Abstract
Abnormal placental function is well-established as a major cause for poor pregnancy outcome. Placental blood flow within the maternal uteroplacental compartment, the fetoplacental circulation, or both, is a vital factor in mediating placental function. Impairment in flow in either or both vasculatures is a significant risk factor for adverse pregnancy outcome, potentially impacting maternal well-being, affecting immediate neonatal health, and even influencing the long-term health of the infant.
Much remains unknown regarding the mechanistic underpinnings of proper placental blood flow. This review highlights the currently recognized molecular and cellular mechanisms in the development of normal uteroplacental and fetoplacental blood flow. Utilizing the entities of preeclampsia (PE) and fetal growth restriction (FGR) as clinical phenotypes that are often evident downstream of abnormal placental blood flow, mechanisms underlying impaired uteroplacental and fetoplacental blood flow are also discussed. Deficiencies in knowledge, which limit the efficacy of clinical care, are also highlighted, underscoring the need for continued research on normal and abnormal placental blood flow.
Keywords: Placental angiogenesis, fetoplacental, uteroplacental, preeclampsia, fetal growth restriction
INTRODUCTION
Placental blood flow is a critical component of pregnancy outcome. Human placental blood flow is comprised of two separate compartments – the maternally-derived uteroplacental circulation and the fetoplacental vasculature. This hemochorial villous placenta allows for maternal blood to directly bathe syncytiotrophoblast (STB), which becomes progressively apposed to the fetoplacental endothelium as gestation progresses, allowing for nutrient/waste and gas exchange to occur. While trophoblast, stromal, and endothelial function and interaction are also vital factors influencing pregnancy outcome, abnormal uteroplacental and fetoplacental blood flow, both individually and in combination, are strongly associated with adverse perinatal outcome, posing risks to maternal well-being and to immediate and long-term health of the infant.
CLINICAL IMPLICATIONS OF ABNORMAL PLACENTAL BLOOD FLOW
Abnormal placental blood flow, either on the maternal side, fetal side, or both, has been implicated in various complications of pregnancy such as preeclampsia (PE) and fetal growth restriction (FGR). For instance, one key component to the pathogenesis of PE and perhaps placentally-mediated FGR, as well, is thought to be defective trophoblast invasion into the maternal spiral arteries (Kaufmann, et al. 2003; Zhou, et al. 1993; Zhou, et al. 1997a). This results in abnormally narrow and tortuous uterine vessels that result in placental hypoperfusion and uteroplacental ischemia.
Within the fetal compartment, placental vascular resistance should normally be low, allowing for forward flow through the umbilical arteries during both fetal cardiac systole and diastole (Giles, et al. 1985; Trudinger, et al. 1985c). In high-risk pregnancies that can be seen in PE and/or FGR, placental findings that lead to abnormally elevated placental vascular resistance include vasoconstricted stem villous vessels and impaired fetoplacental vascular angiogenesis can be seen in PE and/or FGR (Kingdom, et al. 1997; Salafia, et al. 1995; Salafia, et al. 1997; Su, et al. 2015). This is seen clinically through abnormal umbilical artery Doppler velocimetric indices, where diastolic forward flow is initially impaired and can eventually becoming absent or reversed (Giles et al. 1985). When severe enough, the fetus is at high risk for prolonged exposure to in utero hypoxemia/acidemia or stillbirth (Alfirevic, et al. 2017; Baschat and Weiner 2000).
Clinically, the “treatment” for both entities is delivery. With PE, it is removal of the placenta after delivery that cures the maternal manifestations of the disease. With isolated FGR, delivery is the only method by which to prevent prolonged exposure to an abnormal in utero environment and stillbirth (Group 2003; Lees, et al. 2015). The problem, though, is that in severe cases, delivery oftentimes needs to occur at very preterm gestational ages with the attendant consequences of prematurity such as blindness, deafness, mental retardation, cerebral palsy, and chronic medical problems (Group 2003; Thornton, et al. 2004). Furthermore, even if these infants are fortunate enough to evade the potentially severe complications of the perinatal period, they are at increased risk for developing obesity, cardiovascular disease, and metabolic syndrome later in life (Barker and Thornburg 2013). Thus, a better understanding of the mechanisms underlying normal and abnormal placental blood flow in both the maternal and fetoplacental compartments is vital if clinical outcomes are truly to be improved.
DEVELOPMENT OF UTEROPLACENTAL BLOOD FLOW
Uteroplacental blood flow develops soon after implantation. By post-fertilization day 6, the blastocyst has attached to the endometrial surface, with partial embedding and contact with the endometrial stroma by day 8 (Sadler 1995; Schlafke and Enders 1975). Lacunae, spaces within the early syncytiotrophoblastic layer of the chorion, begin to appear within the syncytium, and these fuse to maternal sinusoids by day 12 after fertilization (Enders 1989). Once maternal blood flows through this compartment, the rudimentary uteroplacental circulation is established. The fusion of lacunae to maternal sinusoids eventually forms the intervillous space of the placenta, and ultimately, the spiral arteries of the uterus directly communicate with this intervillous space, resulting in uteroplacental blood flow that is clinically evident during pregnancy.
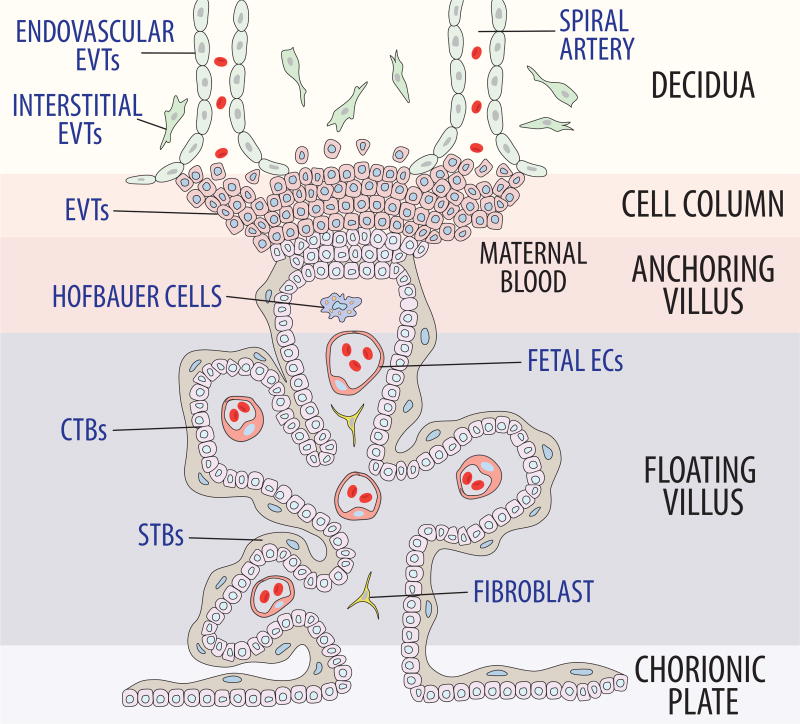
Approximately two to three weeks after fertilization, interstitial trophoblasts, one type of extravillous trophoblasts (EVTs), further migrate through the endometrial stroma, penetrate the decidua and adjacent myometrium, and gather around spiral arteries. This is thought to prepare the spiral artery for endovascular trophoblast invasion (Pijnenborg, et al. 1980). Endovascular trophoblasts, the other type of EVTs, then invade and migrate down the lumens of the spiral arteries (Figure 1) (Fisher 2015; Red-Horse, et al. 2005; Zhou et al. 1997a). They initially form cell plugs within the terminal portions of the spiral arteries, which results in destruction of maternal vascular endothelium via apoptotic mechanisms (Harris, et al. 2006; Kaufmann et al. 2003). Vascular smooth muscle cells and elastic fibers of the vascular media are replaced with fibrinoid, aiding transformation into a low-resistance circuit (Brosens, et al. 1967; Harris 2010). Simultaneously, these plugs are dissolved, and the functional maternal circulatory component of the placenta is established.
Figure 1. Formation of the uteroplacental circulation.
EVTs migrate from the cytotrophoblastic shell into the endometrium. Interestitial EVTs prepare the spiral artery for endovascular EVTs. Endovascular EVTs then invade and migrate down the lumens of spiral arteries as they approach the decidua. The cell plugs that are initially formed destroy maternal vascular endothelium via apoptosis and replace vascular smooth muscle cells and elastic fibers from the media with fibrinoid. After dissolution of these plugs, the maternal circulatory component of the placenta is established.
From a molecular perspective, multiple coordinated events that have yet to be completely elucidated are occurring. For successful invasion to occur, trophoblastic phenotype must change from an epithelial-to-endothelial composition. For instance, during normal pregnancy, down-regulation of epithelial-like adhesion molecules such as α6β4 integrin and concomitant up-regulation of adhesion molecules more typical of endothelial cells, including α5β1 and α1β1 occurs in cytotrophoblasts (Damsky, et al. 1992; Damsky, et al. 1994; Zhou, et al. 1997b). Invasive EVTs also produce various proteases, such as matrix metalloproteinases (MMP) to degrade the extracellular matrix (ECM), but excessive trophoblast invasion is also being limited by EVT production of protease inhibitors, including tissue inhibitors of metalloproteinases (TIMPs) and plasminogen activator inhibitors (PAIs) (Damsky et al. 1992; Librach, et al. 1991; Shimonovitz, et al. 1994). Together, these underlying mechanisms allow endovascular EVTs to migrate through the endothelium, where maternal uterine artery endothelial and vascular smooth muscle cells then undergo apoptosis and are replaced by these trophoblasts, which have assumed an endothelial-like phenotype (Harris et al. 2006; Kam, et al. 1999; Pijnenborg, et al. 1983). Ultimately, these normal processes allow for development of a low-resistance maternal vascular compartment with maternal blood flowing from maternal spiral arteries into the intervillous space by about 10–12 weeks of pregnancy (Hustin and Schaaps 1987; Jaffe, et al. 1997; Rodesch, et al. 1992).
EXPANSION OF THE FETOPLACENTAL CIRCULATION
With regard to the fetoplacental circulation, development of the placental villi starts around day 13 after conception. Mesodermal cells within the villus core initiate the process of vasculogenesis by differentiating into small blood vessels starting approximately 21 days post-fertilization (Castellucci and Kaufmann 1982; Demir, et al. 1989; Sadler 1995). These capillaries within the villus fuse with capillaries developing within the mesoderm of the chorionic plate and connecting stalk, and ultimately they establish contact with the intraembryonic circulation, forming the fetoplacental circulation (Castellucci, et al. 1990; Sadler 1995).
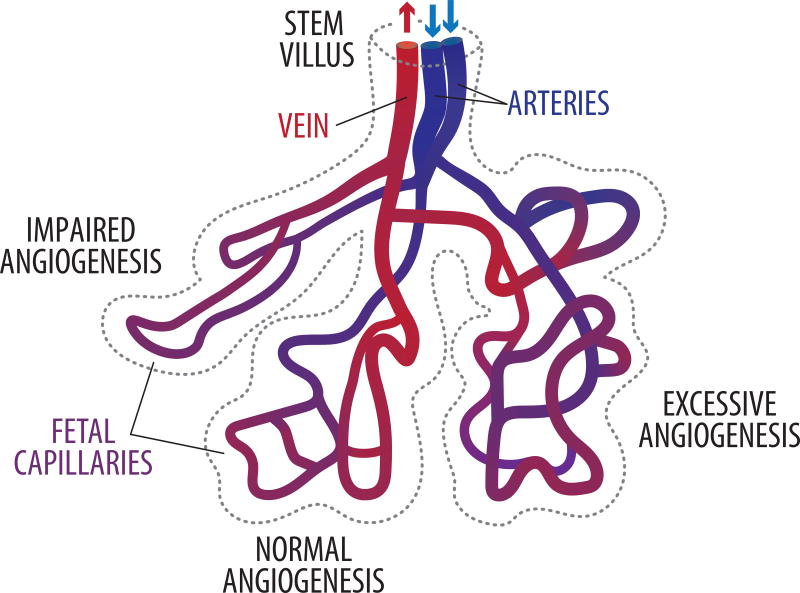
The fetoplacental circulation then continues to expand via a gradual transition from vasculogenesis to angiogenesis between 32 days post-conception to approximately 9 weeks after fertilization (Castellucci et al. 1990; Kaufmann, et al. 1985; van Oppenraaij, et al. 2009). Thereafter, angiogenesis continues throughout gestation but exponentially accelerates beginning at approximately 25 weeks’ gestation (23 weeks post-fertilization), resulting in a vascular bed that is approximately 550 km in length and 15 m2 in surface area (Figure 2) (Burton and Jauniaux 1995; Mayhew 2002). Physiologically, this corresponds to decreasing fetoplacental vascular resistance as demonstrated by increasing end-diastolic velocities within the umbilical artery as gestation progresses (Guiot, et al. 1992; Thompson and Trudinger 1990; Todros, et al. 1992). Much remains unknown regarding the mechanistic underpinnings of normal human fetoplacental vascular development, but various growth factors have been shown to be key players in both vasculogenesis and angiogenesis.
Figure 2. Fetoplacental angiogenesis.
Throughout the second half of pregnancy, fetoplacental angiogenesis continues, resulting in villous capillary beds that are appropriately branched (normal angiogenesis). This allows for a structurally-mediated decrease in fetoplacental vascular resistance. In pregnancies subjected to hypoxia such as high altitude or severe maternal anemia, excessive angiogenesis occurs, although placental vascular resistance is not adversely affected in these scenarios. When severe FGR and/or PE are present, villous capillary angiogenesis is impaired, resulting in thin, elongated vessels that anatomically contribute to abnormally elevated fetoplacental vascular resistance.
Vascular endothelial growth factor (VEGF) family
One key group of factors is the vascular endothelial growth factor (VEGF) family, and most notably for placental angiogenesis, VEGFA. The critical importance of VEGF and its two main receptors – (1) Vascular endothelial growth factor receptor 1, also known as FMS-like tyrosine kinase 1 (FLT1) and (2) Vascular endothelial growth factor receptor 2 or kinase insert domain (KDR) – is demonstrated by the finding of embryonic lethality due to abnormal blood vessel formation during embryogenesis in: (1) Vegf knock-out mice, (2) Flt1 knock-out mice, and (3) Kdr knock-out mice (Carmeliet, et al. 1996; Fong, et al. 1995; Shalaby, et al. 1995). Even mice lacking a single Vegf allele demonstrate abnormal blood vessel development and embryonic lethality (Carmeliet et al. 1996). Within humans, VEGFA is highly expressed in cytotrophoblast cells early in pregnancy, whereas FLK1 and KDR are primarily expressed within the hemangiogenic cords, with a predominance of KDR (Demir, et al. 2004). This suggests that paracrine signaling may help to drive fetoplacental vasculogenesis. As gestation progresses, VEGFA expression decreases within trophoblast while increasing within fetoplacental endothelial, mesenchymal, and Hofbauer cells (Demir et al. 2004).
Based upon animal studies, Vegfa binding to Flt1 and/or Kdr within endothelial cells triggers various signal transduction cascades that result in endothelial proliferation and migration. For instance, in ovine fetoplacental artery endothelial cells, VEGFA induces activation of several signal transduction cascades including the PI3-kinase (PI3K)/AKT1 pathway (Liao, et al. 2010; Zheng, et al. 2008). PI3K inhibition results in suppressed nitric oxide (NO) production and completely blocks VEGFA-mediated cell proliferation and migration (Zheng et al. 2008). In Akt1 knock-out mice, there is also a marked reduction in endothelial nitric oxide synthase (NOS3) phosphorylation (Lee, et al. 2014). In vivo findings also demonstrate significance of the PI3K/AKT1 pathway. For example, in endothelial-specific, postnatal deletion of Akt1, retinal angiogenesis is impaired with delayed radial outgrowth and reduced endothelial coverage (Lee et al. 2014). Furthermore, the placentas of Akt1-null mice show decreased placental vascularization, which is thought to contribute to both growth restriction and neonatal mortality in the pups (Chen, et al. 2001; Yang, et al. 2003).
Placental growth factor (PGF) is also a member of the VEGF family and is expressed in trophoblast. Unlike VEGFA isoforms, however, it is only able to bind to FLT1 and not KDR. Initially considered a simple competitive inhibitor of VEGFA effects through FLT1, it is also capable of stimulating the growth of endothelial cells in vitro, promoting proliferation, survival, and migration (Maglione, et al. 1991). This was thought to occur by increasing VEGFA availability to bind to KDR (Park, et al. 1994). More contemporary data, especially within the cancer literature, suggests that PGF also has direct angiogenic effects, with PGF homodimers and PGF/VEGFA heterodimers being pro-angiogenic both in vitro and in vivo (Autiero, et al. 2003a; Autiero, et al. 2003b; Fischer, et al. 2007; Yang, et al. 2013). However, other studies suggest that PGF, especially when elevated, can decrease angiogenic activity within tumors (Eriksson, et al. 2002; Xu, et al. 2006).
Fibroblast growth factors
The family of fibroblast growth factors (FGFs) are known to regulate several cellular processes, including proliferation, differentiation, migration, and survival (Beenken and Mohammadi 2009). Specific to the human placenta, FGF2 is currently considered the main FGF. Its expression has been localized to the cytotrophoblast in the first trimester and primarily to the fetoplacental endothelium at term, with low expression in STBs (Arany and Hill 1998; Ferriani, et al. 1994; Shams and Ahmed 1994). FGF10 has also been described in the placenta, but its role appears to primarily mediate chorionic villous growth (Natanson-Yaron, et al. 2007). FGF activity is mediated by a family of FGF receptors (FGFR1–4) that are members of the receptor tyrosine kinases family. While the human placenta has been shown to express all the FGFRs, FGFR1 appears to be the only FGFR expressed within the fetoplacental vasculature to-date (Anteby, et al. 2005; Arany and Hill 1998).
The majority of knowledge surrounding the role of FGFs and placental angiogenesis is derived from animal studies. Ovine fetoplacental arterial endothelial cells, which also express FGF2 in vivo and in vitro, have been demonstrated to undergo activation of ERK1/2 and PI3K/AKT1 pathways via phosphorylation of FGFR1 (Feng, et al. 2012). In contrast to VEGFA stimulation of these endothelial cells, inhibition of either signaling pathway only partially inhibits FGF2-stimulated proliferation (Zheng et al. 2008). Interestingly, FGFR1 is confined within plasma membrane caveolae, with the regulatory protein caveolin-1 interacting with FGFR1 (Feng et al. 2012). This allows FGF2/FGFR1-mediated activation of both MAPK and PI3K/AKT1 cascades, which in turn, enhances various measures of angiogenesis including endothelial cell migration and tube formation (Feng et al. 2012).
Angiopoietins
Angiopoietins are a group of four angiogenic growth factors (ANGPT1, ANGPT2, ANGPT3, ANGPT4) that regulate angiogenesis via binding to the TEK receptor tyrosine kinase (TEK, also known as TIE2). ANGPT1, ANGPT2, and TEK are expressed within the human placenta (De, et al. 2016; Dunk, et al. 2000; Geva, et al. 2002; Goldman-Wohl, et al. 2000; Seval, et al. 2008; Zhang, et al. 2001). There is some discrepancy between studies regarding the exact localization of expression of ANGPT1, ANGPT2, and TEK during the course of gestation. However, there is consensus that TEK is expressed within endothelium of placental blood vessels, and the majority of studies suggest that the ANGPT1:ANGPT2 ratio increases as gestation progresses (Dunk et al. 2000; Zhang et al. 2001). More recently, data suggest that there are intraplacental variations, with ANGPT1 and TEK expression being higher in the periphery of the placenta and higher expression of ANGPT2 within the central regions of the placenta (De et al. 2016). Taken in the context where ANGPT2 activation of TEK results in destabilization of the vessels, allowing them to remain plastic and capable of responding to other angiogenic factors such as VEGFA, this suggests that in early pregnancy and within the more central portion of the villous vascular tree, high levels of ANGPT2 allow for progression of angiogenesis. In contrast, as gestation progresses or as vessels grow outward to the periphery of the placenta, increases in ANGPT1-mediated phosphorylation of TEK allows for these newly formed vessels to be stabilized via EC survival and EC-EC interactions (Dunk et al. 2000; Geva et al. 2002; Zhang et al. 2001).
MECHANISMS UNDERLYING ABNORMAL PLACENTAL BLOOD FLOW
Clinically, abnormal placental blood flow can be detected in the maternal uteroplacental compartment, the fetoplacental vasculature, or both. Aberrant uterine artery and/or umbilical artery Doppler velocimetry are suggestive of placental insufficiency and are individually associated with an increased risk for adverse pregnancy outcome (Alfirevic, et al. 2013; Garcia, et al. 2016). Based upon Doppler studies, impairment of flow in either vascular compartment can occur in isolation or concurrently (Brosens, et al. 1977; Trudinger, et al. 1985a, b; Trudinger et al. 1985c). Ovine studies, however, suggest more interdependence between the maternal and fetoplacental circulations. With decreases in uterine perfusion, there is a corresponding reduction in fetoplacental blood flow (Stock, et al. 1980). Similarly, the umbilical circulation has also been shown to locally regulate uterine blood flow (Rankin, et al. 1975). Nevertheless, based upon clinical studies and for the purpose of clarity in this review, the two vascular compartments are discussed separately.
The maternal uteroplacental vasculature
Abnormalities of maternal uteroplacental blood flow often manifest clinically as PE with and without FGR. It is now well-established that defects in both EVT invasion and spiral artery remodeling that are characteristic of hypertensive disorders in pregnancy and FGR lead to abnormal placentation (Kaufmann et al. 2003; Pijnenborg, et al. 2006). In turn, the placenta becomes ischemic, which is then thought to result in release of soluble factors that cause maternal systemic endothelial dysfunction, ultimately leading to the phenotype of PE (Kaufmann et al. 2003; Pijnenborg et al. 2006).
Deficient EVT invasion and impaired spiral artery remodeling
Although likely not the only culprit, much of the impairment in maternal uteroplacental blood flow is initially triggered by compromised EVT invasion of the uterine stroma and vasculature. This is evidenced by histologic sections of placental bed biopsies in patients with PE, where cytotrophoblasts were located farther from uterine vessels than in sections of control placentas (Zhou et al. 1997a). Furthermore, when spiral arteriole endovascular cytotrophoblasts were detected, invasion within the vessel did not exceed the depth of the superficial decidua (Zhou et al. 1997a). These findings have been shown to be mediated, at least in part, by failure of transformation from epithelial to endothelial-like phenotype with abnormally persistent expression of epithelial adhesion molecules such as E-cadherin and α6β4 integrin (Brosens, et al. 1972; Zhou et al. 1993; Zhou et al. 1997a).
Although impaired trophoblast invasion is an important source for abnormal spiral artery remodeling, a defective decidualization process has also been proposed to affect uteroplacental blood flow. Some evidence indicates that changes in the endometrium before EVT invasion are distinct in preeclamptic women. For instance, decidua-dependent secretion of insulin-like growth factor bind protein-1 (IGFBP1) is reduced during the first trimester of women who later develop PE as compared to normal pregnancies (Hietala, et al. 2000; Vatten, et al. 2008). This suggests a maternal component for altered uteroplacental perfusion. Other studies have shown that maternal macrophages are concentrated around suboptimally remodeled spiral arteries from preeclamptic women and that those macrophages secrete tumor necrosis factor-alpha (TNF) and indoleamine 2,3-dioxygenase (IDO1) to induce apoptosis in EVTs (Reister, et al. 1999; Reister, et al. 2001)
Invasive trophoblasts are further regulated by their interaction with other cells including decidual natural killer (NK) cells. NK cells secrete various chemokines, and for NK cells and trophoblast to interface, the trophoblast must express matching chemokine receptors (Hanna, et al. 2006). Additionally, NK cell function is also mediated by NK receptors that bind to MHC class I molecules among other ligands. EVTs express HLA-C as their sole polymorphic classical MHC class 1 molecule, and given paternal contributions, HLA-C alleles can be different to that from the mother. Furthermore, NK cells express an incredibly diverse range of killer immunoglobulin receptors (KIRs) resulting from differences in gene number between individuals and allelic diversity at individual KIR loci (Parham and Moffett 2013). Specific maternal KIR haplotypes have been found to increase the risk of preeclampsia, demonstrating that immune recognition also plays a role in regulation of uteroplacental blood flow (Hiby, et al. 2010).
Uteroplacental hypoperfusion resulting in maternal systemic EC dysfunction
Several lines of evidence support that abnormal uteroplacental blood flow with subsequent poor placental perfusion strongly contribute to the phenotype of PE. For example, the incidence of PE is higher in women who live at high altitudes, where there is some degree of relative hypoxia (Palmer, et al. 1999; Zamudio 2007). Additionally, medical comorbidities resulting in vascular insufficiency, such as pre-existing hypertension, acquired thrombophilias and renal disease increase the risk for impaired placentation, PE, and FGR (Bartsch, et al. 2016; Dekker 1999). Perhaps most compellingly, animal models whose uteroplacental blood flow has been reduced demonstrate phenotypes strongly suggestive of PE (Bird, et al. 2003; Ianosi-Irimie, et al. 2005; Karumanchi and Stillman 2006; Khalil and Granger 2002).
Much of the maternal clinical phenotype of PE is thought to arise from maternal endothelial dysfunction. During normal pregnancy, uterine artery vasodilation has been found to be mediated, at least in part, by enhanced NO production. This occurs not only via NOS3 phosphorylation, but also through other mechanisms including enhanced gap junction communication which contributes to amplified and sustained intracellular calcium bursts (Tran, et al. 2009; Yi, et al. 2010; Yi, et al. 2011). In PE, uterine artery ECs are unable to produce the normally augmented levels of NO that occur during normal pregnancy, and this is thought to be one major mechanism resulting in insufficient vasodilation (Bird et al. 2003; Savvidou, et al. 2003; Sladek, et al. 1997).
The fetoplacental vasculature
As noted earlier, fetoplacental vascular resistance should normally decrease as gestation progresses, allowing for increased forward flow through the umbilical arteries during fetal systole. For this to occur, there are at least two main requirements: (1) Proper placental vasomotor tone, and (2) Appropriate expansion of the fetoplacental vasculature via angiogenesis. When either or both of these core principles are disrupted, fetoplacental vascular resistance may become abnormally elevated.
Constricted vasomotor tone
In contrast to small arterioles of other vascular beds, vasomotor tone of placental chorionic plate and stem villous vessels, which are similar in size to these arterioles, are not under autonomic control as placental vessels lack innervation (Fox and Khong 1990; Poston, et al. 1995; Reilly and Russell 1977; Sabry, et al. 1995). Not only are these larger placental vessels controlled primarily by humoral influences, they also respond differently than other vessels outside of the placenta. For instance, the response of the placental vasculature to factors such as acetylcholine, angiotensin II, and bradykinin is blunted (Mak, et al. 1984; McCarthy, et al. 1994). Issues such as vessel diameter has also been shown to affect sensitivity to factors such as thromboxane A2 (TXA2), with a selective decrease to TXA2 with decreasing vascular diameter in stem villous arteries (Broegger, et al. 2016). Placental vessels also vasoconstrict when subjected to prostaglandin E2 (PGE2), whereas all other vascular beds, including maternal uterine vessels, vasodilate under the influence of PGE2 (Allen, et al. 1989; Boura and Walters 1991; Glance, et al. 1986; Sastry, et al. 1997).
Much of the current knowledge surrounding regulation of human fetoplacental vasomotor tone is extrapolated from in vitro models and studies of humoral factors in cord blood. For example, results from a dual-perfused single cotyledon model showed that vessels within FGR placentas had reduced reactivity to PGE2 stimulation in comparison to control placentas (Luria, et al. 2012). In a study where cordocentesis was performed in pregnancies complicated by FGR, all of whom had umbilical artery Dopplers suggestive of abnormally elevated placental vascular resistance, significantly higher fetal concentrations of endothelin-1 were found in contrast to gestational age-matched, appropriately grown controls (Rizzo, et al. 1996). These same authors also found that the stable metabolite of the vasodilator prostacyclin, 6-keto prostaglandin F1-alpha, were lower in cord blood of FGR pregnancies.
The specific molecular mechanisms underlying the changes in prostanoid levels or of these unique placental vascular responses remain unidentified. There is a small body of literature suggesting that cyclooxygenase-2 (PTGS2) may play a role, although the data are conflicting as to whether it is upregulation or downregulation of PTGS2 that is resulting in abnormal vasoconstriction. In general, PTGS2 inhibition has been shown to alter the prostacyclin to thromboxane A2 ratio, leading to a prostanoid phenotype that favors vasoconstriction (Howard, et al. 2003). A different study found that polymorphisms of the fetal PTGS2 gene that correlate to decreased PTGS2 expression is associated with abnormal placental blood flow and FGR (Polydorides, et al. 2007). Chronic glucocorticoid treatment of chorionic plate arteries results in decreased PTGS2 expression and increases vascular resistance, although glucocorticoid treatment was shown to have effects on several other vasoactive mediators (Nugent, et al. 2013). In contrast, we have found an up-regulation of PTGS2 expression and activity within the endothelium of stem villous vessels in pregnancies complicated by FGR with abnormally elevated fetoplacental vascular resistance in comparison to gestational age-matched controls (Su, et al. 2011). However, our studies also suggest that this may be regulated by estrogen receptor-beta, which concomitantly regulates downstream prostanoid synthases resulting in a vasoconstrictive prostanoid profile (Su et al. 2011; Su, et al. 2009). These differences in mechanistic findings may be explained by disparities in types of samples collected (e.g. normal versus pathologic, cell versus tissue studies) or may represent compensatory, rather than pathologic, mechanisms. As one example, NO also regulates placental blood flow and is produced primarily via NOS3 in the placenta. One group of investigators found that NOS3 expression was significantly higher in endothelium of stem villous vessels in pregnancies complicated by FGR in comparison to controls, suggesting that NOS3 does not contribute to the pathologic phenotype of abnormal placental blood flow but may be an adaptive response to increased fetoplacental vascular resistance (Myatt, et al. 1997).
Deficient formation of the fetoplacental vasculature
Even when there is no disturbance in vasomotor tone, vascular resistance of the fetoplacental circulation is also mediated by the structure of the placental vasculature itself. When fetoplacental vascular resistance is abnormally elevated, as clinically manifested by decreased, absent, or reversed umbilical artery end-diastolic velocities, several groups of investigators have previously shown that there are fewer, more slender, and substantially longer and unbranched villous capillary loops than in placentas of uncomplicated pregnancies (Chen, et al. 2002; Jackson, et al. 1995; Krebs, et al. 1996; Macara, et al. 1996; Macara, et al. 1995; Todros, et al. 1999) (Figure 2). This results in fewer vascular conduits, resulting in a structural cause for abnormally elevated placental vascular resistance. These findings have been attributed to excessive non-branching angiogenesis (Benirschke 2012; Mayhew 2002; Mayhew, et al. 2004). In actuality, however, whether the cause is too much non-branching angiogenesis, impaired branching angiogenesis, or a combination of both entities remains unknown.
There is both limited and conflicting data regarding expression of angiogenic factors in placental pathologies associated with abnormal fetoplacental blood flow. For instance, within a hyperthermic ovine model of placental insufficiency-derived FGR where these fetuses manifest abnormal umbilical artery Doppler indices, cotyledonary VEGFA mRNA expression was actually increased in placentas of FGR fetuses at the equivalent gestational age of the late first trimester (50 days post-conception) (Barry, et al. 2008; Regnault, et al. 2002). This difference, however, disappeared by what could be considered the late second trimester (90 days post-conception) (Regnault et al. 2002). This suggests that there may be gestational age-dependent roles of certain angiogenic mediators. In a study of human placentas, one group found that total placental RNA expression of VEGFA was actually increased in the FGR group as compared to the control group (Szentpeteri, et al. 2013). This study, however, did not discuss several key maternal characteristics, including gestational age at delivery and number of subjects with abnormal umbilical artery Doppler velocimetry. Additionally, use of total placental RNA further limits interpretation of this finding. Cell-specific VEGFA has also been investigated, primarily through immunohistochemistry, with various studies showing no difference in VEGFA protein expression in pregnancies complicated by FGR, unlike the previously mentioned studies that investigated mRNA expression (Gurel, et al. 2003; Helske, et al. 2001). Umbilical cord blood levels have also been investigated, and in a cohort of FGR from Spain that, on average, required a late preterm delivery, there was also no difference in umbilical arterial or umbilical venous VEGFA and sFLT1 concentrations (Borras, et al. 2014). However, in this study, umbilical artery Doppler pulsatility indices did not significantly differ between the two groups and at worst, were only mildly elevated in the FGR group, suggesting that these were not the fetuses that suffered from abnormally high fetoplacental vascular resistance. Taken together, these data suggest that the mechanisms underlying the development of abnormal fetoplacental vascular resistance is much more complex than just VEGFA mediation.
With regard to PGF, data also differ. In the hyperthermic ewe model of FGR that also demonstrates abnormal umbilical artery Doppler velocimetry, there was no difference in PLGF at either 50 or 90 days post-conception (Regnault et al. 2002). Recently, one study found that total placental PGF expression was diminished in cases of severe FGR requiring preterm delivery, and the authors concluded that this may indicate a role of PGF in placental angiogenesis (Joo, et al. 2017). No other specific data regarding PGF and fetoplacental angiogenesis has been described to our knowledge, but it warrants comment, especially given its potential effects on angiogenesis and its interaction with VEGFA.
Knowledge surrounding FGF, the angiopoietins, and placental blood flow is also limited. Based upon cordocentesis data, FGF2, which again is considered the primary placental FGF, appears to peak at approximately 18–20 weeks with a slow decrease all the way until term (Hill, et al. 1995). There was also a trend toward lower FGF2 levels in cord serum of pregnancies that resulted in SGA fetuses, but this was not statistically significant (Hill et al. 1995). As for the angiopoeitins, in a model of FGR derived from overnourished, adolescent, pregnant ewes, the vascularity of the placental cotyledon was decreased, with elevated ANGPT2 expression within these cotyledons in comparison to appropriately grown controls (Carr, et al. 2016). In humans, data are more discrepant. For instance, studies have found diminished ANGPT2 expression within total placenta of pregnancies complicated by severe FGR or PE as compared to gestational age-matched controls (Dunk et al. 2000; Zhang et al. 2001). In contrast, a more recent study showed that ANGPT2 and TEK expression was lower while ANGPT1 was higher in preeclamptic or preeclamptic/FGR pregnancies (Kappou, et al. 2014). This study, however, utilized cases that were all mild enough to be delivered at term, suggesting that these fetuses and placentas were less likely to have experienced abnormal placental blood flow.
In addition to the gaps in knowledge surrounding levels of angiogenic mediators and deficient placental vascularization, the molecular mechanisms underlying this impairment also remain incompletely understood. Murine studies demonstrate that knock-out of aryl hydrocarbon receptor nuclear translocator (ARNT), a heterodimeric partner to hypoxia inducible factor 1-alpha (HIF1A) that induces transcription of angiogenic genes such as VEGFA, results in embryonic lethality secondary to deficient placental vascularization (Kozak, et al. 1997; Maltepe, et al. 1997). Within human fetoplacental endothelial cells isolated from severely growth-restricted fetuses with absent or reversed umbilical artery end-diastolic velocities, expression of ARNT is decreased in comparison to gestational age-matched, appropriately grown controls (Su et al. 2015). Deficient ARNT expression leads to decreased binding of the ARNT/HIF1A transcription factor to hypoxia response elements (HREs) within the VEGFA proximal promoter, diminished VEGFA expression, and impaired proxies of angiogenesis such as tube formation (Su et al. 2015).
CONCLUSION
Placental blood flow, both within the fetoplacental and the maternal uteroplacental vasculatures, is critical for pregnancy outcome. Knowledge surrounding the mechanisms underlying both normal and impaired human placental blood flow is limited for several reasons. First, much of the determinants of uteroplacental blood flow are established at or soon after implantation, oftentimes prior to the parturient even knowing she is pregnant. Second, clinical modalities such as Doppler velocimetry of the uterine or umbilical circulations may identify pregnancies at high-risk for placental insufficiency, but their utility is limited by the fact that mechanistic investigation cannot occur until after delivery of the infant and placenta. Finally, utilizing the diagnosis of FGR or PE to better investigate mechanisms of abnormal placental function has its limitations. For example, there are likely various mechanisms leading to the same clinical phenotype such as PE or FGR, which is likely why prophylactic measures such as heparin or baby aspirin has limited efficacy, even in high risk pregnancies. (Bujold, et al. 2010; Dodd, et al. 2013; Odibo, et al. 2015). This is supported by the discrepancies in expression or mechanistic findings in FGR and/or PE. Continued future investigation is warranted in mechanisms underlying maternal and fetoplacental blood flow.
Acknowledgments
We are grateful to KIMEN Design for Research (kimendesign4research.com) for their assistance with the figures.
DECLARATION OF INTEREST
Dr. Su is a co-investigator on a multicenter, industry-sponsored grant (Progenity, Inc., Evaluation of Preeclampsia Biomarkers).
FUNDING
This work was supported by the National Institutes of Health HL119846 (EJS).
Contributor Information
Yingchun Li, University of Colorado School of Medicine, Department of Obstetrics and Gynecology, Division of Reproductive Sciences, 12700 E. 19th Ave, MS 8613, Aurora, CO 80045.
Ramón A Lorca, University of Colorado School of Medicine, Department of Obstetrics and Gynecology, Division of Reproductive Sciences, 12700 E. 19th Ave, MS 8613, Aurora, CO 80045.
Emily J Su, University of Colorado School of Medicine, Department of Obstetrics and Gynecology, Division of Maternal-Fetal Medicine/Division of Reproductive Sciences, 12631 E. 17th Ave, B198-5, Aurora, CO 80045.
References
- Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2017;6:CD007529. doi: 10.1002/14651858.CD007529.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfirevic Z, Stampalija T, Gyte GM. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2013:CD007529. doi: 10.1002/14651858.CD007529.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J, Lauridsen V, Hansen V, Andersson KE, Forman A. Effects of indomethacin on human placental stem villous arteries. Gynecol Obstet Invest. 1989;27:118–121. doi: 10.1159/000293635. [DOI] [PubMed] [Google Scholar]
- Anteby EY, Natanson-Yaron S, Hamani Y, Sciaki Y, Goldman-Wohl D, Greenfield C, Ariel I, Yagel S. Fibroblast growth factor-10 and fibroblast growth factor receptors 1–4: expression and peptide localization in human decidua and placenta. Eur J Obstet Gynecol Reprod Biol. 2005;119:27–35. doi: 10.1016/j.ejogrb.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Arany E, Hill DJ. Fibroblast growth factor-2 and fibroblast growth factor receptor-1 mRNA expression and peptide localization in placentae from normal and diabetic pregnancies. Placenta. 1998;19:133–142. doi: 10.1016/s0143-4004(98)90001-7. [DOI] [PubMed] [Google Scholar]
- Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003a;1:1356–1370. doi: 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003b;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clin Obstet Gynecol. 2013;56:511–519. doi: 10.1097/GRF.0b013e31829cb9ca. [DOI] [PubMed] [Google Scholar]
- Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol. 2008;32:225–230. doi: 10.1053/j.semperi.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Bartsch E, Medcalf KE, Park AL, Ray JG High Risk of Pre-eclampsia Identification G. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschat AA, Weiner CP. Umbilical artery doppler screening for detection of the small fetus in need of antepartum surveillance. Am J Obstet Gynecol. 2000;182:154–158. doi: 10.1016/s0002-9378(00)70505-9. [DOI] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benirschke K, Burton GJ, Baergen RN. Pathology of the Human Placenta. Berlin: Springer-Verlag; 2012. [Google Scholar]
- Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol. 2003;284:R245–258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- Borras D, Perales-Puchalt A, Ruiz Sacedon N, Perales A. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated with intrauterine growth restriction. J Obstet Gynaecol. 2014;34:218–220. doi: 10.3109/01443615.2013.834304. [DOI] [PubMed] [Google Scholar]
- Boura AL, Walters WA. Autacoids and the control of vascular tone in the human umbilical-placental circulation. Placenta. 1991;12:453–477. doi: 10.1016/0143-4004(91)90023-9. [DOI] [PubMed] [Google Scholar]
- Broegger T, Andersson KE, Aalkjaer C, Forman A, Boedtkjer DB. Sensitivity to the thromboxane A2 analog U46619 varies with inner diameter in human stem villous arteries. Placenta. 2016;39:111–115. doi: 10.1016/j.placenta.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977;84:656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest JC, Giguere Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Sonographic, stereological and Doppler flow velocimetric assessments of placental maturity. Br J Obstet Gynaecol. 1995;102:818–825. doi: 10.1111/j.1471-0528.1995.tb10849.x. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Carr DJ, David AL, Aitken RP, Milne JS, Borowicz PP, Wallace JM, Redmer DA. Placental vascularity and markers of angiogenesis in relation to prenatal growth status in overnourished adolescent ewes. Placenta. 2016;46:79–86. doi: 10.1016/j.placenta.2016.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci M, Kaufmann P. A three-dimensional study of the normal human placental villous core: II. Stromal architecture. Placenta. 1982;3:269–285. doi: 10.1016/s0143-4004(82)80004-0. [DOI] [PubMed] [Google Scholar]
- Castellucci M, Scheper M, Scheffen I, Celona A, Kaufmann P. The development of the human placental villous tree. Anat Embryol (Berl) 1990;181:117–128. doi: 10.1007/BF00198951. [DOI] [PubMed] [Google Scholar]
- Chen CP, Bajoria R, Aplin JD. Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms. Am J Obstet Gynecol. 2002;187:764–769. doi: 10.1067/mob.2002.125243. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- De A, Maulik D, Lankachandra K, Mundy DC, Ye SQ, Gerkovich MM. Fetoplacental regional variations in the expression of angiopoietin-1, angiopoietin-2, and Tie2 in normal-term and near-term pregnancies. J Matern Fetal Neonatal Med. 2016;29:3421–3428. doi: 10.3109/14767058.2015.1136282. [DOI] [PubMed] [Google Scholar]
- Dekker GA. Risk factors for preeclampsia. Clin Obstet Gynecol. 1999;42:422–435. doi: 10.1097/00003081-199909000-00002. [DOI] [PubMed] [Google Scholar]
- Demir R, Kaufmann P, Castellucci M, Erbengi T, Kotowski A. Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anat (Basel) 1989;136:190–203. doi: 10.1159/000146886. [DOI] [PubMed] [Google Scholar]
- Demir R, Kayisli UA, Seval Y, Celik-Ozenci C, Korgun ET, Demir-Weusten AY, Huppertz B. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta. 2004;25:560–572. doi: 10.1016/j.placenta.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Dodd JM, McLeod A, Windrim RC, Kingdom J. Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction. Cochrane Database Syst Rev. 2013:CD006780. doi: 10.1002/14651858.CD006780.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunk C, Shams M, Nijjar S, Rhaman M, Qiu Y, Bussolati B, Ahmed A. Angiopoietin-1 and angiopoietin-2 activate trophoblast Tie-2 to promote growth and migration during placental development. Am J Pathol. 2000;156:2185–2199. doi: 10.1016/S0002-9440(10)65089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders AC. Trophoblast differentiation during the transition from trophoblastic plate to lacunar stage of implantation in the rhesus monkey and human. Am J Anat. 1989;186:85–98. doi: 10.1002/aja.1001860107. [DOI] [PubMed] [Google Scholar]
- Eriksson A, Cao R, Pawliuk R, Berg SM, Tsang M, Zhou D, Fleet C, Tritsaris K, Dissing S, Leboulch P, et al. Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell. 2002;1:99–108. doi: 10.1016/s1535-6108(02)00028-4. [DOI] [PubMed] [Google Scholar]
- Feng L, Liao WX, Luo Q, Zhang HH, Wang W, Zheng J, Chen DB. Caveolin-1 orchestrates fibroblast growth factor 2 signaling control of angiogenesis in placental artery endothelial cell caveolae. J Cell Physiol. 2012;227:2480–2491. doi: 10.1002/jcp.22984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriani RA, Ahmed A, Sharkey A, Smith SK. Colocalization of acidic and basic fibroblast growth factor (FGF) in human placenta and the cellular effects of bFGF in trophoblast cell line JEG-3. Growth Factors. 1994;10:259–268. doi: 10.3109/08977199409010992. [DOI] [PubMed] [Google Scholar]
- Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213:S115–122. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Fox SB, Khong TY. Lack of innervation of human umbilical cord. An immunohistological and histochemical study. Placenta. 1990;11:59–62. doi: 10.1016/s0143-4004(05)80443-6. [DOI] [PubMed] [Google Scholar]
- Garcia B, Llurba E, Valle L, Gomez-Roig MD, Juan M, Perez-Matos C, Fernandez M, Garcia-Hernandez JA, Alijotas-Reig J, Higueras MT, et al. Do knowledge of uterine artery resistance in the second trimester and targeted surveillance improve maternal and perinatal outcome? UTOPIA study: a randomized controlled trial. Ultrasound Obstet Gynecol. 2016;47:680–689. doi: 10.1002/uog.15873. [DOI] [PubMed] [Google Scholar]
- Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB. Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab. 2002;87:4213–4224. doi: 10.1210/jc.2002-020195. [DOI] [PubMed] [Google Scholar]
- Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol. 1985;92:31–38. doi: 10.1111/j.1471-0528.1985.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Glance DG, Elder MG, Myatt L. The actions of prostaglandins and their interactions with angiotensin II in the isolated perfused human placental cotyledon. Br J Obstet Gynaecol. 1986;93:488–494. [PubMed] [Google Scholar]
- Goldman-Wohl DS, Ariel I, Greenfield C, Lavy Y, Yagel S. Tie-2 and angiopoietin-2 expression at the fetal-maternal interface: a receptor ligand model for vascular remodelling. Mol Hum Reprod. 2000;6:81–87. doi: 10.1093/molehr/6.1.81. [DOI] [PubMed] [Google Scholar]
- Group GS. A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG. 2003;110:27–32. doi: 10.1046/j.1471-0528.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- Guiot C, Pianta PG, Todros T. Modelling the feto-placental circulation: 1. A distributed network predicting umbilical haemodynamics throughout pregnancy. Ultrasound Med Biol. 1992;18:535–544. doi: 10.1016/0301-5629(92)90068-l. [DOI] [PubMed] [Google Scholar]
- Gurel D, Ozer E, Altunyurt S, Guclu S, Demir N. Expression of IGR-IR and VEGF and trophoblastic proliferative activity in placentas from pregnancies complicated by IUGR. Pathol Res Pract. 2003;199:803–809. doi: 10.1078/0344-0338-00499. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Harris LK. Review: Trophoblast-vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta. 2010;31(Suppl):S93–98. doi: 10.1016/j.placenta.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Harris LK, Keogh RJ, Wareing M, Baker PN, Cartwright JE, Aplin JD, Whitley GS. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am J Pathol. 2006;169:1863–1874. doi: 10.2353/ajpath.2006.060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helske S, Vuorela P, Carpen O, Hornig C, Weich H, Halmesmaki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod. 2001;7:205–210. doi: 10.1093/molehr/7.2.205. [DOI] [PubMed] [Google Scholar]
- Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietala R, Pohja-Nylander P, Rutanen EM, Laatikainen T. Serum insulin-like growth factor binding protein-1 at 16 weeks and subsequent preeclampsia. Obstet Gynecol. 2000;95:185–189. doi: 10.1016/s0029-7844(99)00489-5. [DOI] [PubMed] [Google Scholar]
- Hill DJ, Tevaarwerk GJ, Arany E, Kilkenny D, Gregory M, Langford KS, Miell J. Fibroblast growth factor-2 (FGF-2) is present in maternal and cord serum, and in the mother is associated with a binding protein immunologically related to the FGF receptor-1. J Clin Endocrinol Metab. 1995;80:1822–1831. doi: 10.1210/jcem.80.6.7539816. [DOI] [PubMed] [Google Scholar]
- Howard BC, Kovac CM, Calhoun BC, Hoeldtke NJ, Napolitano PG. The effects of a cyclo-oxygenase II inhibitor on placental artery production of thromboxane and prostacyclin. Am J Obstet Gynecol. 2003;189:835–838. doi: 10.1067/s0002-9378(03)00844-5. [DOI] [PubMed] [Google Scholar]
- Hustin J, Schaaps JP. Echographic [corrected] and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol. 1987;157:162–168. doi: 10.1016/s0002-9378(87)80371-x. [DOI] [PubMed] [Google Scholar]
- Ianosi-Irimie M, Vu HV, Whitbred JM, Pridjian CA, Nadig JD, Williams MY, Wrenn DC, Pridjian G, Puschett JB. A rat model of preeclampsia. Clin Exp Hypertens. 2005;27:605–617. doi: 10.1080/10641960500298608. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Walsh AJ, Morrow RJ, Mullen JB, Lye SJ, Ritchie JW. Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms. Am J Obstet Gynecol. 1995;172:518–525. doi: 10.1016/0002-9378(95)90566-9. [DOI] [PubMed] [Google Scholar]
- Jaffe R, Jauniaux E, Hustin J. Maternal circulation in the first-trimester human placenta--myth or reality? Am J Obstet Gynecol. 1997;176:695–705. doi: 10.1016/s0002-9378(97)70572-6. [DOI] [PubMed] [Google Scholar]
- Joo JG, Rigo J, Jr, Borzsonyi B, Demendi C, Kornya L. Placental gene expression of the placental growth factor (PlGF) in intrauterine growth restriction. J Matern Fetal Neonatal Med. 2017;30:1471–1475. doi: 10.1080/14767058.2016.1219993. [DOI] [PubMed] [Google Scholar]
- Kam EP, Gardner L, Loke YW, King A. The role of trophoblast in the physiological change in decidual spiral arteries. Hum Reprod. 1999;14:2131–2138. doi: 10.1093/humrep/14.8.2131. [DOI] [PubMed] [Google Scholar]
- Kappou D, Sifakis S, Androutsopoulos V, Konstantinidou A, Spandidos DA, Papantoniou N. Placental mRNA expression of angiopoietins (Ang)-1, Ang-2 and their receptor Tie-2 is altered in pregnancies complicated by preeclampsia. Placenta. 2014;35:718–723. doi: 10.1016/j.placenta.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Stillman IE. In vivo rat model of preeclampsia. Methods Mol Med. 2006;122:393–399. doi: 10.1385/1-59259-989-3:393. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Bruns U, Leiser R, Luckhardt M, Winterhager E. The fetal vascularisation of term human placental villi. II. Intermediate and terminal villi. Anat Embryol (Berl) 1985;173:203–214. doi: 10.1007/BF00316301. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283:R29–45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- Kingdom JC, Burrell SJ, Kaufmann P. Pathology and clinical implications of abnormal umbilical artery Doppler waveforms. Ultrasound Obstet Gynecol. 1997;9:271–286. doi: 10.1046/j.1469-0705.1997.09040271.x. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Abbott B, Hankinson O. ARNT-deficient mice and placental differentiation. Dev Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996;175:1534–1542. doi: 10.1016/s0002-9378(96)70103-5. [DOI] [PubMed] [Google Scholar]
- Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, Kyriakides TR, Morales-Ruiz M, Sessa WC. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci U S A. 2014;111:12865–12870. doi: 10.1073/pnas.1408472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees CC, Marlow N, van Wassenaer-Leemhuis A, Arabin B, Bilardo CM, Brezinka C, Calvert S, Derks JB, Diemert A, Duvekot JJ, et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet. 2015;385:2162–2172. doi: 10.1016/S0140-6736(14)62049-3. [DOI] [PubMed] [Google Scholar]
- Liao WX, Feng L, Zheng J, Chen DB. Deciphering mechanisms controlling placental artery endothelial cell migration stimulated by vascular endothelial growth factor. Endocrinology. 2010;151:3432–3444. doi: 10.1210/en.2009-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria O, Bar J, Barnea O, Golan A, Kovo M. Reactivity of blood vessels in response to prostaglandin E2 in placentas from pregnancies complicated by fetal growth restriction. Prenat Diagn. 2012;32:417–422. doi: 10.1002/pd.3827. [DOI] [PubMed] [Google Scholar]
- Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair J, More IA, Lyall F, Greer IA. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta. 1996;17:37–48. doi: 10.1016/s0143-4004(05)80642-3. [DOI] [PubMed] [Google Scholar]
- Macara L, Kingdom JC, Kohnen G, Bowman AW, Greer IA, Kaufmann P. Elaboration of stem villous vessels in growth restricted pregnancies with abnormal umbilical artery Doppler waveforms. Br J Obstet Gynaecol. 1995;102:807–812. doi: 10.1111/j.1471-0528.1995.tb10847.x. [DOI] [PubMed] [Google Scholar]
- Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak KK, Gude NM, Walters WA, Boura AL. Effects of vasoactive autacoids on the human umbilical-fetal placental vasculature. Br J Obstet Gynaecol. 1984;91:99–106. doi: 10.1111/j.1471-0528.1984.tb05890.x. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- Mayhew TM. Fetoplacental angiogenesis during gestation is biphasic, longitudinal and occurs by proliferation and remodelling of vascular endothelial cells. Placenta. 2002;23:742–750. doi: 10.1016/s0143-4004(02)90865-9. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- McCarthy AL, Woolfson RG, Evans BJ, Davies DR, Raju SK, Poston L. Functional characteristics of small placental arteries. Am J Obstet Gynecol. 1994;170:945–951. doi: 10.1016/s0002-9378(94)70311-6. [DOI] [PubMed] [Google Scholar]
- Myatt L, Eis AL, Brockman DE, Greer IA, Lyall F. Endothelial nitric oxide synthase in placental villous tissue from normal, pre-eclamptic and intrauterine growth restricted pregnancies. Hum Reprod. 1997;12:167–172. doi: 10.1093/humrep/12.1.167. [DOI] [PubMed] [Google Scholar]
- Natanson-Yaron S, Anteby EY, Greenfield C, Goldman-Wohl D, Hamani Y, Hochner-Celnikier D, Yagel S. FGF 10 and Sprouty 2 modulate trophoblast invasion and branching morphogenesis. Mol Hum Reprod. 2007;13:511–519. doi: 10.1093/molehr/gam034. [DOI] [PubMed] [Google Scholar]
- Nugent JL, Wareing M, Palin V, Sibley CP, Baker PN, Ray DW, Farrow SN, Jones RL. Chronic glucocorticoid exposure potentiates placental chorionic plate artery constriction: implications for aberrant fetoplacental vascular resistance in fetal growth restriction. Endocrinology. 2013;154:876–887. doi: 10.1210/en.2012-1927. [DOI] [PubMed] [Google Scholar]
- Odibo AO, Goetzinger KR, Odibo L, Tuuli MG. Early prediction and aspirin for prevention of pre-eclampsia (EPAPP) study: a randomized controlled trial. Ultrasound Obstet Gynecol. 2015;46:414–418. doi: 10.1002/uog.14889. [DOI] [PubMed] [Google Scholar]
- Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol. 1999;180:1161–1168. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Polydorides AD, Kalish RB, Witkin SS, Baergen RN. A fetal cyclooxygenase-2 gene polymorphism is associated with placental malperfusion. Int J Gynecol Pathol. 2007;26:284–290. doi: 10.1097/01.pgp.0000236950.56785.a8. [DOI] [PubMed] [Google Scholar]
- Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacol Ther. 1995;65:215–239. doi: 10.1016/0163-7258(94)00064-a. [DOI] [PubMed] [Google Scholar]
- Rankin JH, Goodman A, Phernetton T. Local regulation of the uterine blood flow by the umbilical circulation. Proc Soc Exp Biol Med. 1975;150:690–694. doi: 10.3181/00379727-150-39107. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development. 2005;132:4097–4106. doi: 10.1242/dev.01971. [DOI] [PubMed] [Google Scholar]
- Regnault TR, Orbus RJ, de Vrijer B, Davidsen ML, Galan HL, Wilkening RB, Anthony RV. Placental expression of VEGF, PlGF and their receptors in a model of placental insufficiency-intrauterine growth restriction (PI-IUGR) Placenta. 2002;23:132–144. doi: 10.1053/plac.2001.0757. [DOI] [PubMed] [Google Scholar]
- Reilly FD, Russell PT. Neurohistochemical evidence supporting an absence of adrenergic and cholinergic innervation in the human placenta and umbilical cord. Anat Rec. 1977;188:277–286. doi: 10.1002/ar.1091880302. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schroder W, Kaufmann P, Rath W. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta. 1999;20:229–233. doi: 10.1053/plac.1998.0373. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–1152. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Capponi A, Rinaldo D, Arduini D, Romanini C. Release of vasoactive agents during cordocentesis: differences between normally grown and growth-restricted fetuses. Am J Obstet Gynecol. 1996;175:563–570. doi: 10.1053/ob.1996.v175.a74253. [DOI] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- Sabry S, Mondon F, Ferre F, Dinh-Xuan AT. In vitro contractile and relaxant responses of human resistance placental stem villi arteries of healthy parturients: role of endothelium. Fundam Clin Pharmacol. 1995;9:46–51. doi: 10.1111/j.1472-8206.1995.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Sadler TW. Langman's Meical Embryology. Baltimore, MD: Williams and Wilkins; 1995. [Google Scholar]
- Salafia CM, Minior VK, Pezzullo JC, Popek EJ, Rosenkrantz TS, Vintzileos AM. Intrauterine growth restriction in infants of less than thirty-two weeks' gestation: associated placental pathologic features. Am J Obstet Gynecol. 1995;173:1049–1057. doi: 10.1016/0002-9378(95)91325-4. [DOI] [PubMed] [Google Scholar]
- Salafia CM, Pezzullo JC, Minior VK, Divon MY. Placental pathology of absent and reversed end-diastolic flow in growth-restricted fetuses. Obstet Gynecol. 1997;90:830–836. doi: 10.1016/S0029-7844(97)00473-0. [DOI] [PubMed] [Google Scholar]
- Sastry BV, Hemontolor ME, Chance MB, Johnson RF. Dual messenger function for prostaglandin E2 (PGE2) in human placenta. Cell Mol Biol (Noisy-le-grand) 1997;43:417–424. [PubMed] [Google Scholar]
- Savvidou MD, Hingorani AD, Tsikas D, Frolich JC, Vallance P, Nicolaides KH. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003;361:1511–1517. doi: 10.1016/S0140-6736(03)13177-7. [DOI] [PubMed] [Google Scholar]
- Schlafke S, Enders AC. Cellular basis of interaction between trophoblast and uterus at implantation. Biol Reprod. 1975;12:41–65. doi: 10.1095/biolreprod12.1.41. [DOI] [PubMed] [Google Scholar]
- Seval Y, Sati L, Celik-Ozenci C, Taskin O, Demir R. The distribution of angiopoietin-1, angiopoietin-2 and their receptors tie-1 and tie-2 in the very early human placenta. Placenta. 2008;29:809–815. doi: 10.1016/j.placenta.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shams M, Ahmed A. Localization of mRNA for basic fibroblast growth factor in human placenta. Growth Factors. 1994;11:105–111. doi: 10.3109/08977199409001052. [DOI] [PubMed] [Google Scholar]
- Shimonovitz S, Hurwitz A, Dushnik M, Anteby E, Geva-Eldar T, Yagel S. Developmental regulation of the expression of 72 and 92 kd type IV collagenases in human trophoblasts: a possible mechanism for control of trophoblast invasion. Am J Obstet Gynecol. 1994;171:832–838. doi: 10.1016/0002-9378(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- Stock MK, Anderson DF, Phernetton TM, McLaughlin MK, Rankin JH. Vascular response of the fetal placenta to local occlusion of the maternal placental vasculature. J Dev Physiol. 1980;2:339–346. [PubMed] [Google Scholar]
- Su EJ, Ernst L, Abdallah N, Chatterton R, Xin H, Monsivais D, Coon J, Bulun SE. Estrogen receptor-beta and fetoplacental endothelial prostanoid biosynthesis: a link to clinically demonstrated fetal growth restriction. J Clin Endocrinol Metab. 2011;96:E1558–1567. doi: 10.1210/jc.2011-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su EJ, Lin ZH, Zeine R, Yin P, Reierstad S, Innes JE, Bulun SE. Estrogen receptor-beta mediates cyclooxygenase-2 expression and vascular prostanoid levels in human placental villous endothelial cells. Am J Obstet Gynecol. 2009;200:427, e421–428. doi: 10.1016/j.ajog.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Su EJ, Xin H, Yin P, Dyson M, Coon J, Farrow KN, Mestan KK, Ernst LM. Impaired fetoplacental angiogenesis in growth-restricted fetuses with abnormal umbilical artery doppler velocimetry is mediated by aryl hydrocarbon receptor nuclear translocator (ARNT) J Clin Endocrinol Metab. 2015;100:E30–40. doi: 10.1210/jc.2014-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentpeteri I, Rab A, Kornya L, Kovacs P, Joo JG. Gene expression patterns of vascular endothelial growth factor (VEGF-A) in human placenta from pregnancies with intrauterine growth restriction. J Matern Fetal Neonatal Med. 2013;26:984–989. doi: 10.3109/14767058.2013.766702. [DOI] [PubMed] [Google Scholar]
- Thompson RS, Trudinger BJ. Doppler waveform pulsatility index and resistance, pressure and flow in the umbilical placental circulation: an investigation using a mathematical model. Ultrasound Med Biol. 1990;16:449–458. doi: 10.1016/0301-5629(90)90167-b. [DOI] [PubMed] [Google Scholar]
- Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M, group Gs. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet. 2004;364:513–520. doi: 10.1016/S0140-6736(04)16809-8. [DOI] [PubMed] [Google Scholar]
- Todros T, Guiot C, Pianta PG. Modelling the feto-placental circulation: 2. A continuous approach to explain normal and abnormal flow velocity waveforms in the umbilical arteries. Ultrasound Med Biol. 1992;18:545–551. doi: 10.1016/0301-5629(92)90069-m. [DOI] [PubMed] [Google Scholar]
- Todros T, Sciarrone A, Piccoli E, Guiot C, Kaufmann P, Kingdom J. Umbilical Doppler waveforms and placental villous angiogenesis in pregnancies complicated by fetal growth restriction. Obstet Gynecol. 1999;93:499–503. doi: 10.1016/s0029-7844(98)00440-2. [DOI] [PubMed] [Google Scholar]
- Tran QK, Leonard J, Black DJ, Nadeau OW, Boulatnikov IG, Persechini A. Effects of combined phosphorylation at Ser-617 and Ser-1179 in endothelial nitric-oxide synthase on EC50(Ca2+) values for calmodulin binding and enzyme activation. J Biol Chem. 2009;284:11892–11899. doi: 10.1074/jbc.M806205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger BJ, Giles WB, Cook CM. Flow velocity waveforms in the maternal uteroplacental and fetal umbilical placental circulations. Am J Obstet Gynecol. 1985a;152:155–163. doi: 10.1016/s0002-9378(85)80016-8. [DOI] [PubMed] [Google Scholar]
- Trudinger BJ, Giles WB, Cook CM. Uteroplacental blood flow velocity-time waveforms in normal and complicated pregnancy. Br J Obstet Gynaecol. 1985b;92:39–45. doi: 10.1111/j.1471-0528.1985.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Trudinger BJ, Giles WB, Cook CM, Bombardieri J, Collins L. Fetal umbilical artery flow velocity waveforms and placental resistance: clinical significance. Br J Obstet Gynaecol. 1985c;92:23–30. doi: 10.1111/j.1471-0528.1985.tb01044.x. [DOI] [PubMed] [Google Scholar]
- van Oppenraaij RH, Koning AH, Lisman BA, Boer K, van den Hoff MJ, van der Spek PJ, Steegers EA, Exalto N. Vasculogenesis and angiogenesis in the first trimester human placenta: an innovative 3D study using an immersive Virtual Reality system. Placenta. 2009;30:220–222. doi: 10.1016/j.placenta.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Vatten LJ, Nilsen TI, Juul A, Jeansson S, Jenum PA, Eskild A. Changes in circulating level of IGF-I and IGF-binding protein-1 from the first to second trimester as predictors of preeclampsia. Eur J Endocrinol. 2008;158:101–105. doi: 10.1530/EJE-07-0386. [DOI] [PubMed] [Google Scholar]
- Xu L, Cochran DM, Tong RT, Winkler F, Kashiwagi S, Jain RK, Fukumura D. Placenta growth factor overexpression inhibits tumor growth, angiogenesis, and metastasis by depleting vascular endothelial growth factor homodimers in orthotopic mouse models. Cancer Res. 2006;66:3971–3977. doi: 10.1158/0008-5472.CAN-04-3085. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang Y, Yang Y, Lim S, Cao Z, Rak J, Cao Y. Vascular endothelial growth factor-dependent spatiotemporal dual roles of placental growth factor in modulation of angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 2013;110:13932–13937. doi: 10.1073/pnas.1309629110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod. 2010;82:66–75. doi: 10.1095/biolreprod.109.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi FX, Boeldt DS, Magness RR, Bird IM. [Ca2+]i signaling vs. eNOS expression as determinants of NO output in uterine artery endothelium: relative roles in pregnancy adaptation and reversal by VEGF165. Am J Physiol Heart Circ Physiol. 2011;300:H1182–1193. doi: 10.1152/ajpheart.01108.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S. High-altitude hypoxia and preeclampsia. Front Biosci. 2007;12:2967–2977. doi: 10.2741/2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EG, Smith SK, Baker PN, Charnock-Jones DS. The regulation and localization of angiopoietin-1, -2, and their receptor Tie2 in normal and pathologic human placentae. Mol Med. 2001;7:624–635. [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Wen Y, Song Y, Wang K, Chen DB, Magness RR. Activation of multiple signaling pathways is critical for fibroblast growth factor 2- and vascular endothelial growth factor-stimulated ovine fetoplacental endothelial cell proliferation. Biol Reprod. 2008;78:143–150. doi: 10.1095/biolreprod.107.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997a;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997b;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]