Abstract
Purpose
Alcohol-use disorders (AUDs) have been associated with increased sepsis-related mortality. As patients with AUDs are often thiamine deficient, we investigated practice patterns relating to thiamine administration in patients with AUDs presenting with septic shock and explored the association between receipt of thiamine and mortality.
Materials
We performed a retrospective cohort study of patients presenting with septic shock between 2008 and 2014 at a single tertiary care center. We identified patients with an AUD diagnosis, orders for microbial cultures and use of antibiotics, vasopressor dependency, and lactate levels ≥ 4 mmol/L. We excluded those who received thiamine later than 48 hours of sepsis onset.
Results
We included 53 patients. Thirty-four (64%) patients received thiamine. Five patients (15%) received their first thiamine dose in the emergency department. The median time to thiamine administration was 9 (quartiles: 4, 18) hours. The first thiamine dose was most often given parenterally (68%) and for 100 mg (88%). In those receiving thiamine, 15/34 (44%) died, compared to 15/19 (79%) of those not receiving thiamine, p = 0.02.
Conclusions
A considerable proportion of patients with AUDs admitted for septic shock do not receive thiamine. Thiamine administration in this patient population was associated with decreased mortality.
Keywords: Alcohol use disorder, EtOH, Sepsis, Septic shock, Thiamine, Vitamin B1
INTRODUCTION
Alcohol use disorders (AUDs) and septic shock are both significant contributors to morbidity and mortality worldwide.[1, 2] Previous studies have shown AUDs to be associated with an increased incidence of sepsis and sepsis-related mortality.[3–5]
Thiamine (vitamin B1) is an essential co-factor for pyruvate dehydrogenase (PDH) and alpha-ketoglutarate, without which glucose cannot enter the Krebs-Cycle.[6, 7] Inadequate PDH function halts mitochondrial aerobic metabolism, forcing energy production to proceed via anaerobic metabolism. This metabolic derangement may lead to refractory acidosis, cardiovascular collapse, and potentially death.[8–11]
Several studies have found thiamine deficiency to be prevalent in both critically ill patients and patients with AUDs.[12, 13] A recent prospective randomized trial from our group illustrated that many septic shock patients without AUDs are thiamine deficient and that thiamine administration may be able to reduce mortality in this deficient population.[13] Septic shock is known to cause PDH inhibition resulting in impaired cellular oxygen utilization, lactate elevation, and acidosis, similar to what is seen for thiamine deficiency alone.[14, 15] While guidelines surrounding thiamine administration exist to prevent devastating neurologic sequelae in patients with AUDs (i.e. the Wernicke-Korsakoff syndrome), thiamine deficiency in critically ill patients with AUDs may go unrecognized.[16, 17]
The objectives of the current study were to (1) investigate practice patterns at our institution as they relate to thiamine administration in patients with AUDs presenting with septic shock, and (2) determine if those not receiving thiamine have increased mortality compared to those receiving thiamine.
MATERIALS AND METHODS
Study design and data source
This was a retrospective cohort study of septic shock patients admitted to the intensive care unit (ICU) at Beth Israel Deaconess Medical Center (BIDMC), an urban tertiary care center in Boston, Massachusetts, USA, between January 2008 and December 2014. Patient data was extracted from archived local electronic medical records using Microsoft SQL, a structural querying language used to host and manage data. Reasons for death were determined via individual chart review based on prior work.[18] The study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center.
Study population
We included adult patients (≥ 18 years at time of admission) with septic shock and an ICD-9 code (see Table S1 in the Supplement) for AUDs or AUD-attributable conditions as defined by the Centers for Disease Control and Prevention.[19] Septic shock was defined as vasopressor dependence (defined as any continuous intravenous infusion [i.e. not a bolus] of norepinephrine, phenylephrine, dopamine, vasopressin, and/or epinephrine), a lactate above 4 mmol/L within 4 hours of vasopressor use, and a suspected infection based on documented initiation of antibiotics and orders for any microbial culture within 24 hours of hospital admission.[20] We included only patients admitted to the intensive care units. Although the Sepsis III definitions of septic shock include a lactate of ≥ 2 mmol/L[20], a lactate threshold of 4 mmol/L was chosen a priori to enrich the population with patients most likely to exhibit thiamine deficiency.[12] We excluded non-index events and patients with seizures during their hospital stay based on ICD-9 codes[21, 22], since seizures are a cause of elevated lactate and common in alcoholics.[23] Moreover, we excluded patients who received thiamine prior to the development of septic shock determined by review of Emergency Department (ED) drug administration records, and those who received thiamine ≥ 48 hours of sepsis onset to limit the extended potential exposure time for patients who survive the initial septic insult and subsequently have long hospital stays. Hospital-free days and ICU-free days were defined as days alive and not requiring hospitalization or intensive care until day 28, respectively. Patients who expired before day 28 were assigned a score of zero days.
Statistical methods
Descriptive statistics were used to characterize the study population and to calculate the proportion of septic shock patient with AUDs who received thiamine. Categorical variables are presented as counts with frequencies, and continuous variables as medians with 1st and 3rd quartiles. Continuous data were compared between the groups using Wilcoxon rank sum test and categorical data were compared between groups using Fisher’s exact test. All analyses were two-sided with a significance level of p < 0.05. Statistical analyses were conducted using STATA, version 14 (College Station, TX, StataCorp LP, USA).
RESULTS
Out of 1,380 patients with a suspected infection and AUD diagnosis, 53 patients met all inclusion and no exclusion criteria and were included in the final cohort (Figure 1). The median age was 57 (quartiles: 46, 67) years, and 64% were male. Patient characteristics were similar between groups (Table 1), except for a significant difference in platelets (p = 0.04).
Figure 1. Flow diagram for inclusion and exclusion criteria.
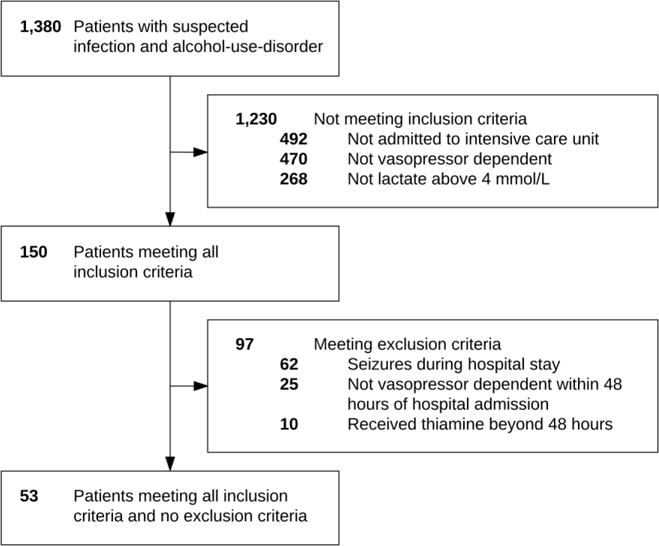
Out of 1,380 patients with a suspected infection, 53 patients met all inclusion and no exclusion criteria.
Table 1.
Patient characteristicsa
| Thiamine n = 34 |
No Thiamine n = 19 |
P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 57 (49, 64) | 62 (44, 68) | 0.74 |
| Gender (female) | 11 (32) | 8 (42) | 0.56 |
| Race (white) | 23 (68) | 10 (53) | 0.47 |
| Body mass index (kg/m2) | 28 (22, 33) | 25 (24, 26) | 0.19 |
| Alcohol use disordersb | |||
| Alcohol dependence syndrome | 26 (76) | 13 (68) | 0.53 |
| Alcoholic liver disease | 22 (65) | 11 (58) | 0.77 |
| Alcohol-induced mental disorders | 5 (15) | 0 (0) | 0.15 |
| Alcoholic cardiomyopathy | 0 (0) | 1 (5) | 0.36 |
| Co-morbidities | |||
| Heart failure | 5 (15) | 2 (11) | 1.00 |
| Hypertension | 18 (53) | 8 (42) | 0.57 |
| Diabetes | 8 (24) | 5 (26) | 1.00 |
| Renal disease | 3 (9) | 3 (16) | 0.65 |
| Liver disease | 23 (68) | 11 (58) | 0.56 |
| COPD | 7 (21) | 2 (11) | 0.46 |
| Laboratory values | |||
| Hemoglobin (g/dL) | 11.0 (10.2, 13.5) | 10.7 (8.1, 13.8) | 0.83 |
| White blood cells (103) | 10.8 (5.2, 14.0) | 11.7 (9.0, 17.0) | 0.17 |
| Bicarbonate (mEq/L) | 15 (11, 19) | 16 (12, 21) | 0.43 |
| Lactate (mmol/L) | 7.1 (5.2, 10.2) | 7.9 (5.5, 10.5) | 0.67 |
| Platelets (103) | 92 (53, 159) | 178 (80, 280) | 0.04 |
| Creatinine (mg/dL) | 2.0 (1.1, 3.1) | 1.6 (1.3, 3.5) | 0.60 |
| Glucose (mg/dL) | 119 (83, 170) | 133 (103, 202) | 0.37 |
| Potassium (mEq/L) | 4.2 (3.7, 4.7) | 4.4 (3.8, 4.9) | 0.56 |
| AST (IU/L) | 153 (34, 611) | 131 (39, 206) | 0.84 |
| ALT (IU/L) | 49 (20, 174) | 64 (34, 91) | 0.59 |
| Bilirubin (mg/dL) | 4.2 (1.0, 8.7) | 1.9 (0.7, 5.4) | 0.50 |
| Interventions/Vital signs | |||
| Intubated | 27 (79) | 18 (95) | 0.23 |
| SOFA score | 11 (8, 16) | 12 (10, 13) | 0.54 |
Categorical variables are presented as count (frequency) and continuous variables as median (quartiles). Abbreviations: COPD, chronic obstructive pulmonary disease; AST, aspartate transaminase; ALT, alanine transaminase; SOFA, sepsis-related organ failure assessment.
Patients may have more than one diagnosis of alcohol use disorder (see Table S1 in the Supplement).
Thirty-four (64%) patients received thiamine. In those receiving thiamine, the median time from hospital admission to first thiamine administration was 9 (quartiles: 4, 18) hours. Twenty-nine patients (85%) received their first thiamine dose in the ICU, and five patients (15%) in the ED. Six (18%) patients only received a single dose of thiamine. The median number of thiamine administrations per hospital stay was 4 (quartiles: 3, 8). The most commonly prescribed dose of thiamine was 100 mg accounting for 88% of the initial doses and 97% of the total doses. Parenteral thiamine accounted for 68% of the initial doses and 33% of the total doses administered, while oral thiamine accounted for the remaining doses.
The overall mortality was 30/53 (57%). Refractory shock was the overall most common (37%) reason for death. We found a significantly lower mortality for those receiving thiamine compared to those not receiving thiamine (15 [44%] vs. 15 [79%], p = 0.02, Table 2). The median time to death was 64 [IQR: 26 – 148] hours in the non-thiamine group, and 68 [quartiles: 40, 143] hours in the thiamine group. We found no statistically significant difference between the thiamine and non-thiamine group for hospital-free days (12 [0, 19] vs. 18 [13, 19] days, p = 0.36, Table 2) and ICU-free days (21 [17, 24] vs. 21 [20, 24] days, p = 0.71, Table 2) during the first 28 days after hospital admission among those who survived to hospital discharge. Patient characteristics of survivors and non-survivors are provided in Table S2 in the Supplement.
Table 2.
Clinical outcomes for the thiamine and non-thiamine group
| Thiamine | No thiamine | P value | |
|---|---|---|---|
| Full cohort | n = 34 | n = 19 | |
| Mortalitya | 15 (44) | 15 (79) | .02 |
|
| |||
| Survivors | n = 19 | n = 4 | |
| Hospital-free daysb | 12 (0, 19) | 18 (13, 19) | .36 |
| Intensive care unit-free daysb,c | 21 (17, 24) | 21 (20,24) | .71 |
Presented as count (frequency)
Presented as median (quartiles). Hospital-free days and intensive care unit-free days were defined as days alive and not requiring hospitalization or intensive care until day 28, respectively.
All non-survivors died prior to ICU discharge
DISCUSSION
In this retrospective observational pilot study, we found that thiamine was administered in 64% of patients with an AUD and septic shock, and that those who received thiamine had lower mortality compared to those who did not receive thiamine. Among patients who receive thiamine, the median time to first administration was 9 hours and just 15% received thiamine in the ED.
To our knowledge, this is the first descriptive study of thiamine administration in patients with AUDs and septic shock. Chronic alcohol consumption combined with critical illness has previously been shown to be associated with worse outcomes, including an increase in the risk for sepsis, multiple organ dysfunction, and acute respiratory distress syndrome.[3] In 2007, O’Brien et al. studied 11,651 patients admitted to the ICU and found alcohol dependence to be associated with higher rates of sepsis (12.9% vs. 7.6%, p < 0.001) and sepsis-related mortality (9.4% vs. 7.5%, p = 0.02).[3]
In the current study, we found a significant difference in mortality between those receiving thiamine compared to those not receiving thiamine. In a recent prospective randomized trial, our group found that a sub-group of septic patients with thiamine deficiency had lower mortality over time when randomized to thiamine as compared to placebo.[13] In the population of septic shock patients meeting the inclusion/exclusion for that trial, 35% were thiamine deficient despite the intentional exclusion of those with AUDs. Even though thiamine has minimal harmful effects, low cost, and known neurologic benefits, our data suggests that thiamine administration to AUD patients may be overlooked when patients present with septic shock.
The high number of patients at our facility who did not receive thiamine was somewhat unexpected given that our group has advocated for thiamine and conducted extensive research in this area for over a decade.[12, 13] This selection bias could have skewed our results toward higher thiamine administration rates than other institutions. Guidelines in both Europe[16] and the United States[24] recommend treatment with thiamine to prevent neurologic complications in patients with AUDs. However, there are no clear consensus surrounding the dosage, timing, or route of administration, and there are no standardized guidelines for management of alcohol withdrawal in critically ill patients. We speculate that the failure to administer thiamine in critically ill patients may be related to a shift in clinician focus toward the septic insult and away from the AUD history.
Our data on administration frequency, dosage amount, and administration route are largely consistent with that of research in patients with evident alcohol abuse[25], only our study differed in that the patient population was critically ill and had high mortality. European guidelines suggest provision of 200 mg or 500 mg parenteral thiamine three times daily, however the optimal dosage of thiamine remains unknown and the 100 mg dosage is traditional in the United States.[16, 26] Given that patients in our study had septic shock and were on vasopressors (which may alter intestinal uptake), the use of oral thiamine in 32% of cases is notable.
Our findings should be interpreted in the context of the study limitations. First, a potential problem when assessing mortality in observational studies is that of immortal time bias, which states that patients with early death may exaggerate the mortality benefit of the treatment since they must survive long enough to receive or not receive treatment.[27] We have attempted to partly mitigate this effect by excluding patients who received thiamine later than 48 hours from admission. Further adjustment for this potential bias was not possible given the small sample size. Second, while the groups appeared to have similar levels of illness severity based on data in Table 1, the possibility remains that there were differences in illness severity between groups – given the small sample size and no apparent difference between groups, we did not control for severity of illness. Third, there is a possibility that some eligible patients may have been missed due to reliance on ICD-9 codes to identify patients with AUDs. Fourth, our data is derived from a single center, which limits generalizability to other settings. Finally, the effect of thiamine on all-cause mortality may have been biased by unmeasured and measured factors. Due to above limitation, the retrospective design, and the small sample size from a single institution our findings should be interpreted with caution and be considered exploratory.
CONCLUSION
In conclusion, we found that a considerable proportion of patients at our institution with AUDs admitted for septic shock do not receive thiamine and the strong majority do not receive any in the ED setting. Further, failure to receive thiamine was associated with increased mortality in this population, though the sample size prohibited adjustment for potential confounders.
Supplementary Material
Highlights.
Many patients with alcohol-use-disorders and septic shock do not receive thiamine
Thiamine was most often given in the ICU, rather than the emergency department
Failure to receive thiamine may be associated with increased mortality
Acknowledgments
The authors would like to thank Francesca Montillo M.M. and Lisa O’Rourke for assisting with preparation of the manuscript.
SOURCES OF FUNDING
Dr. Donnino is supported by the American Heart Association (14GRNT2001002) and the National Institute of Health (1K24HL127101). Dr. Moskowitz is supported by the National Institute of Health (2T32HL007374-37). None of the funding sources were involved in the design of the study, data collection, analysis, writing or submission of this paper.
ABBREVIATIONS
- AUD
alcohol-use disorder
- ICU
intensive care unit
- PDH
pyruvate dehydrogenase
- BIDMC
Beth Israel Deaconess Medical Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien JM, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35(2):345–50. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 4.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31(3):869–77. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 5.McPeake JM, Shaw M, O’Neill A, Forrest E, Puxty A, Quasim T, et al. Do alcohol use disorders impact on long term outcomes from intensive care? Crit Care. 2015;19:185. doi: 10.1186/s13054-015-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34(Pt 2):217–22. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 7.Linn TC, Pettit FH, Reed LJ. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969;62(1):234–41. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oriot D, Wood C, Gottesman R, Huault G. Severe lactic acidosis related to acute thiamine deficiency. JPEN J Parenter Enteral Nutr. 1991;15(1):105–9. doi: 10.1177/0148607191015001105. [DOI] [PubMed] [Google Scholar]
- 9.Blanc P, Henriette K, Boussuges A. Severe metabolic acidosis and heart failure due to thiamine deficiency. Nutrition. 2002;18(1):118. doi: 10.1016/s0899-9007(01)00731-6. [DOI] [PubMed] [Google Scholar]
- 10.Donnino M. Gastrointestinal beriberi: a previously unrecognized syndrome. Ann Intern Med. 2004;141(11):898–9. doi: 10.7326/0003-4819-141-11-200412070-00035. [DOI] [PubMed] [Google Scholar]
- 11.Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312(16):1035–9. doi: 10.1056/NEJM198504183121606. [DOI] [PubMed] [Google Scholar]
- 12.Donnino MW, Carney E, Cocchi MN, Barbash I, Chase M, Joyce N, et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care. 2010;25(4):576–81. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Donnino MW, Andersen LW, Chase M, Berg KM, Tidswell M, Giberson T, et al. Randomized, Double-Blind, Placebo-Controlled Trial of Thiamine as a Metabolic Resuscitator in Septic Shock: A Pilot Study. Crit Care Med. 2016;44(2):360–7. doi: 10.1097/CCM.0000000000001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuzzo E, Berg KM, Andersen LW, Balkema J, Montissol S, Cocchi MN, et al. Pyruvate Dehydrogenase Activity Is Decreased in the Peripheral Blood Mononuclear Cells of Patients with Sepsis. A Prospective Observational Trial. Ann Am Thorac Soc. 2015;12(11):1662–6. doi: 10.1513/AnnalsATS.201505-267BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacalone M, Martinelli R, Abramo A, Rubino A, Pavoni V, Iacconi P, et al. Rapid reversal of severe lactic acidosis after thiamine administration in critically ill adults: a report of 3 cases. Nutr Clin Pract. 2015;30(1):104–10. doi: 10.1177/0884533614561790. [DOI] [PubMed] [Google Scholar]
- 16.Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408–18. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]
- 17.Thomson AD, Cook CC, Touquet R, Henry JA, Royal College of Physicians Ln The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002;37(6):513–21. doi: 10.1093/alcalc/37.6.513. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz A, Omar Y, Chase M, Lokhandwala S, Patel P, Andersen LW, et al. Reasons for death in patients with sepsis and septic shock. J Crit Care. 2016;38:284–8. doi: 10.1016/j.jcrc.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Alcohol Related Disease Impact (ARDI) Available: https://nccd.cdc.gov/DPH_ARDI/Info/ICDCodes.aspx [Accessed Feb 28 2017]
- 20.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaitatzis A, Carroll K, Majeed A, Sander JW. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613–22. doi: 10.1111/j.0013-9580.2004.17504.x. [DOI] [PubMed] [Google Scholar]
- 22.Jetté N, Reid AY, Quan H, Hill MD, Wiebe S. How accurate is ICD coding for epilepsy? Epilepsia. 2010;51(1):62–9. doi: 10.1111/j.1528-1167.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 23.Lipka K, Bülow HH. Lactic acidosis following convulsions. Acta Anaesthesiol Scand. 2003;47(5):616–8. doi: 10.1034/j.1399-6576.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- 24.Practice guideline for the treatment of patients with substance use disorders: alcohol, cocaine, opioids. American Psychiatric Association. Am J Psychiatry. 1995;152(11 Suppl):1–59. doi: 10.1176/ajp.152.11.1. [DOI] [PubMed] [Google Scholar]
- 25.Isenberg-Grzeda E, Chabon B, Nicolson SE. Prescribing thiamine to inpatients with alcohol use disorders: how well are we doing? J Addict Med. 2014;8(1):1–5. doi: 10.1097/01.ADM.0000435320.72857.c8. [DOI] [PubMed] [Google Scholar]
- 26.Donnino MW, Vega J, Miller J, Walsh M. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6):715–21. doi: 10.1016/j.annemergmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–9. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


