Abstract
Objective
To examine the baseline prevalence and longitudinal evolution in non-motor symptoms (NMS) in a prospective cohort of, at baseline, patients with de novo Parkinson’s disease (PD) compared with healthy controls (HC).
Methods
Parkinson’s Progression Markers Initiative (PPMI) is a longitudinal, ongoing, controlled study of de novo PD participants and HC. NMS were rated using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part I score and other validated NMS scales at baseline and after 2 years. Biological variables included cerebrospinal fluid (CSF) markers and dopamine transporter imaging.
Results
423 PD subjects and 196 HC were enrolled and followed for 2 years. MDS-UPDRS Part I total mean (SD) scores increased from baseline 5.6 (4.1) to 7.7 (5.0) at year 2 in PD subjects (p<0.001) versus from 2.9 (3.0) to 3.2 (3.0) in HC (p=0.38), with a significant difference between the groups (p<0.001). In the multivariate analysis, higher baseline NMS score was associated with female sex (p=0.008), higher baseline MDS-UPDRS Part II scores (p<0.001) and more severe motor phenotype (p=0.007). Longitudinal increase in NMS severity was associated with the older age (0.008) and lower CSF Aβ1–42 (0.005) at baseline. There was no association with the dose or class of dopaminergic therapy.
Conclusions
This study of NMS in early PD identified clinical and biological variables associated with both baseline burden and predictors of progression. The association of a greater longitudinal increase in NMS with lower baseline Aβ1–42 level is an important finding that will have to be replicated in other cohorts.
Trial registration
ClinicalTrials.gov identifier: NCT01141023.
Keywords: parkinson’s disease, non-motor symptoms, biomarkers
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative condition associated with a broad range of motor and non-motor symptoms (NMS). A number of NMS, including hyposmia, rapid eye movement behaviour disorder (RBD), depression, anxiety and constipation can occur in the earliest stages of PD and indeed may precede the onset of motor disability,1–5 theoretically reflecting Braak’s staging of PD that demonstrates involvement of the brain stem early in the disease pathological process.6 The prevalence and clinical correlates of NMS in advanced PD population are well established,7 8 but NMS are also one of the major factors impairing quality of life in early PD.9 10 In the last 5 years, there has been a growing body of literature on NMS in early untreated PD, which consistently demonstrates a high prevalence across the spectrum of NMS and generally a lack of correlation with the degree of motor disability.5 11–14 Only a subset of these studies included a control group, which is essential considering the increasing burden of NMS with ageing. Only two groups have reported longitudinal data on the evolution of NMS in an initially untreated PD population and only one of them had a control group for comparison.9 11 13 15 Thus, there are limited data from large, controlled, prospective studies on the evolution of NMS in early PD. As was highlighted in the recent review, there is an urgent need to establish the biological underpinnings of NMS early in the disease course.16
The aim of this analysis was to systematically explore the prevalence, clinical spectrum, 2-year longitudinal evolution, and biological correlates of NMS in a large group of subjects with early untreated PD (at baseline) compared with healthy controls (HC) enrolled in the Parkinson’s Progression Markers Initiative (PPMI).
Methods
Study design
In brief, PPMI is an ongoing observational, international, multicenter study aimed at identifying serological, genetic, spinal fluid and imaging biomarkers of PD progression in a large cohort of participants with early untreated (de novo) PD at enrolment, compared with HC. The aims and methodology of the study have been published elsewhere and are available at www.ppmi-info.org/study-design.17 The data used for this paper constitute the analysis of the baseline and 2-year follow-up dataset downloaded 4 January 2016. At the time of data download, all enrolled subjects still in the study had completed at least 2 years of follow-up, with an early study dropout rate of approximately 10%.
Participants
Newly diagnosed, untreated patients with PD (n=423) were enrolled in PPMI. At baseline PD participants were required to: (1)have a recent idiopathic PD diagnosis, (2) be untreated for PD, (3) have a dopamine transporter deficit on the 123-I Ioflupane dopamine transporter (DatScan) on imaging and (4) not have dementia as determined by the site investigator. HC were matched by age and gender and were required to have no significant neurological dysfunction, no first-degree family member with PD and no cognitive impairment based on a Montreal Cognitive Assessment (MoCA) score >26. Participants were recruited at the participating sites and via central recruitment strategies, including online advertising and Fox Trial Finder (https://foxtrialfinder.michaeljfox.org/), the latter an online recruitment tool as previously reported.18 The study was approved by the institutional review board at each site, and participants provided written informed consent.
Study outcomes
For these analyses, NMS were rated with the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)19 Part I score (0–52) that we subdivided into the subscores for cognitive and psychiatric symptoms ((Q 1.1–1.6)(score range 0–24)), sleep (Q 1.7–1.8) (score range 0–8)), pain (Q 1.9 (0–4)), autonomic dysfunction (Q 1.10–1.12 (0–12)) and fatigue (Q 1.13 (0–4)). Each item is rated on a scale of 0–4: (0 none, 1 slight, 2 mild, 3 moderate and 4 severe). The MDS-UPDRS Part I scale has been shown to have strong convergent validity with the Non-Motor Symptoms Scale (NMSS).20
Other validated NMS scales available in the PPMI dataset include Montreal Cognitive Assessment (MoCA) for assessment of global cognitive abilities,21 the 15-item Geriatric Depression Scale (GDS-15),22 the Scale for Outcomes for PD-autonomic function (SCOPA-AUT),23 State and Trait Anxiety Scale (STAI),24 the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP),25 the Epworth Sleepiness Scale (ESS),26 the Rapid Eye Movement Behaviour Disorder (RBD) Questionnaire (RBDSQ)27 and the University of Pennsylvania Smell Identification Test (UPSIT)28; the latter was performed only at the baseline. In addition to MoCA, the participants undergo a detailed neuropsychiatric assessment. Of note, we included analysis of the cognitive domain of the MDS-UPDRS I as part of overall NMS, but a detailed analysis of baseline and longitudinal evolution of cognition in the PPMI cohort is out of the scope of this publication and has been previously reported.29 30
Other measures included basic demographic variables and the MDS-UPDRS total and subscale scores. Tremor score was calculated as a mean of 11 tremor items (2.10 and 3.15–3.18) and Postural instability Gait Disorder (PIGD) score as a mean of five items (2.12, 2.13 and 3.10–3.12).31 All subjects underwent DatScan measuring the dopamine transporter (DAT) that was analysed according to the imaging technical operations manual (http://ppmi-info.org/). Biological sample tests included cerebrospinal fluid (CSF) biomarkers (β-amyloid 1–42 (Aβ1–42), total tau (T-tau), phosphorylated tau (P-tau181) and unphosphorylated total α-synuclein (α-syn)) in all participants. The details of sample collection, processing and biomarker analyses have been previously reported.32 These measures are currently available only for the baseline and 12 months follow-up samples. Once participants start dopaminergic therapy (DT), the dose is reported as cumulative levodopa equivalent daily dose (LEDD) as well as LEDD by DT subclasses.33
Statistical methods
All statistical analyses were performed using SAS V.9.4 (SAS Institute, Cary, North Carolina, USA). Χ2 or t-tests were used to compare baseline demographics and PD characteristics between groups. Generalised linear mixed (GLM) models were used to test for changes in various non-motor scores over time separately in each group and test for differences between groups over time. In the latter models, an interaction for visit and group was tested before testing for a group difference over time. GLM models were also used to compare rates of progression among the three NMS subdomain scores over time. Repeated-measures linear mixed models were used to examine the impact of PD medications on NMS total and subdomain scores over time, in the subset of PD subjects who started treatment by year 1. Since no PD subjects were treated at baseline, only year 1 and year 2 time points were included in these models, while adjusting for baseline NMS score.
Linear models were used to examine the univariate and multivariable relationships between baseline demographic, clinical and biological predictors and either baseline NMS score or 2 year change in NMS score. Any variables with univariate associations with p-values <0.20 were included in a multivariable model, and a backwards selection process was used to remove variables individually until all remaining variables were significant at the 0.10 level. Additionally, linear models were used to examine whether baseline NMS total or subdomain scores were predictive of 2 year changes in the following motor disability scores: MDS-UPDRS motor score, tremor and PIGD scores.
Finally, logistic generalised estimating equations (GEE) were used to find estimates of time for each MDS-UPDRS Part 1 item in PD subjects, using a cut-off score of 2 (mild) or above for each item. A significance level of p=0.01 was used as the cut-off to account for multiple comparisons. A more formal method of adjustment for multiple comparisons was not used, as the authors felt this would have been too stringent given the exploratory nature of these analyses. See online supplementary file 1 for further details on the statistical analysis.
jnnp-2017-316213supp001.pdf (47.9KB, pdf)
Results
There were 423 PD subjects and 196 HC at baseline, and 2-year data were available for 380 PD and 174 HC subjects, with the decrease in sample size due to early study terminations. Baseline demographics and PD characteristics of the cohort are presented in table 1. MDS-UPDRS Part I total mean (SD) scores increased from baseline 5.6 (4.1) to 7.7 (5.0) at year 2 in PD subjects (p<0.001) versus from 2.9 (3.0) to 3.2 (3.0) in HC (p=0.38), with a significant difference between the groups in rates of change over time (p<0.001) (table 2). There was a significant increase over time in every MDS-UPDRS Part I subscore in PD but not HC. The most common NMSs in the PD cohort at baseline (present in >25% based on MDS-UPDRS responses) were sleep dysfunction (53%), pain and other sensation (52%), fatigue (50%), urinary problems (51%), excessive daytime sleepiness (EDS) (50%), anxiety (36%), constipation (33%) and fatigue (50%), though the majority of these were rated as slight (score=1) with a modest increase to mild severity (score=2) in years 1 and 2 (table 2). Sleep dysfunction and daytime sleepiness were the only NMS symptoms with the score of 2 or more in more than 10% of PD participants at baseline. Interestingly, the sleep problems score (Q 1.7) did not change significantly over time while EDS did increase.
Table 1.
Baseline demographics and PD characteristics
| Variable | PD subjects (n=423) | Healthy controls (HC) (n=196) | p Value (PD vs HC) |
| Age | |||
| Mean (SD) | 61.7 (9.7) | 60.9 (11.2) | 0.34 |
| (Min, Max) | (33.5, 84.9) | (30.6, 83.7) | |
| Missing | 0 | 0 | |
| Age | |||
| <56 years | 115 (27.2%) | 55 (28.1%) | 0.65 |
| 56–65 years | 152 (35.9%) | 76 (38.8%) | |
| >65 years | 156 (36.9%) | 65 (33.2%) | |
| Missing | 0 | 0 | |
| Gender | |||
| Male | 277 (65.5%) | 126 (64.3%) | 0.77 |
| Female | 146 (34.5%) | 70 (35.7%) | |
| Missing | 0 | 0 | |
| Education | |||
| <13 years | 76 (18.0%) | 29 (14.8%) | 0.33 |
| 13–23 years | 344 (81.3%) | 166 (84.7%) | |
| >23 years | 3 (0.7%) | 1 (0.5%) | |
| Missing | 0 | 0 | |
| Ethnicity | |||
| Hispanic/Latino | 9 (2.1%) | 3 (1.5%) | 0.62 |
| Not Hispanic/Latino | 414 (97.9%) | 193 (98.5%) | |
| Missing | 0 | 0 | |
| Race | |||
| White | 391 (92.4%) | 182 (92.7%) | 0.85 |
| Black/African-American | 6 (1.4%) | 9 (4.6%) | |
| Asian | 8 (1.9%) | 1 (0.5%) | |
| Other | 18 (4.3%) | 4 (2.0%) | |
| Missing | 0 | 0 | |
| Family history of PD | |||
| Family members w/PD | 103 (24.4%) | 10 (5.1%) | <0.0001 |
| No family members w/PD | 319 (75.6%) | 186 (94.9%) | |
| Missing | 1 | 0 | |
| Disease duration (Mon.) | |||
| Mean (SD) | 6.7 (6.5) | N/A | N/A |
| (Min, Max) | (0.4, 35.8) | N/A | |
| Missing | 0 | N/A | |
| Age of PD onset | |||
| Mean (SD) | 59.6 (10.0) | N/A | N/A |
| (Min, Max) | (25.4, 83.0) | N/A | |
| Missing | 8 | N/A | |
| Side most affected | |||
| Left | 179 (42.3%) | N/A | N/A |
| Right | 234 (55.3%) | N/A | |
| Symmetric | 10 (2.4%) | N/A | |
| Missing | 0 | N/A | |
| MDS-UPDRS mean (SD) score and subscores | |||
| MDS-UDPRS total score | 32.4 (13.1) | 4.6 (4.4) | <0.0001 |
| MDS-UDPRS Part I score | 5.6 (4.1) | 2.9 (3.0) | |
| MDS-UDPRS Part II score | 5.9 (4.2) | 0.5 (1.0) | |
| MDS-UDPRS Part III score | 20.9 (8.9) | 1.2 (2.2) | |
| Missing | 1 | 1 | |
| Hoehn and Yahr | |||
| Stage 0 | 0 (0.0%) | 193 (99.0%) | <0.0001 |
| Stage 1 | 185 (43.7%) | 2 (1.0%) | |
| Stage 2 | 236 (55.8%) | 0 (0.0%) | |
| Stages 3–5 | 2 (0.5%) | 0 (0.0%) | |
| Missing | 0 | 1 | |
| Modified Schwab and England Activities of Daily Living Scale | |||
| Mean (SD) | 93.2 (5.9) | N/A | N/A |
| (Min, Max) | (70.0, 100.0) | N/A | |
| Missing | 0 | N/A | |
| TD/non-TD classification | |||
| TD | 299 (70.9%) | 26 (13.3%) | <0.0001 |
| Non-TD | 123 (29.1%) | 169 (86.7%) | |
| Missing | 1 | 1 | |
| PIGD score | |||
| Mean (SD) | 0.23 (0.2) | 0.02 (0.1) | <0.0001 |
| (Min, Max) | (0.0, 1.4) | (0.0, 0.8) | |
| Missing | 1 | 1 | |
| Tremor score | |||
| Mean (SD) | 0.49 (0.3) | 0.03 (0.1) | <0.0001 |
| (Min, Max) | (0.0, 1.8) | (0.0, 0.6) | |
| Missing | 1 | 1 | |
Report generated on data submitted as of 4 January 2016.
MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale; PD, Parkinson’s disease; PIGD, postural instability gait disorder; TD, tremor dominant.
Table 2.
MDS-UPDRS Part I at baseline, year 1 and year 2
| Variable | PD subjects | Healthy controls (HC) | p-Values | |||||||
| Baseline (n=423) | Year 1 (n=398) | Year 2 (n=380) | p Value (change over time) | Baseline (n=196) | Year 1 (n=185) | Year 2 (n=174) | p Value (change over time) | Group*Visit Interaction |
PD versus HC | |
| MDS-UPDRS Part I score | ||||||||||
| Mean (SD) | 5.57 (4.06) | 6.78 (4.60) | 7.68 (5.02) | <0.0001 | 2.94 (2.96) | 3.21 (3.21) | 3.18 (3.03) | 0.3817 | <0.0001 | N/A* |
| (Min, Max) | (0.00, 24.00) | (0.00, 29.00) | (0.00, 26.00) | (0.00, 17.00) | (0.00, 16.00) | (0.00, 14.00) | ||||
| Missing | 1 | 3 | 4 | 1 | 0 | 1 | ||||
| Cognitive impairment | ||||||||||
| Normal | 315 (74.64%) | 279 (71.36%) | 242 (64.36%) | 0.0007 | 176 (90.26%) | 170 (91.89%) | 149 (86.13%) | 0.1354 | 0.5412 | <0.0001 |
| Slight | 94 (22.27%) | 95 (24.30%) | 102 (27.13%) | 18 (9.23%) | 15 (8.11%) | 24 (13.87%) | ||||
| Mild | 13 (3.08%) | 15 (3.84%) | 25 (6.65%) | 1 (0.51%) | 0 (0.00%) | 0 (0.00%) | ||||
| Moderate | 0 (0.00%) | 2 (0.51%) | 7 (1.86%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Severe | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Hallucinations and psychosis | ||||||||||
| Normal | 409 (96.92%) | 372 (95.14%) | 347 (92.29%) | 0.0057 | 194 (99.49%) | 185 (100.00%) | 171 (98.84%) | N/A† | N/A† | <0.0001 |
| Slight | 13 (3.08%) | 19 (4.86%) | 24 (6.38%) | 1 (0.51%) | 0 (0.00%) | 2 (1.16%) | ||||
| Mild | 0 (0.00%) | 0 (0.00%) | 4 (1.06%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Moderate | 0 (0.00%) | 0 (0.00%) | 1 (0.27%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Severe | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Depressed mood | ||||||||||
| Normal | 322 (76.30%) | 277 (70.84%) | 260 (69.15%) | 0.0111 | 171 (87.69%) | 163 (88.11%) | 153 (88.44%) | 0.9070 | 0.2815 | <0.0001 |
| Slight | 85 (20.14%) | 86 (21.99%) | 81 (21.54%) | 21 (10.77%) | 16 (8.65%) | 16 (9.25%) | ||||
| Mild | 14 (3.32%) | 23 (5.88%) | 23 (6.12%) | 2 (1.03%) | 4 (2.16%) | 4 (2.31%) | ||||
| Moderate | 0 (0.00%) | 5 (1.28%) | 10 (2.66%) | 0 (0.00%) | 2 (1.08%) | 0 (0.00%) | ||||
| Severe | 1 (0.24%) | 0 (0.00%) | 2 (0.53%) | 1 (0.51%) | 0 (0.00%) | 0 (0.00%) | ||||
| Anxious mood | ||||||||||
| Normal | 271 (64.22%) | 257 (65.73%) | 253 (67.29%) | 0.5020 | 157 (80.51%) | 155 (83.78%) | 147 (84.97%) | 0.2748 | 0.6480 | <0.0001 |
| Slight | 130 (30.81%) | 101 (25.83%) | 95 (25.27%) | 34 (17.44%) | 24 (12.97%) | 23 (13.29%) | ||||
| Mild | 17 (4.03%) | 29 (7.42%) | 18 (4.79%) | 3 (1.54%) | 6 (3.24%) | 3 (1.73%) | ||||
| Moderate | 3 (0.71%) | 4 (1.02%) | 9 (2.39%) | 1 (0.51%) | 0 (0.00%) | 0 (0.00%) | ||||
| Severe | 1 (0.24%) | 0 (0.00%) | 1 (0.27%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Apathy | ||||||||||
| Normal | 351 (83.18%) | 301 (76.98%) | 278 (73.94%) | 0.0002 | 186 (95.38%) | 178 (96.22%) | 165 (95.38%) | 0.8919 | 0.3936 | <0.0001 |
| Slight | 60 (14.22%) | 62 (15.86%) | 63 (16.76%) | 8 (4.10%) | 6 (3.24%) | 7 (4.05%) | ||||
| Mild | 10 (2.37%) | 25 (6.39%) | 27 (7.18%) | 1 (0.51%) | 1 (0.54%) | 1 (0.58%) | ||||
| Moderate | 0 (0.00%) | 3 (0.77%) | 7 (1.86%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Severe | 1 (0.24%) | 0 (0.00%) | 1 (0.27%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Dopamine dysregulation syndrome | ||||||||||
| Normal | 413 (97.87%) | 382 (97.70%) | 353 (94.13%) | 0.0188 | 192 (98.46%) | 185 (100.00%) | 171 (98.84%) | N/A† | N/A† | 0.0006 |
| Slight | 8 (1.90%) | 7 (1.79%) | 17 (4.53%) | 3 (1.54%) | 0 (0.00%) | 2 (1.16%) | ||||
| Mild | 1 (0.24%) | 2 (0.51%) | 5 (1.33%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Moderate | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Severe | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Sleep problems | ||||||||||
| Normal | 198 (46.92%) | 163 (41.69%) | 152 (40.43%) | 0.0655 | 110 (56.41%) | 94 (50.81%) | 95 (54.91%) | 0.2950 | 0.3815 | 0.0010 |
| Slight | 125 (29.62%) | 106 (27.11%) | 100 (26.60%) | 53 (27.18%) | 55 (29.73%) | 39 (22.54%) | ||||
| Mild | 62 (14.69%) | 79 (20.20%) | 80 (21.28%) | 19 (9.74%) | 23 (12.43%) | 24 (13.87%) | ||||
| Moderate | 26 (6.16%) | 35 (8.95%) | 31 (8.24%) | 10 (5.13%) | 11 (5.95%) | 13 (7.51%) | ||||
| Severe | 11 (2.61%) | 8 (2.05%) | 13 (3.46%) | 3 (1.54%) | 2 (1.08%) | 2 (1.16%) | ||||
| Daytime sleepiness | ||||||||||
| Normal | 213 (50.47%) | 150 (38.36%) | 118 (31.38%) | <0.0001 | 127 (65.13%) | 117 (63.24%) | 102 (58.96%) | 0.2332 | 0.0228 | N/A* |
| Slight | 123 (29.15%) | 133 (34.02%) | 132 (35.11%) | 49 (25.13%) | 42 (22.70%) | 46 (26.59%) | ||||
| Mild | 84 (19.91%) | 106 (27.11%) | 119 (31.65%) | 18 (9.23%) | 25 (13.51%) | 25 (14.45%) | ||||
| Moderate | 2 (0.47%) | 1 (0.26%) | 4 (1.06%) | 1 (0.51%) | 1 (0.54%) | 0 (0.00%) | ||||
| Severe | 0 (0.00%) | 1 (0.26%) | 3 (0.80%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Pain and other sensations | ||||||||||
| Normal | 201 (47.63%) | 168 (42.97%) | 142 (37.77%) | 0.0034 | 130 (66.67%) | 114 (61.96%) | 112 (64.74%) | 0.4874 | 0.2124 | <0.0001 |
| Slight | 168 (39.81%) | 151 (38.62%) | 171 (45.48%) | 53 (27.18%) | 55 (29.89%) | 46 (26.59%) | ||||
| Mild | 32 (7.58%) | 39 (9.97%) | 38 (10.11%) | 5 (2.56%) | 7 (3.80%) | 9 (5.20%) | ||||
| Moderate | 17 (4.03%) | 30 (7.67%) | 19 (5.05%) | 6 (3.08%) | 7 (3.80%) | 6 (3.47%) | ||||
| Severe | 4 (0.95%) | 3 (0.77%) | 6 (1.60%) | 1 (0.51%) | 1 (0.54%) | 0 (0.00%) | ||||
| Urinary problems | ||||||||||
| Normal | 207 (49.05%) | 170 (43.48%) | 160 (42.55%) | 0.0228 | 148 (75.90%) | 139 (75.54%) | 133 (76.88%) | 0.9005 | 0.3128 | <0.0001 |
| Slight | 165 (39.10%) | 160 (40.92%) | 142 (37.77%) | 39 (20.00%) | 33 (17.93%) | 31 (17.92%) | ||||
| Mild | 35 (8.29%) | 45 (11.51%) | 52 (13.83%) | 6 (3.08%) | 11 (5.98%) | 8 (4.62%) | ||||
| Moderate | 11 (2.61%) | 14 (3.58%) | 19 (5.05%) | 2 (1.03%) | 1 (0.54%) | 1 (0.58%) | ||||
| Severe | 4 (0.95%) | 2 (0.51%) | 3 (0.80%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Constipation | ||||||||||
| Normal | 282 (66.82%) | 206 (52.69%) | 187 (49.73%) | <0.0001 | 171 (87.69%) | 161 (87.03%) | 153 (88.44%) | 0.7779 | 0.0272 | N/A* |
| Slight | 109 (25.83%) | 139 (35.55%) | 135 (35.90%) | 21 (10.77%) | 22 (11.89%) | 18 (10.40%) | ||||
| Mild | 26 (6.16%) | 36 (9.21%) | 40 (10.64%) | 2 (1.03%) | 2 (1.08%) | 2 (1.16%) | ||||
| Moderate | 5 (1.18%) | 10 (2.56%) | 14 (3.72%) | 1 (0.51%) | 0 (0.00%) | 0 (0.00%) | ||||
| Severe | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Lightheadedness | ||||||||||
| Normal | 311 (73.70%) | 274 (70.08%) | 244 (64.89%) | 0.0061 | 175 (89.74%) | 163 (88.11%) | 157 (90.75%) | 0.6703 | 0.2232 | <0.0001 |
| Slight | 96 (22.75%) | 97 (24.81%) | 99 (26.33%) | 20 (10.26%) | 19 (10.27%) | 16 (9.25%) | ||||
| Mild | 12 (2.84%) | 18 (4.60%) | 23 (6.12%) | 0 (0.00%) | 3 (1.62%) | 0 (0.00%) | ||||
| Moderate | 3 (0.71%) | 2 (0.51%) | 9 (2.39%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Severe | 0 (0.00%) | 0 (0.00%) | 1 (0.27%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Fatigue | ||||||||||
| Normal | 210 (49.76%) | 181 (46.29%) | 161 (42.82%) | 0.0524 | 146 (74.87%) | 131 (70.81%) | 119 (68.79%) | 0.2992 | 0.9097 | <0.0001 |
| Slight | 165 (39.10%) | 144 (36.83%) | 138 (36.70%) | 44 (22.56%) | 46 (24.86%) | 48 (27.75%) | ||||
| Mild | 34 (8.06%) | 51 (13.04%) | 62 (16.49%) | 2 (1.03%) | 5 (2.70%) | 6 (3.47%) | ||||
| Moderate | 10 (2.37%) | 13 (3.32%) | 12 (3.19%) | 3 (1.54%) | 3 (1.62%) | 0 (0.00%) | ||||
| Severe | 3 (0.71%) | 2 (0.51%) | 3 (0.80%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||||
| Cognitive/psychiatric subdomain | ||||||||||
| Mean (SD) | 1.23 (1.57) | 1.53 (1.85) | 1.87 (2.27) | <0.0001 | 0.54 (1.08) | 0.48 (1.13) | 0.52 (1.01) | 0.6261 | <0.0001 | N/A* |
| (Min, Max) | (0.00, 13.00) | (0.00, 10.00) | (0.00, 14.00) | (0.00, 8.00) | (0.00, 8.00) | (0.00, 5.00) | ||||
| Missing | 1 | 7 | 4 | 1 | 0 | 1 | ||||
| Score=0 | 177 (41.94%) | 150 (38.36%) | 129 (34.31%) | 137 (70.26%) | 140 (75.68%) | 122 (70.52%) | ||||
| One or above | 245 (58.06%) | 241 (61.64%) | 247 (65.69%) | 0.0290 | 58 (29.74%) | 45 (24.32%) | 51 (29.48%) | 0.2169 | 0.1156 | <0.0001 |
| Two or above | 134 (31.75%) | 148 (37.85%) | 164 (43.62%) | 0.0001 | 27 (13.85%) | 18 (9.73%) | 20 (11.56%) | 0.2925 | 0.0204 | N/A* |
| Sleep subdomain | ||||||||||
| Mean (SD) | 1.58 (1.49) | 1.93 (1.55) | 2.13 (1.60) | <0.0001 | 1.13 (1.30) | 1.28 (1.30) | 1.33 (1.39) | 0.0737 | 0.0089 | N/A* |
| (Min, Max) | (0.00, 7.00) | (0.00, 8.00) | (0.00, 8.00) | (0.00, 6.00) | (0.00, 5.00) | (0.00, 6.00) | ||||
| Missing | 1 | 7 | 4 | 1 | 0 | 1 | ||||
| Score=0 | 123 (29.15%) | 87 (22.25%) | 70 (18.62%) | 83 (42.56%) | 63 (34.05%) | 64 (36.99%) | ||||
| One or above | 299 (70.85%) | 304 (77.75%) | 306 (81.38%) | 0.0003 | 112 (57.44%) | 122 (65.95%) | 109 (63.01%) | 0.1001 | 0.2159 | <0.0001 |
| Two or above | 187 (44.31%) | 221 (56.52%) | 235 (62.50%) | <0.0001 | 63 (32.31%) | 63 (34.05%) | 67 (38.73%) | 0.2330 | 0.0259 | N/A* |
| Autonomic subdomain | ||||||||||
| Mean (SD) | 1.40 (1.37) | 1.74 (1.48) | 1.99 (1.70) | <0.0001 | 0.54 (0.81) | 0.59 (0.86) | 0.51 (0.85) | 0.4648 | <0.0001 | N/A* |
| (Min, Max) | (0.00, 7.00) | (0.00, 10.00) | (0.00, 8.00) | (0.00, 4.00) | (0.00, 4.00) | (0.00, 5.00) | ||||
| Missing | 1 | 7 | 4 | 1 | 0 | 1 | ||||
| Score=0 | 127 (30.09%) | 88 (22.51%) | 75 (19.95%) | 120 (61.54%) | 111 (60.00%) | 113 (65.32%) | ||||
| One or above | 295 (69.91%) | 303 (77.49%) | 301 (80.05%) | 0.0002 | 75 (38.46%) | 74 (40.00%) | 60 (34.68%) | 0.3327 | 0.0060 | N/A* |
| Two or above | 162 (38.39%) | 198 (50.64%) | 209 (55.59%) | <0.0001 | 21 (10.77%) | 26 (14.05%) | 19 (10.98%) | 0.4175 | 0.0432 | N/A* |
Report generated on data submitted as of 04 January 2016.
p Values for individual Part I items are from logistic models considering response=0 versus any other response.
*PD versus HC comparison is not applicable if test of interaction was significant. A significant test of interaction means the rates of change over time are different between the two groups.
†Test could not be performed because all subjects from one group are in only one category for at least one time point.
MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale; N/A, not applicable;PD, Parkinson’s disease.
Figure 1 shows the probability of being positive (score of 2 or above) for each MDS-UDPRS Part 1 subdomain over time, while the inset table shows the ORs for a 1-year increase in follow-up time compared with baseline. Sleep and autonomic subdomains showed similar rates of change over time, and both subdomains showed greater changes over time compared with the cognitive subdomain. However, there was not sufficient evidence to conclude that the rates were significantly different (p=0.29).
Figure 1.
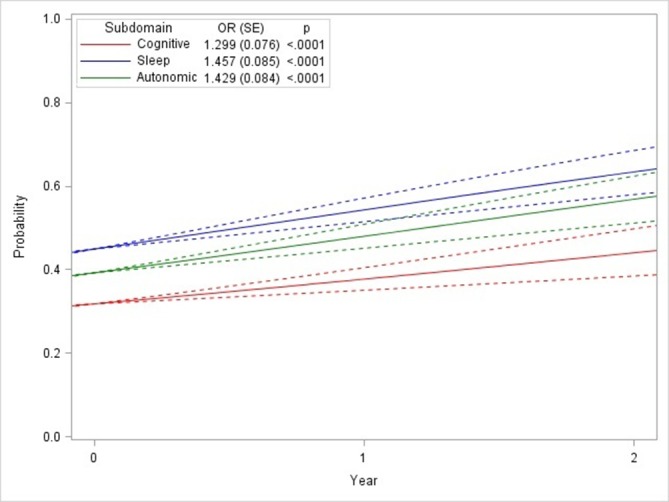
Probabilities (95% CI) and ORs of progression rates for non-motor symptom (NMS) subdomains. NMS domains are defined as Movement Disorder Society Unified Parkinson’s Disease Rating Scale Part I subscores as outlined in the publication narrative.
We then analysed evolution of the NMS in PD versus HC using individual domain NMS scales (table 3). Consistent with the MDS-UPDRS data, there was a significant longitudinal worsening in the scores of global cognition (MoCA), depression (GDS-15), autonomic dysfunction (SCOPA-AUT), and impulse control disorders (QUIP) in PD subjects, while HC worsened only in cognition and trait anxiety (STAI). There was a significant difference in the rate of change of SCOPA-AUT and QUIP between PD subjects (worse) and HC. As per statistical model, the overall PD vs HC group difference was reported only for variables that did not have significant group/visit interactions (table 3).
Table 3.
Non-motor scores at baseline, year 1 and year 2
| Variable | PD subjects | Healthy controls (HC) | p Values | |||||||
| Baseline (n=423) | Year 1 (n=398) | Year 2 (n=380) | p-Value (change over time) | Baseline (n=196) | Year 1 (n=185) | Year 2 (n=174) | p-Value (change over time) | Group*Visit interaction | PD versus HC | |
| MoCA | ||||||||||
| Mean (SD) | 27.13 (2.32) | 26.30 (2.83) | 26.26 (3.16) | <0.0001 | 28.23 (1.11) | 27.27 (2.19) | 27.25 (2.34) | <0.0001 | 0.8095 | <0.0001 |
| (Min, Max) | (17.00, 30.00) | (15.00, 30.00) | (9.00, 30.00) | (26.00, 30.00) | (20.00, 30.00) | (21.00, 30.00) | ||||
| Missing | 3 | 6 | 8 | 0 | 0 | 1 | ||||
| GDS | ||||||||||
| Mean (SD) | 2.32 (2.44) | 2.57 (2.92) | 2.63 (2.87) | 0.0049 | 1.29 (2.10) | 1.41 (2.36) | 1.16 (1.92) | 0.3403 | 0.1341 | <0.0001 |
| (Min, Max) | (0.00, 14.00) | (0.00, 15.00) | (0.00, 15.00) | (0.00, 15.00) | (0.00, 15.00) | (0.00, 12.00) | ||||
| Missing | 0 | 3 | 5 | 0 | 0 | 0 | ||||
| SCOPA-AUT | ||||||||||
| Mean (SD) | 9.51 (6.18) | 10.92 (6.44) | 11.53 (6.56) | <0.0001 | 5.83 (3.69) | 5.84 (4.40) | 6.04 (4.37) | 0.8642 | <0.0001 | NA* |
| (Min, Max) | (0.00, 39.00) | (0.00, 45.00) | (0.00, 42.00) | (0.00, 20.00) | (0.00, 22.00) | (0.00, 22.00) | ||||
| Missing | 8 | 8 | 9 | 2 | 1 | 1 | ||||
| STAI—state subscore | ||||||||||
| Mean (SD) | 32.96 (10.24) | 32.45 (10.02) | 32.48 (10.09) | 0.2572 | 28.02 (8.03) | 27.43 (8.59) | 27.94 (8.67) | 0.5887 | 0.9003 | <0.0001 |
| (Min, Max) | (20.00, 76.00) | (20.00, 77.00) | (20.00, 76.00) | (20.00, 58.00) | (20.00, 64.00) | (20.00, 78.00) | ||||
| Missing | 1 | 3 | 4 | 0 | 0 | 0 | ||||
| STAI—trait subscore | ||||||||||
| Mean (SD) | 32.37 (9.45) | 32.69 (9.68) | 32.58 (9.55) | 0.1596 | 29.11 (7.47) | 28.78 (8.77) | 28.00 (7.27) | 0.0041 | 0.0282 | NA* |
| (Min, Max) | (20.00, 63.00) | (20.00, 73.00) | (20.00, 66.00) | (20.00, 53.00) | (20.00, 64.00) | (20.00, 58.00) | ||||
| Missing | 1 | 3 | 4 | 0 | 0 | 0 | ||||
| Epworth Sleepiness Scale (ESS) | ||||||||||
| Negative (less than 10) | 357 (84.40%) | 329 (83.29%) | 290 (77.33%) | 0.4782 | 171 (87.69%) | 165 (89.19%) | 151 (86.78%) | 0.6304 | 0.3868 | 0.0145 |
| Positive (10 or above) | 66 (15.60%) | 66 (16.71%) | 85 (22.67%) | 24 (12.31%) | 20 (10.81%) | 23 (13.22%) | ||||
| Missing | 0 | 3 | 5 | 1 | 0 | 0 | ||||
| REM sleep behaviour disorder (RBDQ) | ||||||||||
| Negative (less than 5) | 261 (62.14%) | 256 (65.14%) | 218 (58.13%) | 0.4137 | 157 (80.10%) | 150 (81.08%) | 145 (83.33%) | 0.5163 | 0.1060 | <0.0001 |
| Positive (five or above) | 159 (37.86%) | 137 (34.86%) | 157 (41.87%) | 39 (19.90%) | 35 (18.92%) | 29 (16.67%) | ||||
| Missing | 3 | 5 | 5 | 0 | 0 | 0 | ||||
| QUIP | ||||||||||
| No disorders | 335 (79.38%) | 342 (86.58%) | 300 (79.79%) | 0.0025 | 160 (81.63%) | 149 (80.54%) | 145 (83.33%) | 0.5375 | 0.0135 | NA* |
| Any one or more disorders | 87 (20.62%) | 53 (13.42%) | 76 (20.21%) | 36 (18.37%) | 36 (19.46%) | 29 (16.67%) | ||||
| Missing | 1 | 3 | 4 | 0 | 0 | 0 | ||||
| Age-adjusted/sex-adjusted UPSIT | ||||||||||
| Normosmia | 39 (9.22%) | NA | NA | NA | 122 (62.24%) | NA | NA | NA | NA | <0.001† |
| Hyposmia | 237 (56.03%) | NA | NA | 69 (35.20%) | NA | NA | ||||
| Anosmia | 147 (34.75%) | NA | NA | 5 (2.55%) | NA | NA | ||||
| Missing | 0 | 0 | ||||||||
See text for the description of the scales. Higher scores are worse for GDS, SCOPA-AUT, STAI, ESS, RBDQ and QUIP. Higher scores are better for MoCA.
Report generated on data submitted as of 4January 2016.
*PD versus HC comparison is not applicable if test of interaction was significant. A significant test of interaction means the rates of change over time are different between the two groups.
†UPSIT p-value comes from a Χ2 test.
GDS, Geriatric Depression Scale; MoCA, Montreal Cognitive Assessment; PD, Parkinson’s disease; N/A, not applicable; QUIP, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease; REM, rapid eye movement; SCOPA-AUT Scale for Outcomes for Parkinson disease-autonomic function; STAI, State and Trait Anxiety Scale; UPSIT, University of Pennsylvania Smell Identification Test.
Variables associated with NMS at baseline, as measured by MDS-UPDRS Part I score, in the PD cohort are shown in table 4. The following baseline variables were significantly associated with the higher NMS score in the multivariate analysis model: female sex (p=0.008), higher MDS-UPDRS Part II scores (p<0.0001) and PIGD subscore (p=0.007). There was also a non-significant association (p=0.06) for higher t-tau/Aβ1–42 ratio predicting total NMS. There was no association with the level of CSF α-synuclein (p=0.44). There was no association of NMS with the degree of motor disability as measured by MDS-UPDRS Part III score, degree, distribution or laterality of presynaptic loss of dopaminergic function as measured by a DatScan. When the same multivariate statistical model was applied for the analysis of the baseline predictors of the longitudinal change of NMS, only older age at enrolment (p=0.008) and lower Aβ1–42 (p=0.005) were significant (table 5).
Table 4.
Baseline predictors of baseline NMS* in PD subjects
| Variable | Univariate analysis | Number of observations missing | Multivariable analysis | ||
| Parameter estimate (95% CI) | p-Value | Parameter estimate (95% CI) | p-Value | ||
| Age | 0.022 (−0.018 to 0.063) | 0.2760 | 0 | – | – |
| Gender (male) | −0.828 (−1.644 to 0.013) | 0.0465 | 0 | −0.952 (−1.659 to 0.245) | 0.0084 |
| Education (<13 years) | −0.491 (−1.503 to 0.521) | 0.3409 | 0 | – | – |
| Ethnicity (hispanic) | −0.472 (−3.166 to 2.221) | 0.7305 | 0 | – | – |
| Race (white) | −0.833 (−2.302 to 0.635) | 0.2651 | 0 | – | – |
| Family history of PD | 0.020 (−0.887 to 0.927) | 0.9656 | 1 | – | – |
| Age-adjusted/sex-adjusted UPSIT (Anosmia) | 0.832 (0.019 to 1.645) | 0.0449 | 0 | NS | NS |
| MDS-UPDRS Part II Score | 0.497 (0.417 to 0.577) | <0.0001 | 0 | 0.443 (0.351 to 0.534) | <0.0001 |
| MDS-UDPRS Part III Score | 0.091 (0.048 to 0.134) | <0.0001 | 0 | NS | NS |
| Modified Schwab and England Activities of Daily Living Scale | −0.209 (−0.273 to 0.146) | <0.0001 | 0 | NS | NS |
| Hoehn and Yahr (Stage 1) | −1.002 (−1.780 to 0.223) | 0.0118 | 0 | NS | NS |
| TD/non-TD classification | −1.382 (−2.228 to 0.536) | 0.0014 | 0 | Not included | Not included |
| PIGD score | 6.522 (4.896 to 8.149) | <0.0001 | 0 | 2.358 (0.645 to 4.072) | 0.0071 |
| Tremor score | 0.857 (−0.371 to 2.084) | 0.1708 | 0 | NS | NS |
| Side most affected (Left) | −0.038 (−0.765 to 0.688) | 0.9172 | 0 | – | – |
| Disease duration | 0.033 (−0.028 to 0.093) | 0.2870 | 0 | – | – |
| Age of PD onset | 0.021 (−0.019 to 0.060) | 0.3027 | 8 | – | – |
| Contralateral caudate | −0.698 (−1.400 to 0.003) | 0.0509 | 5 | NS | NS |
| Ipsilateral caudate | −0.460 (−1.122 to 0.202) | 0.1725 | 5 | Not included | Not included |
| Contralateral putamen | 0.162 (−1.289 to 1.613) | 0.8262 | 5 | – | – |
| Ipsilateral putamen | −0.412 (−1.436 to 0.612) | 0.4299 | 5 | – | – |
| A-Beta | −0.001 (−0.005 to 0.002) | 0.4458 | 11 | – | – |
| t-tau | 0.002 (−0.001, to 0.005) | 0.2257 | 15 | – | – |
| p-tau | −0.001 (−0.005 to 0.002) | 0.4738 | 13 | – | – |
| t-tau/A-Beta | 0.004 (0.0001 to 0.007) | 0.0389 | 15 | 0.003 (−0.0001 to 0.006) | 0.0611 |
| p-tau/A-Beta | −0.000 (−0.004 to 0.003) | 0.7923 | 13 | – | – |
| p-tau/t-tau | −0.003 (−0.007 to 0.0001) | 0.0462 | 17 | NS | NS |
| α-Synuclein | 0.001 (−0.002 to 0.005) | 0.4370 | 11 | – | – |
Report generated on data submitted as of 4 January 2016.
*NMS is defined as MDS-UPDRS Part I Score.
Note: TD/non-TD classification was not included in the multivariable model because tremor and PIGD scores were already being considered.
Similarly, ipsilateral caudate was not included in the multivariable model because contralateral caudate was already being considered.
MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale; NMS, non-motor symptoms; PD, Parkinson’s disease; PIGD, postural instability gait disorder; TD, tremor dominant; UPSIT, University of Pennsylvania Smell Identification Test.
Table 5.
Baseline predictors of change in NMS* from baseline to year 2 in PD subjects
| Variable | Univariate analysis | Number of observations missing | Multivariable analysis | ||
| Parameter estimate (95% CI) | p Value | Parameter estimate (95% CI) | p-Value | ||
| Age | 0.064 (0.023 to 0.106) | 0.0024 | 0 | 0.057 (0.015 to 0.099) | 0.0078 |
| Gender (male) | 0.722 (−0.134 to 1.578) | 0.0982 | 0 | NS | NS |
| Education (<13 years) | 0.623 (−0.461 to 1.706) | 0.2590 | 0 | – | – |
| Ethnicity (hispanic) | −2.354 (−5.011 to 0.302) | 0.0822 | 0 | NS | NS |
| Race (white) | 0.457 (−1.048 to 1.961) | 0.5510 | 0 | – | – |
| Family history of PD | −0.664 (−1.601 to 0.274) | 0.1649 | 1 | NS | NS |
| Age-adjusted/sex-adjusted UPSIT (anosmia) | 0.332 (−0.523 to 1.186) | 0.4458 | 0 | – | – |
| MDS-UPDRS Part II score | 0.061 (−0.039 to 0.161) | 0.2312 | 0 | – | – |
| MDS-UDPRS Part III score | 0.000 (−0.046 to 0.047) | 0.9884 | 0 | -– | – |
| Modified Schwab and England Activities of Daily Living Scale | −0.006 (−0.077 to 0.065) | 0.8631 | 0 | – | – |
| Hoehn and Yahr (Stage 1) | −0.053 (−0.873 to 0.767) | 0.8991 | 0 | – | – |
| TD/non-TD classification | 0.236 (−0.665 to 1.137) | 0.6067 | 0 | – | – |
| PIGD score | −0.987 (−2.797 to 0.824) | 0.2845 | 0 | – | – |
| Tremor score | −0.111 (−1.383 to 1.162) | 0.8641 | 0 | – | – |
| Side most affected (left) | 0.565 (−0.194 to 1.323) | 0.1440 | 0 | NS | NS |
| Disease duration | 0.033 (−0.029 to 0.095) | 0.2905 | 0 | – | – |
| Age of PD onset | 0.060 (0.019 to 0.101) | 0.0041 | 7 | NS | NS |
| Contralateral caudate | −0.329 (−1.088 to 0.431) | 0.3952 | 4 | – | – |
| Ipsilateral caudate | −0.530 (−1.224 to 0.164) | 0.1341 | 4 | NS | NS |
| Contralateral putamen | −0.887 (−2.565 to 0.791) | 0.2993 | 4 | – | – |
| Ipsilateral putamen | −1.298 (−2.381 to 0.215) | 0.0189 | 4 | NS | NS |
| A-Beta | −0.005 (−0.009 to 0.002) | 0.0024 | 9 | −0.005 (−0.008 to 0.002) | 0.0046 |
| t-tau | −0.003 (−0.006 to 0.001) | 0.0966 | 13 | NS | NS |
| p-tau | −0.001 (−0.004 to 0.003) | 0.6422 | 11 | – | – |
| t-tau/A-Beta | −0.001 (−0.004 to 0.003) | 0.6951 | 13 | – | – |
| p-tau/A-Beta | 0.002 (−0.001 to 0.006) | 0.2024 | 11 | – | – |
| p-tau/t-tau | 0.001 (−0.002 to 0.005) | 0.5213 | 15 | – | – |
| α-Synuclein | −0.002 (−0.005 to 0.002) | 0.3010 | 9 | – | – |
Report generated on data submitted as of 4 January 2016.
*NMS is defined as MDS-UPDRS Part 1 score.
MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale; NMS, non-motor symptoms; PD, Parkinson’s disease;N.S, not significant; PIGD, postural instability gait disorder; TD, tremor dominant; UPSIT, University of Pennsylvania Smell Identification Test.
Given the substantial literature on the potential impact of DT on manifestations of different domains of NMS, we ran a number of analyses. We first compared the degree of change in NMS in treated (n=306) versus untreated (n=69) PD subjects at 2 years and there was no significant difference (p=0.42) (data not shown). We then explored the impact of cumulative dose of DT and specific classes of DT (both expressed as LEDD)33 on progression of NMS in the subset of treated PD subjects and there were no significant differences (see table 1 in the online supplementary file 1), though LEDD was low overall, 292 (222) mg/day at year 1 and 420 (295) mg/day at year 2. We reran the latter analysis looking at subdomains of NMS, specifically cognition, sleep and autonomic dysfunction, and there was no significant impact of DT on any NMS subdomains.
We also ran analyses to assess the impact of baseline NMS total and subdomain scores on rate of change of motor disability (MDS-UPDRS Part III), as well as tremor and PIGD scores, and did not find an association (see table 2 in the online supplementary file 1).
Discussion
Our study represents one of the largest observational studies of the baseline prevalence and longitudinal evolution of NMS in participants with newly diagnosed, at baseline untreated, PD compared with non-PD controls. Consistent with the prior reports,5 11–15 our data demonstrate a higher NMS burden and a significant increase over 2 years in NMS in early at baseline untreated PD compared with HC. Our data demonstrate high prevalence and significant change over time but mild severity overall in total NMS scale over time (5.6 (4.1) vs 7.7 (5.0) (range 0–52) (p<0.0001)). We also affirm prior reports that progression of NMS is not associated with the dose or class of DT.9 Our preplanned analysis was focused on overall NMS burden. Detailed analysis of the predictors of progression of specific cognitive tests or domains in the PPMI cohort is beyond the scope of this publication and has been recently published.34
There are a number of novel aspects of the study to be highlighted. This is to our knowledge the largest controlled longitudinal study of the evolution of NMS in early untreated PD versus HC. Erro et al reported initially 2 year and more recently 4-year longitudinal data on the baseline prevalence and longitudinal evolution of NMS in a cohort of 91 patients with PD untreated at baseline using the NMS questionnaire,9 13 but the study did not include a control group. Similarly, they found that the majority of patients (97.8%) reported at least one NMS at baseline, with limited overall longitudinal change. They did not see a major impact of DT on overall NMS. In their 4-year follow-up data on a subset of that cohort (n=61), they again demonstrated progression of majority of NMS and lack of association with any class of DT or total LEDD, and lack of association with the motor disability as measured by UPDRS III.9 Thus, both longitudinal studies provide consistent data. However, our data offer an additional important component of comparison to HC as NMS are also related to general ageing. So far, there has been only one other controlled longitudinal study of clinical and biological predictors of PD progression in a baseline de novo PD population.11 15 Mollenhauer et al reported a 2-year single centre follow-up study with extensive phenotypic characterisation and biological ascertainment that included MRI and CSF biomarkers. In contrast to our analysis, they did not identify an association of NMS with the CSF measures but the numbers were smaller and the CSF assay was different. That underscores the exploratory nature of the CSF biomarker findings that will require replication in other cohorts.
Another novel aspect is that we included a multivariate analysis of the baseline variables associated with the higher baseline NMS score and baseline predictors of the longitudinal change of NMS. Such analyses are important as they can provide the clues to the biological underpinnings of NMS as well as help with stratification factors or inclusion criteria that may be useful in designing clinical trials. For the analysis of the baseline NMS burden, we identified three variables: female sex (p=0.008), higher MDS-UPDRS II scores (p<0.0001) and PIGD subscore (p=0.007). Association with the PIGD score and lack of association with the motor disability (MDS-UPDRS III) are not surprising and have been reported by others both in advanced PD cohorts and in the de novo PD population (see Marras and Chaudhari16 for review). Association of specific domains of NMS (depression, cognitive scores) with the female sex in early PD has also been previously reported though the data have been inconsistent (see Picillo et al for review).35–39
Association of older age with the higher burden of NMS is not unexpected and has been well established as a risk factor for overall rate of progression of PD disability.40 In the analysis of the baseline predictors of the longitudinal change of NMS, only two variables were significant, age at the time of analysis but not age of PD onset and lower CSF Aβ1–42. Interestingly, age was not significantly associated with baseline severity of NMS but was with the rate of progression. The opposite was true for female sex, as that was not a significant predictor of the longitudinal change in NMS but was associated with the baseline NMS severity. Difference in the variables associated with the baseline NMS burden versus longitudinal progression of NMS might reflect difference in biological underpinnings of the disease trait versus state and deserve further exploration with the longer follow-up and validation in other cohorts.
The major novel aspect of this study is the inclusion of biomarker data. We did not find an association of baseline burden or rate of NMS progression with the DAT imaging. That could be related to the hypothesis that NMS are largely driven by non-dopaminergic pathways or alternatively that the analysis did not tease out specific domains of NMS that could be more linked to the degree of dopamine deficiency. In that regard, it would be instructive to also have serotonin and cholinergic ligand imaging, but these are not part of the dataset.
In the multivariate analysis of CSF biomarkers, we identified a trend for higher t-tau/Aβ1–42 ratio (p=0.06) association with NMS at baseline and lower level of Aβ1–42 (p=0.005) association with longitudinal progression of NMS. There was no association with α-synuclein. The role of β-amyloid and tau protein as biomarkers in PD is not well established, but number of publications including a pilot PPMI data analysis reported reduction of levels of CSF Aβ1–42, t-tau or p-tau in patients with PD with or without dementia compared with HC.30 32 41–44 A lower level of Aβ1–42 was associated with the baseline level of somnolence in the PPMI cohort, though again at a marginal level of significance.45 One possibility is that the low CSF Aβ1–42 reflects the presence of early stages of cortical Aβ1–42 deposition in PD, manifesting with various NMS, analogous to the occurrence of various neuropsychiatric symptoms and sleep disturbances prior to the diagnosis of Alzheimer’s dementia.46 47 It remains to be determined if the association with the lower level of Aβ1–42 will persist with longer follow-up and if an association with α-synuclein will emerge at more advanced staged of PD.
There are a number of study limitations to be acknowledged. PPMI cohort might not be reflective of the general PD population, as based on the fairly demanding nature of the study ascertainments the study attracts younger and relatively cognitively intact at baseline. Indeed, the age of the cohort is younger than the average age of PD onset though is consistent with the cohorts recruited in majority of the disease modification trials. The same can be true of the healthy control cohort that might be representative of the ‘super controls’ though inclusion criteria for HC were fairly broad aside from the cognitive cut-offs. Our results will have to be confirmed in longer duration follow-up that is planned. In addition, NMS assessment was based on MDS-UPDRS Part 1 and selected domain-specific scales. As such it lacked in-depth assessment of some domains like sleep or RBD with polysomnography, comprehensive objective assessment of autonomic dysfunction, or other specific domain scales and could have missed other variables. Lastly, NMS assessments were based on PD-specific scales and might not be optimal for assessment of HC cohort. Despite that, we believe that PPMI has a sufficiently rich ascertainment of the NMS domain of PD and HC to provide meaningful data analysis. In conclusion, our study confirms previous reports of high prevalence and significant longitudinal increase even over a relatively short time period of essentially all domains of NMS in early at baseline untreated PD participants. Increase of overall NMS in early PD was not linked to either cumulative dose or specific classes of DT, though it remains to be determined if this will remain true with the increase of the LEDD as the disease progresses. Association of NMS progression with the lower baseline levels of Aβ1–42 is intriguing and can contribute to the knowledge of the potential biological underpinnings of NMS in PD, though this will have to be replicated in other cohorts. Further longitudinal data will establish impact of early NMS on the long-term progression of PD disability and could be helpful for subtyping of PD population and potentially for stratification into clinical trial.
Acknowledgments
Melanie Chitwood: editorial review of the manuscript and submisison.
Footnotes
Contributors: TS, CCG, DW, SL, BM, CMT, DJ, LC and KN: drafting/revising manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. CCG: statistical analysis. CC: study concept or design, analysis or interpretation of data and acquisition of data. SL, KM: study supervision or coordination.
Funding: Study Funding: PPMIis sponsored by the Michael J. Fox Foundation for Parkinson’s Research (MJFF)and is co-funded by MJFF, Abbvie, Avid Radiopharmaceuticals, Biogen Idec,Bristol-Myers Squibb, Biolegend, Eli Lilly & Co., F. Hoffman-La Roche, Ltd.,GE Healthcare, Genentech, GlaxoSmithKline, Lundbeck, Merck, MesoScale, Piramal,Pfizer, Servier. TEVA, Takeda, Sanofi Genzyme and UCB.
Disclaimer: Statistical analysis: CCG, Clinical Trials Statistical and Data Management Center, University of Iowa, Iowa City, IA, USA. Search Terms: Parkinson’s disease, excessive daytime sleepiness, case control study, biomarkers. Melanie Chitwood: editorial review of the manuscript and submission. Dr Tanya Simuni had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. PPMI is sponsored by the Michael J. Fox Foundation for Parkinson’s Research (MJFF) and is cofunded by MJFF, Abbvie, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Covance, EliLilly and Company, F. Hoffman-La Roche, GE Healthcare, Genentech, GlaxoSmithKline, Lundbeck, Merck, MesoScale, Piramal, Pfizer and UCB.
Competing interests: TS reports grants and non-financial support from Michael J Fox Foundation during the conduct of the study; personal fees from Acadia, Abbvie, Allergan, Anavex, Avid, GE Medical, Eli Lilly and Company, Harbor, Ibsen, IMPAX, Merz, Voyager, US World Meds, Pfizer and UCB; grants and personal fees from Lundbeck, National Parkinson’s Foundation, Navidea, Teva and Michael J Fox Foundation; grants from NINDS, Northwestern Foundation, NIH, Auspex, Biotie, Civitas, Acorda and Neuroderm, outside the submitted work. CC-G reports grants from The Michael J Fox Foundation, during the conduct of the study; grants and personal fees from Michael J Fox Foundation, outside the submitted work. CC reports grants from The Michael J Fox Foundation, during the conduct of the study; grants and personal fees from NIH/NINDS (NIH/NINDS, U01 NS077352, PI, 10/01/11-09/30/18 (2) NIH/NINDS, U01 NS077108, PI, 10/01/11-09/30/16(3) NIH/NHLBI, U01 HL091843, PI, 08/01/09-02/28/15(4) NIH/NHLBI, U01 NS038529, PI, 12/01/09-12/31/13 NIH/NINDS,(5) U01 NS079163, 08/05/2012-07/31/2015 (6) NIH/NINDS, U01 NS082329, 07/15/2013-06/30/2018 (7) NIH/NINDS, U01 NS084495, 09/15/2013-07/31/2018), personal fees from NIA, Rho Inc. and ZZ Biotech, outside the submitted work. DW reports grants from The Michael J Fox Foundation, during the conduct of the study; grants from Michael J Fox Foundation, NIH/NINDS, Dept. of Veterans Affairs, Avid Radiopharmaceuticals, Alzheimer’s Disease Cooperative Study, and International Parkinson’s Disease and Movement Disorder Society; grants and personal fees from Novartis; personal fees from AbbVie, Acadia, Biogen , Biotie (Acorda), Clintrex LLC, Eisai, Eli Lilly , Janssen, Merck, Pfizer, Takeda, Teva, UCB, CHDI Foundation, other from U Penn and Wolters Kluweland, outside the submitted work. Weintraub receives fees for legal consultation for lawsuits related to medication prescribing in patients with Parkinson’s disease. BM is employed by Parcacelsus Kliniken Germany and the University medical center Goettingen. BM reports grants and personal fees from Teva Pharma; grants from Desitin, Boehringer Ingelheim , GE Healthcare, BMBF, EU, Parkinson Fond Deutschland, Deutsche Parkinson Vereinigung and Stifterverband für die deutsche Wissenschaft; personal fees from Bayer Schering, Pharma AG, Roche, AbbVie, Glaxo Smith Kline, Orion Pharma and Michael J Fox Foundation; personal fees and scientific collaborations with Biogen; scientific collaborations with Roche, Bristol Myers Squibb, Eli Lilly and Covance, outside the submitted work. SL is employed by Molecular NeuroImaging, LLC. CMT is an employee of the San Francisco Veterans Affairs Medical Center and the University of California – San Francisco. CMT reports grants from Michael J Fox Foundation, during the conduct of the study; grants from Michael J Fox Foundation, Parkinson’s Disease Foundation, Dept. of Defence, Sage Bionetworks, NIH; personal fees from Biotie Therapeutics, Voyager Therapeutics, Neurocrine Biosciences, Adamas Pharmaceuticals , Intec Pharmaceuticals, outside the submitted work. DJ is an employee of Eli Lilly. KK reports personal fees from NIH/NIND, Acord, Astellas Pharma, AstraZeneca, Auspex, Biotie, Britannia, Cangene, CHDI, Civitas, Clearpoint Strategy Group, Clintrex, Cynapsus, INC Research, IntecIsis, Eli Lilly, Lundbeck, Medavante, Medivation , Melior Discovery , Neuroderm, Neurmedix, Omeros, Otsuka, Pfizer, Pharma2B, Prothena/Neotope/Elan Pharmaceutical, Raptor Pharmaceutical, Roche/Genentech, Sage Bionetworks, Stealth Peptides, Synagile, Teikoku Pharma, Titan, Turing Pharmaceuticals, Upsher-Smith, US WorldMeds, Vaccinex, Voyager and Westeon Brain Institut; grants from NIH, Micheal J Fox Foundation and Teva, outside the submitted work. LC reports grants from Michael J Fox Foundation, during the conduct of the study; grants from Michael J Fox Foundation, personal fees from Wolters Kluwel (for book authorship), outside the submitted work. KM reports grants from Michael J Fox Foundation, during the conduct of the study; personal fees from Pfizer, GE Healthcare, Merck, Lilly, BMS, Piramal, Prothena, Neurophage, nLife, Roche; grants from Michael J Fox Foundation, outside the submitted work; and ownership in Molecular NeuroImaging, LLC.
Patient consent: The study was approved by the institutional review board at each site.
Ethics approval: TheResearch Subjects Review Board at the University of Rochesterapproved the PPMI study protocol.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
Collaborators: The Parkinson’s Progressive Marker Initiative—PPMI: PPMI Steering Committee: Kenneth Marek, MD (Principal Investigator); Shirley Lasch, MBA; Caroline Tanner, MD, PhD (Site Investigator); Tanya Simuni, MD (Site Investigator); Christopher Coffey, PhD (Statistics Core, PI); Karl Kieburtz, MD, MPH (Clinical Core, PI); Renee Wilson; Brit Mollenhauer, MD (Bioanalytics Core, co-PI; Site Investigator); Douglas Galasko, MD (Bioanalytics Core, co-PI; Site Investigator); Tatiana Foroud, PhD (Genetics Coordination Core and Biorepository, PI); Lana Chahine, MD (Site Investigator); Andrew Siderowf, MD, MSCE; John Seibyl, MD (Imaging Core, PI); Arthur Toga, PhD (Bioinformatics Core, PI); Andrew Singleton, PhD (Genetics Core, PI); Daniel Weintraub, MD (Cognitive and Behavioral); John Trojanowski, MD, PhD; Leslie Shaw, PhD; Duygu Tosun-Turgut, PhD (DTI, PI); Kathleen Poston, MD, MS (fMRI, PI); Susan Bressman, MD; Kalpana M Merchant, MD; Werner Poewe, MD (Site Investigator); Todd Sherer, PhD; Sohini Chowdhury; Mark Frasier, PhD; Catherine Kopil, PhD; Anna Naito, PhD and Vanessa Arnedo were responsible for the scientific rationale, study design, site selection, logistics, data management and analysis planning for the study. PPMI Study Cores: Clinical Coordination Core: Ray Dorsey, PhD; Cynthia Casaceli, MBA—The Clinical Coordination Core was responsible for study concept or design and analysis or interpretation of clinical including collection, tracking and performance of quality control. Imaging Core: Nichole Daegele; Justin Albani—The Imaging Core was responsible for study concept or design and analysis or interpretation of imaging data. Statistics Core: Chelsea Caspell-Garcia; Liz Uribe, MS; Eric Foster; Jeff Long, PhD; Nick Seedorff—The Statistics Core led the statistical analyses and supported study concept or design and analysis or interpretation of data. Bioinformatics Core: Karen Crawford, MLIS—The Bioinformatics core was responsible for concept or design and maintained the PPMI study database to contribute to the support of the analytic analysis and data quality. Bio Repository: Danielle Elise Smith; Paola Casalin; Giulia Malferrari—The Biorepository Core was responsible for study concept or design, analysis or interpretation of biosamples, collection quality and data analysis. Genetics Coordination and Pathology Core: Cheryl Halter; Laura Heathers—The Genetics Coordination Core contributed to study concept or design and analysis or interpretation of cohort data. PPMI Site Investigators: David Russell, MD, PhD; Stewart Factor, DO; Penelope Hogarth, MD; David Standaert, MD, PhD; Amy Amara, MD, PhD; Robert Hauser, MD, MBA; Joseph Jankovic, MD; Matthew Stern, MD; Shu-Ching Hu, MD PhD; Gretchen Todd; Rachel Saunders-Pullman MD; Irene Richard, MD; Marie H Saint-Hilaire, MD; Klaus Seppi, MD; Holly Shill, MD; Hubert Fernandez, MD; Claudia Trenkwalder, MD; Wolfgang Oertel MD; Daniela Berg, MD; Kathrin Brockman, MD; Isabel Wurster MD; Liana Rosenthal, MD; Yen Tai, MD; Nicola Pavese, MD; Paolo Barone, MD, PhD; Stuart Isaacson, MD; Alberto Espay, MD, MSc; Dominic Rowe, MD, PhD; Melanie Brandabur MD; James Tetrud MD; GraceLiang MD; Alex Iranzo, MD; Eduardo Tolosa MD; Karen Marder, MD; Maria de ArribaSanchez, MD; Leonidis Stefanis, MD, PhD; Maria Jose Marti, MD, PhD; Javier Ruiz Martinez, MD, PhD; Jean-Christophe Corvol, MD; Jan O Assly, MD; Salima Brillman, MD and Nir Giladi, MD were responsible for data collection and contribution to data quality and analysis. PPMI Site Coordinators: Debra Smejdir; Julia Pelaggi; Farah Kausar, PhD; Linda Rees, MPH; Barbara Sommerfield, MSN, RN; Madeline Cresswell; Courtney Blair, MA; Karen Williams; Grace Zimmerman; Stephanie Guthrie, MSN; Ashlee Rawlins; Leigh Donharl; Christine Hunter, RN; Baochan Tran; Abigail Darin; Carly Linder; Marne Baca; Heli Venkov; Cathi-Ann Thomas, RN, MS; Raymond James, RN; Beatrice Heim, MD; Paul Deritis; Fabienne Sprenger, MD; Deborah Raymond; Diana Willeke; Zoran Obradov, CRC; Jennifer Mule; Nancy Monahan; Katharina Gauss; Deborah Fontaine, BSN, MS; Daniel Szpak; Arita McCoy; Becky Dunlop; Laura Marie Payne; Susan Ainscough; Lisbeth Carvajal; Rebecca Silverstein; Kristy Espay; Madelaine Ranola; Elisabet Mondragon Rezola; Helen Mejia Santana; Maria Stamelou, MD, PhD; Alicia Garrido, MD; Stephanie Carvalho, MS; Anne Grete Kristiansen; Krista Specketer Anat Mirlman were responsible for data collection and contribution to data quality. ISAB (Industry Scientific Advisory Board): Maurizio Facheris, MD; Holly Soares, PhD; Mark A Mintun, MD; Jesse Cedarbaum, MD; Peggy Taylor, ScD; Danna Jennings, MD; Lawrence Slieker, PhD; Brian McBride, PhD; Colin Watson, PhD; Etienne Montagut, MBA; Zulfiqar Haider Sheikh; Baris Bingol, PhD; Remi Forrat; PabloSardi, PhD; Tanya Fischer, MD, PhD; Alastair D Reith, PhD; Jan Egebjerg, PhD; Lone Frydelund Larsen; Nathalie Breysse, PhD; Didier Meulien, MD; Barbara Saba,MD; Vera Kiyasova, MD, PhD; Chris Min, MD, PhD; Thomas McAvoy, PhD; Robert Umek, PhD; Philip Iredale, PhD; Jeremy Edgerton, PhD; Susan De Santi, PhD;Christian Czech, PhD; Frank Boess, PhD; Jeffrey Sevigny, MD; Thomas Kremer, PhD; Igor Grachev, MD, PhD; Kaplana Merchant, PhD; Andreja Avbersek, MD; Pierandrea Muglia, MD; Alexandra Stewart, MBA; Rene Prashad, PhD and JohannesTaucher, MD were responsible for funding and scientific rationale.
References
- 1. Abbott RD, Ross GW, White LR, et al. . Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 2005;65:1442–6. 10.1212/01.wnl.0000183056.89590.0d [DOI] [PubMed] [Google Scholar]
- 2. Breen DP, Williams-Gray CH, Mason SL, et al. . Excessive daytime sleepiness and its risk factors in incident Parkinson’s disease. J Neurol Neurosurg Psychiatry 2013;84:233–4. 10.1136/jnnp-2012-304097 [DOI] [PubMed] [Google Scholar]
- 3. Abbott RD, Petrovitch H, White LR, et al. . Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 2001;57:456–62. 10.1212/WNL.57.3.456 [DOI] [PubMed] [Google Scholar]
- 4. Ross GW, Petrovitch H, Abbott RD, et al. . Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol 2008;63:167–73. 10.1002/ana.21291 [DOI] [PubMed] [Google Scholar]
- 5. Pont-Sunyer C, Hotter A, Gaig C, et al. . The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov Disord 2015;30:229–37. 10.1002/mds.26077 [DOI] [PubMed] [Google Scholar]
- 6. Braak H, Ghebremedhin E, Rüb U, et al. . Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 2004;318:121–34. 10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- 7. Antonini A, Barone P, Marconi R, et al. . The progression of non-motor symptoms in Parkinson’s disease and their contribution to motor disability and quality of life. J Neurol 2012;259:2621–31. 10.1007/s00415-012-6557-8 [DOI] [PubMed] [Google Scholar]
- 8. Barone P, Antonini A, Colosimo C, et al. . The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009;24:1641–9. 10.1002/mds.22643 [DOI] [PubMed] [Google Scholar]
- 9. Erro R, Picillo M, Vitale C, et al. . The non-motor side of the honeymoon period of Parkinson’s disease and its relationship with quality of life: a 4-year longitudinal study. Eur J Neurol 2016;23:1673–9. 10.1111/ene.13106 [DOI] [PubMed] [Google Scholar]
- 10. Duncan GW, Khoo TK, Yarnall AJ, et al. . Health-related quality of life in early Parkinson’s disease: the impact of nonmotor symptoms. Mov Disord 2014;29:195–202. 10.1002/mds.25664 [DOI] [PubMed] [Google Scholar]
- 11. Mollenhauer B, Trautmann E, Sixel-Döring F, et al. . Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013;81:1226–34. 10.1212/WNL.0b013e3182a6cbd5 [DOI] [PubMed] [Google Scholar]
- 12. Ongre SO, Larsen JP, Tysnes OB, et al. . Fatigue in early Parkinson’s disease: the Norwegian ParkWest study. Eur J Neurol 2017;24:105–11. 10.1111/ene.13161 [DOI] [PubMed] [Google Scholar]
- 13. Erro R, Picillo M, Vitale C, et al. . Non-motor symptoms in early Parkinson’s disease: a 2-year follow-up study on previously untreated patients. J Neurol Neurosurg Psychiatry 2013;84:14–17. 10.1136/jnnp-2012-303419 [DOI] [PubMed] [Google Scholar]
- 14. Zis P, Martinez-Martin P, Sauerbier A, et al. . Non-motor symptoms burden in treated and untreated early Parkinson’s disease patients: argument for non-motor subtypes. Eur J Neurol 2015;22:1145–50. 10.1111/ene.12733 [DOI] [PubMed] [Google Scholar]
- 15. Mollenhauer B, Zimmermann J, Sixel-Döring F, et al. . Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology 2016;87:168–77. 10.1212/WNL.0000000000002651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov Disord 2016;31:1095–102. 10.1002/mds.26510 [DOI] [PubMed] [Google Scholar]
- 17. Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011;95:629–35. 10.1016/j.pneurobio.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malek N, Swallow DM, Grosset KA, et al. . Tracking Parkinson’s: study design and baseline patient data. J Parkinsons Dis 2015;5:947–59. 10.3233/JPD-150662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goetz CG. [Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): a new scale for the evaluation of Parkinson’s disease]. Rev Neurol 2010;166:1–4. 10.1016/j.neurol.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 20. Martinez-Martin P, Chaudhuri KR, Rojo-Abuin JM, et al. . Assessing the non-motor symptoms of Parkinson’s disease: MDS-UPDRS and NMS Scale. Eur J Neurol 2015;22:37–43. 10.1111/ene.12165 [DOI] [PubMed] [Google Scholar]
- 21. Nasreddine ZS, Phillips NA, Bédirian V, et al. . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 22. Weintraub D, Oehlberg KA, Katz IR, et al. . Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry 2006;14:169–75. 10.1097/01.JGP.0000192488.66049.4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez-Blazquez C, Forjaz MJ, Frades-Payo B, et al. . Independent validation of the scales for outcomes in Parkinson’s disease-autonomic (SCOPA-AUT). Eur J Neurol 2010;17:194–201. 10.1111/j.1468-1331.2009.02788.x [DOI] [PubMed] [Google Scholar]
- 24. Kendall PC, Finch AJ, Auerbach SM, et al. . The state-trait anxiety inventory: a systematic evaluation. J Consult Clin Psychol 1976;44:406–12. 10.1037/0022-006X.44.3.406 [DOI] [PubMed] [Google Scholar]
- 25. Weintraub D, Hoops S, Shea JA, et al. . Validation of the questionnaire for impulsive–compulsive disorders in Parkinson’s disease. Mov Disord 2009;24:1461–7. 10.1002/mds.22571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 27. Stiasny-Kolster K, Mayer G, Schäfer S, et al. . The REM sleep behavior disorder screening questionnaire – a new diagnostic instrument. Mov Disord 2007;22:2386–93. 10.1002/mds.21740 [DOI] [PubMed] [Google Scholar]
- 28. Doty RL, Shaman P, Kimmelman CP, et al. . University of pennsylvania smell identification test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 1984;94:176–8. 10.1288/00005537-198402000-00004 [DOI] [PubMed] [Google Scholar]
- 29. Weintraub D, Simuni T, Caspell-Garcia C, et al. . Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov Disord 2015;30:919–27. 10.1002/mds.26170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schrag A, Siddiqui UF, Anastasiou Z, et al. . Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: a cohort study. Lancet Neurol 2017;16:66–75. 10.1016/S1474-4422(16)30328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stebbins GT, Goetz CG, Burn DJ, et al. . How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord 2013;28:668–70. 10.1002/mds.25383 [DOI] [PubMed] [Google Scholar]
- 32. Kang JH, Irwin DJ, Chen-Plotkin AS, et al. . Association of cerebrospinal fluid β-amyloid 1-42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 2013;70:1277–87. 10.1001/jamaneurol.2013.3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomlinson CL, Stowe R, Patel S, et al. . Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 2010;25:2649–53. 10.1002/mds.23429 [DOI] [PubMed] [Google Scholar]
- 34. Caspell-Garcia C, Simuni T, Tosun-Turgut D, et al. . Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One 2017;12:e0175674 10.1371/journal.pone.0175674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Picillo M, Nicoletti A, Fetoni V, et al. . The relevance of gender in Parkinson’s disease: a review. J Neurol 2017;264:1583–607. 10.1007/s00415-016-8384-9 [DOI] [PubMed] [Google Scholar]
- 36. Picillo M, Amboni M, Erro R, et al. . Gender differences in non-motor symptoms in early, drug naïve Parkinson’s disease. J Neurol 2013;260:2849–55. 10.1007/s00415-013-7085-x [DOI] [PubMed] [Google Scholar]
- 37. Picillo M, Erro R, Amboni M, et al. . Gender differences in non-motor symptoms in early Parkinson’s disease: a 2-years follow-up study on previously untreated patients. Parkinsonism Relat Disord 2014;20:850–4. 10.1016/j.parkreldis.2014.04.023 [DOI] [PubMed] [Google Scholar]
- 38. Picillo M, Palladino R, Moccia M, et al. . Gender and non motor fluctuations in Parkinson’s disease: a prospective study. Parkinsonism Relat Disord 2016;27:89–92. 10.1016/j.parkreldis.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 39. Song Y, Gu Z, An J, et al. . Gender differences on motor and non-motor symptoms of de novo patients with early Parkinson’s disease. Neurol Sci 2014;35:1991–6. 10.1007/s10072-014-1879-1 [DOI] [PubMed] [Google Scholar]
- 40. Jankovic J, McDermott M, Carter J, et al. . Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson study group. Neurology 1990;40:1529–34. 10.1212/WNL.40.10.1529 [DOI] [PubMed] [Google Scholar]
- 41. Mollenhauer B, Trenkwalder C, von Ahsen N, et al. . Beta-amlyoid 1-42 and tau-protein in cerebrospinal fluid of patients with Parkinson’s disease dementia. Dement Geriatr Cogn Disord 2006;22:200–8. 10.1159/000094871 [DOI] [PubMed] [Google Scholar]
- 42. Mollenhauer B, Trautmann E, Taylor P, et al. . Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett 2013;532:44–8. 10.1016/j.neulet.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 43. Compta Y, Martí MJ, Ibarretxe-Bilbao N, et al. . Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord 2009;24:2203–10. 10.1002/mds.22594 [DOI] [PubMed] [Google Scholar]
- 44. Shi M, Bradner J, Hancock AM, et al. . Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol 2011;69:570–80. 10.1002/ana.22311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simuni T, Caspell-Garcia C, Coffey C, et al. . Correlates of excessive daytime sleepiness in de novo Parkinson’s disease: a case control study. Mov Disord 2015;30:1371–81. 10.1002/mds.26248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pietrzak RH, Lim YY, Neumeister A, et al. . Amyloid-β, anxiety, and cognitive decline in preclinical Alzheimer disease: a multicenter, prospective cohort study. JAMA Psychiatry 2015;72:284–91. 10.1001/jamapsychiatry.2014.2476 [DOI] [PubMed] [Google Scholar]
- 47. Ju YE, McLeland JS, Toedebusch CD, et al. . Sleep quality and preclinical Alzheimer disease. JAMA Neurol 2013;70:587–93. 10.1001/jamaneurol.2013.2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2017-316213supp001.pdf (47.9KB, pdf)


