Abstract
The immune system is our interface with the environment, and immune molecules such as cytokines and chemokines and the cells that produce them within the brain, notably microglia, are critical for normal brain development. This recognition has in recent years led to the working hypothesis that inflammatory events during pregnancy or the early postnatal period, e.g. in response to infection, may disrupt the normal developmental trajectory of microglia and consequently their interactions with neurons, thereby contributing to the risk for neurological disorders. The current article outlines recent findings on the impact of diverse, pervasive environmental challenges, beyond infection, including air pollution and maternal stress; and their impact on microglial development and its broad implications for neural pathologies.
Introduction
Increasingly, animal and human studies show a link between early life/maternal immune activation (MIA) and adverse neural outcomes in offspring; the former involving diverse environmental factors, including infections; but also toxin exposures, maternal stress, and metabolic disruptions like obesity, each of which may profoundly impact inflammatory or immune pathways. Microglia, the primary immune and cytokine-producing cells of the central nervous system (CNS), are critical for normal brain development via the phagocytosis of extraneous synapses [1–4] and apoptotic cells [5–7], learning dependent synapse formation [8,9], cortical wiring [10], neuronal survival [11], and the induction of programmed cell death [7,12]; topics that have been reviewed in detail elsewhere [13,14] (see Figure 1). Impairment of these developmental functions of microglia – primarily via genetic manipulations of critical genes - has been shown to adversely impact brain connectivity and behavior [15]. Due to their macrophage/antigen presenting cell lineage, microglia are exquisitely sensitive to disruptions of homeostasis and thus environmental influences as well, perhaps more so than any other CNS cell type. The perinatal period is a time of intense microglial proliferation, development, and activity, and hence may be particularly vulnerable to the induction of long-term changes in microglial cell number or function [16,17]. Indeed, our work has demonstrated significant and persistent impacts of perinatal inflammation on microglial function in rodents, which produce exaggerated cytokine responses to subsequent insults later in life that are directly implicated in behavioral abnormalities such as cognitive dysfunction [18–20]. In this brief review, we focus on the broader implications of this work for microglial activation by additional non-infectious environmental factors early in life – factors that have become increasingly pervasive in our modern world - and the potential contribution of such activation to a significant proportion of neurodevelopmental disorders, which if true has implications for a wide segment of the population, but also for treatment and prevention strategies.
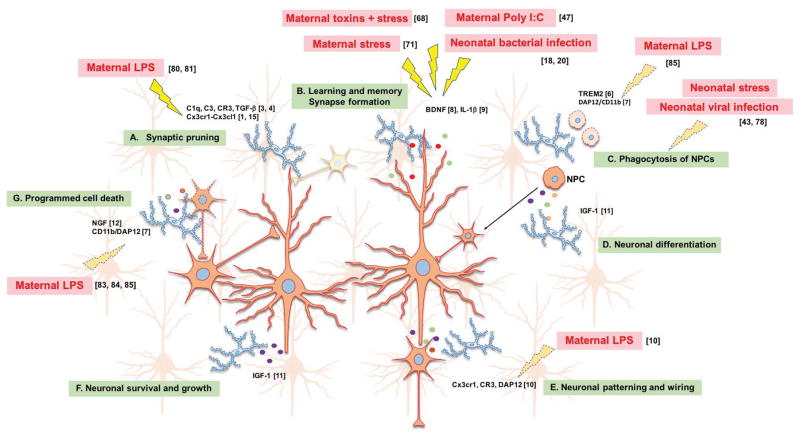
Figure 1. Microglia homeostatic functions during development, and in response to diverse environmental factors.
Microglia, the primary immune and cytokine-producing cells of the central nervous system (CNS), are also critical for normal brain development via (A) the phagocytosis of extraneous synapses using the complement pathway; (B) releasing factors such as BDNF and IL1β critical for synapse formation during learning and memory; (C) phagocytosis of neural progenitor cells (NPCs) via the TREM2 and DAP12/CD11b pathway; (D) assisting in neuronal differentiation via the release of insulin-like growth factor-1 (IGF-1); (E) Neuronal patterning and wiring using Cx3cr1 signaling; (F) supporting neuronal growth and survival by releasing trophic factors; and (G) inducing programmed cell death via nerve growth factor (NGF), CD11b integrin DAP12 immunoreceptor. Diverse environmental challenges with known (solid lightning bolts) or suspected (dotted lightning bolts) impacts on microglial development are depicted in red text. Refer to Table 1 for additional references.
Microglial development and function
Microglial development is a unique process starting with colonization of the brain by fetal yolk sac-derived myeloid precursor cells in early embryonic stages, followed by migration and spread throughout the CNS, which is accompanied by morphological transition to a ramified state coinciding with transcriptional maturation in most brain regions [21,22]. As microglia develop, there is simultaneous maturation of neurons, astrocytes, and finally oligodendrocytes, and we now recognize that microglia perform numerous functions in response to (or perhaps initiating) the dynamic changing needs of their CNS microenvironments (see [23] in this issue). Microglia are CNS-restricted and self-renewing cells, i.e. without contribution from the hematopoietic cells in the periphery under normal conditions [24,25]. Due to the critical role that microglia play in normal brain development, we have hypothesized that activation or “programming” of these dynamic, long-living cells in response to immune activation, especially early in life, can have persistent consequences for brain function throughout the lifespan [16].
Environmental factors affecting microglial development
Infection during prenatal or early-postnatal development is increasingly identified as a risk factor for a number of neurodevelopmental disorders such as autism [26] and schizophrenia [27,28], and neuropsychiatric disorders such as psychosis [29]. A growing body of work has illustrated some of the mechanisms by which MIA with viral/bacterial infection or their mimetics can persistently alter offspring immune function, disrupt fetal brain development, and induce the onset of behavioral abnormalities in animal models [30,31]. For instance, influenza virus administered to pregnant mice increases cytokine levels in placental tissue and amniotic fluid, in particular IL-6, and results in abnormal development of the offspring [32], as does the viral mimic polyinosinic:polycytidylic acid (poly I:C) [33]. Importantly, the infectious agents themselves most often do not enter the fetal circulation, and experiments demonstrating that specific cytokine antagonists in the presence of infection protect the fetal brain from adverse outcomes have implicated the critical role of the maternal immune response itself [26]. A more recent series of studies using this model pinpoints the role of maternal Th17 cell-derived IL-17a as critical in producing a striking cortical patching phenotype in offspring, alongside autism-like behavioral abnormalities [34–36]. In contrast to these pre-clinical data, a recent study in humans links high levels of maternal IL-17a during the first trimester to better cognitive outcomes in children by 4 years of age, rather than the opposite [37]. Nonetheless, this is a promising avenue of research, as IL-17a could be an important therapeutic target to pursue.
Despite the intensifying interest in this research area, and the elucidation of mechanisms critical for maternal-to-fetal transmission of inflammatory stimuli, few studies have examined the mechanisms by which brain function is directly and/or persistently disrupted. We have demonstrated over a series of experiments that bacterial infection in newborn rats – roughly comparable to the late second trimester in humans – enduringly alters the function of microglia such that a subsequent immune challenge - systemic injection with a low dose of lipopolysaccharide (LPS) - results in exaggerated cytokine production within the brain, which is causally linked to profound cognitive deficits if the LPS occurs around the time of learning later in life [20]. This exaggerated immune response to the “second hit”, the LPS challenge, has been described in the literature previously as microglial priming, e.g. within the neurodegenerative disease literature [38], which simply refers to the phenomenon that the inflammatory response is exaggerated compared to an individual that does not receive a prior infection (i.e., the “first hit”). This pattern is striking given growing recognition that some neuropsychiatric disorders are linked to an underlying vulnerability, which likely begins perinatally, and a “second hit” that unmasks the full pathology [39]. Microglia are ideal candidates as a mechanism underlying priming, as they are macrophages that shift their function following immune stimulation, potentially permanently, in common with many immune cells; but they are also long-living cells [40,41], in contrast to most innate immune cell populations outside the brain. Notably, infection at a later stage of development, postnatal day (P)30, does not have the same persistent impact, implicating a critical window [42]. Early-life viral infection of piglets, who demonstrate similar brain growth trajectories and time course as humans, similarly induces a long-term priming effect on microglia [43]. Specifically, microglia collected at P28 from piglets infected with virus at P7, display enhanced phagocytic and migratory activity, increased sensitivity to a second challenge with LPS and Poly I:C, as well as increased soma size compared to controls, closely supporting our findings in rodents [20,44]. Conversely, a few prenatal infection models have failed to demonstrate overt microglial activation in the offspring [45,46]. Timing – and resulting alterations in developmental trajectory - are undoubtedly key (see [23] in this issue).
An interesting recent study investigating transcriptional and epigenomic regulation of microglia throughout brain development demonstrates that microglial development proceeds through three distinct temporal stages: early microglia (until E14), pre-microglia (E14 to early postnatal weeks), and adult microglia [47]. At each stage of development, microglia evolve distinct pathways for processing relevant signals from the environment and to maintain homeostasis within the brain [47]. Notably, MIA with the viral mimic poly I:C can disrupt the pre-microglial developmental stage in offspring and push the microglial transcription state to a more advanced developmental stage [47]. Similarly, we have demonstrated that LPS challenge in late adolescent mice accelerates microglial transcriptional maturation, but only in males [48]. Impacts on microglial transcriptional development likely have significant adverse consequences on neuronal development (e.g. by disrupting normal processes like pruning or phagocytosis), increasing the risk of disease (Figure 2). Sex differences in the incidence of neurodevelopmental disorders such as autism (4:1 males to females) are well recognized [49], suggesting sex differences in microglial development is an important disease-modifying factor [50], and we return to this concept in a later section.
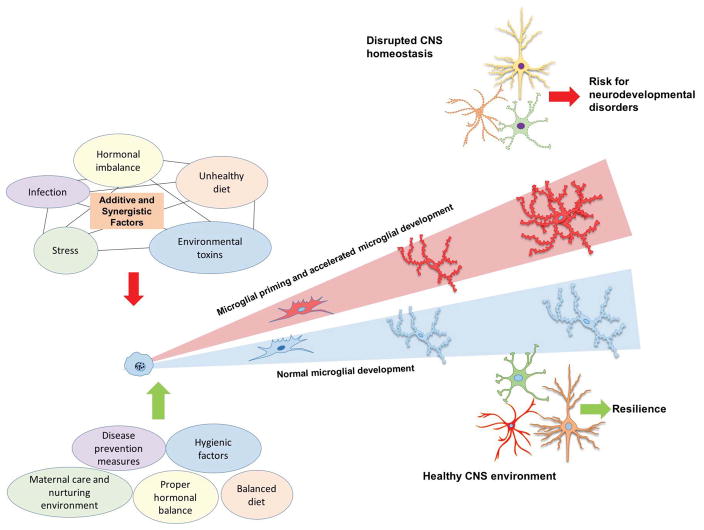
Figure 2. Effect of early-life environmental challenge on microglial developmental trajectory.
A recent study investigating transcriptional and epigenomic regulation of microglia throughout brain development demonstrated that microglial development proceeds through distinct temporal stages: early microglia, pre-microglia, and adult microglia [47]. Further, accumulating data suggests that environmental exposures increases vulnerability in the offspring by accelerating microglial development [47,48]. Impacts on microglial transcriptional development likely have significant adverse consequences on neuronal development (e.g. by disrupting normal processes like pruning or phagocytosis), increasing the risk of disease.
Beyond Infection
Neurodevelopmental disorders have increased over the past two decades at an alarming rate, strongly suggesting an environmental component beyond infections which have been pervasive throughout our evolutionary history. Indeed, diverse exposures to modern environmental factors are increasingly recognized as a risk factor for neurodevelopmental disorders (reviewed in [51]), including pesticides, various components of air pollution (diesel exhaust, NO2, heavy metals), phthalates, polychlorinated biphenyls (e.g., BPA), and even dietary components such as saturated fats and common food allergens (e.g., gluten, casein) [52] (see Table 1). Air pollution, of which diesel exhaust is a primary toxic component [53,54] is the 4th leading cause of mortality worldwide [55,56] and poses a serious and growing health concern for countries where industrial growth is exponential. Maternal air pollutant chemical exposures during pregnancy may be especially harmful and have been linked with asthma in offspring, even independent of postnatal exposures [57], suggesting similar mechanisms may be impacting the brain. Indeed, air levels of diesel exhaust at the time and place of birth is one of the strongest and most consistent environmental links to autism spectrum disorder [58,59].
Table 1.
Diverse environmental factors impact neurodevelopmental outcomes dependent on agent, time of exposure, and interactions with other factors or variables.
| Developmental age | Model | Agent | Primary neuroimmune and behavioral findings | References |
|---|---|---|---|---|
| Prenatal | Infection | Live bacteria | ↓ IL-10 and increase in white-matter damage | [81] |
| Live virus | ↑ microglial phagocytic and migratory activity in offspring. ↑ microglial soma size. ↑ sensitivity to LPS and Poly I:C (second hit) | [43,46] | ||
| LPS | ↑ corticosterone, anxiety, depression in male offspring ↓ Cx3cr1 microglia marker in male offspring, ASD- like behavior observed in males only. Alteration of synaptic development (likely DAP12- dependent mechanism) |
[82] [83,84] [85] |
||
| Poly I:C | No persistent microglial activation or morphology changes in offspring. HP-dependent cognitive deficits and ↓ BDNF observed Accelerated microglial transcriptional maturation |
[45] [47] |
||
| Toxins | Diesel exhaust particles | ↑ CC size, ↓HP area in male and female offspring; hypermyelination, microglial activation in male and female offspring. Altered microglial maturation and microglia-neuron interactions in male offspring |
[64] [63] |
|
| Stress | Restraint stress | ↑ IL-1β mRNA levels in HP, ↑ microglia number, ↑ in proportion of amoeboid cells | [86] | |
| Exposure to bright light | ↑ in ex vivo microglial IL-1β, IL-18, IL-6, TNF-α, CCL2, or NO production. | [79] | ||
| Sleep deprivation | ↑ microglial density, deficits in spatial learning and memory in male offspring. Persistent ↑ in proinflammatory cytokines. | [87] | ||
| Combinations | Diesel exhaust particles + restricted bedding material | ↑ microglial IL-1β release and behavioral deficits in male offspring. | [68] | |
| Diesel exhaust particles + postnatal high fat diet | ↑ anxiety and ↑ microglial activation in male offspring. ↑ microglial activation in male and female offspring. ↓ activity and ↑anxiety in males. |
[77] [76] |
||
| Prenatal poly I:C + peripubertal sub-chronic unpredictable stress | ↑ hippocampal and prefrontal microglia soma size, CD68, and interleukin-1β protein expression | [88] | ||
| Neonatal | Infection | Live bacteria | Persistent microglial morphological and functional changes; Memory impairment (following LPS second hit) in males ↑ IL-1b, ↓ BDNF following learning ↓ motor coordination, ↑ hypomyelination in males |
[18,20] [89] [90] |
| Live virus | ↑ microglial size and number, ↑ microglial migratory and phagocytic activity | [43] | ||
| LPS | Transient ↑ in microglia number, long-term dysregulation of immune gene expression, ↓ number of neuronal precursors in hippocampus. | [91,92] | ||
| Toxins | Diesel exhaust particles | ↑ in microglia number in CC and HP, in male offspring only. ↑ hypomyelination in CC in males. E:I imbalance in males and females. | [65] | |
| Combinations | Brief daily separation + restricted bedding material | Transient ↑ in microglia density and altered morphology. Persistent ↑ in microglial phagocytic activity and proinflammatory cytokines expression. | [78] |
Key: IL-10: Interleukin 10; LPS: Lipopolysaccharide; ASD: Autism spectrum disorders; HP: Hippocampus; BDNF: Brain-derived neurotrophic factor; CC: Corpus callosum; IL-1β: Interleukin 1 beta; IL-18: Interleukin 18; IL-6: Interleukin 6; TNF-α: Tumor necrosis factor alpha; CCL2: aka MCP-1, monocyte chemoattractant protein; NO: Nitric oxide
Though the evidence is primarily derived from large scale epidemiological studies with their inherent caveats regarding causation, the animal literature in support of these associations at a mechanistic level is steadily increasing. Diesel exhaust particles markedly activate microglia in vitro and in vivo, in adult rodents and canines [60,61]. We developed a model to assess the impact of maternal exposure to diesel exhaust on microglial development in the offspring mouse brain. Pregnant mice receive aspirations of vehicle (VEH) or diesel exhaust particles (DEP) intermittently (every 2–3 days) throughout gestation, a regimen which induces significant lung inflammation in the mother similar to that observed in humans living in polluted areas [62]. Because there is no evidence that DE particles cross the placenta, systemic inflammation in the mother is hypothesized to induce an inflammatory reaction within the uterine environment/placenta, and thereby the fetal brain, to alter development. Consistent with this hypothesis, we found a significant impact of maternal DEP on microglial morphology in fetal brains, consistent with activation and/or a delay in maturation in several brain regions, but notably only in males, along with striking changes in neural-glial interactions in males suggestive of a defect in phagocytosis [63].
Another recent study showed that exposure of pregnant dams to ultrafine particles (UFP), a component of air pollution, leads to increases in corpus callosum size, hypermyelination and microglial activation in offspring [64]. Similarly, administration of UFP to male and female mouse pups from P4-13 led to a persistent increase in microglia number in males only, as far out as P270 [65]. Further there were changes in corpus callosum size and reduced myelination in males, and an excitatory-inhibitory imbalance in the frontal cortex in both males and females– consistent with many patients with autism [66,67].
Based on the hypothesis that perturbations of normal microglial development have significant consequences for neural development and thus behavior, we assessed cognitive abilities in young adult mice exposed prenatally to DEP or VEH in our model; however, there was no impact of DEP alone [68]. These data suggest that maternal exposure to air pollution, even at a relatively high dose that induces inflammation in the mother and alters microglial development in the fetal (male) brain, is insufficient to cause changes in behavior. Notably, the effect sizes of even the largest single environmental factor associations with disorders like autism are quite small. This strongly suggests that it is a combination of multiple perinatal exposures (including genetics) that increases vulnerability in the offspring and tips the balance to dysfunction (Figure 2).
Building better models and conclusions
Most studies from the pre-clinical literature suffer from oversimplification, with single factors studied in isolation, which lends little insight into mechanisms by which diverse environmental exposures likely combine or synergize to cause pathology. Growing research suggests that maternal well-being during pregnancy is a crucial determinant of lifelong physical and mental health of the offspring [69,70]. The epidemiological literature demonstrates parental stress can significantly modulate the effect of in utero toxin exposures (e.g., air pollution, tobacco smoke) on childhood health outcomes such as asthma risk [71]. A large literature links stress-induced physiological changes to specific impacts on microglia, particularly stress during the prenatal period. For instance, prenatal stress induced by exposure to 45-min of restraint in pregnant mice increased microglia-mediated inflammatory responses in adult female offspring [72]. Similarly, sleep deprivation in pregnant rat dams during the last gestational trimester increased microglial density in the hippocampus of pre-pubertal male offspring, accompanied by deficits in spatial learning and memory [73]. These effects were reversed by minocycline, an imperfect microglial modulator that nonetheless suggests microglial activity is central to stress-induced behavioral deficits. An interesting and very useful meta-analysis on stress studies [74] found that in all cases studied, early-life or prenatal stress led to elevated microglial activity (assessed by microglial morphological changes and the release of pro-inflammatory immune molecules) in the hippocampus. Thus, early-life stress leads to sensitization of microglia in the offspring, potentially making them more vulnerable to concurrent/subsequent challenges.
Based on this literature, we combined our model of air pollution with a novel model of maternal stress, in which the nesting material within the cage is restricted during the final week of pregnancy, a psychological stressor designed to model poor housing conditions that often co-occur with high exposures to toxicants [75]. Using this 2-hit model, we show that prenatal exposure to the combined stressors leads to robust behavioral deficits later in life, which strongly correlate with microglial-derived levels of proinflammatory cytokines, but again only in males [68].
These collective data demonstrated that microglia may be “primed” by DEP, and subsequently over respond to the secondary challenge of maternal stress, with persistent consequences for neural development and function. Notably, the “second hit” extends to very different environmental factors as well. Prenatal exposure to DEP followed by postnatal exposure to high fat diet reveals striking changes in metabolism and microglial activation in male offspring [76,77]. Again, only minor effects were observed in females. Another model of brief daily separation (BDS) in mouse pups during the first 3 weeks after birth - in conjunction with reduced nesting material - led to a transient but significant increase in microglial density at P14, which normalized at P28 [78]. In rodents, P14 falls within the critical window of development when microglia are actively pruning synapses within certain brain regions like hippocampus – a process critical for proper circuit formation and wiring. Indeed, microglial phagocytic activity was significantly increased in BDS mice, which might be related to their pruning ability. The mechanisms underlying prenatal stress-induced changes in microglia remain largely unclear and warrant further investigation, though a recent study showed evidence that the cross-talk between early-life stress, microglia and neurons might be driven by the fractalkine receptor- ligand pathway [79].
In conclusion, both the associations between immune system dysfunction and neurological disorders, and the list of environmental contributors to these disorders, appear to be increasing, presenting an urgent need for animal models that incorporate multiple factors in a rigorous and mechanistic way. Though the literature is growing (see Table 1), mechanisms remain largely unknown. One prominent challenge for the microglial field is to understand the functional relevance of observed transitions among different stages of microglial morphology, i.e. so-called “activation”, which remains largely descriptive. We have recently demonstrated a close positive correlation between developmental changes in microglial gene expression and morphology, in mice [48]. Specifically, global transcriptional changes in microglia over normal development correlate strongly with changes in microglial morphology in the same individual. Moreover, the changes in microglial whole transcriptome in response to an immune challenge can be used to predict the canonical transition to an “activated” morphology commonly referenced in the literature. That is, an upregulation of inflammatory genes is strongly correlated with a withdrawal of processes and decreased process branching. However, this association - the close tracking of gene expression with morphology in response to immune challenge - was only true in males, and not in females, a striking sexual dimorphism that likely has implications for many neurological disorders, and certainly in the interpretation of the extant literature on microglia. Clearly, characterizations of microglial morphology alone are insufficient, and a recognition of sex differences in these outcomes is absolutely critical.
Moving forward, with validated models in place, neurobiologists are poised to make great strides in the understanding of the consequences of pathological changes in glial function, both acutely and persistently, and their implications for neurological disorders. This said, not all environmental challenges are harmful. Exposure to low levels of infectious agents such as symbiotic bacteria or multicellular organisms such as intestinal worms during early development is important for building a healthy and robust immune system. For instance, “parasitic” helminth colonization of pregnant rat dams and post-weaned offspring confers a striking protection from the effects of early-life bacterial infection on later-life microglial hypersensitivity and cognitive dysfunction [80]. This protection was associated with alterations to the gut microbiome of the offspring, resulting in a more “anti-inflammatory” environment. Similarly, the impact of MIA with poly I:C on maternal IL-17a and fetal outcomes was recently demonstrated to depend on the precise microbiota present in the maternal gut [34,35]. In sum, these data point toward an inter-relatedness of various components of the developing nervous and immune systems, environment, and microglial function, and suggest potential mechanisms by which a greater understanding of environmental impacts on glial development and biology might lead to therapeutic benefits in the treatment and prevention of neurological disorders.
HIGHLIGHTS.
Microglia are critical for normal brain development.
Infection, environmental toxins and stress can all contribute to aberrant microglial development.
Perturbation of normal microglial development can have long-lasting effects on brain function, increasing the risk of disease.
Rigorous models are needed to study combined effects of modern environmental factors on microglia.
Acknowledgments
This work was supported by R01 MH101183 and R01 ES025549 to SDB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 2.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Fraser DA, Pisalyaput K, Tenner AJ. C1q enhances microglial clearance of apoptotic neurons and neuronal blebs, and modulates subsequent inflammatory cytokine production. J Neurochem. 2010;112:733–743. doi: 10.1111/j.1471-4159.2009.06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- **10.Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. doi: 10.1016/j.celrep.2014.07.042. Microglia modulate outgrowth of dopaminergic axons and the laminar positioning of subsets of neocortical interneurons during embryonic forebrain formation. This microglia activity is dependent on Cx3cr1 signaling. [DOI] [PubMed] [Google Scholar]
- 11.Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 12.Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 13.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 14.Schafer DP, Stevens B. Microglia Function in Central Nervous System Development and Plasticity. Cold Spring Harb Perspect Biol. 2015;7:a020545. doi: 10.1101/cshperspect.a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature neuroscience. 2014;17:400. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 16.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilbo SD, Smith SH, Schwarz JM. A Lifespan Approach to Neuroinflammatory and Cognitive Disorders: A Critical Role for Glia. Journal of neuroimmune pharmacology: the official journal of the Society on Neuro Immune Pharmacology. 2011 doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 20.Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (New York, NY) 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Hölscher C. Microglia emerge from erythromyeloid precursors via Pu.1-and Irf8-dependent pathways. Nature neuroscience. 2013;16:273. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 23.Thion MS, Garel S. On place and time: Microglia in embryonic and perinatal brain development. Current Opinion in Neurobiology. doi: 10.1016/j.conb.2017.10.004. in press 47, Glial Biology. [DOI] [PubMed] [Google Scholar]
- 24.Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 26.Patterson PH. Maternal infection and immune involvement in autism. Trends in molecular medicine. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown AS. The risk for schizophrenia from childhood and adult infections. Am J Psychiatry. 2008;165:7–10. doi: 10.1176/appi.ajp.2007.07101637. [DOI] [PubMed] [Google Scholar]
- 28.Boksa P. Maternal infection during pregnancy and schizophrenia. J Psychiatry Neurosci. 2008;33:183–185. [PMC free article] [PubMed] [Google Scholar]
- 29.Dalman C, Allebeck P, Gunnell D, Harrison G, Kristensson K, Lewis G, Lofving S, Rasmussen F, Wicks S, Karlsson H. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am J Psychiatry. 2008;165:59–65. doi: 10.1176/appi.ajp.2007.07050740. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behavioural brain research. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain, behavior, and immunity. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017 doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin Yim Y, Park A, Berrios J, Lafourcade M, Pascual LM, Soares N, Yeon Kim J, Kim S, Kim H, Waisman A, et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature. 2017 doi: 10.1038/nature23909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dozmorov MK, Bilbo SD, Kollins S, Zucker N, Schechter JC, Do EK, Zhang J, Murphy S, Hoyo C, Fuemmeler BF. Associations between maternal cytokine levels during gestation and child neurodevelopment. Brain Behav Immun. doi: 10.1016/j.bbi.2018.03.029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72–93. doi: 10.1016/j.neubiorev.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuger P, Hefendehl JK, Veeraraghavalu K, Wendeln AC, Schlosser C, Obermuller U, Wegenast-Braun BM, Neher JJ, Martus P, Kohsaka S, et al. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat Neurosci. 2017 doi: 10.1038/nn.4631. [DOI] [PubMed] [Google Scholar]
- 41.Reu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H, et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017;20:779–784. doi: 10.1016/j.celrep.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- *43.Ji P, Schachtschneider KM, Schook LB, Walker FR, Johnson RW. Peripheral viral infection induced microglial sensome genes and enhanced microglial cell activity in the hippocampus of neonatal piglets. Brain Behav Immun. 2016;54:243–251. doi: 10.1016/j.bbi.2016.02.010. Early-life viral infection in pigs leads to upregulation of microglial “sensome” genes in the hippocampus later in life, accompanied by increases in microglial phagocytic activity in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, Bilbo SD. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329–338. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giovanoli S, Notter T, Richetto J, Labouesse MA, Vuillermot S, Riva MA, Meyer U. Late prenatal immune activation causes hippocampal deficits in the absence of persistent inflammation across aging. J Neuroinflammation. 2015;12:221. doi: 10.1186/s12974-015-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antonson AM, Radlowski EC, Lawson MA, Rytych JL, Johnson RW. Maternal viral infection during pregnancy elicits anti-social behavior in neonatal piglet offspring independent of postnatal microglial cell activation. Brain Behav Immun. 2017;59:300–312. doi: 10.1016/j.bbi.2016.09.019. [DOI] [PubMed] [Google Scholar]
- **47.Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada Gonzalez F, Perrin P, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353:aad8670. doi: 10.1126/science.aad8670. Authors identified three stages of microglia development, which are characterized by distinct gene expression and linked with chromatin changes, occurring in sync with the developing brain. Furthermore, they demonstrate that the proper development of microglia is affected by the microbiome. Maternal immune activation resulted in a transcriptional shift of “pre-microglia” toward a more advanced developmental stage. [DOI] [PubMed] [Google Scholar]
- **48.Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia. 2017;65:1504–1520. doi: 10.1002/glia.23176. The authors describe sex differences in microglial developmental trajectory in mice in which female microglia mature more quickly than male microglia during normal development. Further, microglial transcriptional responses to LPS differed between male and female mice, which were strongly correlated to changes in morphology. Finally, using these transcriptome data from purified mouse microglia, authors show accelerated microglial development in human brain disease such as autism and Alzheimer’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanamsagar R, Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. 2016;160:127–133. doi: 10.1016/j.jsbmb.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carter CJ, Blizard RA. Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol A, phthalates and many others in food, cosmetics or household products. Neurochem Int. 2016 doi: 10.1016/j.neuint.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Lin X, Cheng C, Terry P, Chen J, Cui H, Wu J. Rapid and sensitive detection of bisphenol a from serum matrix. Biosens Bioelectron. 2016;91:104–109. doi: 10.1016/j.bios.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 53.Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008;22:2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, Johnson JA, Duke L, Kodavanti P, Surace MJ. Diesel exhaust activates and primes microglia: Air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environmental health perspectives. 2011;119:1149. doi: 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brauer M, Freedman G, Frostad J, van Donkelaar A, Martin RV, Dentener F, van Dingenen R, Estep K, Amini H, Apte JS, et al. Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ Sci Technol. 2016;50:79–88. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- 57.Jedrychowski W, Bendkowska I, Flak E, Penar A, Jacek R, Kaim I, Spengler JD, Camann D, Perera FP. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: an epidemiologic prospective cohort study in Poland. Environ Health Perspect. 2004;112:1398–1402. doi: 10.1289/ehp.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry. 2014;4:e360. doi: 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, Koenen KC, Ascherio A, Weisskopf MG. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect. 2013;121:978–984. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2015 doi: 10.1016/j.neuro.2015.11.008. [DOI] [PMC free article] [PubMed]
- 61.Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, Cory-Slechta DA, Costa D, Diaz-Sanchez D, Dorman DC, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972–984. doi: 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghio AJ, Smith CB, Madden MC. Diesel exhaust particles and airway inflammation. Curr Opin Pulm Med. 2012;18:144–150. doi: 10.1097/MCP.0b013e32834f0e2a. [DOI] [PubMed] [Google Scholar]
- 63.Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD. Gestational Exposure to Air Pollution Alters Cortical Volume, Microglial Morphology, and Microglia-Neuron Interactions in a Sex-Specific Manner. Front Synaptic Neurosci. 2017;9:10. doi: 10.3389/fnsyn.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klocke C, Allen JL, Sobolewski M, Mayer-Proschel M, Blum JL, Lauterstein D, Zelikoff JT, Cory-Slechta DA. Neuropathological Consequences of Gestational Exposure to Concentrated Ambient Fine and Ultrafine Particles in the Mouse. Toxicol Sci. 2017;156:492–508. doi: 10.1093/toxsci/kfx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allen JL, Oberdorster G, Morris-Schaffer K, Wong C, Klocke C, Sobolewski M, Conrad K, Mayer-Proschel M, Cory-Slechta DA. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2017;59:140–154. doi: 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nickel K, Tebartz van Elst L, Perlov E, Endres D, Muller GT, Riedel A, Fangmeier T, Maier S. Altered white matter integrity in adults with autism spectrum disorder and an IQ >100: a diffusion tensor imaging study. Acta Psychiatr Scand. 2017;135:573–583. doi: 10.1111/acps.12731. [DOI] [PubMed] [Google Scholar]
- 67.Nelson SB, Valakh V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron. 2015;87:684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, Bilbo SD. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect. 2013;121:1075–1082. doi: 10.1289/ehp.1306560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Case A, Fertig A, Paxson C. The lasting impact of childhood health and circumstance. J Health Econ. 2005;24:365–389. doi: 10.1016/j.jhealeco.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A. 2009;106:12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diz-Chaves Y, Pernia O, Carrero P, Garcia-Segura LM. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J Neuroinflammation. 2012;9:71. doi: 10.1186/1742-2094-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *73.Zhao Q, Xie X, Fan Y, Zhang J, Jiang W, Wu X, Yan S, Chen Y, Peng C, You Z. Phenotypic dysregulation of microglial activation in young offspring rats with maternal sleep deprivation-induced cognitive impairment. Sci Rep. 2015;5:9513. doi: 10.1038/srep09513. Maternal stress, in the form of sleep deprivation in pregnant rats, results in increased microglia number and activation markers, decreased hippocampal neurogenesis and impaired hippocampus-dependent spatial learning and memory in offspring. These effects can be reversed by prepubertal administration of minocycline in the offspring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *74.Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 2016;233:1637–1650. doi: 10.1007/s00213-016-4218-9. This is a systematic review of effects of stress on microglia using pre-defined search criteria on PubMed and EMBASE. Authors found consistent evidence that a range of psychosocial stressors lead to elevated microglial activity in the hippocampus and other brain regions. These effects were seen with early-life/prenatal stress, as well as stressors in adulthood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bolton JL, Auten RL, Bilbo SD. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain Behav Immun. 2014;37:30–44. doi: 10.1016/j.bbi.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 77.Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, Bilbo SD. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012;26:4743–4754. doi: 10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- **78.Delpech JC, Wei L, Hao J, Yu X, Madore C, Butovsky O, Kaffman A. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav Immun. 2016;57:79–93. doi: 10.1016/j.bbi.2016.06.006. In this study, authors used brief daily separation (BDS) as a model for early-life stress. In BDS offspring, microglial cell density decreases, and inflammatory and cell migration gene expression increases. Microglia harvested from the hippocampus of 28-day old BDS mice showed an increase in phagocytic activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Slusarczyk J, Trojan E, Glombik K, Chamera K, Roman A, Budziszewska B, Basta-Kaim A. Fractalkine Attenuates Microglial Cell Activation Induced by Prenatal Stress. Neural Plast. 2016;2016:7258201. doi: 10.1155/2016/7258201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Williamson LL, McKenney EA, Holzknecht ZE, Belliveau C, Rawls JF, Poulton S, Parker W, Bilbo SD. Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early-life infection. Brain Behav Immun. 2016;51:14–28. doi: 10.1016/j.bbi.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Pang Y, Rodts-Palenik S, Cai Z, Bennett WA, Rhodes PG. Suppression of glial activation is involved in the protection of IL-10 on maternal E. coli induced neonatal white matter injury. Brain Res Dev Brain Res. 2005;157:141–149. doi: 10.1016/j.devbrainres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Enayati M, Solati J, Hosseini MH, Shahi HR, Saki G, Salari AA. Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring. Brain Res Bull. 2012;87:295–302. doi: 10.1016/j.brainresbull.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 83.Fernandez de Cossio L, Guzman A, van der Veldt S, Luheshi GN. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 84.Schaafsma W, Basterra LB, Jacobs S, Brouwer N, Meerlo P, Schaafsma A, Boddeke E, Eggen BJL. Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood. Neurobiol Dis. 2017;106:291–300. doi: 10.1016/j.nbd.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 85.Roumier A, Pascual O, Bechade C, Wakselman S, Poncer JC, Real E, Triller A, Bessis A. Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PLoS One. 2008;3:e2595. doi: 10.1371/journal.pone.0002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diz-Chaves Y, Pernía O, Carrero P, Garcia-Segura L. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. Journal of Neuroinflammation. 2012;9:71. doi: 10.1186/1742-2094-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Q, Xie X, Fan Y, Zhang J, Jiang W, Wu X, Yan S, Chen Y, Peng C, You Z. Phenotypic dysregulation of microglial activation in young offspring rats with maternal sleep deprivation-induced cognitive impairment. Sci Rep. 2015;5:9513. doi: 10.1038/srep09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giovanoli S, Engler H, Engler A, Richetto J, Feldon J, Riva MA, Schedlowski M, Meyer U. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl Psychiatry. 2016;6:e772. doi: 10.1038/tp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22:451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Lieblein-Boff JC, McKim DB, Shea DT, Wei P, Deng Z, Sawicki C, Quan N, Bilbo SD, Bailey MT, McTigue DM, et al. Neonatal E. coli infection causes neuro-behavioral deficits associated with hypomyelination and neuronal sequestration of iron. J Neurosci. 2013;33:16334–16345. doi: 10.1523/JNEUROSCI.0708-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pang Y, Dai X, Roller A, Carter K, Paul I, Bhatt AJ, Lin RC, Fan LW. Early Postnatal Lipopolysaccharide Exposure Leads to Enhanced Neurogenesis and Impaired Communicative Functions in Rats. PLoS One. 2016;11:e0164403. doi: 10.1371/journal.pone.0164403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Claypoole LD, Zimmerberg B, Williamson LL. Neonatal lipopolysaccharide treatment alters hippocampal neuroinflammation, microglia morphology and anxiety-like behavior in rats selectively bred for an infantile trait. Brain Behav Immun. 2017;59:135–146. doi: 10.1016/j.bbi.2016.08.017. [DOI] [PubMed] [Google Scholar]