Abstract
Insulin resistance is the hallmark of type 2 diabetes; however, the mechanism underlying the development of insulin resistance is still not completely understood. Previous reports showed that posttranslational modifications of IRS play a critical role in insulin signaling, especially the phosphorylation of IRS by distinct kinases. While it is known that increasing Sirtuin 1 deacetylase activity improves insulin sensitivity in the liver, the identity of its counterpart, an acetyl-transferase, remains unknown. Our recent study shows that elevated endotoxin (LPS) levels in the liver of obese mice lead to the induction of the acetyl-transferase P300 through the IRE1-XBP1s pathway. Subsequently, induced P300 impairs insulin signaling by acetylating IRS1 and IRS2 in the insulin signaling pathway. Therefore, P300 acetyl-transferase activity appears to be a promising therapeutic target for the treatment of diabetes.
1. Activation of the insulin signaling pathway regulates nutrient metabolism
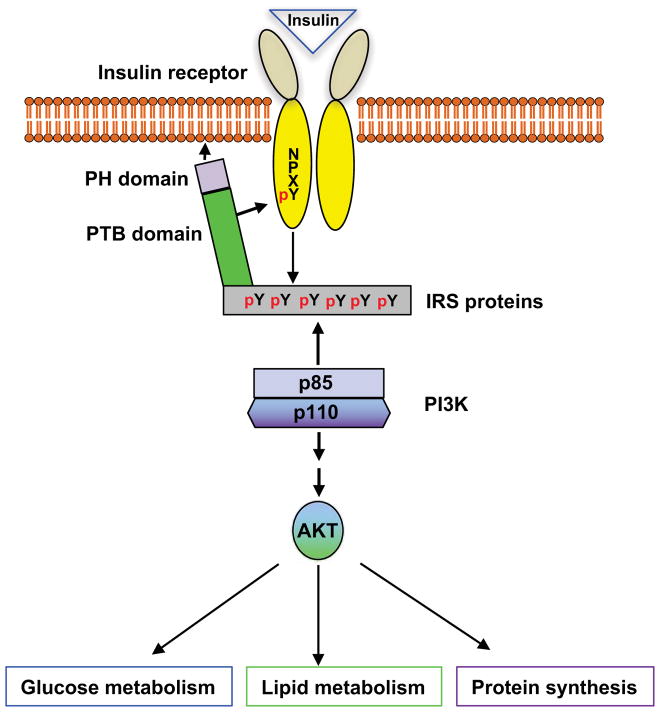
Insulin, secreted by the β-cells of the islets of Langerhans in the pancreas, is the most powerful anabolic hormone known to be involved in regulating glucose, lipid, and amino acid metabolism. The regulation of anabolic metabolism by insulin is through the activation of the insulin signaling pathway(Avruch 1998; Taniguchi, et al. 2006). The insulin receptor (IR) is a tetrameric complex consisting of two extracellular α-subunits and two transmembrane β-subunits that are covalently linked through disulfide bonds(Cheatham and Kahn 1995; Sweet, et al. 1987). Both α- and β-subunits are generated by proteolytic cleavage from a single precursor. Insulin binding to the α-subunits leads to a conformational change, the activation of tyrosine kinase activity in the β-subunits, and the transphosphorylation of β-subunits. Phosphorylation at Y972 of the β-subunit generates a NPXpY motif that is critical for the recognition and binding of insulin receptor substrate (IRS) proteins(White, et al. 1988). Several IRS proteins have been identified, including IRS1-6, Grb-2-associated protein (GAB1), and Shc1-3(Taniguchi et al. 2006; White 2003). IRS binds to the IR through its pleckstrin-homology domains (PH domains) and phosphotyrosine-binding domains (PTB domains). The tyrosine-phosphorylation of IRS by the IRβ, resulting in the recruitment of intracellular molecules that contain Src-homology-2 domains (SH2 domains) and the activation of the PI3K-AKT signaling cascade(Guo 2014). The initiation of this cascade of phosphorylation events results in the suppression of gluconeogenic enzyme gene expression along with the augmentation of lipogenic enzyme gene expression and protein synthesis and glycogen storage (Assimacopoulos-Jeannet, et al. 1995; Guo 2014; He, et al. 2009; Horton, et al. 1998; Koo, et al. 2005) (Figure 1).
Figure 1. Activation of insulin signaling regulates the metabolisms of glucose and lipids, and protein synthesis.
Phosphorylation at Y972 of the β-subunit generates a NPXpY motif, leading to the increase in the affinity of the IRS binding to the IR and the tyrosine phosphorylation of IRS.
2. IRS1 and IRS2 play a key role in mediating insulin’s effect on the regulation of glucose metabolism
Both IRS1 and IRS2 are ubiquitously expressed and are the primary mediators of IRS proteins in insulin-dependent regulation of glucose metabolism in most cells(White 2002). Mice lacking both IRS1 and IRS2 are embryonically lethal and die before implantation(Withers, et al. 1999). Mice with IRS1 knockout exhibit mild insulin resistance without diabetes(Araki, et al. 1994; Tamemoto, et al. 1994). Inactivation of IRS2 in mice results in the development of diabetes due to insulin resistance and decreased insulin secretion from pancreatic β-cells(Kido, et al. 2000; Tamemoto et al. 1994). Since defective hepatic insulin sensitivity in type 2 diabetes results in increased glucose production, which is the major cause of hyperglycemia in diabetic patients(Kunert, et al. 2003; Magnusson, et al. 1992), mice with liver-specific double IRS1 and IRS2 knockout display severe hyperglycemia and hyperinsulinemia(Dong, et al. 2006). These data suggest that IRS1 and IRS2 are critical mediators of insulin’s regulation of glucose metabolism.
3. Phosphorylation of IRS1 and IRS2 proteins affects their functions
Following the landmark discoveries that insulin mediates the tyrosine phosphorylation of IRS1(White, et al. 1985), and that this phosphorylation event is critical for the recruitment of downstream mediators to the plasma membrane(Guo 2014), multiple tyrosine residues were found to be phosphorylated in these two proteins after insulin treatment. The phosphorylation of tyrosine residues at 460, 546, 608, 628, 658, 727, 935, 983, and 1006 in IRS1 (mouse) and tyrosine residues at 536, 594, 649, 734, 814, and 1061 in IRS2 (mouse) leads to the generation of the YXXM motif(Sun, et al. 1991). Subsequently, an SH2 domain containing downstream mediator, such as the adaptor protein p85 in PI3K, binds to these YXXM motifs, resulting in the activation of PI3K(Myers, et al. 1992).
On the other hand, it was proposed that there are more than 40 potential serine/threonine phosphorylation sites, and phosphorylation of these sites serves to negatively regulate insulin signaling(Cheatham and Kahn 1995; Rui, et al. 2001). To define the posttranslational modification sites in IRS1 and IRS2, we generated adenoviral FLAG-tagged IRS1 and IRS2 (mouse) expression vectors and transduced these adenoviral vectors into Hepa1-6 cells. After the purification of these proteins(Cao J 2017), we subjected these proteins to mass spectrometry to identify the modification sites. In good agreement with previous findings that tyrosine residues are not phosphorylated in untreated cells(White et al. 1985), we could not detect any tyrosine phosphorylation sites in these two proteins, as Hepa1-6 cells were harvested without insulin treatment. However, we found that 40 serine/threonine residues are phosphorylated in IRS1 protein, and 19 serine/threonine residues are phosphorylated in IRS2 (Table 1).
Table 1.
Serine/Threonine phosphorylation sites in IRS1 and IRS2
| Serine residue | Threonine residue | |
|---|---|---|
| IRS1 (mouse) | 263, 265, 290, 298, 302, 307, 324, 325, 302, 336, 340, 343, 369, 439, 444, 458, 528, 542, 632, 857, 887, 994, 999, 1074, 1096, 1097, 1157, 1214 | 300, 304, 306, 330, 346, 441, 448, 525, 547, 985, 998, 1099 |
| IRS2 (mouse) | 61, 66, 171, 234, 301, 303, 305, 306, 338, 343, 362, 381, 385, 388 | 55, 341, 347, 360, 374 |
3.1. Endoplasmic reticulum (ER) stress impairs insulin signaling via JNK phosphorylation of IRS
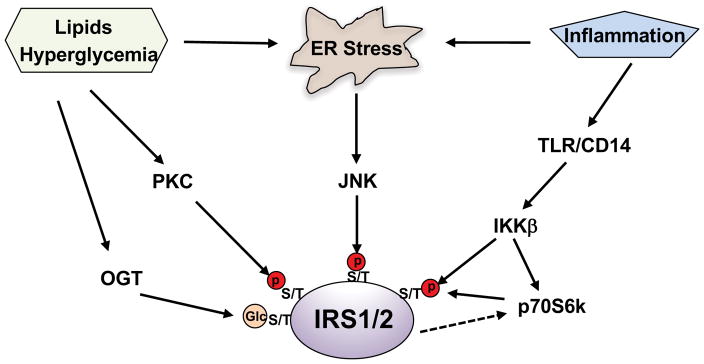
The ER is the primary site of protein synthesis, maturation, folding, and transport. Disturbance of these processes triggers the unfolded protein response (UPR) and leads to the activation of three canonical pathways: inositol-requiring enzyme 1(IRE1)-XBP1s, PKR-like ER-regulating kinase (PERK) and activating transcription factor 6 (ATF6)(Ron and Walter 2007). The activation of these pathways can alleviate ER stress by placing a brake on protein synthesis, and/or by increasing the production of protein chaperones needed for protein folding, or by degrading unfolded proteins. Excess nutrient intake causes ER stress and insulin resistance in obese animal models and obese humans subjects. ER stress results in the formation of the IRE1α-TRAF 2 complex, which leads to the phosphorylation and activation of JNK. Subsequently, activated JNK induces insulin resistance through serine phosphorylation of insulin receptor substrates 1 and 2(Hirosumi, et al. 2002) (Figure 2). In an elegant study, Dr. White’s group showed that the activation of ER stress by thapsigargin or anisomycin-mediated activation of JNK caused broad IRS serine/threonine phosphorylation in CHOIR /IRS1 cells(Hancer, et al. 2014).
Figure 2. Phosphorylation of IRS at serine and threonine residues by Ser/Thr kianses impairs insulin signaling.
Activation of Ser/Thr kianses by hyperglycemia, lipids, inflammation, and ER stress leads to the inhibitory serine and threonine phosphorylation of IRS, causing insulin resistance. O-GlcNAc modification of IRS can affect its phosphorylation.
3.2. Hyperglycemia and abnormal intracellular accumulation of lipids result in IRS serine/threonine phosphorylation by PKC
In diabetic and obese patients, hyperglycemia is the consequence of insufficient insulin secretion from β cells and insulin resistance in target tissues/organs; however, hyperglycemia can in turn aggravate insulin resistance. In cultured hepatocytes, treatment with high concentrations of glucose augmented IRS serine phosphorylation and impaired insulin-stimulated AKT phosphorylation(Nakajima, et al. 2000). Inhibition of protein kinase C (PKC) activity abolished the serine phosphorylation of IRS resulting from high glucose treatment, suggesting that PKC can phosphorylate IRS. Moreover, intracellular lipid accumulation was correlated with insulin resistance, and infusion of lipids into patients caused insulin resistance(Boden, et al. 2001; Pan, et al. 1997). The cumulative evidence has shown that lipids such as diacylglycerols and ceramides can activate conventional and novel PKC isoforms and impair insulin signaling by inducing multiple serine phosphorylation of IRS(Itani, et al. 2002) (Figure 2). Corroborating these findings, deletion of PKC family members decreased IRS serine phosphorylation and ameliorated insulin resistance in skeletal muscle and liver(Kim, et al. 2004; Samuel, et al. 2007).
3.3. Activation of the IKK/NF-κB pathway causes insulin resistance via increasing IRS serine/threonine phosphorylation
Obesity is associated with a low-grade chronic inflammation in different metabolic tissues, including adipose tissue and the liver. Low-grade chronic inflammation triggers the pathogenesis of insulin resistance. We found that mice fed a high-fat diet for 2 weeks exhibited insulin resistance and elevated glucose production in the liver. Since low-grade chronic inflammation can activate TLR/CD14 signaling, and we tested the importance of this signaling pathway in glucose metabolism. Our studies showed that high-fat diet feeding did not increase liver glucose production in CD14 knockout mice(Cao J 2017). Furthermore, studies in IKKβ transgenic mice indicate that NF-kB may be linked to the pathogenesis of insulin resistance(Cai, et al. 2005), which was proven in NF-kB p50 knockout mice, as this mouse model exhibited improved insulin sensitivity and produced significantly less glucose in the liver(Gao, et al. 2009). Further studies have revealed that IKKβ can phosphorylate multiple serine/threonine sites in IRS(Cai et al. 2005; Gao et al. 2009). In addition, IKKβ activation could augment serine phosphorylation of IRS via activation of the mTORC1/P70S6K pathway(Cai et al. 2005; Gao et al. 2009) (Figure 2).
Overall, due to the fact that IRS1/2 are heavily phosphorylated at serine and threonine residues (Table 1), it has been proposed that serine and threonine phosphorylation in IRS is a general mechanism to negatively modulate insulin signaling and contribute to pathological insulin resistance. In this respect, mice harboring muscle-specific mutated IRS1, in which three serine residues were changed to alanine at 302, 307, and 612, were protected from fat-induced insulin resistance and exhibited improved insulin sensitivity in muscle(Morino, et al. 2008). Of note, the activation of downstream Ser/Thr kinases ERK1/2 and P70S6K by insulin can also modulate IRS serine and threonine phosphorylation(Copps and White 2012), thus causing the signal to be self-limited. Analogous to phosphorylation, O-GlcNAc molecule can be introduced to IRS serine and threonine residues by O-GlcNAc transferase (OGT), this O-GlcNAc modification can block IRS phosphorylation and may affect the association of downstream mediators with IRS(Klein, et al. 2009) (Figure 2).
4. Acetyl-transferase P300 is a negative regulator of insulin signaling
The deacetylase Sirtuin1 is able to restore insulin sensitivity in cells or tissue with insulin resistance(Schug, et al. 2010; Sun, et al. 2007). Moreover, activation of Sirtuin1 by resveratrol improved insulin sensitivity(Kang, et al. 2012). This evidence indicates that acetylation of mediators in the insulin signaling pathway downregulates insulin signaling. Several mechanisms have been proposed to explain the upregulation of insulin signaling by the deacetylase Sirtuin1, including deacetylation of the insulin receptor substrate(Frojdo, et al. 2011; Zhang 2007). While it is known that increasing Sirtuin1 deacetylase activity improves insulin sensitivity, the identity of its counterpart, an acetyl-transferase, remains unknown.
It has been known for nearly a decade that curcumin, a specific inhibitor of P300 and CBP acetyl-transferase activity(Balasubramanyam, et al. 2004; Marcu, et al. 2006; Morimoto, et al. 2008), can improve hyperglycemia in diabetic patients and in animal models(Chuengsamarn, et al. 2012; Perez-Torres, et al. 2013; Shehzad, et al. 2011). We found that curcumin treatment increased the phosphorylation of AKT and GSK3 in a concentration-dependent manner in Hepa1-6 cells(Cao J 2017). Given that both P300 and CBP are acetyl-transferases and critical co-activators in the regulation of hepatic glucose production(He, et al. 2014; He, et al. 2012; He et al. 2009), we examined the protein levels of these co-activators in a diet-induced obese mouse model and found that P300 protein levels were dramatically induced by high-fat diet (HFD) feeding(Cao J 2017). Furthermore, we found that ob/ob mice have elevated P300 protein levels in their liver when compared to heterozygous lean control mice. In comparison, the protein levels of CBP remained unchanged in the liver of HFD-feeding mice and ob/ob mice. We tested whether the acetylation of mediators in the insulin signaling pathway by P300 acetyl-transferase led to downregulation of insulin signaling. First, we found that overexpression of P300 reduced insulin-mediated AKT and GSK3 phosphorylation. Second, depletion of P300, but not its closely related protein CBP, improved insulin sensitivity. Third, treatment with C646, a chemical P300-specific acetyl-transferase inhibitor(Bowers, et al. 2010; Wondisford, et al. 2014), improved insulin signaling/sensitivity in HFD-feeding mice and ob/ob mice. These data suggest that P300 is an acetyl-transferase that functions as a negative regulator of insulin signaling.
5. Acetylation of IRS1 and IRS2 by P300 impairs insulin signaling
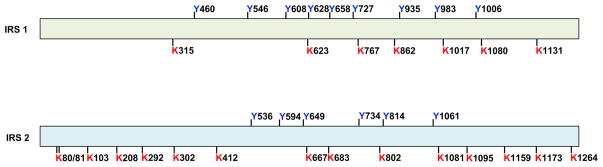
Treatment with the P300 acetyl-transferase specific inhibitor C646 led to a significant increase in the phosphorylation levels of AKT and GSK3 and increased PI3K activity by 6-fold. These data indicate that the IRS or insulin receptor might be the targets of P300. Indeed, we found that IRS1 and IRS2, but not the insulin receptor, were heavily acetylated in the liver of ob/ob mice. To mimic the effect of P300 induction on IRS acetylation, we co-transfected P300 and IRS1 and IRS2 expression vectors into Hepa1-6 cells. IRS1 and IRS2 proteins were purified and used to map acetylation sites by mass spectrometry. Seven lysine residues in IRS1 and fifteen in IRS2 can be acetylated (Figure 3).
Figure 3. Identified acetylation sites in IRS1 and IRS2.
Seven lysine residues in IRS1 and fifteen lysine residues in IRS2 can be acetylated. The tyrosine residues in IRS1 and IRS2, which are important for the generation of YXXM motif after phosphorylation, are also shown.
Interestingly, the blockade of a single acetylation site in either IRS1 or IRS2 by the substitution of the lysine residue with arginine as a mimic of non-acetylated lysine (K to R mutation) had a mild effect on AKT and GSK phosphorylation. However, combined mutations (K to R mutation) at the 315/767/862, 1017/1080, and 1017/1080/1131 lysine residues in IRS1, and the 683/802, 683/802/1081, 1173/1264, and 683/802/1081/1173/1264 lysine residues in IRS2 significantly increased insulin signaling. In particular, combined triple KR mutations at the 1017/1080/1131 lysine residues in IRS1 and combined double KR mutations at the 1173/1264 lysine residues in IRS2 had the strongest effect on the augmentation of insulin signaling(Cao J 2017). We further assessed the effects of IRS1/2 KR mutations on insulin signaling in HFD-fed mice using adenoviral expression vectors to express similar amounts of wild type IRS1 and IRS2, or IRS1 and IRS2 KR mutants in the liver. Mice with injections of IRS1 and IRS2 KR mutants exhibited improved insulin sensitivity, suggesting that the acetylation of IRS1 and IRS2 impairs insulin signaling. Of note, combined KR mutation in either IRS1 or IRS2 dramatically decreased P300-mediated IRS1 and IRS2 acetylation, indicating that these identified acetylation sites in IRS1 and IRS2 are P300 target sites.
6. Acetylation of IRS1 and IRS2 by P300 results in the reduction of their tyrosine phosphorylation
Our studies showed that inhibition of P300 acetyl-transferase activity by C646 led to increased tyrosine phosphorylation of IRS1 and IRS2 in cultured Hepa1-6 cells and in the liver of ob/ob mice. To validate these data, we overexpressed IRS1 or IRS2 to similar levels as their corresponding IRS1-panKR or IRS2-panKR (all identified acetylation sites in IRS1 or IRS2 lysine residues were substituted with arginine residues) in Hepa1-6 cells. We found KR mutations in IRS1 or IRS2 resulted in elevated IRS tyrosine phosphorylation. Intriguingly, we observed increased association of IRS1/2-panKR with IRβ when compared to IRS1/2 (wild type), suggesting that acetylation of IRS by P300 blocks the association of IRS with IRβ, such that IRS cannot be phosphorylated at tyrosine residues. To test this hypothesis, Hepa1-6 cells were treated with C646, and specific antibodies of IRS1, IRS2, and IRβ were used to immunoprecipitate these proteins and their associated proteins. Inhibition of P300 acetyl-transferase activity by C646 drastically increased the tyrosine phosphorylation of IRS1 and IRS2 and increased association of IRS1 and IRS2 with IRβ. However, C646 had a minimal effect on IRβ’s tyrosine phosphorylation. Our data suggest that the acetylation of IRS may function as a “gatekeeper” to prevent the association of IRS with IRβ in the unstimulated state. In accordance with our findings, inhibition of deacetylase activity increased IRS2 acetylation, resulting in a reduction of IRS2 tyrosine phosphorylation(Kawada, et al. 2017). However, it has been reported that IRS1 acetylation had a permissive effect on its tyrosine phosphorylation(Kaiser and James 2004; Tan, et al. 2015), suggesting that IRS can be acetylated by other acetyltransferases at different sites; these acetylation sites might have distinct functions in regulating insulin signaling and controlling cell growth.
7. Endotoxemia-mediated P300 induction through the activation of the IRE1-XBP1s pathway
A high-fat, western-style diet is an important predisposing factor for the onset of diabetes and obesity. HFD feeding results in changes in gut microbiota that initiate endotoxin (LPS)-induced inflammation and endoplasmic reticulum (ER) stress(Cani, et al. 2007; Cani, et al. 2008; Li, et al. 2012). We found that LPS levels were significantly increased in the liver of mice fed an HFD, and treatment with LPS triggered the activation of the IRE1-XBP1s pathway in the ER stress response and increased P300 protein levels in the liver and cultured hepatocytes. Although P300 is a nuclear protein, we found substantial amounts of P300 relocated into the cytoplasm in the liver cells of mice fed an HFD. Moreover, LPS treatment or overexpression of XBP1s resulted in P300 induction and cytoplasmic localization in hepatocytes, disrupting insulin signaling by acetylating IRS1/2 in the cytoplasm(Cao J 2017). Since LPS treatment significantly decreased ubiquitin-conjugated P300 and increased P300 protein levels, and XBP1s can bind directly to P300, it could be speculated that the binding of XBP1s to P300 prevents P300 ubiquitination and nuclear localization. Collectively, our study proposes a mechanism underlying the disruption of insulin signaling by elevated LPS in obese animal models through the acetylation of IRS1 and IRS2 by induced P300.
8. P300 acetyl-transferase activity is a potential therapeutic target
Previous studies showed that the inhibition of P300 and CBP acetyl-transferase activity by curcumin alleviated hyperglycemia in diabetic patients and animal models(Balasubramanyam et al. 2004; Marcu et al. 2006; Morimoto et al. 2008). We found that the inhibition of P300 acetyl-transferase activity by C646 significantly alleviated hyperglycemia in obese ob/ob mice and improved insulin sensitivity in HFD-fed mice(Cao J 2017). The improvement of insulin signaling by the inhibition of P300 acetyl-transferase activity leads to an increase in AKT-mediated phosphorylation and degradation of FoxO1 and CRTC2(Accili and Arden 2004; Koo et al. 2005), two important transcription factors for gluconeogenic gene expression, which should lead to the suppression of hepatic glucose production. However, the complex of CREB-CBP/P300 is critical for glucagon stimulation of hepatic glucose production(He et al. 2009). Therefore, inhibition of P300 acetyl-transferase activity by C646 or curcumin can affect the function of the CREB-CBP/P300 complex, resulting in the suppression of hepatic glucose production as well. Furthermore, inhibition of histone acetyltransferase activity of P300 by C646 significantly decreased hepatic Foxo1 mRNA through the regulation of Foxo1 gene transcription(Wondisford et al. 2014). This line of evidence suggests that P300 acetyl-transferase activity is a therapeutic target for the treatment of diabetes and obesity. Since P300 is an important co-activator in the activation of gene transcription and participates in many critical developmental processes, a key priority is undoubtedly the development of a drug targeting P300 acetyltransferase activity in the liver. Previously, C646 was found to be a promising agent for cancer treatment(Santer, et al. 2011; Yang, et al. 2013), and we have found that this agent can be used to treat diabetes, with its primary target being hepatic P300(Cao J 2017). However, it is conceivable that more work needs to be done before this agent can be used in clinic.
9. Perspective
Insulin resistance is the hallmark of type 2 diabetes, and defective hepatic insulin sensitivity in type 2 diabetes results in increased glucose production, which is the major cause of hyperglycemia in diabetic patients(Doria, et al. 2008; Magnusson et al. 1992; Wajngot, et al. 2001). Elevated serum LPS levels from changes in gut microbiota cause low-grade inflammation and ER stress, which play a critical role in the development of insulin resistance. We believe that the induction of P300 links inflammation and metabolic diseases. Even though we found that acetylation of IRS1 and IRS2 by P300 led to the impairment of insulin signaling by decreasing the association of IRS with IRβ, it is also possible that the acetylation of IRS might impede its tyrosine phosphorylation by receptor kinase IRβ, therefore resulting in insulin resistance. Because protein tyrosine phosphatase 1B (PTP1B) is the protein phosphatase responsible for the dephosphorylation of tyrosine residues in mediators of insulin signaling(Elchebly, et al. 1999; Kenner, et al. 1996), it is also possible that acetylation of IRS1 and IRS2 will facilitate the dephosphorylation of tyrosine residues in these proteins. Since the cardinal feature of diabetes and obesity is insulin resistance, improving insulin resistance is a critical step in combating these metabolic diseases. Thus, P300 acetyl-transferase activity appears to be a promising therapeutic target for the treatment of diabetic patients.
Acknowledgments
Funding
This work was supported in part by grant from the National Institute of Diabetes and Digestive and Kidney Diseases R01DK107641.
Footnotes
Authors have no conflict of interest to declare.
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, 3rd, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F, Brichard S, Rencurel F, Cusin I, Jeanrenaud B. In vivo effects of hyperinsulinemia on lipogenic enzymes and glucose transporter expression in rat liver and adipose tissues. Metabolism. 1995;44:228–233. doi: 10.1016/0026-0495(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Avruch J. Insulin signal transduction through protein kinase cascades. Mol Cell Biochem. 1998;182:31–48. [PubMed] [Google Scholar]
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- Bowers EM, Yan G, Mukherjee C, Orry A, Wang L, Holbert MA, Crump NT, Hazzalin CA, Liszczak G, Yuan H, et al. Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chem Biol. 2010;17:471–482. doi: 10.1016/j.chembiol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Cao JPJ, An H, He Q, Boronina T, Guo S, White MF, Glimcher LH, Cole PA, He L. Endotoxemia-mediated activation of acetyltransferase P300 impairs insulin signaling in obesity. Nature Communications. 2017;8:1–12. doi: 10.1038/s41467-017-00163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham B, Kahn CR. Insulin action and the insulin signaling network. Endocr Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35:2121–2127. doi: 10.2337/dc12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Park S, Lin X, Copps K, Yi X, White MF. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest. 2006;116:101–114. doi: 10.1172/JCI25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria A, Patti ME, Kahn CR. The emerging genetic architecture of type 2 diabetes. Cell Metab. 2008;8:186–200. doi: 10.1016/j.cmet.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Frojdo S, Durand C, Molin L, Carey AL, El-Osta A, Kingwell BA, Febbraio MA, Solari F, Vidal H, Pirola L. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol. 2011;335:166–176. doi: 10.1016/j.mce.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Gao Z, Yin J, Zhang J, He Q, McGuinness OP, Ye J. Inactivation of NF-kappaB p50 leads to insulin sensitization in liver through post-translational inhibition of p70S6K. J Biol Chem. 2009;284:18368–18376. doi: 10.1074/jbc.M109.007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. 2014;220:T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancer NJ, Qiu W, Cherella C, Li Y, Copps KD, White MF. Insulin and metabolic stress stimulate multisite serine/threonine phosphorylation of insulin receptor substrate 1 and inhibit tyrosine phosphorylation. J Biol Chem. 2014;289:12467–12484. doi: 10.1074/jbc.M114.554162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Meng S, Germain-Lee EL, Radovick S, Wondisford FE. Potential biomarker of metformin action. J Endocrinol. 2014;221:363–369. doi: 10.1530/JOE-14-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Naik K, Meng S, Cao J, Sidhaye AR, Ma A, Radovick S, Wondisford FE. Transcriptional co-activator p300 maintains basal hepatic gluconeogenesis. J Biol Chem. 2012;287:32069–32077. doi: 10.1074/jbc.M112.385864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- Kaiser C, James SR. Acetylation of insulin receptor substrate-1 is permissive for tyrosine phosphorylation. BMC Biol. 2004;2:23. doi: 10.1186/1741-7007-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Hong HJ, Guan J, Kim DG, Yang EJ, Koh G, Park D, Han CH, Lee YJ, Lee DH. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2012;61:424–433. doi: 10.1016/j.metabol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Kawada Y, Asahara SI, Sugiura Y, Sato A, Furubayashi A, Kawamura M, Bartolome A, Terashi-Suzuki E, Takai T, Kanno A, et al. Histone deacetylase regulates insulin signaling via two pathways in pancreatic beta cells. PLoS One. 2017;12:e0184435. doi: 10.1371/journal.pone.0184435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenner KA, Anyanwu E, Olefsky JM, Kusari J. Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J Biol Chem. 1996;271:19810–19816. doi: 10.1074/jbc.271.33.19810. [DOI] [PubMed] [Google Scholar]
- Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105:199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O’Brien WR, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AL, Berkaw MN, Buse MG, Ball LE. O-linked N-acetylglucosamine modification of insulin receptor substrate-1 occurs in close proximity to multiple SH2 domain binding motifs. Mol Cell Proteomics. 2009;8:2733–2745. doi: 10.1074/mcp.M900207-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Kunert O, Stingl H, Rosian E, Krssak M, Bernroider E, Seebacher W, Zangger K, Staehr P, Chandramouli V, Landau BR, et al. Measurement of fractional whole-body gluconeogenesis in humans from blood samples using 2H nuclear magnetic resonance spectroscopy. Diabetes. 2003;52:2475–2482. doi: 10.2337/diabetes.52.10.2475. [DOI] [PubMed] [Google Scholar]
- Li J, Huang J, Li JS, Chen H, Huang K, Zheng L. Accumulation of endoplasmic reticulum stress and lipogenesis in the liver through generational effects of high fat diets. J Hepatol. 2012;56:900–907. doi: 10.1016/j.jhep.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu MG, Jung YJ, Lee S, Chung EJ, Lee MJ, Trepel J, Neckers L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med Chem. 2006;2:169–174. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. 2008;118:868–878. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K, Neschen S, Bilz S, Sono S, Tsirigotis D, Reznick RM, Moore I, Nagai Y, Samuel V, Sebastian D, et al. Muscle-specific IRS-1 Ser->Ala transgenic mice are protected from fat-induced insulin resistance in skeletal muscle. Diabetes. 2008;57:2644–2651. doi: 10.2337/db06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Backer JM, Sun XJ, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White MF. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci U S A. 1992;89:10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Yamauchi K, Shigematsu S, Ikeo S, Komatsu M, Aizawa T, Hashizume K. Selective attenuation of metabolic branch of insulin receptor down-signaling by high glucose in a hepatoma cell line, HepG2 cells. J Biol Chem. 2000;275:20880–20886. doi: 10.1074/jbc.M905410199. [DOI] [PubMed] [Google Scholar]
- Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- Perez-Torres I, Ruiz-Ramirez A, Banos G, El-Hafidi M. Hibiscus sabdariffa Linnaeus (Malvaceae), curcumin and resveratrol as alternative medicinal agents against metabolic syndrome. Cardiovasc Hematol Agents Med Chem. 2013;11:25–37. doi: 10.2174/1871525711311010006. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer FR, Hoschele PP, Oh SJ, Erb HH, Bouchal J, Cavarretta IT, Parson W, Meyers DJ, Cole PA, Culig Z. Inhibition of the acetyltransferases p300 and CBP reveals a targetable function for p300 in the survival and invasion pathways of prostate cancer cell lines. Mol Cancer Ther. 2011;10:1644–1655. doi: 10.1158/1535-7163.MCT-11-0182. [DOI] [PubMed] [Google Scholar]
- Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad A, Ha T, Subhan F, Lee YS. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr. 2011;50:151–161. doi: 10.1007/s00394-011-0188-1. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Sweet LJ, Morrison BD, Pessin JE. Isolation of functional alpha beta heterodimers from the purified human placental alpha 2 beta 2 heterotetrameric insulin receptor complex. A structural basis for insulin binding heterogeneity. J Biol Chem. 1987;262:6939–6942. [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- Tan HW, Sim AY, Huang SL, Leng Y, Long YC. HC toxin (a HDAC inhibitor) enhances IRS1-Akt signalling and metabolism in mouse myotubes. J Mol Endocrinol. 2015;55:197–207. doi: 10.1530/JME-15-0140. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Wajngot A, Chandramouli V, Schumann WC, Ekberg K, Jones PK, Efendic S, Landau BR. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metabolism. 2001;50:47–52. doi: 10.1053/meta.2001.19422. [DOI] [PubMed] [Google Scholar]
- White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- White MF, Livingston JN, Backer JM, Lauris V, Dull TJ, Ullrich A, Kahn CR. Mutation of the insulin receptor at tyrosine 960 inhibits signal transmission but does not affect its tyrosine kinase activity. Cell. 1988;54:641–649. doi: 10.1016/s0092-8674(88)80008-4. [DOI] [PubMed] [Google Scholar]
- White MF, Maron R, Kahn CR. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985;318:183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet. 1999;23:32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- Wondisford AR, Xiong L, Chang E, Meng S, Meyers DJ, Li M, Cole PA, He L. Control of Foxo1 gene expression by co-activator P300. J Biol Chem. 2014;289:4326–4333. doi: 10.1074/jbc.M113.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Pinello CE, Luo J, Li D, Wang Y, Zhao LY, Jahn SC, Saldanha SA, Chase P, Planck J, et al. Small-molecule inhibitors of acetyltransferase p300 identified by high-throughput screening are potent anticancer agents. Mol Cancer Ther. 2013;12:610–620. doi: 10.1158/1535-7163.MCT-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem. 2007;282:34356–34364. doi: 10.1074/jbc.M706644200. [DOI] [PubMed] [Google Scholar]