Abstract
Myelin increases the speed and efficiency of action potential propagation. Yet, not all axons are myelinated and some axons are discontinuously myelinated, prompting the question of how myelinating glia select axons for myelination. Whereas myelination by Schwann cells depends on axonal induction, oligodendrocytes can form myelin membrane in the absence of axons. However, oligodendrocytes alone cannot architect the complex myelination patterns of the central nervous system and recent advances have implicated axonal signaling in this process. This review considers how oligodendrocytes and their precursors could be influenced by inductive, attractive, permissive, repulsive, and preventative cues, and discusses recent evidence identifying synaptic activity and membrane-bound adhesion molecules as such cues directing axon selection.
The myelin landscape
Myelin, the insulating membrane concentrically wrapped around axons, accelerates impulse propagation and offloads neuronal energy expenditure. Despite these advantages, not all axons are myelinated and some axons are only intermittently myelinated. In the peripheral nervous system (PNS), Schwann cells (SCs) myelinate solely large diameter axons [1], with individual SCs each myelinating only one axon segment. By contrast, central nervous system (CNS) myelination patterns are more complicated. During development, myelination proceeds in a stereotyped sequence with different regions being myelinated at different times as oligodendrocyte precursor cells (OPCs), which are distributed throughout the CNS, differentiate into myelinating oligodendrocytes (OLs) [2,3]. OPC differentiation and myelination continues into adulthood, albeit at a much slower rate [4–6]. Unlike SCs, each OL extends many processes to myelinate several different axon segments. The smallest caliber CNS axons are never myelinated but there is significant overlap in the diameters of myelinated and unmyelinated axons [7], whereas other CNS structures geometrically similar to axons – dendrites, blood vessels, and glial processes – remain unmyelinated, with rare exceptions.
Recent volumetric reconstructions of cortical tissue have further refined our understanding of CNS myelination. Intriguingly, myelination may depend on neuron subtype: a recent study found that parvalbumin-positive basket cells are essentially the only type of GABAergic interneuron with myelinated axons in the adult mouse neocortex [8•]. Another study contested the prevailing dogma of myelination as an all-or-none phenomenon; rather, many pyramidal axons in the adult mouse neocortex are intermittently myelinated, with large unmyelinated gaps between myelinated sections [9], a configuration distinct from nodes of Ranvier that separate individual myelin sheaths (internodes) to permit saltatory conduction.
This partial myelin coverage likely reflects the need to balance the advantages of myelination with overall energy [10] and space constraints, and likely contributes to the precise control of circuit timing [11], underscoring the importance of appropriate myelin localization. In the case of intermittent myelination along a single axon, unmyelinated gaps could provide space for new myelin formation to fine-tune myelination patterns as circuit activity changes in the adult. This review will focus on our current understanding of how these intricate patterns of myelination are established.
The intrinsic nature of OL myelination
Given that sensory and motor axons that pass between the CNS and PNS are myelinated by both OLs and SCs, it seems intuitive that CNS and PNS axons would use similar mechanisms to control myelin formation. In the PNS, axons play a necessary and inductive role in their own myelination. Neuregulin-1 type III is required for SC maturation and myelination of PNS axons [12] and its expression level determines myelin sheath thickness [13]. Importantly, its ectopic suprathreshold expression induces the myelination of normally unmyelinated axons [12], demonstrating its importance in determining myelination fate.
However, neuregulin-1 type III is largely dispensable for CNS myelination [14,15]. In fact, several aspects of OL myelination proceed successfully without molecular axonal signaling altogether. Cultured OPCs intrinsically differentiate into OLs, which, remarkably, can form compact myelin around inert polystyrene fibers [16]. In fact, certain properties of in vivo myelination can be explained by how OL-lineage cells intrinsically interact with axonal geometries. Neither OPCs or OLs will ensheathe or wrap synthetic fibers with a diameter smaller than or equal to 0.3µm, a similar threshold to that observed in vivo [16], indicating that OL-lineage cells directly sense fiber geometries. Geometric sensing by OL-lineage cells may also explain the general correlation between internode length and axon diameter in vivo [17,18] as this correlation persists with synthetic fibers [19]. Exactly how OL-lineage cells detect radial axon size remains elusive, but they may utilize properties such as curvature or circumference to do so. Curvature-inducing components could impose a physical limit on OL-lineage cell membrane curvature that prevents wrapping of highly curved, subthreshold diameter axons. Alternatively, OL-lineage cells could sense their own membrane curvature as they wrap (for an example of curvature sensing at a similar scale, see [20]) and avoid wrapping axons above a threshold curvature. Another approach could be for OL-lineage cells to infer axonal circumference by sensing the length of one wrap of their membrane around an axon (for a potential mechanism of length sensing, see [21]). Upon completing one wrap, an OL-lineage cell process would contact itself. If the process length is subthreshold, the process would retract upon self-contact, preventing wrapping. Retraction of an extending OPC process upon contact with another process of the same OPC has been observed in the adult mouse cortex [6]; the same mechanism could operate in this context as well. To avoid retraction upon self-contact, the OL-lineage process would have to exceed a threshold length. At this threshold, the length sensing machinery would initiate a rapid downregulation of the self-repulsion mechanism locally within the wrapping process (e.g. by internalizing cell adhesion molecules), enabling self-contact and myelination of suprathreshold circumference axons. Similar mechanisms could also be used to correlate internode length with radial axon size.
Regional differences in OL-lineage cells, which likely emerge after OPC specification [22], may explain some differences in myelination between CNS regions. OPCs isolated from developing spinal cord form longer internodes upon differentiation than do those isolated from neocortex in cultures with synthetic fibers or neurons [19], reproducing the differences in internode length observed between these regions in vivo [23]. Another study demonstrated that the higher levels of differentiation and myelination in white matter (WM) [24] might be partially explained by differences between WM and grey matter (GM) OPCs. Adult WM OPCs transplanted into either adult WM or GM differentiated more efficiently than adult GM OPCs transplanted into either region [25].
Extrinsic factors regulating OL axon selection
Whereas intrinsic properties of OL-lineage cells might be helpful for setting up some basic features of the myelin landscape, they cannot independently produce the complex and specific myelination patterns observed in the CNS. For example, the relationship between myelin sheath thickness and axonal diameter [26] is not reproduced with synthetic fibers [19], and the ratio of internode length to axon diameter is actually highly variable in vivo [17,18,27]. In particular, since OLs are not programmed to myelinate a specific type of axon [28–30] and many suprathreshold diameter axons are not myelinated in vivo [7], other factors must contribute to the regulation of axon selection. Indeed, extrinsic factors have an important role in this process. For myelination of the appropriate axons at the appropriate time, OLs must be present and they must know where to form myelin – two components that can be influenced by extrinsic factors. By influencing virtually every aspect of OL development, extrinsic signals can affect the distribution of OLs at any time. Furthermore, extrinsic signals can influence where individual OLs localize their myelin sheaths.
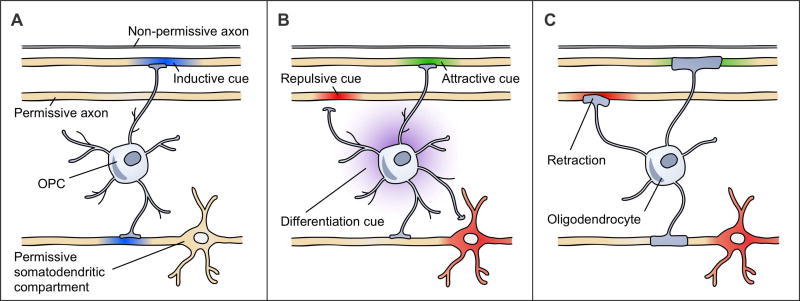
Axons, as the substrates for myelination, are logical candidates for being the source of these extrinsic cues. Axons might precisely regulate the timing of their myelination by expressing dynamic cues. An inductive cue could turn on, or a converse preventative cue could turn off, in an axon to initiate its myelination. OPCs, which actively survey their environment [6,31] and receive synaptic inputs from axons [32], are well-positioned to integrate such cues from multiple axons. A threshold level of change in these axons could cause OPCs to differentiate and to myelinate these axons. By utilizing a combined differentiation/axon selection cue (Figure 1A), this system would be extremely efficient in matching the number of OLs and their internodes to the axons requiring myelination at any time.
Figure 1. Different mechanisms of axon selection could produce the same CNS myelination patterns.
These three models are non-exhaustive and not mutually-exclusive. Structures with suprathreshold diameters are shown in yellow as permissive. The first axon has a subthreshold diameter, rendering it non-permissive. (a) An inductive cue (blue) turns on in axons to initiate their myelination. Shown here, an OPC senses a threshold myelination need from the second and fourth axons that causes the OPC to differentiate and myelinate these axons. These axons are among the permissive structures. (b) OPCs respond to attractive (green), permissive (yellow), and repulsive (red) cues on axons and other cellular compartments that regulate the contacts that OPCs make and the eventual placement of myelin sheaths. The OPC processes retract from repulsive cues on the third axon and the somatodendritic compartment. Upon differentiation, this cell will myelinate the second and fourth axons. The differentiation cue (purple) is uncoupled from axon selection. (c) OLs respond to attractive (green), permissive (yellow), and repulsive (red) cues that affect sheath initiation or stability. Retraction of an early sheath in response to a repulsive cue on the third axon is shown. This OL myelinates the second and fourth axons.
Alternatively, differentiation and axon selection could be uncoupled (Figure 1B, C) [33•]. Differentiation could occur either through an actively regulated mechanism or through one that is indiscriminately applied wherein OLs that differentiated in excess of available axons would eventually die [34–36]. Axon selection could be performed by either OPCs (Figure 1B) or OLs (Figure 1C). OPCs could identify axons to myelinate upon their eventual differentiation (Figure 1B), or, once differentiated, OLs could become receptive to axonal cues that regulate either sheath initiation or stability (Figure 1C). The axon selection cues described below could apply to either cell type. Inductive or preventative axon selection cues could operate in this context (although they are not illustrated in Figure 1B, C). In the case of unregulated OPC differentiation, both the patterns and timing of myelination would be controlled solely by the axons that become available for myelination based on their changing expressions of inductive or preventative cues. In the case of actively regulated OPC differentiation, the timing of inductive (or preventative) cue onset (or offset) would have to precisely coordinate with the timing of the regulated differentiation mechanism to be able to initiate myelination of an axon during the seemingly short period between OPC differentiation and the termination of new sheath formation [6,37,38] (although, see [5]). With regulated OPC differentiation, it would instead be simpler for OL-lineage cells to respond to axon selection cues that are not dynamically regulated, perhaps having existed on axons long before differentiation occurs. Such cues would influence myelin localization but not its timing. These cues could be termed attractive or repulsive, positively or negatively biasing an axon’s selection for myelination by either altering the distribution or number of OL internodes (Figure 1B, C).
Finally, regardless of whether differentiation and axon selection are coupled or not, axons must be permissive for myelination – a feature that permits their myelination but does not guarantee it (e.g. suprathreshold axon diameters). Axon permissiveness may be static or dynamically regulated (e.g. as subthreshold axons grow). An outstanding question in the field is whether structures that are simply permissive – lacking any inductive or attractive cues – are myelinated in the CNS (as illustrated in Figure 1B, C). While the models of axon selection presented here are not mutually exclusive, their conceptual separation is useful for establishing a framework to evaluate research in this field.
A role for axons in OL myelination
Efforts to elucidate the role of CNS axons in their own myelination have greatly benefited from innovations in sparse (low-efficiency recombination), targeted genetic manipulations and live imaging with single-cell resolution. A creative genetic approach generating zebrafish with supernumerary large caliber spinal cord Mauthner axons provided compelling evidence for the influence of axons on an individual OL’s myelination patterns. These extra axons caused Mauthner axon-associated OLs to extend more internodes to myelinate more Mauthner axons than normal, and even prompted non-Mauthner axon-associated OLs to myelinate Mauthner axons. Notably, OL numbers did not change [29], supporting a model wherein axonal cues alter axon selection independent of OPC differentiation (Figure 1B, C).
A recent study in mice demonstrated that axonal cues can have a considerably broader impact on OL development and myelination. Knocking-out Pten specifically from cerebellar granule cells, which increased axon caliber to suprathreshold diameters and altered gene expression, caused ectopic proliferation and differentiation of OPCs and myelination of granule cell axons in the normally unmyelinated cerebellar molecular layer [39••]. It is unclear whether all of these effects are mediated through the same or disparate mechanisms – or exactly what those mechanisms are. Could these effects be entirely attributable to increasing axon caliber to a permissive level, or are molecular changes also involved? Intriguingly, ectopic myelination was still observed when Pten was only sparsely knocked-out [39••]. Identifying whether the axons lacking PTEN were the ones that were myelinated in this sparse manipulation will provide important information on the spatial nature of this change. Moving forward, this genetic model could be a tractable system to investigate the mechanisms by which axons regulate their own myelination.
Axonal cues for axon selection
An axon selection cue must be sufficiently localized to facilitate the distinction between axons as well as geometrically similar substrates in close proximity. Membrane-bound cues or those released at synapses both meet this requirement.
Axonal cues for axon selection: synaptic activity
OPCs receive glutamatergic and GABAergic synaptic inputs from axons and express a myriad of other neurotransmitter receptors [32,40], perhaps to inform OPCs of a myelination need. A model often termed “activity-dependent myelination” posits that changes in synaptic inputs to OPCs could serve as an inductive cue, causing OPC differentiation and myelination of the axons providing modified inputs, perhaps even converting axon-OPC synapses into a myelinating sheath (Figure 1A) [41]. This process could shape brain development based on experience or contribute to learning by modifying circuit timing.
Two landmark in vivo studies energized this hypothesis. Optogenetic stimulation of neurons in the mouse premotor cortex increased proliferation and differentiation of OPCs [42], which presumably generate new myelin. Similarly, training mice to run on a complex wheel increases OPC proliferation and differentiation in the corpus callosum. In fact, generating new OLs, and presumably new myelin, was required for mice to properly learn the task [43]. Since simply increasing OPC density can enhance differentiation [44], the direct effects of neuronal activity on OPC differentiation in these studies is unclear. A follow-up study using the same motor learning paradigm showed that differentiation occurs from the existing pool of OPCs [45•], suggesting that subsequent OPC proliferation may occur in response to local OPC differentiation, as was observed in live imaging of cortical OPC dynamics [6]. An important next step is to analyze where new myelin is localized in these paradigms; are axons with increased activity inducing their own myelination by expressing a combined differentiation/axon selection cue (Figure 1A) or might axonal activity simply act as a differentiation cue without affecting axon selection (Figure 1B)?
A recent study supports the latter. Social isolation of adult mice reduced OPC differentiation, the number of myelinated axons, and myelin thickness in the prefrontal cortex (PFC) and caused social withdrawal, all of which were rescued by oral administration of the pro-differentiation compound clemastine [46]. If the presumably reduced activity of specific sociability-related axons during social isolation prevented these axons from expressing an inductive cue that would normally cause OPC differentiation and their selection for myelination (Figure 1A), it’s unlikely that the ostensibly indiscriminate upregulation of OPC differentiation by clemastine would rescue these deficits. More plausibly, PFC activity from social interaction upregulates overall OPC differentiation without affecting axon selection, and is important for providing sufficient numbers of OLs to myelinate the relevant axons, which utilize other cues to regulate their selection for myelination (Figure 1B, C).
Notably, other studies have found no [47] or opposite [48] effects of neuronal activity on OPC differentiation, while others have observed differences in properties such as OL internode number [47,49••], length [48,50••,51•], or thickness [42,46,47,52]. Of these, two pioneering studies found a direct role for the synaptic activity of individual axons in regulating their selection for myelination. Treatment of zebrafish with tetrodotoxin (TTX) to block action potentials reduced the myelination of phox2b+ axons in the spinal cord without affecting total internode or OL numbers, suggesting that these axons use neuronal activity to facilitate their myelination [50••]. Indeed, expressing tetanus toxin (TeNT), which prevents synaptobrevin/VAMP2-mediated synaptic exocytosis, in individual phox2b+ axons substantially reduced the myelination of these axons [50••]. The same was true for TeNT expression in single reticulospinal axons [49••]. TeNT expression globally or specifically in neurons caused individual OLs to form 30% fewer internodes [49••,50••].
The results with TTX versus TeNT suggest different mechanisms of activity-mediated axon selection, highlighting the importance of elucidating the specific mechanisms involved to resolve the sources of these discrepancies. The TTX data support a model of competition between axons for myelination. When neuronal activity was abolished, phox2b+ axons lost their competitive advantage, and so other axons were myelinated in their place. Conversely, the TeNT data suggests that the axon selection cue used by these axons promotes their myelination by increasing the number of internodes per OL, perhaps by affecting downstream OL Fyn kinase signaling [37]. Live imaging showed that very early retractions of presumably nascent sheaths [50••] caused a decrease in sheaths per OL 6h after the beginning of sheath formation in the TeNT condition [49••], and that increased sheath retractions continued for many hours [50••]. Despite the reduction in excitatory synaptic input as OPCs differentiate into early OLs, these cells continue to respond to glutamate [53,54] and maintain some glutamate receptor expression [55,56]. The increase in sheath retractions in response to blocking synaptic exocytosis along with the observation that synaptic vesicles preferentially stopped at nascent ensheathment sites [50••] imply that early myelinating OLs do respond to synaptic inputs and that these inputs are important for axon selection (Figure 1C).
Could synaptic activity-mediated axon selection account for CNS myelination patterns? Different neuronal subtypes utilize different neurotransmitters and have distinct firing rates, features OL-lineage cells could use to identify specific axonal subtypes to myelinate [8•]. But since these features do not differ within a single neuron, how could synaptic activity produce intermittent myelination along a single axon [9]? Axons could synapse with OPCs unevenly along their lengths. Alternatively, cues from an individual axon could remain consistent but the surrounding environment could affect that axon’s competitiveness for myelination.
Axonal cues for axon selection: membrane-bound adhesion molecules
Not surprisingly, not all axons utilize synaptic activity to regulate their selection for myelination. While individual reticulospinal neurons expressing TeNT had reduced myelin coverage, TeNT expression in individual commissural primary ascending axons had no effect on their myelination [51•]. What other cues might axons use to regulate their selection for myelination?
Adhesion molecules, which mediate cell-cell interactions, are logical candidates. In fact, several axonal adhesion molecules negatively regulate OPC differentiation and/or myelination, and seem to downregulate their expression as myelination proceeds (e.g. Jagged1 [57], PSA-NCAM [58], or Lsamp [59]). It is tempting to regard these molecules as potential preventative cues for myelination, wherein their removal from an axon would initiate myelination of that axon, potentially in conjunction with causing OPC differentiation. Future studies genetically manipulating these molecules sparsely in axons will elucidate their potential roles in axon selection.
Recently, the adhesion molecule JAM2 was identified as a negative regulator of myelination with no effect on OPC differentiation. It is expressed by the somatodendritic compartment of cultured spinal cord neurons and prevents myelination of this cellular compartment. Oligodendrocytes ectopically myelinate the somata and dendrites of cultured Jam2 knock-out spinal cord neurons, and increased ectopic myelination of PAX2+ neuronal somata was found in the dorsal spinal cord of Jam2 knock-out mice [60•]. This study confirms the presence of repulsive cues that shape where OLs form internodes. It also suggests either that neuronal somata express sufficiently attractive cues such that they become myelinated when inhibition is reduced, or that simply permissive structures can be myelinated in the CNS (Figure 1B, C). Intriguingly, JAM2-Fc coated micropillars are repulsive to both cultured OPCs and OPC-derived OLs [60•], indicating that OPCs respond to substrate selection cues that influence their eventual internode placement, independent of that cue’s ability to affect differentiation (Figure 1B). Likewise, contact-mediated self-repulsion by OPCs [6,31] supports the idea that these cells are well-equipped with adhesion molecules to regulate the placement of their processes.
Could similar repulsive cues exist that prevent the myelination of glia or blood vessels? Might there be adhesion molecules, attractive or repulsive, that distribute unevenly along axons or differentially between different neuronal subtypes, shaping CNS myelination patterns [8•,9]?
Wrapping it up
The question of how axons are selected for myelination has been historically difficult to answer. However, new research efforts utilizing sparse genetic manipulations and high-resolution live imaging have begun to shed light on this complex issue and will be essential moving forward. Despite in vitro work establishing the intrinsic nature of OL myelination, current research has clearly demonstrated that axons have a role in their selection for myelination. The extent of their contribution and the molecular players remain to be determined and will be fruitful grounds for future studies.
This review has focused primarily on the mechanisms through which axons might communicate with OL-lineage cells to regulate their initial myelin coverage. While myelin sheath retractions have been observed in early OLs, it is not yet clear how ongoing communication between axons and OLs might alter myelin coverage in the long term. In addition to affecting axon selection, evidence suggests that axons can regulate internode length and sheath thickness, properties that – like axon selection – affect conduction velocity and energy usage. It remains a beautiful mystery as to how these properties are regulated in concert with one another, and to what extent axons are active players in this coordination, functioning as grand architects of the ever-changing myelin landscape.
Highlights.
-
-
Not all axons are myelinated and some axons are discontinuously myelinated.
-
-
Oligodendrocytes do not require molecular axonal signals to form myelin membranes.
-
-
Axon selection can occur at various stages of oligodendrocyte development.
-
-
Axons can express various cues to regulate their selection for myelination.
-
-
Synaptic activity and membrane-bound adhesion molecules influence axon selection.
Acknowledgments
This review was supported by the National Institutes of Heath/National Institute of Neurological Disorders and Stroke [R01NS062796, R01NS097428, R01NS095889], the National Multiple Sclerosis Society [RG5203A4] and the Rachleff family endowment to JRC and the Natural Sciences and Engineering Research Council of Canada [PGS D] to LAO. We would like to thank Justin Berot-Burns for his technical assistance with the figure and the members of the Chan laboratory for their critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI
The authors declare no conflict of interests.
References
- 1.Voyvodic JT. Target size regulates calibre and myelination of sympathetic axons. Nature. 1989;342:430–433. doi: 10.1038/342430a0. [DOI] [PubMed] [Google Scholar]
- 2.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Foran DR, Peterson AC. Myelin acquisition in the central nervous system of the mouse revealed by an MBP-Lac Z transgene. J Neurosci. 1992;12:4890–4897. doi: 10.1523/JNEUROSCI.12-12-04890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young KM, Psachoulia K, Tripathi RB, Dunn S-JJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturrock RR. Myelination of the mouse corpus callosum. Neuropathol Appl Neurobiol. 1980;6:415–420. doi: 10.1111/j.1365-2990.1980.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 8•.Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, Bock DD. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife. 2016;5:e15784. doi: 10.7554/eLife.15784. Using volumetric reconstructions of cortical tissue correlating electron microscopy and immunofluorescence images, these researchers were able to identify the intermittent myelination of parvalbumin-positive basket cells in the adult mouse neocortex. The authors suggest that myelination of these axons might mainly serve to reduce axonal energy consumption. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomassy G, Berger D, Chen H-H, Kasthuri N, Hayworth K, Vercelli A, Seung S, Lichtman J, Arlotta P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344:319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012;32:356–371. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Hoz L, Simons M. The emerging functions of oligodendrocytes in regulating neuronal network behaviour. Bioessays. 2015;37:60–69. doi: 10.1002/bies.201400127. [DOI] [PubMed] [Google Scholar]
- 12.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave K-AA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 14.Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- 15.Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Müller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, Feng Z-QQ, Corey JM, Chan JR. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Na. Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray JA, Blakemore WF. The relationship between internodal length and fibre diameter in the spinal cord of the cat. J Neurol Sci. 1980;45:29–41. doi: 10.1016/s0022-510x(80)80004-9. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim M, Butt AM, Berry M. Relationship between myelin sheath diameter and internodal length in axons of the anterior medullary velum of the adult rat. J Neurol Sci. 1995;133:119–127. doi: 10.1016/0022-510x(95)00174-z. [DOI] [PubMed] [Google Scholar]
- 19.Bechler ME, Byrne L ffrench-Constant C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr Biol. 2015;25:2411–2416. doi: 10.1016/j.cub.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridges AA, Jentzsch MS, Oakes PW, Occhipinti P, Gladfelter AS. Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J Cell Biol. 2016;213:23–32. doi: 10.1083/jcb.201512029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rishal I, Kam N, Perry RB, Shinder V, Fisher EM, Schiavo G, Fainzilber M. A motor-driven mechanism for cell-length sensing. Cell Rep. 2012;1:608–616. doi: 10.1016/j.celrep.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen Y-AA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI, et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A. 2012;109:1299–1304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 25.Viganò F, Möbius W, Götz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci. 2013;16:1370–1372. [Google Scholar]
- 26.Bishop GH, Clare MH, Landau WM. The relation of axon sheath thickness to fiber size in the central nervous system of vertebrates. Int J Neurosci. 1971;2:69–77. doi: 10.3109/00207457109146994. [DOI] [PubMed] [Google Scholar]
- 27.Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, Pecka M, Attwell D, Grothe B. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat Commun. 2015;6:8073. doi: 10.1038/ncomms9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanarraga ML, Griffiths IR, Zhao M, Duncan ID. Oligodendrocytes are not inherently programmed to myelinate a specific size of axon. J Comp Neurol. 1998;399:94–100. [PubMed] [Google Scholar]
- 29.Almeida RG, Czopka T, ffrench-Constant C. Lyons DA. Individual axons regulate the myelinating potential of single oligodendrocytes in vivo. Development. 2011;138:4443–4450. doi: 10.1242/dev.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osanai Y, Shimizu T, Mori T, Yoshimura Y, Hatanaka N, Nambu A, Kimori Y, Koyama S, Kobayashi K, Ikenaka K. Rabies virus-mediated oligodendrocyte labeling reveals a single oligodendrocyte myelinates axons from distinct brain regions. Glia. 2017;65:93–105. doi: 10.1002/glia.23076. [DOI] [PubMed] [Google Scholar]
- 31.Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- 32.Maldonado PP, Angulo MCC. Multiple modes of communication between neurons and oligodendrocyte precursor cells. Neuroscientist. 2015;21:266–276. doi: 10.1177/1073858414530784. [DOI] [PubMed] [Google Scholar]
- 33•.Almeida R, Lyons D. Oligodendrocyte development in the absence of their target axons in vivo. PLoS ONE. 2016;11:e0164432. doi: 10.1371/journal.pone.0164432. By preventing the normal outgrowth of reticulospinal axons into the ventral spinal cord of zebrafish, these researcher were able to assess the effect these axons normally have on oligodendrocyte development. With a dramatic reduction in reticulospinal axons, OL numbers are only modestly reduced, implying that much of oligodendrocyte development is not dependent on target axon signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 35.Trapp BD, Nishiyama A, Cheng D, Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 37.Czopka T, ffrench-Constant C. Lyons DA. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell. 2013;25:599–609. doi: 10.1016/j.devcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crawford AH, Tripathi RB, Foerster S, McKenzie I, Kougioumtzidou E, Grist M, Richardson WD, Franklin RJ. Pre-existing mature oligodendrocytes do not contribute to remyelination following toxin-induced spinal cord demyelination. Am J Pathol. 2016;186:511–516. doi: 10.1016/j.ajpath.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Goebbels S, Wieser GL, Pieper A, Spitzer S, Weege B, Yan K, Edgar JM, Yagensky O, Wichert SP, Agarwal A, et al. A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination. Nat Neurosci. 2017;20:10–15. doi: 10.1038/nn.4425. Knocking out Pten specifically from cerebellar granule cells caused OPC proliferation and differentation and ectopic myelination of granule cell axons in the cerebellar molecular layer, which is normally void of oligodendrocytes. Ectopic myelination was also observed when Pten was only sparsely knocked out. [DOI] [PubMed] [Google Scholar]
- 40.Larson VA, Zhang Y, Bergles DE. Electrophysiological properties of NG2+ cells: Matching physiological studies with gene expression profiles. Brain Res. 2016;1638:138–160. doi: 10.1016/j.brainres.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almeida RG, Lyons DA. On the resemblance of synapse formation and CNS myelination. Neuroscience. 2014;276:98–108. doi: 10.1016/j.neuroscience.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 42.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero B, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci. 2016;19:1210–1217. doi: 10.1038/nn.4351. In a follow-up to Ref [43], this study finds that mice unable produce new oligodendrocytes are worse than controls at learning to run on a complex wheel after just a few hours. Using a novel marker of newly differentiated OLs, they find that during this short time frame, learning this task normally induces differentiation from the existing pool of OPCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Dupree JL, Gacias M, Frawley R, Sikder T, Naik P, Casaccia P. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J Neurosci. 2016;36:957–962. doi: 10.1523/JNEUROSCI.3608-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, Redmond SA, Ullian EM, Chan JR. Dynamic modulation of myelination in response to visual stimuli alters optic nerve conduction velocity. J Neurosci. 2016;36:6937–6948. doi: 10.1523/JNEUROSCI.0908-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18:628–630. doi: 10.1038/nn.3991. This study demonstrated a role for synaptic activity in regulating the selection of axons for myelination and the number of myelin sheaths formed per oligodendrocyte in the zebrafish spinal cord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. Using various techniques to alter neuronal activity, this study showed that phox2b+ axons in the zebrafish spinal cord use neuronal activity to increase their selection for myelination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, Lyons DA. Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr Biol. 2016;26:1447–1455. doi: 10.1016/j.cub.2016.03.070. By expressing tetanus toxin in individual neurons and using a clever method to visualize myelination along a single axon, they show that reticulospinal neurons – but not commissural primary ascending neurons – depend on synaptic synaptobrevin/VAMP2-mediated exocytosis for their selection for myelination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature Neuroscience. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 2010;30:3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kukley M, Nishiyama A, Dietrich D. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci. 2010;30:8320–8331. doi: 10.1523/JNEUROSCI.0854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marques S, Zeisel A, Codeluppi S, van Bruggen D, Falcao AM, Xiao L, Li H, Haring M, Hochgerner H, Romanov RA, et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science. 2016;352:1326–1329. doi: 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 58.Charles P, Hernandez MP, Stankoff B, Aigrot MS, Colin C, Rougon G, Zalc B, Lubetzki C. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci U S A. 2000;97:7585–7590. doi: 10.1073/pnas.100076197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma K, Schmitt S, Bergner CG, Tyanova S, Kannaiyan N, Manrique-Hoyos N, Kongi K, Cantuti L, Hanisch U-KK, Philips M-AA, et al. Cell type- and brain region-resolved mouse brain proteome. Nat Neurosci. 2015;18:1819–1831. doi: 10.1038/nn.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Redmond SA, Mei F, Eshed-Eisenbach Y, Osso LA, Leshkowitz D, Shen Y-AA, Kay JN, Aurrand-Lions M, Lyons DA, Peles E, et al. Somatodendritic expression of JAM2 inhibits oligodendrocyte myelination. Neuron. 2016;91:824–836. doi: 10.1016/j.neuron.2016.07.021. This study identifies the membrane-bound adhesion molecule JAM2 as a repulsive cue preventing the myelination of the neuronal somatodendritic compartment. [DOI] [PMC free article] [PubMed] [Google Scholar]