Abstract
Background
Although active surveillance is increasingly used for the management of low-risk prostate cancer, many eligible patients are still nonetheless subject to curative treatment. One argument for considering surgery rather than active surveillance is that the probability of postoperative recovery of erectile function is age dependent, that is, patients who delay surgery may lose the window of opportunity to recover erectile function after surgery.
Objective
To model erectile function over a 10-yr period for immediate surgery versus active surveillance.
Design, setting, and participants
Data from 1103 men who underwent radical prostatectomy at a tertiary referral center were used.
Outcome measurements and statistical analysis
Patients completed the International Index of Erectile Function (IIEF-6) pre- and postoperatively as a routine part of clinical care. Preoperative IIEF-6 scores were plotted against age to assess the natural rate of functional decline due to aging. Reported erectile scores in the 2-yr period following surgery were used to assess post-surgical recovery.
Results and limitations
Each year increase in patient age resulted in a 0.21 reduction in IIEF scores. In addition to IIEF reducing with increased age, the amount of erectile function that is recovered from presurgery to 12-mo postsurgery also decreases (−0.18 IIEF points/yr, 95% confidence interval: −0.29, −0.08, p = 0.001). However, delayed radical prostatectomy increased the mean IIEF-6 score over a 10-yr period compared with immediate surgery (p = 0.001), even under the assumption that all men placed on active surveillance are treated within 5 yr.
Conclusions
Small differences in erectile function recovery in younger men are offset by a longer period of time living with decreased postoperative function. Better erectile recovery in younger men should not be a factor used to recommend immediate surgery in patients suitable for active surveillance, even if crossover to surgery is predicted within a short period of time.
Patient summary
Younger men have better recovery of erectile function after surgery for prostate cancer. This has led to the suggestion that delaying surgery for low-risk disease may lead patients to miss a window of opportunity to recover erectile function postoperatively. We conducted a modeling study and found that predicted erectile recovery was far superior on delayed treatment because slightly better recovery in younger men is offset by a longer period of time living with poorer postoperative function in those choosing immediate surgery.
Keywords: Prostate neoplasm, Radical prostatectomy, Erectile dysfunction, Active surveillance, Patient-reported outcomes
1. Introduction
There is widespread agreement in the academic community that men with low-risk prostate cancer should be managed by active surveillance. The approach is recommended in guidelines [1] based on evidence that risk of prostate cancer mortality is very low [2] and not improved by treatment [3]. Yet resistance to active surveillance remains, with one recent study demonstrating that while rates of active surveillance have increased, a majority of men with low-risk prostate cancer are nonetheless treated curatively [4].
A key rationale for conservative management is the avoidance of treatment-related morbidities, such as erectile dysfunction. However, an argument can be made that the possibility of such morbidities actually justifies immediate surgery. This argument starts from the premise that many men managed conservatively do end up being treated: in one well-known study, the probability of radical treatment was 57% by 15 yr [2]. Given that the risk of postoperative erectile dysfunction increases with age [5], patients who delay treatment may lose the window of opportunity to recover erectile function after surgery. We have heard this argument expressed as: “I’m probably going to have to operate on you eventually, so I might as well treat you now while you are young and have a good chance of recovery.”
Numerous studies have compared erectile function between men undergoing surgery versus active surveillance, the randomized ProtecT study being a notable example [6]. A challenge of such studies is that functional outcomes in conservatively managed patients depend on the rate of crossover to surgery: a more aggressive approach to monitoring will lead to more treatment and hence poorer functional outcomes. An alternative is to use a statistical modeling approach, which allows the rate of surgery to be varied. At Memorial Sloan Kettering Cancer Center, patients undergoing radical prostatectomy (RP) complete pretreatment and post-treatment patient-reported outcomes as a routine part of clinical care. These data allow us to model a quantitative comparison between immediate and delayed surgery: comparing pretreatment function at different ages gives an estimate of how erectile function would change in an untreated man; comparing postsurgery recovery at different ages allows us to evaluate the marginal benefit of early surgery. Here we report a novel modeling study to compare long-term erectile function comparing immediate surgery versus active surveillance.
2. Materials and methods
2.1. Patients and outcomes
Following institutional review board approval, we identified 5865 patients through our prospectively maintained database who underwent open or minimally-invasive nerve-sparing RP from an experienced attending surgeon from 2009 through 2013, and who did not receive adjuvant therapy. Given that we were interested in patients potentially eligible for active surveillance, we excluded men with any clinical features of high-risk prostate cancer, including prostate-specific antigen ≥20 ng/ml, biopsy Gleason grade group ≥3, and clinical tumor stage >cT2b. Men who did not undergo bilateral nerve-sparing surgery were also omitted, leaving a cohort of 1581. Note that although we included some patients (eg, Gleason grade group 2) who are not considered eligible for active surveillance in some institutions, this does not affect our findings, as functional outcomes are not importantly different in these patients compared with those with lower risk disease. Four hundred and fifty men without preoperative erectile function scores were excluded, as well as 28 men without a postoperative score (final cohort n = 1103). There were no significant differences in patient age, preoperative prostate-specific antigen, comorbidities, number of positive biopsy cores, surgical margin status, extracapsular extension, seminal vesical invasion, lymph node invasion, pathology Gleason, or pathology stage between the men with and without preoperative erectile data (all p > 0.05).
Patient-reported erectile function was electronically collected through our web-based platform using the International Index of Erectile Function 6 (IIEF-6; range, 1–30). The surveys are administered at baseline (preoperative period) and postoperatively at 3 mo, 6 mo, 9 mo, 12 mo, 18 mo, and 24 mo or shortly before any postoperative appointments. As is standard, patients were asked to report function on oral medication—if they took Viagra or other phosphodiesterase type 5 inhibitors—but without the use of injection therapy or other erectile function aids.
2.2. Statistical analysis
Our primary aim was to model the effects of immediate surgery versus active surveillance on long-term erectile function. We plotted preoperative IIEF-6 scores against age to assess the natural rate of functional decline due to aging. Reported erectile scores in the 2-yr period following surgery were used to assess postsurgical recovery. Due to more limited data on erectile function by age after 2 yr, we assumed that recovery plateaus after 2 yr, and thereafter function declines with age at the same rate as for preoperative function.
It is well known that comorbidities have an adverse effect on erectile function. We sought to assess whether function declined at a faster rate due to aging for men with more comorbidities. Using linear regression models, we investigated the effect of patient age and comorbidity status on baseline and postoperative IIEF-6 scores, with an interaction term in the model to examine the effect of patient comorbidities on the rate of decline of erectile function with increasing age. Data on major comorbidities known to affect erectile function (cardiovascular disease, hypertension requiring medical therapy, diabetes mellitus, and peripheral vascular disease) were captured and included in the model categorized as none, one, two, or three or more major comorbidities. A significant interaction between age and the number of comorbidities would indicate that function declines faster for unhealthy patients as they age, and that the above analyses investigating the effects of delayed RP would need to be stratified by comorbidity status.
Using patient age, baseline, and postoperative IIEF-6 scores from the study cohort, we estimated the expected postoperative erectile function recovery using locally weighted scatterplot smoothing for immediate surgery versus surgery after varying periods of observation. An average IIEF-6 score over 10-yr duration was calculated for the two scenarios by calculating the area under the erectile function curve. Bootstrap resampling was used to 95% confidence intervals (CIs). Our plan was to use prior data [2] on the distribution of time to treatment to give expected erectile function on active surveillance. In brief, the predicted IIEF score over 10 yr for a patient undergoing surgery after n yr would be multiplied by the actuarial probability of surgery at n yr with results then summed over all n. However, this analysis was found not to be warranted. All statistical analyses were conducted using Stata 13 (Stata Corp., College Station, TX, USA).
3. Results
Table 1 summarizes the clinicopathologic characteristics of the study cohort from which patient-reported data were obtained. Median patient age in this cohort was 62 (interquartile range: 57–67) yr, with a median reported baseline IIEF-6 score of 28 (interquartile range: 21–30).
Table 1.
Clinicopathologic characteristics of cohort (n = 1103; data are given as median and quartiles or frequency and percentage)
| Age (yr) | 62 (57, 67) |
| Preoperative IIEF-6 | 28 (21, 30) |
| Preoperative PSA (ng/ml) | 4.9 (3.6, 6.5) |
| Biopsy cores (N = 1193) | 12 (6, 13) |
| Positive biopsy cores (N = 1197) | 3 (1, 5) |
| Biopsy Gleason grade group | |
| 1 | 566 (51%) |
| 2 | 537 (49%) |
| RP Gleason sum | |
| 1 | 264 (22%) |
| 2 | 681 (62%) |
| 3 | 153 (14%) |
| 4 | 13 (1.2%) |
| 5 | 12 (1.1%) |
| T Stage | |
| ≤pT2c | 810 (73%) |
| pT3a | 259 (23%) |
| pT3b | 34 (3.1%) |
| Positive surgical margins | 151 (14%) |
| Unknown | 1 (<0.1%) |
| Comorbidities | |
| 0 | 372 (34%) |
| 1 | 390 (35%) |
| 2 | 234 (21%) |
| 3+ | 107 (10%) |
| Type of surgery | |
| Laproscopic | 288 (26%) |
| Open | 294 (27%) |
| Robotically-assisted laproscopic | 521 (47%) |
IIEF-6 = International Index of Erectile Function-6; PSA = prostate-specific antigen.
Each year increase in patient age resulted in a 0.21 (95% CI: 0.10, 0.31, p < 0.0001) point reduction in baseline IIEF scores (Fig. 1). Comorbidity reduced baseline scores, with a −0.80 (95% CI: −1.93, +0.16, p = 0.16), −2.93 (95% CI: −4.22, −1,64, p < 0.0001), and −3.66 (95% CI: −5.39, −1.91, p < 0.0001) point change in IIEF for one, two, or three or more comorbidities. However, there was no evidence that erectile function declined faster with increasing age in patients with comorbidities (p = 0.2 for the interaction term). As such, comorbidities were excluded from the model estimating postoperative recovery.
Fig. 1.
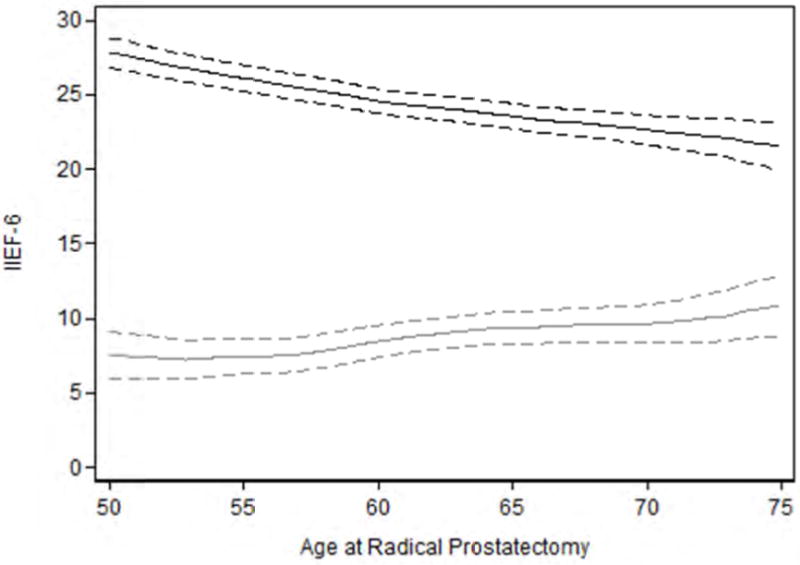
Effects of age on erectile function and recovery of erectile function after radical prostatectomy. Black line International Index of Erectile Function-6 (IIEF-6) measured erectile function by age. Grey line is loss in IIEF-6 measured erectile function from pre- to 12-mo postradical prostatectomy by age. Dashed lines are 95% confidence intervals. Older patients have lower baseline IIEF scores and experience larger losses in erectile function compared to younger patients after surgery.
The degree of erectile function recovery after RP also decreases (−0.18 IIEF points/yr, 95% CI: −0.29, −0.08, p = 0.001). For example, a typical 55-yr-old patient would lose 7.5 points from pre-RP to 12-mo post-RP; a 60-yr-old patient who would lose 8.4 points (Fig. 1).
Figure 2 illustrates the predicted erectile function scores over time for a 55-yr-old patient with a baseline IIEF-6 score of 26 with immediate versus surgery delayed by 5 yr, a timepoint at which about a third of men initially managed on active surveillance have been treated [2]. The average 10-yr IIEF score was higher on delayed surgery (2.1 points, 95% CI: 0.4, 3.9). Indeed, even if we assume a very short delay to surgery of only 3 yr, we continue to see a significant improvement compared with immediate treatment (1.5 points, 95% CI: 0.2, 3.0).
Fig. 2.
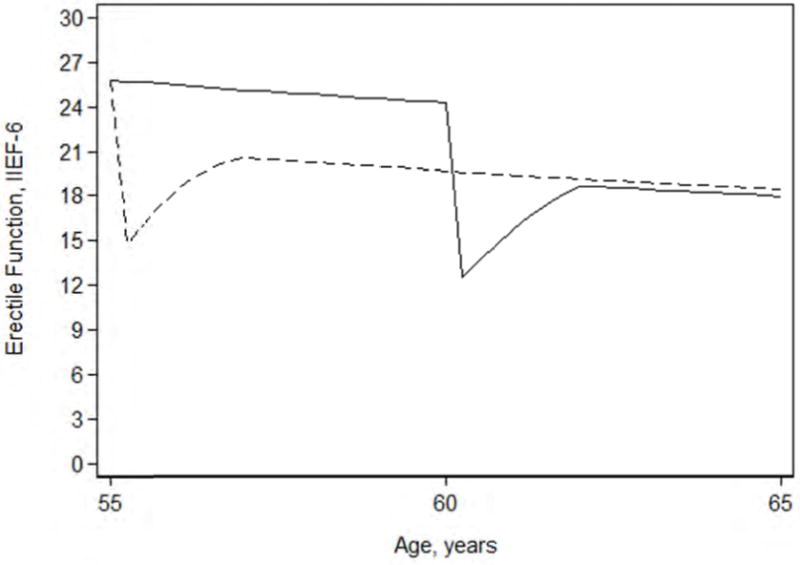
Erectile function scores over time for a 55-yr-old patient with a baseline International Index of Erectile Function-6 (IIEF-6) score of 26 with immediate (dashed line) versus surgery delayed by 5 yr (solid line).
We repeated these analyses for men aged 60 yr and 65 yr looking over 10-yr and 15-yr timeframes with similar results (Table 2). For example, a man diagnosed with prostate cancer at 65 yr old would have an average of 3.3 more points on the IIEF-6 over a 10-yr period if RP were delayed for 5 yr (95% CI: 1.8, 4.6). In each scenario, delayed RP was estimated to lead to an increase in mean IIEF-6 score, although differences between groups were not statistically significant in all cases.
Table 2.
Difference in mean International Index of Erectile Function-6 (IIEF-6) score over a 10-yr or 15-yr time frame after prostate cancer diagnosis (comparing immediate radical prostatectomy to 3-yr and 5-yr delays in surgery)
| Age at diagnosis (yr) | RP delay (yr) | Time frame (yr) | Difference in mean IIEF-6 score | 95% CI for difference |
|---|---|---|---|---|
| 55 | 3 | 10 15 |
1.5 1.0 |
0.2, 3.0 −0.2, 2.8 |
| 5 | 10 15 |
2.1 1.3 |
0.4, 3.9 −0.5, 3.3 |
|
| 60 | 3 | 10 15 |
0.8 0.2 |
−0.6, 1.8 −1.3, 1.3 |
| 5 | 10 15 |
1.9 0.8 |
0.3, 3.1 −0.9, 2.1 |
|
| 65 | 3 | 10 15 |
1.9 1.3 |
0.9, 3.0 0.1, 2.4 |
| 5 | 10 15 |
3.3 2.2 |
1.8, 4.6 0.4, 3.6 |
CI = confidence interval; RP = radical prostatectomy.
As erectile function was superior on active surveillance even if a man was subject to surgery after a relatively short duration, incorporation of longer delays to surgery, or no surgery at all, would have no effect on our conclusion that erectile function is superior on delayed surgery. Accordingly, these planned analyses were not conducted.
4. Discussion
Using a robust database of baseline and postoperative patient reported outcomes for men with low-and intermediate-risk prostate cancer, we found that the predicted average long-term erectile function is higher for men who undergo delayed compared with immediate RP at a younger age. Our findings disconfirm the hypothesis that early surgery compared with active surveillance improves sexual function by utilizing a window of opportunity for postoperative recovery. Indeed, the results suggest that a man should opt for active surveillance even if he knew that surgery would be required within a few years.
Our data can be compared with other reports in the literature. First, there is clear evidence for our finding that recovery is age-related. Brajtbord et al [7] report erectile recovery after RP in two age groups, ≤60 yr and >60 yr. Older men were more likely to have a “clinically significant” decrease in sexual outcomes, particularly bother. In a paper describing a prediction model for postoperative erectile dysfunction [5], Alemozaffar et al [5] reported that increasing age was associated with a decreased probability of erectile function, even after adjusting for baseline function. The time course of erectile function we report can be compared with data from the Prostate Cancer Outcomes Study [8]. Results are similar except that erectile function recovery appears better in the current study, possibly due to the differences between specialist and community settings. Poorer recovery overall would push our results even further in favor of delayed surgery.
Donovan et al [6] report erectile function over a 6-yr period in the ProtecT randomized trial comparing surgery with active surveillance. Men in the surveillance group were subject to treatment on progression—with approximately 35% being treated by 6 yr [9]—and hence the outcome reported includes declines associated both with aging and with treatment. Erectile function was superior in the active surveillance arm compared with surgery, supporting our principal findings. The other major randomized trial that compares surgery with conservative management is SPGC4. The authors report rates of erectile dysfunction of 66% versus 24% and 81% versus 75% at 4 yr and 12 yr, respectively [10]. While this lends general support to our findings, SPCG4 is not directly relevant to current practice as patients in the conservative management group were not as aggressively followed as would be typical for contemporary active surveillance approaches. As such, the rate of treatment was lower—less than 20% at 10 yr—leading to lower rates of treatment-related erectile dysfunction.
The advantage of using a modeling approached based on empirical data compared with studies, such as ProtecT, based on empirical data alone, is our ability to model follow-up over a 10–15-yr period. That said, modeling approaches do have limitations. One major assumption we make is that cross-sectional data on preoperative function by age can be used to estimate how erectile function would change over time on an active surveillance program. As there is no strong evidence that aspects of active surveillance such as repeat biopsy [11,12] have a strong effect on erectile function, age-related declines seem the most reasonable hypothesis.
Due to limited data on long-term recovery by age, we assume that recovery is complete by 2 yr, even though there is evidence that erectile function continues to recover in some men after this time point [13]. This type of underestimation of recovery would constitute a bias against immediate surgery. However, we do not incorporate discounting, the principle that having something now is more valuable than having it later. One recommended rate of discounting for health outcomes is 1.5–2% [14], equivalent to 14–18% over 10 yr. The theory behind immediate surgery is that although a patient will suffer short-term morbidity, long-term recovery will be superior. Failure to incorporate discounting is therefore a bias towards immediate surgery. Our best guess is that these two biases—longer-term recovery and discounting—are approximately equal and so would not have a large effect on our conclusions.
We also assume that IIEF is linearly associated with erectile function, that is, a given change in IIEF is equally important to a man at all levels of IIEF. This is unlikely to be true as, say, a difference between an IIEF of 5 versus 8 has no impact on a man’s sex life, whereas a difference between 21 and 24 might reflect a man being able to have sex most of the time when he wants to compared with about half of the time. That said, there is no reason to believe that this assumption would differentially favor either delayed or immediate intervention. Moreover, the effects we describe are so large—better function on active surveillance even if we assume that all men are treated relatively rapidly—that alternative methods of characterizing IIEF scores are highly unlikely to influence our findings.
We note that our findings are not a direct argument for active surveillance. Although the data suggest that the approach is a safe one [2], further data may modify our understanding of the oncologic outcome of conservative management. Similarly, there may be reasons for a man with low-risk cancer to choose immediate surgery, including concurrent benign prostate disease, or baseline erectile dysfunction coupled with other factors such as excessive anxiety or strong family history. Our point is only that better recovery of erectile function in younger men cannot be used as an argument for early surgery.
5. Conclusions
In conclusion, we found no evidence to support the claim that immediate RP in younger men leads to better erectile function outcomes compared with active surveillance. In fact, predicted average erectile function over 10 yr was estimated to be better with surveillance due to preservation of baseline function. This was the case even under the assumption that all men on active surveillance are treated within a relatively short period of time. Therefore, age-related recovery of erectile function following RP should not be used to justify immediate surgery for men eligible for active surveillance.
Acknowledgments
Funding/Support and role of the sponsor: This work was supported by David H. Koch provided through the Prostate Cancer Foundation; the Sidney Kimmel Center for Prostate and Urologic Cancers; SPORE grant from the National Cancer Institute to Dr. H. Scher (grant number P50-CA92629); and a National Institutes of Health/National Cancer Institute Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center (grant number P30-CA008748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Andrew J. Vickers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Vickers, Miller, Ehdaie.
Acquisition of data: None.
Analysis and interpretation of data: Sjoberg, Vickers.
Drafting of the manuscript: Vickers, Lee, Miller.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Sjoberg.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: None.
Other: None.
Financial disclosures: Andrew J. Vickers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. 2017 doi: 10.6004/jnccn.2010.0012. https://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf. [DOI] [PubMed]
- 2.Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol. 2015;33:3379–85. doi: 10.1200/JCO.2015.62.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990 – 2013. JAMA. 2015;314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 5.Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306:1205–14. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–37. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brajtbord JS, Punnen S, Cowan JE, Welty CJ, Carroll PR. Age and baseline quality of life at radical prostatectomy–who has the most to lose? J Urol. 2014;192:396–401. doi: 10.1016/j.juro.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–45. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 10.Johansson E, Steineck G, Holmberg L, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891–9. doi: 10.1016/S1470-2045(11)70162-0. [DOI] [PubMed] [Google Scholar]
- 11.Anderson CB, Tin AL, Sjoberg DD, et al. Association between number of prostate biopsies and patient-reported functional outcomes after radical prostatectomy: implications for active surveillance protocols. BJU Int. 2016;117:E46–51. doi: 10.1111/bju.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun K, Ahallal Y, Sjoberg DD, Ghoneim T, et al. Effect of repeated prostate biopsies on erectile function in men on active surveillance for prostate cancer. J Urol. 2014;191:744–9. doi: 10.1016/j.juro.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 13.Lee JK, Assel M, Thong AE, et al. Unexpected long-term improvements in urinary and erectile function in a large cohort of men with self-reported outcomes following radical prostatectomy. Eur Urol. 2015;68:899–905. doi: 10.1016/j.eururo.2015.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torgerson DJ, Raftery J. Discounting. BMJ. 1999;319:914–5. doi: 10.1136/bmj.319.7214.914. [DOI] [PMC free article] [PubMed] [Google Scholar]


