Abstract
The vertebrate nervous system is divided into two functional halves; the central nervous system (CNS), which includes the brain and spinal cord, and the peripheral nervous system (PNS), which consists of nerves and ganglia. Incoming peripheral stimuli transmitted from the periphery to the CNS and subsequent motor responses created because of this information, require efficient communication between the two halves that make up this organ system. Neurons and glial cells of each half of the nervous system, which are the main actors in this communication, segregate across nervous system transition zones and never mix, allowing for efficient neurotransmission. Studies aimed at understanding the cellular and molecular mechanisms governing the development and maintenance of these transition zones have predominantly focused on mammalian models. However, zebrafish has emerged as a powerful model organism to study these structures and has allowed researchers to identify novel glial cells and mechanisms essential for nervous system assembly. This review will highlight recent advances into the important role that glial cells play in building and maintaining the nervous system and its boundaries.
Introduction
The vertebrate nervous system is a remarkably complex organ that consists of an intricate network of distinct populations of neurons and supporting glial cells that form neural circuits that allow for organismal survival. This elaborated system allows for peripheral sensory input and its ultimate central integration as well as the generation and fine-tuning of motor output. Communication between the central and peripheral nervous systems (CNS and PNS, respectively) occurs via peripheral nerves, which are composed of motor and/or sensory axons and their associated glia that cross between the two halves of the nervous system via specialized structures known as transition zones (TZ).
In the early nineteenth century, the Scottish surgeon Charles Bell and the French physiologist François Magendie reported from their independent observations the separation of sensory and motor nerves into and out of the spinal cord, disproving the previous belief that spinal ventral nerve roots were a mix of sensory and motor axons capable of signaling in both directions [1]. We now know that in the vertebrate spinal cord, developing motor neurons migrate to reach their final destination and extend their axons into the periphery through permissive gaps in the spinal cord margins known as motor exit point (MEP) TZs, while their cell bodies remain in the CNS. Conversely, sensory afferent axons derived from sensory neurons in the PNS enter the spinal cord in distinct locations known as dorsal root entry zones (DREZ), which are also characterized by gaps in the basal lamina of the spinal cord [2,3]. These transition zones reflect a selectively gated boundary between the two halves of the nervous system, segregating not only neurons and axons, but also central and peripheral glia.
In the CNS, oligodendrocytes myelinate axons, while Schwann cells in the PNS perform the same task along peripheral axons [4,5]. Oligodendrocytes were first described in 1921 by the neuroanatomist Pío del Río Hortega and Schwann cells were named after the anatomist and physiologist Theodor Schwann in 1871 [6,7]. Both were identified with the development of pioneering histological staining methods still used today. While these myelinating glial cells have been extensively studied by neuroscientists for over a century, the first studies dedicated to understanding the boundary between the CNS and PNS only emerged in the late 1960s, with the invention of transmission electron microscopy (TEM), a nanometer-scale resolution technique developed after the end of World War II.
How a selective barrier allows pathfinding axons to freely cross, while neuronal cell bodies and select populations of highly migratory glial cells segregate to their respective half of the nervous system, is intriguing, but unknown. Elucidating the mechanisms that mediate how axons and glia travel across TZs is a fascinating developmental question that also has implications when considering how circuits reconnect and rewire after injury. In the injured adult mammalian nervous system, damaged neurons attempt to regrow their axons to rebuild functional circuits. Peripherally, this regrowth is mostly successful and motor and sensory axons can regrow and reinnervate target organs. However, regrowth of sensory axons into the CNS fails [8]. The disparity between the CNS and PNS in terms of self-repair ability is particularly interesting when you consider that regrowth fails when axons needs to cross through TZs. Both developmentally and after injury and disease, these specialized transition zones have recently spurred many studies which shed light on the cellular mechanisms responsible for TZ development, maintenance and involvement in disease [9–16]. However, the molecular mechanisms that mediate these cellular behaviors are still poorly understood.
In this article, we review new insights into the cellular, molecular and functional aspects of spinal cord TZs, including mammalian boundary cap (BC) cells as well as recent findings that raise the hypothesis that BC cell function may be covered by a combination of different subtypes of glia in zebrafish that include MEP and perineurial glia [11,12,15].
Glia orchestrate the development of the nervous system at TZs
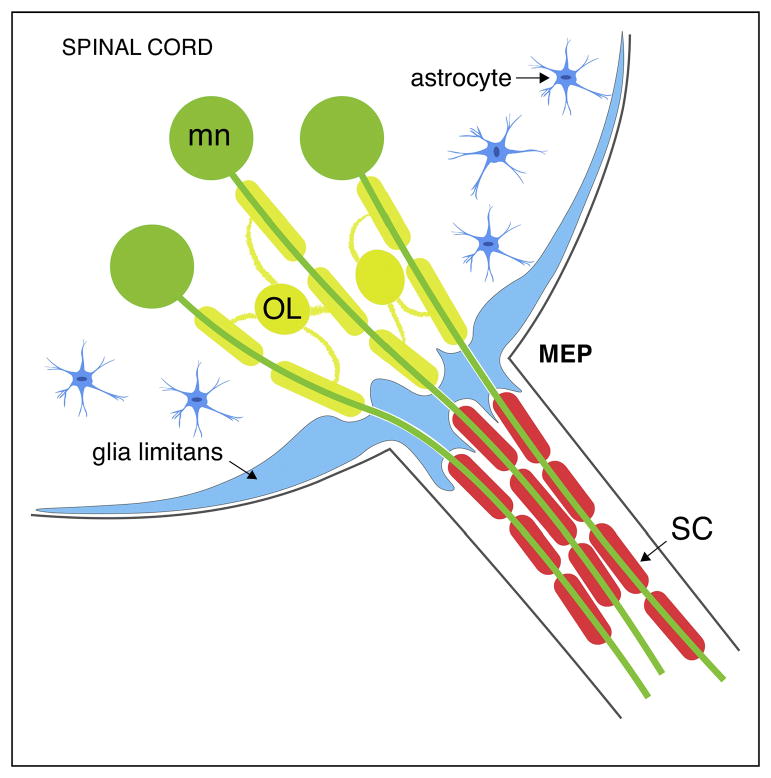
In mammals, an astrocytic barrier is generally found at the TZs between the CNS and the PNS (Figure 1). This barrier, consisting of a thick layer of astrocytic endfeet, is continuous with the glia limitans that covers the surface of the spinal cord but has perforations, allowing axons to cross [2]. Following irradiation of the spinal cord, Schwann cells (SC) are able to pass through the damaged glia limitans (Figure 2c). Additionally, remyelination by SCs in the damaged CNS only occurs in restricted areas where astrocytes are absent, strongly suggesting that astrocytes and SCs repel each other [17,18]. However, oligodendrocyte (OL) and SC intermixing is never seen in normal animals [19]. In line with this, the first electron microscopy ultrastructural study of a rat cranial nerve TZ described the transitional region along myelinated axons as an abrupt change between central OLs and peripheral SCs [8]. While this study did not demonstrate the existence of a cell type other than OLs or SCs, ultrastructural and molecular studies have recently shed light on novel glial populations that reside at TZs and mediate the selective segregation of cells observed under normal conditions [20,21].
Figure 1. Diagram of a transverse section of a mammalian MEP TZ.
Motor neurons (green) project their axons into the periphery through the MEP through an astrocytic glia limitans (blue). Oligodendrocytes (yellow) myelinate the central portion of motor axons while SCs (red) myelinate their peripheral portion. The glia limitans layer, which is thicker at the TZ, segregate OLs from SCs.
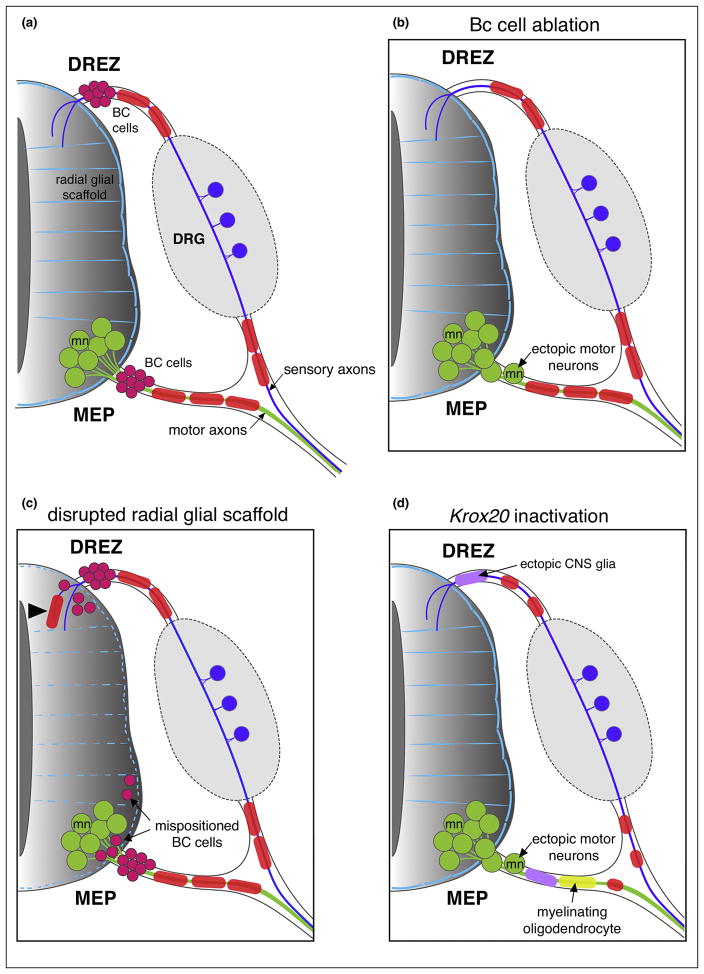
Figure 2. Mammalian TZs.
(a) Schematic of a mammalian neural tube showing BC cells (magenta) sitting at the DREZ and MEP TZs. (b) In the absence of BC cells, motor neurons (mn; green) transgress the MEP TZ and invade the motor root. (c) When the radial glial scaffold (light blue) that covers the spinal cord is absent or disrupted, peripheral glial cells (SCs; red, and BC cells) enter the CNS ectopically. (d) Following Krox20 inactivation, CNS glia (purple) invade the dorsal and ventral roots, oligodendrocytes (yellow) myelinate peripheral axons and SCs (red) fail to develop.
Boundary cap (BC) cells are neural crest derivatives that migrate along the outer edge of the neural tube and are thought to transiently reside in clusters at the TZs between the CNS and PNS, precisely at the sites where axons exit and enter the CNS (Figure 2a) [22]. Electron microscopy studies revealed that BC cells prefigure the DREZ, but were never detected at the presumptive MEP TZ prior to motor axons exiting the ventral spinal cord. They were only observed after axons left the spinal cord and were at a significant distance away from the MEP TZ [21]. Analysis of the temporal and spatial pattern of expression of the zinc finger transcription factor Krox-20 (also known as Egr2) during murine development revealed the presence of an early group of Krox20-expressing neural crest cells that stopped at both the DREZ and MEP TZ [20,23]. Krox-20 is first expressed in rhombomeres 3 and 5 and in early neural crest cells and its expression persists in neural crest-derived SCs and BC cells [20]. This molecular study concluded that BC cells arise from the neural crest at embryonic day 10.5 (E10.5) and migrate along the neural tube to reach the MEP where Krox-20 expression is detected in BC cells [24,25].
In fish and mammals, Krox-20 plays a master role in SC development and function, and in its absence, SCs do not progress past the pro-myelinating stage and do not myelinate axons after radial sorting [26,27]. In spite of being the most widely accepted marker of BC cells, Krox-20 might have a different outcome in these cells, as BC cells do not initiate myelination but generate SCs, which in turn, are capable of myelinating axons. Functionally, BC cells play an essential role in spinal motor nerve development (Figure 2b). Before they become Schwann cells at the MEP, DREZ and in the dorsal root ganglia (DRG), BC cells in mammals also give rise to nociceptive neurons, satellite glial cells and terminal glia, and above all, they act as a gate-keeper [9,10,13,24,28]. Surgical and genetic ablation of BC cells results in ectopic motor neuron cell bodies and CNS glia mispositioned along ventral roots (Figure 2b) [9,10,13,29]. Vermeren et al. showed that ablation of neural crest cells, including both BC cells and SCs, performed by surgically removing the dorsal half of the neural tube of E2 chick embryos, resulted in the abnormal presence of motor neurons along ventral nerve roots [9]. They also stated that the segregating function lost after neural crest cell ablation was due to the loss of BC cells, as conditional genetic ablation of Krox-20-expressing BC cells in mouse embryos, obtained by targeting diphtheria toxin to the Krox-20 locus, also resulted in the emigration of motor neurons from the spinal cord to the root [9]. However, the potential presence of ectopic CNS glia along the motor nerve was not examined in this study and this genetic manipulation also affected neural crest-derived Schwann cells. In a more directed BC ablation study, Coulpier et al. reported that the inactivation of Krox-20 in mouse resulted in oligodendrocytes and astrocytes invading both dorsal and ventral nerve roots, and in oligodendrocytes ultimately myelinating peripheral axons [13]. In the same study, the authors also showed a transient presence of ectopic motor neurons along the nerve root of the Schwann cell depleted mice at E12.5 but not at E17.5. Regrettably, the authors did not look at the motor neuron phenotype in more depth and their work did not lead to a hypothesis that explained the role of Krox-20 in motor neuron segregation at TZs.
Although neural crest-derived BC cells exist and have the same gating role in birds and mammals, they have not been observed, per se, in fish. However, motor neuron cell bodies are perfectly segregated to the spinal cord in fish as well, and various subpopulations of glial cells beautifully orchestrate spinal nerve development, architecture and maintenance (Figure 3a). Several lines of evidence suggest that distinct CNS-derived glial populations work jointly to ensure TZ segregation in zebrafish [11,14–16].
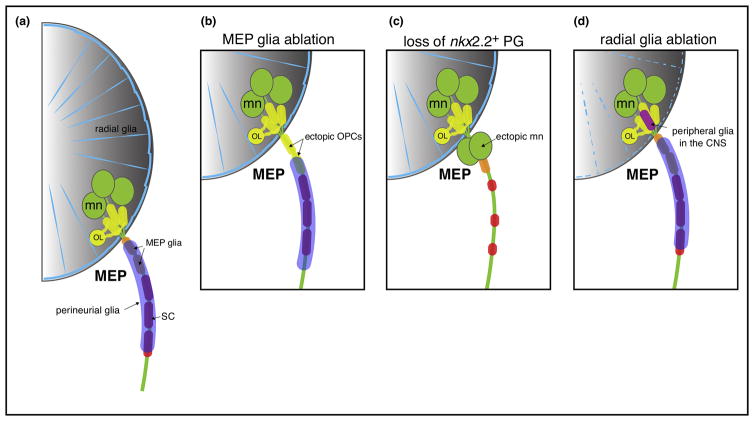
Figure 3. Zebrafish MEP TZs.
(a) Schematic of a zebrafish MEP TZ and the diverse populations of glial cells orchestrating this boundary. MEP glia (orange) restrict the oligodendrocyte lineage (OL; yellow) to the spinal cord. Perineurial glia (purple), which make the perineurium around axon bundles, also constrain motor neurons (mn; green) to the ventral spinal cord. Radial glia (blue) cover the surface of the spinal cord and prevent peripheral glia from entering the CNS. (b) In the absence of MEP glia, OPCs ectopically migrate out of the spinal cord and ensheath motor root axons. (c) In the absence of perineurial glia, motor neurons ectopically exit the spinal cord and are found along the motor root and SCs (red) fail to develop. (d) Following radial glial ablation, peripheral glial cells ectopically enter the spinal cord. Note that MEP and perineurial glia originate in the ventral spinal cord and must migrate into the periphery to restrict CNS components.
Perineurial glia, which also exist in mice, are nkx2.2a-expressing cells that originate from the lateral floor plate (p3 domain) of the spinal cord and migrate into the PNS via MEP TZs (Figure 3a). Ultimately, these cells migrate along motor axons, ensheath the motor nerve by tightly associating with one another via tight-junctions to ultimately form the perineurium, a protective layer that ensheaths both axons and Schwann cells into fascicles [11,14]. Experiments in zebrafish show that perineurial glia prefigure motor axon exit points and form a selective barrier that confines motor neurons to the CNS. Nkx2.2a loss of function leads to ectopic motor axon exit from the spinal cord and motor neuron cell bodies escaping the CNS (Figure 3c) [11]. Consistent with these findings, in mice lacking Nkx2.2, ectopic motor neuron cell bodies were found outside of the spinal cord along peripheral axons and the latter were defasciculated [14]. Together, these data highlight the crucial function that CNS-derived perineurial glia have in motor nerve development and maturation.
Not long ago, another fundamental CNS-derived glial cell type was described in the PNS, regularly spaced at each MEP TZ outside of the spinal cord, where they ensheath and myelinate spinal motor nerve root axons (Figure 3a) [12,15]. These cells, known as MEP glia, originate from the pMN domain of the ventral neural tube, where they acquire olig2 expression, and exit the spinal cord to reach the motor roots at about 50 hours post fertilization (hpf) in zebrafish, after motor axon outgrowth is completed but before oligodendrocyte progenitor cells (OPC) start migrating and filling the CNS [12,15]. MEP glia sit along the ventral motor root like BC cells, and although they share a common marker, wnt-inhibitory factor 1 (wif1), they do not express the hallmark BC cell marker krox-20 [15,30]. Using in vivo imaging coupled with single cell laser ablation of MEP glia, we demonstrated that OPCs exit the spinal cord by pinching through the MEP TZ to colonize motor axons when these cells are absent (Figure 3b) [15]. These findings demonstrate that MEP glia are responsible for restricting central myelinating glia to the CNS. However, there is no evidence that they are involved in motor neuron positioning.
Taken together, these studies investigating perineurial glia and MEP glia demonstrate that oligodendrocytes and motor neurons are restricted to the spinal cord by distinct mechanisms, mediated by specific CNS-derived peripheral glial populations. Overall, these CNS-derived glia share an important gating function with the mammalian neural crest-derived BC cells during nervous system assembly in spite of the noteworthy differences in their origin and gene expression. However, because in vivo live imaging is impossible during murine embryonic development and in situ hybridization does not allow for accurate fate-mapping, we cannot rule out the possibility that a subset of mammalian BC cells are also CNS-derived. Moreover, because in situ hybridization does not offer precise cellular resolution, it is also possible that the wif1 expression observed in mouse BC cells may be restricted to a subset of this population [30].
Molecular mechanisms mediating cell segregation at TZs
BC cells restrict motor neuron cell bodies to the CNS, most likely by releasing repulsive signals, but allow axons to cross (Figure 2a). To identify the molecular mechanisms that mediate this selective permeability at TZs, studies have targeted essential axon pathfinding pathways, including Netrin, Semaphorins and Plexins (Table 1) [29,31–35].
Table 1.
Signaling governing nervous system transition zones
| MUTANT/KNOCK-DOWN | EXPRESSION | PHENOTYPE | ORGANISM | REFERENCES |
|---|---|---|---|---|
| Ntn1/Dcc | Dorsolateral spinal cord | Interneuron exit the DREZ ectopically | Mouse | 28 |
| Ntn5/Dcc | BC cells | Ectopic motor neurons | Mouse | 29 |
| Krox20 | BC cells, SCs | Ectopic motor neurons and CNS glia in the motor nerve root | 11 | |
| Nkx2.2 | Perineurial glia | Ectopic motor neurons in the motor nerve root | Zebrafish/Mouse | 9, 12 |
| Npn1 | Motor neurons | Ectopic motor neurons in the motor nerve root | Zebrafish | 33 |
| Npn2 | Motor neurons | Ectopic motor neurons in the motor nerve root | Chick/Mouse | 8, 27 |
| Sema6a | BC cells | Ectopic motor neurons in the motor nerve root | Mouse | 27 |
| Sema3ab | Posterior half of the somite | Mispositioned motor neurons | Zebrafish | 33 |
| PlexinA3 (sidetracked) | Motor neurons | Mispositioned MEP | Zebrafish | 30–32 |
| Erbb3 | PNS glia | Ectopic oligodendrocytes along motor nerves | Zebrafish | 41 |
The Netrin family is an evolutionarily conserved family of bifunctional secreted guidance molecules that can function as chemoattractants or chemorepellents, depending on the class of receptor expressed by the responding neuron. Recently, Laumonnerie et al. demonstrated that Netrin 1 plays a role in the confinement of spinal interneuron axons to the CNS. Netrin 1 attracts spinal interneurons to the ventral spinal cord, through Deleted in colorectal cancer (DCC), and prevents them from exiting the CNS via the DREZ [31]. A more recent study demonstrated that another member, Netrin 5 (Ntn5), which is expressed by BC cells, prevented motor neuron migration out of the CNS by repulsion, likely mediated by DCC receptors expressed by motor neurons [32].
BC cells also mediate motor neuron confinement to the spinal cord through repulsive semaphorin-plexin interactions [10,29]. Plexins are receptor components for semaphorins and their chemotropic activities are well described in axonal growth and pathfinding. Loss of Neuropilin-2 (Npn-2), a high affinity receptor for class 3 semaphorins in chick and mouse, leads to ectopic motor neuron cell bodies in the PNS. Additionally, BC cells express the transmembrane protein Semaphorin6A (Sema6A), whose loss of function phenocopies Npn-2 mutants, resulting in motor neurons mispositioned along motor axons in the PNS [29]. Studies conducted in zebrafish also reveal the conserved role of semaphorin-plexin signaling in cellular restriction at MEP TZs, as neuropilin 1 and Sema3b knockdown lead to the mispositioning of a subset of primary motor neurons and ectopic axonal exit from the CNS [36].
Interestingly, Bron et al. [10] reported that the ectopic motor neuron cell bodies observed in Sema6A and Npn-2 null mouse embryos were confined to caudal regions of the trunk and that, on the contrary, no significant defects were observed at the forelimb and thoracic levels. Intriguingly, in Ntn5 mutant mice, Garrett et al. observed that the defects were mainly located rostrally. This heterogeneous distribution of the defects along the antero-posterior axis led the authors to suggest that BC cell-derived Netrin 5 might signal towards a specific subtype of motor neuron [32]. An alternative explanation for this spatial pattern is that the proteins cited above and involved in restricting motor neurons within the CNS might be expressed at different levels in the neural tube, following opposite gradients along the antero-posterior axis. Moreover, we cannot rule out the possibility that there might be redundancy with the pathways involved, as it is well known that the semaphorin, plexin and netrin families all consist of numerous members and various combinations of ligands and receptors.
More recently, a study linked defects in radial glial scaffolding to defective CXCL12 signaling and the presence of mispositioned BC cells inside the spinal cord (Figure 2c). The meninges-derived chemokine CXCL12 binds to the CXCR4 receptor expressed by BC cells. In Cxcl12 and Cxcr4 mouse mutants, the pial layer that surrounds the CNS is dotted with gaps and BC cells were observed inside the developing spinal cord at these perforated sites [37]. Further analysis revealed that CXCL12/CXCR4 signaling activated integrin β1, which in turn increased radial glial endfeet adhesiveness to pial basement membrane components. This study demonstrated that CXCL12 signaling was an extrinsic factor regulating radial glial scaffold integrity during spinal cord development. In keeping with previous observations in mouse mutants, Zhu et al. stated that in the Cxcl12 and Cxcr4 null mice, ectopic BC cells were almost exclusively found in the lumbosacral region of the spinal cord and were rare in the rostral region, in spite of the homogeneous presence of radial glia defects [37].
In the mature mammalian nervous system, the presence of peripheral glial cells in the CNS is generally attributed to a disruption of the astrocytic layer that forms the glia limitans on the surface of the spinal cord [19,38]. Peripheral glia, such as SCs, are found in the CNS following spinal cord injury or experimental irradiation and the prevalence of SC invasion in intact spinal cords becomes more prevalent with age [17,19,39,40]. Recently, this was also described in the developing nervous system of zebrafish. Smith et al. demonstrated that before radial glial maturation, peripheral glial precursors can freely transgress the MEP TZ (Figure 3d). However, this migration stops once radial glial endfeet mature [16]. This study, along with the MEP and perineurial glial analyses, demonstrate the synchronization of several glial populations that allow for unidirectional migration across the MEP TZ [11,15]. These studies also highlight an intriguing phenomenon where glia freely pass the boundary between the CNS and PNS during development but have to ultimately differentiate on one half of the TZ. Once they definitively sit in the PNS, they function as a gate-keeper to maintain selective and unidirectional migration across the CNS/PNS boundary, in addition to their wrapping function. Altogether, these studies illustrate a developmental mechanism conserved among vertebrates including mice, chick and fish in motor neuron and glial segregation to the CNS.
Although the question of how neurons become segregated to either the CNS or PNS is old, many recent studies have begun to shed light on the important role of several cell populations in vertebrate models, including BC cells in preventing neurons from crossing the borders of the spinal cord while selectively allowing axonal pass through [9,10,29]. However, how myelinating glia are precisely segregated to the two halves of the vertebrate nervous system is still not thoroughly understood. Fascinatingly, we currently know of two peripheral glial populations that are strictly restricted to the PNS, MEP glia and perineurial glia, but which are generated in the neural tube and must migrate across MEP TZs or spinal motor nerve development and maturation is perturbed. The absence of these glial cells along the nerves causes the ectopic migration of CNS populations, including motor neurons and OPCs, into the periphery [11,14,15]. Understanding how TZs balance cell-specific restriction and selective permeability is an intriguing question that not only sheds light on the mechanisms required for nervous system development, but also those mechanisms that could be perturbed in disease.
Conclusion
The assembly of the nervous system is orchestrated during development by multiple glial cells that migrate freely across spinal cord transition zones. A better understanding of the mechanisms that define these specialized points, as well as the molecular pathways involved in the selective cellular restrictions observed at these boundaries in development and disease will provide a deeper comprehension of the mechanisms underlying neuropathies. Studies in vertebrates such as zebrafish will not only continue to increase our knowledge on TZ structure, but will also allow for the establishment of new therapeutic strategies for neuropathies and demyelinating diseases.
Highlights.
Glial cells segregate neurons and glia at nervous system transition zones.
Glia are essential to build and maintain the nervous system boundaries.
Novel glia described in zebrafish show conserved mechanisms among vertebrates.
Acknowledgments
This work was supported by grants from the NIH/NINDS (R01NS072212 & R21NS092070) and the Hartwell Foundation.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Berkowitz C. Disputed discovery: vivisection and experiment in the 19th century. Endeavour. 2006;30:98–102. doi: 10.1016/j.endeavour.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Fraher JP. The CNS-PNS transitional zone of the rat. Morphometric studies at cranial and spinal levels. Prog Neurobiol. 1992;38:261–316. doi: 10.1016/0301-0082(92)90022-7. [DOI] [PubMed] [Google Scholar]
- 3.Fraher JP. Axon-glial relationships in early CNS–PNS transitional zone development: an ultrastructural study. J Neurocytol. 1997;26:41–52. doi: 10.1023/a:1018511425126. [DOI] [PubMed] [Google Scholar]
- 4.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 5.Emery B. Regulation of Oligodendrocyte Differentiation and Myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 6.Del Rio Hortega P. Estudios sobre la neuroglia. La glia de escasas radiaciones oligodendroglia. Boletin de la Real Sociedad Espanola de Historia Natural. 1921;21:63–92. [Google Scholar]
- 7.Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu Rev Neurosci. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- 8.Doucette R. PNS-CNS transitional zone of the first cranial nerve. J Comp Neurol. 1991;312:451–66. doi: 10.1002/cne.903120311. [DOI] [PubMed] [Google Scholar]
- 9.Vermeren M, Maro GS, Bron R, McGonnell IM, Charnay P, Topilko P, Cohen J. Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron. 2003;37:403–415. doi: 10.1016/s0896-6273(02)01188-1. [DOI] [PubMed] [Google Scholar]
- 10.Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J. Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Dev. 2007;2:21. doi: 10.1186/1749-8104-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Kucenas S, Takada N, Park H, Woodruff E, Broadie K, Appel B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci. 2008;11:143–151. doi: 10.1038/nn2025. These two zebrafish live imaging studies reveal novel major CNS-derived glial cells that have a crucial function in the organization of the TZs and in spinal motor nerve development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Kucenas S, Wang W, Knapik EW, Appel B. A selective glial barrier at motor axon exit points prevents oligodendrocyte migration from the spinal cord. J Neurosci. 2009;29:15187–15194. doi: 10.1523/JNEUROSCI.4193-09.2009. See annotation to Ref 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulpier F, Decker L, Funalot B, Vallat J-M, Garcia-Bragado F, Charnay P, Topilko P. CNS/PNS boundary transgression by central glia in the absence of Schwann cells or Krox20/Egr2 function. J Neurosci. 2010;30:5958–5967. doi: 10.1523/JNEUROSCI.0017-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Clark J, O’keefe A, Mastracci T, Sussel L, Matise M, Kucenas S. Mammalian Nkx2.2+ Perineurial Glia Are Essential for Motor Nerve Development. Dev Dyn. 2014;243:1116–1129. doi: 10.1002/dvdy.24158. This mouse study demonstrates the important role of perineurial glia in motor nerve development and highlights a mechanism conserved among vertebrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Smith CJ, Morris AD, Welsh TG, Kucenas S. Contact-Mediated Inhibition Between Oligodendrocyte Progenitor Cells and Motor Exit Point Glia Establishes the Spinal Cord Transition Zone. PLoS Biol. 2014;12 doi: 10.1371/journal.pbio.1001961. In vivo zebrafish work that describes MEP glia for the first time and demonstrates that these CNS-derived glia cross the MEP TZ and develop to eventually ensheath the motor root axons and restrict OPCs to the spinal cord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Smith CJ, Johnson K, Welsh TG, Barresi MJF, Kucenas S. Radial glia inhibit peripheral glial infiltration into the spinal cord at motor exit point transition zones. Glia. 2016;64:1138–1153. doi: 10.1002/glia.22987. An exploration of how radial glia prevent peripheral glial cells from (re)entering the spinal cord during development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blakemore WF, Patterson RC. Observations on the interactions of Schwann cells and astrocytes following X-irradiation of neonatal rat spinal cord. J Neurocytol. 1975;4:573–85. doi: 10.1007/BF01351538. [DOI] [PubMed] [Google Scholar]
- 18.Shields SA, Blakemore WF, Franklin RJM. Schwann Cell Remyelination Is Restricted to Astrocyte-Deficient Areas After Transplantation Into Demyelinated Adult Rat Brain. J Neurosci Res. 2000;578:571–578. doi: 10.1002/(SICI)1097-4547(20000601)60:5<571::AID-JNR1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Franklin RJM, Blakemore WF. Requirements for schwann cell migration within cns environments: A viewpoint. Int J Dev Neurosci. 1993;11:641–649. doi: 10.1016/0736-5748(93)90052-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson DG, Bhatt S, Chavrier P, Bravo R, Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989;337:461–4. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]
- 21.Fraher JP, Dockery P, O’Donoghue O, Riedewald B, O’Leary D. Initial motor axon outgrowth from the developing central nervous system. J Anat. 2007;211:600–611. doi: 10.1111/j.1469-7580.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golding JP, Cohen J. Border Controls at the Mammalian Spinal Cord•. 1997;396:381–396. doi: 10.1006/mcne.1997.0647. [DOI] [PubMed] [Google Scholar]
- 23.Niederländer C, Lumsden A. Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves. Development. 1996;122:2367–74. doi: 10.1242/dev.122.8.2367. [DOI] [PubMed] [Google Scholar]
- 24.Maro GS, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P, Topilko P. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci. 2004;7:930–938. doi: 10.1038/nn1299. [DOI] [PubMed] [Google Scholar]
- 25.Jacob C. Transcriptional control of neural crest specification into peripheral glia. Glia. 2015;63:1883–1896. doi: 10.1002/glia.22816. [DOI] [PubMed] [Google Scholar]
- 26.Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi A, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 27.Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–5. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gresset A, Coulpier F, Gerschenfeld G, Jourdon A, Matesic G, Richard L, Vallat J-M, Charnay P, Topilko P. Boundary Caps Give Rise to Neurogenic Stem Cells and Terminal Glia in the Skin. Stem Cell Reports. 2015;5:278–290. doi: 10.1016/j.stemcr.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauti O, Domanitskaya E, Andermatt I, Sadhu R, Stoeckli ET. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Dev. 2007;2:28. doi: 10.1186/1749-8104-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulpier F, Le Crom S, Maro GS, Manent J, Giovannini M, Maciorowski Z, Fischer A, Gessler M, Charnay P, Topilko P. Novel features of boundary cap cells revealed by the analysis of newly identified molecular markers. Glia. 2009;57:1450–1457. doi: 10.1002/glia.20862. [DOI] [PubMed] [Google Scholar]
- 31•.Laumonnerie C, Da Silva RV, Kania A, Wilson SI. Netrin 1 and Dcc signalling are required for confinement of central axons within the central nervous system. Development. 2014;141:594–603. doi: 10.1242/dev.099606. This mouse study provides insight into molecular mechanisms that maintain CNS-PNS integrity. [DOI] [PubMed] [Google Scholar]
- 32•.Garrett AM, Jucius TJ, Sigaud LPR, Tang F-L, Xiong W-C, Ackerman SL, Burgess RW. Analysis of Expression Pattern and Genetic Deletion of Netrin5 in the Developing Mouse. Front Mol Neurosci. 2016;9:1–14. doi: 10.3389/fnmol.2016.00003. Identification of a novel signaling essential for motor neuron restriction at MEP TZs and that acts through boundary cap cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palaisa Ka, Granato M. Analysis of zebrafish sidetracked mutants reveals a novel role for Plexin A3 in intraspinal motor axon guidance. Development. 2007;134:3251–3257. doi: 10.1242/dev.007112. [DOI] [PubMed] [Google Scholar]
- 34.Feldner J, Reimer MM, Schweitzer J, Wendik B, Meyer D, Becker T, Becker CG. PlexinA3 Restricts Spinal Exit Points and Branching of Trunk Motor Nerves in Embryonic Zebrafish. J Neurosci. 2007;27:4978–4983. doi: 10.1523/JNEUROSCI.1132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sainath R, Granato M. Plexin A3 and Turnout Regulate Motor Axonal Branch Morphogenesis in Zebrafish. 2013;8:1–12. doi: 10.1371/journal.pone.0054071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato-Maeda M, Obinata M, Shoji W. Position fine-tuning of caudal primary motoneurons in the zebrafish spinal cord. Development. 2008;135:323–332. doi: 10.1242/dev.007559. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, Matsumoto T, Nagasawa T, Mackay F, Murakami F. Chemokine Signaling Controls Integrity of Radial Glial Scaffold in Developing Spinal Cord and Consequential Proper Position of Boundary Cap Cells. J Neurosci. 2015;35:9211–9224. doi: 10.1523/JNEUROSCI.0156-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncan ID, Hoffman RL. Schwann cell invasion of the central nervous system of the myelin mutants. J Anat. 1997;190:35–49. doi: 10.1046/j.1469-7580.1997.19010035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sims TJ, Durgun BM, Gilmore SA. Schwann Cell Invasion of Ventral Spinal Cord: The Effect of Irradiation on Astrocyte Barriers. J Neuropathol Exp Neurol. 1998;57:866–873. doi: 10.1097/00005072-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Adelman LS, Aronson SM. Intramedullary nerve fiber and Schwann cell proliferation within the spinal cord (schwannosis) Neurology. 1972;22:726. doi: 10.1212/wnl.22.7.726. [DOI] [PubMed] [Google Scholar]
- 41.Morris A, Lewis G, Kucenas S. Perineurial Glial Plasticity and the Role of TGF-β in the Development of the Blood-Nerve-Barrier. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.2875-16.2017. [DOI] [PMC free article] [PubMed]