M. What is the meaning of the ATP surge during sleep? Sleep. 2011;34:833–834. doi: 10.5665/SLEEP.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 24.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blomqvist G, Seitz RJ, Sjogren I, Halldin C, Stone-Elander S, Widen L, Solin O, Haaparanta M. Regional cerebral oxidative and total glucose consumption during rest and activation studied with positron emission tomography. Acta Physiol Scand. 1994;151:29–43. doi: 10.1111/j.1748-1716.1994.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 26.Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bülow J, Holm S, Wildschiödtz G, Paulson OB, Lassen NA. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1995;15:485–491. doi: 10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- 27.Maquet P, Dive D, Salmon E, Sadzot B, Franco G, Poirrier R, von Frenckell R, Franck G. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res. 1990;513:136–143. doi: 10.1016/0006-8993(90)91099-3. [DOI] [PubMed] [Google Scholar]
- 28.Heiss WD, Pawlik G, Herholz K, Wagner R, Wienhard K. Regional cerebral glucose metabolism in man during wakefulness, sleep, and dreaming. Brain Res. 1985;327:362–366. doi: 10.1016/0006-8993(85)91537-9. [DOI] [PubMed] [Google Scholar]
- 29.Franck G, Salmon E, Poirrier R, Sadzot B, Franco G. Study of regional cerebral glucose metabolism, in man, while awake or asleep, by positron emission tomography. Rev Electroencephalogr Neurophysiol Clin. 1987;17:71–77. doi: 10.1016/s0370-4475(87)80116-8. [DOI] [PubMed] [Google Scholar]
- 30.Buchsbaum MS, Gillin JC, Wu J, Hazlett E, Sicotte N, Dupont RM, Bunney WE., Jr Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography. Life Sci. 1989;45:1349–1356. doi: 10.1016/0024-3205(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 31.Hoshi Y, Mizukami S, Tamura M. Dynamic features of hemodynamic and metabolic changes in the human brain during all-night sleep as revealed by near-infrared spectroscopy. Brain Res. 1994;652:257–262. doi: 10.1016/0006-8993(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 32.Mangold R, Sokoloff L, Conner E, Kleinerman J, Therman PO, Kety SS. The effects of sleep and lack of sleep on the cerebral circulation and metabolism of normal young men. J Clin Invest. 1955;34:1092–1100. doi: 10.1172/JCI103158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madsen PL, Schmidt JF, Holm S, Vorstrup S, Lassen NA, Wildschiodtz G. Cerebral oxygen metabolism and cerebral blood flow in man during light sleep (stage 2) Brain Res. 1991;557:217–220. doi: 10.1016/0006-8993(91)90137-k. [DOI] [PubMed] [Google Scholar]
- 34.Madsen PL, Schmidt JF, Wildschiodtz G, Friberg L, Holm S, Vorstrup S, Lassen NA. Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid-eye-movement sleep. J Appl Physiol (1985) 1991;70:2597–2601. doi: 10.1152/jappl.1991.70.6.2597. [DOI] [PubMed] [Google Scholar]
- 35.Wisor JP, Rempe MJ, Schmidt MA, Moore ME, Clegern WC. Sleep slow-wave activity regulates cerebral glycolytic metabolism. Cereb Cortex. 2013;23:1978–1987. doi: 10.1093/cercor/bhs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sara SJ. Locus Coeruleus in time with the making of memories. Curr Opin Neurobiol. 2015;35:87–94. doi: 10.1016/j.conb.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **40.Shannon BJ, Vaishnavi SN, Vlassenko AG, Shimony JS, Rutlin J, Raichle ME. Brain aerobic glycolysis and motor adaptation learning. Proc Natl Acad Sci U S A. 2016;113:E3782–3791. doi: 10.1073/pnas.1604977113. The first in vivo human study measuring brain glucose/oxygen utilization and plasticity changes during a visual-motor adaptation task. Combining [18F]-fluorodeoxyglucose and [15O]-water/carbon monoxide/oxygen positron-emission tomography with functional-connectivity magnetic resonance imaging, a correlation between increased aerobic glycolysis, resting-state connectivity and behavioral adaptation was reported. These outcomes strongly support a number of recent observations that identied aerobic glycolysis as a critical mechanism for memory formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Xue Y, Meng S, Luo Y, Liang J, Li J, Ai S, Sun C, Shen H, Zhu W, et al. Inhibition of Lactate Transport Erases Drug Memory and Prevents Drug Relapse. Biol Psychiatry. 2016;79:928–939. doi: 10.1016/j.biopsych.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Tadi M, Allaman I, Lengacher S, Grenningloh G, Magistretti PJ. Learning-Induced Gene Expression in the Hippocampus Reveals a Role of Neuron -Astrocyte Metabolic Coupling in Long Term Memory. PLoS One. 2015;10:e0141568. doi: 10.1371/journal.pone.0141568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. 2014;111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, Teschemacher AG. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun. 2014;5:3284. doi: 10.1038/ncomms4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *48.Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, Deane R, Nedergaard M. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab. 2016;37:2112–2124. doi: 10.1177/0271678X16661202. Role of glymphatic system in brain lactate decrease associated with sleep assessed in mice in vivo. Different procedures known to impair insterstitial-cerebrospinal fluid exchange and glymphatic function were found to abolish the decrease in cerebral lactate as measured by microdialysis. As lymphatic-paravascular fluxes are substantially smaller during wakefulness than sleep, the study suggests that the glymphatic system contributes to maintain waking brain lactate levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ball KK, Cruz NF, Mrak RE, Dienel GA. Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. J Cereb Blood Flow Metab. 2010;30:162–176. doi: 10.1038/jcbfm.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiNuzzo M. Astrocyte-Neuron Interactions during Learning May Occur by Lactate Signaling Rather than Metabolism. Front Integr Neurosci. 2016;10:2. doi: 10.3389/fnint.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. 2012;37:2496–2512. doi: 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherpa AD, Xiao F, Joseph N, Aoki C, Hrabetova S. Activation of beta-adrenergic receptors in rat visual cortex expands astrocytic processes and reduces extracellular space volume. Synapse. 2016;70:307–316. doi: 10.1002/syn.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **54.Ding F, O’Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science. 2016;352:550–555. doi: 10.1126/science.aad4821. In vivo measurements of interstitial fluid ionic composition during sleep-wake cycle in mice. Alterations in the extracellular concentration of several important ions were reported to correlate with sleep and waking in a neuromodulator-dependent manner. Blockade of neuronal activity failed to prevent these changes from occurring during state transitions. Most importantly, topical application or intracisternal injection of artificial cerebrospinal fluid with ion concentrations mimicking sleep and wake states recapitulated the corresponding electrophysiological brain patterns regardless of the actual brain state. The study demonstrates that changes in neuronal discharge associated with sleep and wakefulness are secondary to changes in interstitial ionic levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baskey G, Singh A, Sharma R, Mallick BN. REM sleep deprivation-induced noradrenaline stimulates neuronal and inhibits glial Na-K ATPase in rat brain: in vivo and in vitro studies. Neurochem Int. 2009;54:65–71. doi: 10.1016/j.neuint.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Risher WC, Andrew RD, Kirov SA. Real-Time Passive Volume Responses of Astrocytes to Acute Osmotic and Ischemic Stress in Cortical Slices and in vivo Revealed by Two-Photon Microscopy. Glia. 2009;57:207–221. doi: 10.1002/glia.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrew RD, Labron MW, Boehnke SE, Carnduff L, Kirov SA. Physiological evidence that pyramidal neurons lack functional water channels. Cereb Cortex. 2007;17:787–802. doi: 10.1093/cercor/bhk032. [DOI] [PubMed] [Google Scholar]
- *58.Bellesi M, de Vivo L, Tononi G, Cirelli C. Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC Biol. 2015;13:66. doi: 10.1186/s12915-015-0176-7. Determination of astrocytic gene expression and ultrastructure during sleep-wake cycle. Using a combination of techniques, an association between wakefulness (sleep) and increases (decreases) in elongation, surface area and synaptic coverage of peripheral astrocytic processes was found. The study provides a structural support for state-dependent changes in astrocyte-neuron interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M. Astrocytes modulate neural network activity by Ca(2)+-dependent uptake of extracellular K+ Sci Signal. 2012;5:ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *61.Watson BO, Levenstein D, Greene JP, Gelinas JN, Buzsaki G. Network Homeostasis and State Dynamics of Neocortical Sleep. Neuron. 2016;90:839–852. doi: 10.1016/j.neuron.2016.03.036. In vivo measurements of neuronal firing during sleep-wake cycle in rats. By sampling a large number of neurons using multi-site silicon-probe based recordings, sleep sub-stages were reported to be associated with homeostasis of neuronal firing rate distribution (i.e. homogenization). The study suggests that sleep rebalances learning-induced and homeostatic plastic processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 63.Papouin T, Dunphy J, Tolman M, Foley JC, Haydon PG. Astrocytic control of synaptic function. Philos Trans R Soc Lond B Biol Sci. 2017:372. doi: 10.1098/rstb.2016.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Regehr WG. Short-term presynaptic plasticity. Cold Spring Harb Perspect Biol. 2012;4:a005702. doi: 10.1101/cshperspect.a005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hinckelmann M-V, Virlogeux A, Niehage C, Poujol C, Choquet D, Hoflack B, Zala D, Saudou F. Self-propelling vesicles define glycolysis as the minimal energy machinery for neuronal transport. Nature Communications. 2016;7:13233. doi: 10.1038/ncomms13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ikemoto A, Bole DG, Ueda T. Glycolysis and glutamate accumulation into synaptic vesicles. Role of glyceraldehyde phosphate dehydrogenase and 3-phosphoglycerate kinase. J Biol Chem. 2003;278:5929–5940. doi: 10.1074/jbc.M211617200. [DOI] [PubMed] [Google Scholar]
- 67.Ishida A, Noda Y, Ueda T. Synaptic vesicle-bound pyruvate kinase can support vesicular glutamate uptake. Neurochem Res. 2009;34:807–818. doi: 10.1007/s11064-008-9833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelieres FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 69.Chavan V, Willis J, Walker SK, Clark HR, Liu X, Fox MA, Srivastava S, Mukherjee K. Central presynaptic terminals are enriched in ATP but the majority lack mitochondria. PLoS One. 2015;10:e0125185. doi: 10.1371/journal.pone.0125185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sasaki T, Matsuki N, Ikegaya Y. Action-potential modulation during axonal conduction. Science. 2011;331:599–601. doi: 10.1126/science.1197598. [DOI] [PubMed] [Google Scholar]
- 71.Lujan B, Kushmerick C, Banerjee TD, Dagda RK, Renden R. Glycolysis selectively shapes the presynaptic action potential waveform. J Neurophysiol. 2016;116:2523–2540. doi: 10.1152/jn.00629.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4:413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L. Requirement of Glycogenolysis for Uptake of Increased Extracellular K(+) in Astrocytes: Potential Implications for K (+) Homeostasis and Glycogen Usage in Brain. Neurochem Res. 2013;38:472–485. doi: 10.1007/s11064-012-0938-3. [DOI] [PubMed] [Google Scholar]
- 74.Sickmann HM, Walls AB, Schousboe A, Bouman SD, Waagepetersen HS. Functional significance of brain glycogen in sustaining glutamatergic neurotransmission. J Neurochem. 2009;109(Suppl 1):80–86. doi: 10.1111/j.1471-4159.2009.05915.x. [DOI] [PubMed] [Google Scholar]
- 75.Bittner CX, Valdebenito R, Ruminot I, Loaiza A, Larenas V, Sotelo-Hitschfeld T, Moldenhauer H, San Martin A, Gutierrez R, Zambrano M, et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci. 2011;31:4709–4713. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drulis-Fajdasz D, Wójtowicz T, Wawrzyniak M, Wlodarczyk J, Mozrzymas JW, Rakus D. Involvement of cellular metabolism in age-related LTP modifications in rat hippocampal slices. Oncotarget. 2015;6:14065–14081. doi: 10.18632/oncotarget.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Satrustegui J, Bak LK. Fluctuations in Cytosolic Calcium Regulate the Neuronal Malate-Aspartate NADH Shuttle: Implications for Neuronal Energy Metabolism. Neurochem Res. 2015;40:2425–2430. doi: 10.1007/s11064-015-1652-8. [DOI] [PubMed] [Google Scholar]
- 78.Sobieski C, Fitzpatrick MJ, Mennerick SJ. Differential Presynaptic ATP Supply for Basal and High-Demand Transmission. J Neurosci. 2017;37:1888–1899. doi: 10.1523/JNEUROSCI.2712-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kjaerby C, Rasmussen R, Andersen M, Nedergaard M. Does Global Astrocytic Calcium Signaling Participate in Awake Brain State Transitions and Neuronal Circuit Function? Neurochem Res. 2017;42:1810–1822. doi: 10.1007/s11064-017-2195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levenstein D, Watson BO, Rinzel J, Buzsaki G. Sleep regulation of the distribution of cortical firing rates. Curr Opin Neurobiol. 2017;44:34–42. doi: 10.1016/j.conb.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turrigiano GG. The dialectic of Hebb and homeostasis. Philos Trans R Soc Lond B Biol Sci. 2017:372. doi: 10.1098/rstb.2016.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *82.Hengen Keith B, Torrado Pacheco A, McGregor James N, Van Hooser Stephen D, Turrigiano Gina G. Neuronal Firing Rate Homeostasis Is Inhibited by Sleep and Promoted by Wake. Cell. 2016;165:180–191. doi: 10.1016/j.cell.2016.01.046. In vivo measurements of neuronal firing during sleep-wake cycle in rats. Using tungsten microelectrode arrays neuronal firing was monitored in primary visual cortex during firing rate rebound after monocular visual deprivation. Sleep was reported to inhibit rather than promote firing rate homeostasis, indicating that changes in absolute neuronal firing require wakefulness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 84.Hasselmo ME, Linster C, Patil M, Ma D, Cekic M. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J Neurophysiol. 1997;77:3326–3339. doi: 10.1152/jn.1997.77.6.3326. [DOI] [PubMed] [Google Scholar]
- 85.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haydon PG, Nedergaard M. How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol. 2014;7:a020438. doi: 10.1101/cshperspect.a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]