Abstract
Objective
To test the hypotheses that an innovative skills-based behavioral family clinic and home-based intervention (LAUNCH) would reduce body mass index z-score (BMIz) compared with motivational interviewing (MI) and to standard care (STC) in preschool-aged children with obesity.
Study design
Randomized controlled trial with children between the ages of 2 and 5 years above the 95th percentile for body mass index for age and sex recruited from 27 pediatrician offices across 10 recruitment cycles between March 12, 2012 and June 8, 2015. Children were randomized to LAUNCH (an 18 session clinic and home based behavioral intervention), MI (delivered at the same frequency as LAUNCH), or STC (no formal intervention). Weight and height were measured by assessors blinded to participant assignment. The primary outcome, BMIz at Month 6 after adjusting for baseline BMIz, was tested separately comparing LAUNCH with MI and LAUNCH with STC using regression-based analysis of covariance models.
Results
151 of the 167 children randomized met intent-to-treat criteria and 92% completed the study. Children were 76% White and 57% female, with an average age of 55 months and BMI percentile of 98.57, with no demographic differences between the groups. LAUNCH participants demonstrated a significantly greater decrease in BMIz (mean=−0.32, SD= ±0.33) compared with MI (mean=−0.05, SD= ±0.27), P < .001, ω2 = 0.74 and compared with STC (mean=−0.13, SD= ±0.31), p < 0.004, ω2 = 0.75.
Conclusions
In preschool-age children, an intensive 6 month behavioral skills-based intervention is necessary to reduce obesity.
Keywords: Behavior family therapy, obesity treatment, preschoolers, motivational interviewing, home visits
Nearly 2 million preschool-aged children in the United States meet criteria for obesity,1 yet there are few published treatment studies targeting this age group.2 Young children do not “outgrow” obesity. Obesity in the preschool years dramatically increases the risk of being overweight, obese and even severely obese in later childhood and adulthood.3–5 There is an association between early onset overweight and increased odds of developing diabetes8 and asthma.9 Efficacious treatment of obesity in the preschool years could dramatically change and even reverse this trend.
Despite the need for innovative weight management interventions for younger children, a recent review shows there are few randomized trials targeting weight reduction in preschoolers2 and only one, a pilot study, targeting preschoolers who are already obese. Focusing solely on children with obesity is important because they are at higher risk for mortality compared with overweight peers.11 This study examined a novel clinic and home family-based behavioral intervention (Learning about Activity and Understanding Nutrition for Child Health [LAUNCH]),12 designed to address specific behaviors caregivers report as barriers to establishing and maintaining healthy eating patterns in preschool children,13 including food neophobia and tantruming for food, through home visits designed to consolidate clinic-taught strategies into the home setting using in-vivo practice of these skills. LAUNCH reduced BMIz significantly more than one-session counseling by a pediatrician.12
Since the publication of the behavioral intervention for preschoolers with obesity, two additional studies were published targeting preschoolers above the 85th percentile body mass index (BMI) in the primary care setting. A six-month family-based behavioral intervention with 18 contacts was found to reduce overweight in this age group14 compared with an education only control, while in a separate study a 12-month, 7 session motivational interviewing (MI) intervention was not found to be more effective than usual care15 in reducing BMI. MI is one of the recommended treatment approaches by the AAP Expert Committee on treatment of child and adolescent overweight/obesity16 and is designed to address barriers of motivation and ambivalence. As parents of preschoolers often do not recognize obesity17, 18 and frequently feel it is unfair to implement changes to their child’s diet,19 MI is a credible alternative treatment, addressing parent ambivalence about implementing diet and activity changes for their child.
The objective of this Phase III randomized clinical trial (RCT) (ClinicalTrials.gov: NCT01546727) was to test whether the skills-based behavioral family clinic and home-based intervention (LAUNCH) was superior to MI and to standard care (STC). It was hypothesized a priori that preschoolers receiving LAUNCH would have a greater decrease in their body mass index z-score (BMIz) compared with MI and STC at posttreatment. Changes in parent BMI were examined secondarily.
Methods
Across 10 recruitment periods between March 12, 2012 and June 8, 2015, children and their families were recruited from 27 pediatric practices in the Greater Cincinnati/Northern Kentucky area. The study was approved by the institutional review board at the primary medical center where the study was conducted and written informed consent was obtained from caregivers. Inclusion criteria were: 1) ages 2 to 5 years; 2) BMI percentile for age and sex ≥95th 20 but no more than 100% above the median BMI; 3) medical clearance from their pediatrician; 4) active patient with anthropometric measurements within the previous year; and 5) living within 50 miles from the medical center. Exclusion criteria included 1) developmental disability or medical conditions known to promote obesity (eg, Prader-Willi syndrome); 2) child enrolled in another weight control program; 3) taking weight-affecting medications (e.g., steroids); 4) condition that would preclude full participation in the program; and 5) non-English-speaking.
Introductory letters were sent from the primary care practice with an “opt-out” postcard if families did not want to be contacted for the study. Families not returning the postcard were contacted by study staff. Seven additional practices, belonging to a unified health system whose administrative polices prevented participation in the recruitment procedures described above, were allowed to refer families to the study. Families meeting eligibility screening by phone and interest in study participation were scheduled for two baseline visits, at clinic and home. Children whose families did not complete both baseline visits were not randomized into the study. Intent to treat was defined a priori as being reached for treatment assignment (STC) and attending the first intervention session (LAUNCH and MI).
The study protocol is described in detail elsewhere.21 The randomization sequence was kept by the study statistician, concealed from study personnel, and was not assigned until all baseline measures were obtained from all children in a recruitment cycle. Child baseline BMIz was used as a stratification variable in a randomized stratification design with randomly chosen blocks of size 6 and 9, equal allocation to the three groups within blocks to ensure that BMIz was equivalent across the three arms. Beginning with cycle 8, child race/ethnicity was added to the stratification process to ensure equivalence across the three arms.
The overall goal of LAUNCH and MI was to follow the Expert Committee Recommendations on Prevention, Assessment and Treatment of Child and Adolescent Overweight and Obesity16 for reducing obesity in preschoolers by either stabilizing or slowing the rate of children’s weight gain or to produce a gradual weight loss of 1 lb/month. Both interventions targeted: 1) limiting portion size; 2) limiting consumption of energy-dense foods; 3) limiting eating out; 4) consumption of ≥5 servings of fruit and vegetables per day; 5) minimizing or eliminating sugar-sweetened beverages; 6) limiting screen time to ≤2 hours per day, and no TV in the room where child sleeps; and 7) achieving ≥1hour of moderate to vigorous physical activity per day. All families received $50 for completing the baseline and 6-month assessments. Beginning at recruitment cycle 7 (where practices were farther from the medical center) families traveling ≥20 miles were given an additional $25 to help offset travel costs. Intervention arms are briefly described below and fully elsewhere.21
LAUNCH is an 18 session clinic and home family-based behavioral weight management intervention, consisting of a 3-month intensive treatment phase (weekly sessions) followed by a 3-month maintenance phase (every other week sessions). Intervention sessions alternated between clinic (10 sessions) and home (8 sessions) visits.
Parent clinic-based group sessions were 90 minutes each and led by a licensed clinical psychologist. Sessions consisted of education and problem-solving around parent and child diet, dietary and physical activity changes, and child behavior management strategies such as differential attention (e.g., ignoring complaints about food, praising trying vegetables), contingency management (e.g., rewarding healthy behaviors), limit setting, effective use of timeout to manage tantrums, shaping (e.g., gradually introducing change) and exposure to introduce new foods, and implementing stimulus control measures to improve food choices and physical activity. Sessions 1–7 focused on dietary changes (with dietary tracking conducted throughout treatment), Sessions 8–10 focused on changing sedentary and physical activity, and Sessions 11–18 focused on bringing all the skills together and problem-solving barriers to recommended lifestyle changes. A simultaneously held child group provided education about healthy eating, opportunities for moderate to vigorous physical activity, and exposure to a variety of fruits and vegetables through a meal. LAUNCH incorporated home visits (60 minutes) to facilitate generalization of the clinic taught skills to the home including parenting skills and changing the home environment22, 23 using instruction, modeling and rehearsal of dietary, physical activity, parenting, and stimulus control techniques. Child groups and home visits were conducted by a postdoctoral fellow in clinical psychology or nutrition.
Motivational Interviewing (MI) was a parent only intervention consisting of 18 sessions over 6 months, delivered weekly during the initial 3-months and every other week months 4–6. At the initial 60 minute session parents met with a pediatrician trained in MI during which they completed questionnaires to assess their values and motivation for change, were given information about their child’s weight and BMI percentile, and a packet of publicly available materials/brochures from the “Let’s Go 5-2-1-0” program. Following the tenets of MI, caregivers were asked about their concern about their preschoolers’ weight, diet and physical activity and asked about their desired child outcome, motivation, and confidence to make changes in any area of concern. If receptive, they were asked to select a nutrition or physical activity as a primary target of discussion from a menu of the AAP recommendations and the Let’s Go 5-2-1-0 materials. Subsequent MI intervention sessions were delivered by a licensed clinical psychologist trained in MI in either the families’ home (Sessions 2,12,16) or over the telephone (14 sessions). These MI intervention sessions consisted of a discussion of previous goals selected by the caregiver, exploration of the caregiver’s perception of the success in reaching these goals, determination of caregiver’s confidence and willingness to continue working on existing goal(s) or establishing new behavioral goals, and enhancement of motivation to address ambivalence and readiness to change behaviors in the caregivers, and identification of self-selected strategies for goal attainment. Following the tenets of MI, the length of the phone sessions were determined by parents. The median phone session length was 15 minutes with 22% (135/625 of phone sessions) being ≤10 minutes. All home visits were scheduled for 60 minutes. Standard Care (STC) informed caregivers of their child’s weight status during the recruitment process, but neither the children nor caregivers received any treatment.
Caregivers completed a questionnaire regarding child date of birth, race, ethnicity, and sex as well as caregiver information on these variables and caregiver education, occupation, marital status and family income. Caregivers’ education and occupation were used to calculate a family’s socioeconomic status using the Hollinghead 4-Factor Index of socioeconomic status24 where scores range from 8 to 66 with higher numbers indicating higher socioeconomic status.
Caregiver and children’s weight and height were measured by trained personnel in the Clinical Translational Research Center (CTRC) Bionutrition Core who were unaware of participant treatment assignment using a standard protocol.21, 25 The primary outcome was BMIz calculated using CDC growth charts and the LMS method.26 In addition, BMI percentile, and percent over the 50th percentile BMI (0BMI%)27 were calculated to assess eligibility. All LAUNCH and MI sessions were recorded and 25% were coded for treatment integrity. Attendance at treatment sessions was tracked for LAUNCH and MI. Families were offered a make-up session prior to the next scheduled intervention session if a session was missed. A session was counted as complete if the family attended either session. Adverse events (AE) were assessed at each intervention session (LAUNCH and MI) and at 6 months (all groups) using a standardized protocol and AEs on child height were monitored as described elsewhere.21 Caregivers completed a checklist at the 6 month assessment asking if they sought weight management advice from a healthcare professional (e.g., physician, dietitian) for their preschooler outside of the study.
The primary goal of this trial was to determine if LAUNCH would lead to a greater reduction in BMIz at 6 months when compared with each of 2 comparisons: LAUNCH vs MI and LAUNCH vs STC. A priori power and sample size estimates indicated that we would be sufficiently powered (80%) at 43 children per group using a longitudinal study design, an average expected effect size of 0.67σ between groups, α2 = 0.25, and a 22% attrition rate. Primary analyses were performed using regression-based analysis of covariance models, with BMIz at 6 months serving as the outcome of interest, group assignment as the testable covariate, and BMIz at baseline as the adjusting covariate. Criteria for statistical significance included model and variable specific Wald statistics as well as ω2 and conditional error tests. With respect to model sensitivity, and as an added assurance that findings were stable irrespective of missing and/or extreme observations, all models were run with and without the extreme observations present, and using a series of 10 (multiple) imputations per model to estimate standard errors from the missing data (MIANALYZE). Because missing data were minimal (8%), and all three scenarios produced the same pattern and magnitude of results within each hypothesis, a complete case analysis was deemed prudent and served as the basis for model results presented. All assumptions and distributional properties were tested and deemed amenable for parametric modeling. Both hypotheses were tested at an adjusted α =0.025 level to account for multiplicity of comparisons. All data were analyzed using SAS v93.
Results
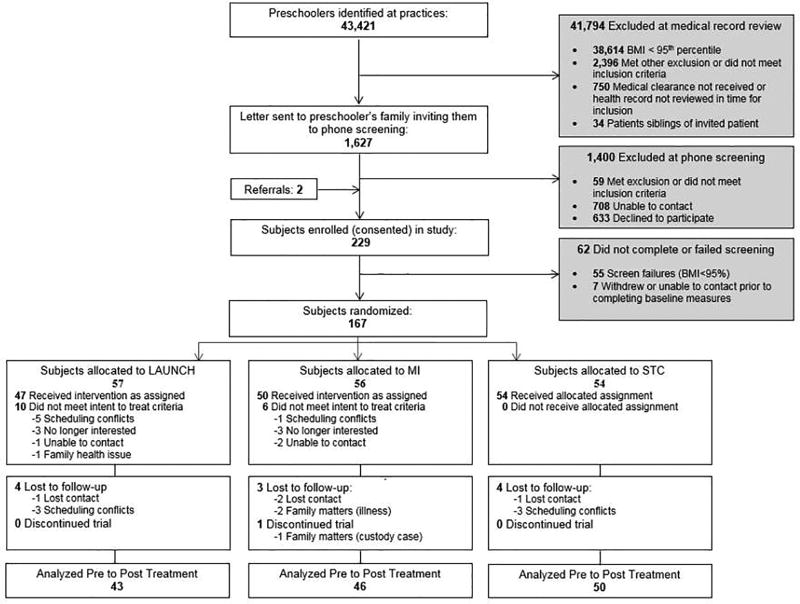
Participant flow through the trial appears in Figure 1 (available at www.jpeds.com). At baseline there were no statistically significant demographic differences between LAUNCH and MI or STC for child age, BMIz, BMI percentile, sex, race, ethnicity, family income, and Hollingshead score, all P > .05. Descriptive information for the sample as whole and by condition is shown in Table I. Across the whole sample, children were primarily white (76.16%) and non-Hispanic (94.04%) with a mean baseline BMIz of 2.44, corresponding to a BMI percentile of 98.57%. Most of the caregivers were mothers (90.07%) and met criteria for being obese (66.89%) or overweight (16.56%).
Figure 1.
CONSORT flow diagram of participants to LAUNCH, Motivational Interviewing and Standard Care
Table 1.
Baseline characteristics of children and caregivers meeting intent to treat criteria in LAUNCH, Motivational Interviewing (MI), Standard of Care (STC), and Overall sample.
| Overall (n=151) |
LAUNCH (n=47) |
MI (n=50) |
STC (n=54) |
|
|---|---|---|---|---|
| Child Demographics | ||||
| Age, months * | 55.14 (11.19) | 55.10 (12.07) | 55.00 (10.67) | 55.30 (11.06) |
| Sex (Female) No. (%)* | 86 (56.95) | 25 (53.19) | 29 (58.00) | 32 (59.26) |
| Race No. (%)* | ||||
| Black | 14 (9.27) | 3 (6.38) | 6 (12.00) | 5 (9.26) |
| White | 115 (76.16) | 37 (78.72) | 38 (76.00) | 40 (74.07) |
| More than One/Other | 22 (14.57) | 7 (14.89) | 6 (12.00) | 9 (16.67) |
| Ethnicity No. (%)* | ||||
| Hispanic or Latino | 9 (5.96) | 1 (2.13) | 3 (6.00) | 5 (9.26) |
| Non-Hispanic | 142 (94.04) | 46 (97.87) | 47 (94.00) | 49 (90.74) |
| Weight, kg | 26.01 (5.52) | 26.15 (6.16) | 25.91 (5.02) | 25.97 (5.47) |
| Height, cm | 111.13 (8.17) | 111.02 (8.71) | 111.62 (8.04) | 110.77 (7.92) |
| Child BMIz* | 2.44 (0.60) | 2.41 (0.53) | 2.41 (0.56) | 2.48 (0.70) |
| % OBMI* | 35.36 (16.66) | 35.64 (17.21) | 34.18 (15.77) | 36.21 (17.23) |
| Child BMI Percentile* | 98.57 (1.28) | 98.60 (1.23) | 98.52 (1.31) | 98.57 (1.30) |
| Caregiver Demographics | ||||
| Age | 35.42 (6.55) | 35.36 (6.56) | 34.78 (5.95) | 36.07 (7.09) |
| Relationship to Child No. (%) | ||||
| Mother | 136 (90.07) | 42 (89.36) | 47 (94.00) | 47 (87.04) |
| Father | 11 (7.28) | 4 (8.41) | 2 (4.00) | 5 (9.26) |
| Grandparent | 3 (1.99) | 1 (2.13) | 1 (2.00) | 1 (1.85) |
| Other | 1 (0.66) | - | - | 1 (1.85) |
| Caregiver Education No. (%) | ||||
| Less Than High School Degree | 2 (1.32) | - | - | 2 (3.70) |
| High School Graduate/ GED | 16 (10.60) | 5 (10.64) | 5 (10.00) | 6 (11.11) |
| Some College/ Specialized training | 53 (35.10) | 21 (44.68) | 17 (34.00) | 15 (27.78) |
| College Degree | 55 (36.42) | 15 (31.91) | 20 (40.00) | 20 (37.04) |
| Graduate Degree | 25 (16.56) | 6 (12.77) | 8 (16.00) | 11 (20.37) |
| Family Income No. (%)* | ||||
| < $30k | 16 (10.60) | 4 (8.51) | 8 (16.00) | 4 (7.41) |
| $30k – 49.9k | 23 (15.23) | 9 (19.15) | 5 (10.00) | 9 (16.67) |
| $50k – 99.9k | 77 (50.99) | 25 (53.19) | 23 (46.00) | 29 (53.70) |
| ≥ $100k | 34 (22.52) | 9 (19.15) | 14 (28.00) | 11 (20.37) |
| Not Reported | 1 (0.66) | - | - | 1 (1.85) |
| Hollingshead Score * | 43.03 (11.68) | 43.21 (11.12) | 42.24 (12.73) | 43.59 (11.31) |
| Marital Status No. (%) | ||||
| Single | 31 (20.53) | 10 (21.28) | 11 (22.00) | 10 (18.52) |
| Married | 108 (71.52) | 35 (74.47) | 34 (68.00) | 39 (72.22) |
| Divorced | 6 (3.97) | 2 (4.26) | 2 (4.00) | 2 (3.70) |
| Separated | 3 (1.99) | - | 2 (4.00) | 1 (1.85) |
| Widowed | 1 (0.66) | - | 1 (2.00) | - |
| Cohabit, Not Married | 2 (1.32) | - | - | 2 (3.70) |
| Caregiver Weight Status No. (%) | ||||
| Healthy Weight (BMI <25) | 19 (12.58) | 6 (12.77) | 8 (16.00) | 5 (9.26) |
| Overweight (BMI 25 to <30) | 25 (16.56) | 7 (14.89) | 7 (14.00) | 11 (20.37) |
| Obese (BMI ≥30) | 101 (66.89) | 33 (70.21) | 32 (64.00) | 36 (66.67) |
| Pregnant | 6 (3.97) | 1 (2.13) | 3 (6.00) | 2 (3.70) |
| Self-Reported Pre-Pregnancy BMI | 32.32 (6.02) | 33.59 (−) | 34.05 (3.47) | 29.07 (11.19) |
denotes variables tested for differences at baseline, all p’s > 0.05
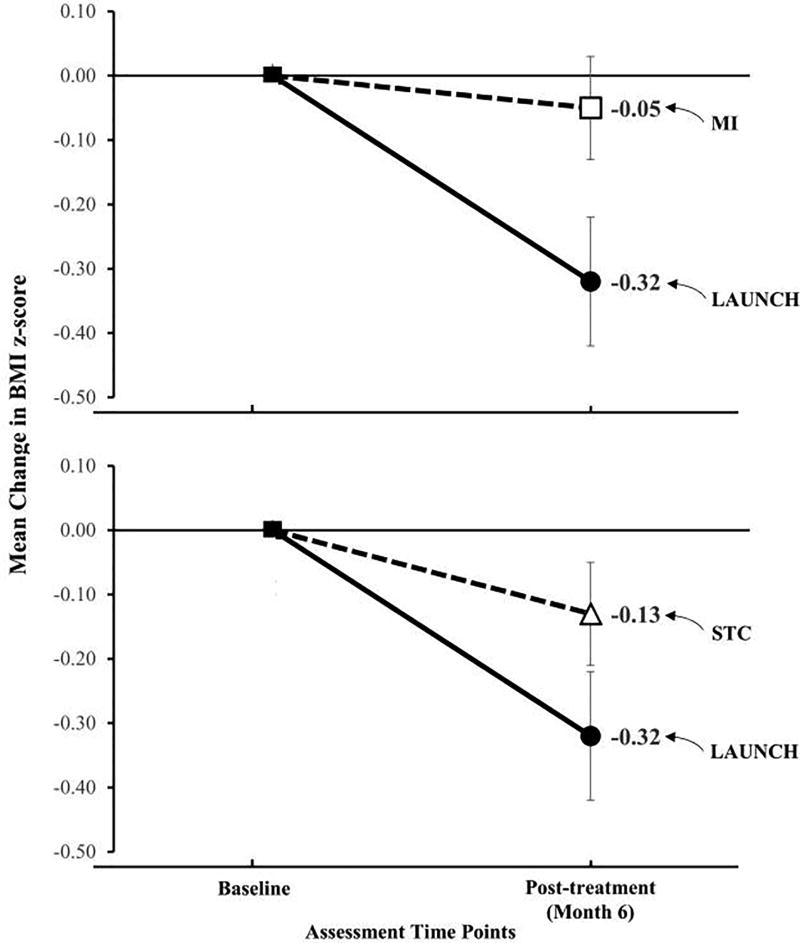
As shown in Figure 2, LAUNCH demonstrated a mean (±SD) decrease in BMIz of −0.32 (±0.33) while MI yielded a decrease of −0.05 (±0.27). LAUNCH participants had a statistically significant reduction in BMIz at the end of the intervention period, as compared with the MI group, p < 0.001 (Table 2).
Figure 2.
Mean change in body mass index z-score for LAUNCH compared to Motivational Interviewing (MI) and Standard Care (STC) from baseline to post-treatment (Month 6
Table 2.
Regression Analyses Comparing LAUNCH with Motivational Interviewing (MI) and with Standard Care (STC)
| LAUNCH vs MI (N = 89) |
LAUNCH vs STC (N = 93) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β Coefficient |
95% CI | Wald Statistic |
p-value | Effect Size (ω2) |
β Coefficient |
95% CI | Wald Statistic |
p-value | Effect Size (ω2) |
| BMIz Baseline | 0.89 | −0.36, 0.22 | 15.53 | < 0.001 | 0.91 | 0.80, 1.02 | 16.40 | < 0.001 | ||
| Intervention Group | 0.27 | 0.15, 0.40 | 4.28 | < 0.001 | 0.74 | 0.19 | 0.06, 0.32 | 2.92 | < 0.004 | 0.75 |
| Intercept | −0.07 | −0.10 | ||||||||
Note. 95% CI = 95% confidence interval of parameter estimates.
Also shown in Figure 2, STC yielded a decrease of −0.13 (±0.31) compared with the mean decrease in BMIz of −0.32 (±0.33) for LAUNCH. LAUNCH participants had a statistically significant reduction in BMIz at the end of the intervention period when compared with the STC group, p = 0.004 (Table 2).
As shown in Table 3 (available at www.jpeds.com), the decrease in BMIz was achieved in LAUNCH by a slowing in weight gain for children to an average gain of 0.67 kg over 6 months compared with MI and STC, both of which had an average weight gain of over 2 kg. Children across groups had similar gains in height. This slowing of weight gain while children grew in height resulted in an average BMI percentile change of −2.0 percentile points for LAUNCH, − 0.21 for MI, and −0.77 for STC. Children in LAUNCH showed a decrease in %OBMI of −4.45%, while MI and STC showed increases of 2.43% and 1.45%, respectively.
Table 3.
Weight, Height, and body mass indexes (BMI) of z-score (BMIz), percentile for age and gender (BMI %ile) and percent over the 50th percentile BMI (%OBMI) at Baseline and 6 Months and Change Scores Over Intervention Period for LAUNCH, Motivational Interviewing (MI) and Standard Care (STC)
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| LAUNCH (n = 43) |
MI (n = 46) |
STC (n = 50) |
|||||||
| Variable | Baseline | Month 6 | Change | Baseline | Month 6 | Change | Baseline | Month 6 | Change |
|
|
|||||||||
| Height | 111.10 (8.64) | 114.74 (8.38) | 3.64 (0.90) | 111.96 (7.89) | 115.85 (7.56) | 3.89 (0.91) | 110.85 (7.94) | 114.64 (7.78) | 3.79 (0.91) |
| Weight | 26.04 (6.10) | 26.71 (6.18) | 0.67 (1.86) | 25.95 (5.02) | 28.16 (5.45) | 2.21 (1.35) | 25.74 (5.17) | 27.76 (6.05) | 2.03 (1.75) |
| BMIz | 2.40 (0.54) | 2.08 (0.62) | −0.32 (0.33) | 2.39 (0.57) | 2.35 (0.54) | −0.05 (0.27) | 2.44 (0.64) | 2.31 (0.63) | −0.13 (0.31) |
| BMI %ile | 98.55 (1.25) | 96.56 (3.64) | −2.00 (2.91) | 98.45 (1.34) | 98.23 (1.80) | −0.21 (1.23) | 98.52 (1.32) | 97.75 (2.68) | −0.77 (1.94) |
| %OBMI | 34.87 (17.10) | 30.43 (17.80) | −4.45 (7.55) | 33.63 (15.60) | 36.07 (16.72) | 2.43 (5.62) | 34.88 (15.24) | 36.33 (17.83) | 1.45 (7.49) |
Caregivers with a BMI ≥25, excluding those who were or became pregnant, underwent or were preparing for bariatric surgery, or lost to follow-up were analyzed (n = 109). A significantly greater reduction in BMI was also observed for parents in LAUNCH (mean = −0.98, SD = 1.79) compared with parents in MI (mean = 0.43, SD = 1.63), βGroup = 1.40 [95% CI 0.55, 2.25] p = 0.002, and in STC (mean 0.21, SD = 1.88), βGroup = 1.17 [95% CI 0.32, 2.02] p = 0.007. LAUNCH demonstrated 96% adherence to the treatment manual checklist. For MI all domain scores were above proficient thresholds as defined by the MITI fidelity scoring and 95% MI adherent. LAUNCH participants attended an average 15.68 of 18 sessions (87%). MI participants attended an average 16.24 of 18 sessions (90%). Two, five, and six caregivers in LAUNCH, MI and STC, respectively, reported consulting a healthcare professional for assistance in weight management for their preschooler outside of the study: child’s pediatrician (LAUNCH 2; MI 3; STC 5), a dietitian (MI 1; STC 1) or both (MI 1). There were no SAEs in this study and no AE for child height. Only one AE, child bumping their head during a LAUNCH group activity, was coded as a “definitely” related, but mild and not requiring treatment.
Discussion
Results of this Phase III RCT demonstrated that the LAUNCH 18 session, skills-based behavioral family clinic and home-based intervention was effective in reducing child BMIz. We used a rigorous three-group design that tested LAUNCH against a credible alternative treatment, MI, that was delivered at the same frequency, but focused on motivation of the child’s caregiver to make diet and activity changes and against a control group followed over the same 6 month period, but not assigned any treatment. The reduction in BMIz for LAUNCH participants was achieved primarily by slowing the rate of weight gain over the 6 months of intervention. Children in MI and STC gained almost triple the amount of weight during the 6-month period as children in LAUNCH. This slowing of weight gain resulted in a 4.45% decrease in percent overweight for LAUNCH, while both MI and STC increased in their percent overweight by 2.43% and 1.45%, respectively. Thus, the decrease in BMIz for LAUNCH was not only statistically significant, but clinically meaningful as well.
Our results add to the burgeoning treatment research in preschoolers14 and extend the findings with school age children,28 that intensive, skills-based behavioral treatment, such as LAUNCH, is necessary to reduce obesity at all ages. Our results are also consistent with Taveras et al and show that an MI intervention that addresses motivation and resolution of ambivalence for parents about dietary change for their child is not sufficient to overcome the barriers parents face in terms of child behaviors to effectively implement dietary changes.15
Home visits in LAUNCH provided an opportunity for research staff to model and coach caregivers to effectively manage child behavior problems (e.g., lengthy tantrums) via ignoring or time-out. Often times, caregivers inadvertently reinforced these behaviors by giving the child the requested food as a means of ending the tantrum. The home visits also provided dedicated time and personal guidance in helping parents identify “unhealthy” foods and making plans to remove them from the home, as well as incorporating exposure to new vegetables on a routine basis. Although home visits increase the cost of obesity treatment compared with clinic only, we have previously estimated that each home visit would cost $65.80 if delivered by a social worker, resulting in a total estimated cost of $1,276 for the intervention.12 There is a growing interest in incorporating home visits into obesity treatment 29 and prevention.30 Our results support this as a fruitful avenue for future obesity research as well.
Despite MI being endorsed by the American Academy of Pediatrics16 for weight management, only one of three studies examining MI for weight management in young children (those aged 4–8 and 2–8 years)31,15, 32 found a significant effect for MI on reducing BMI percentile.32 All studies of MI with preschoolers, including the current study, used a combination of physicians and other healthcare professionals (dietitians, nurses, psychologists), a combination of in-person, but primarily phone based delivery, and sessions were brief (most reporting scheduled 15 minute phone sessions). The primary difference between the Resnicow et al study, where an intensive 10 session MI intervention was found to be superior to a 4 session physician only MI intervention and care as usual, and other MI studies, was that MI was delivered over a 2 year intervention period compared with 6-months in the current study and Schwartz et al and 1-year in Taveras et al.15,31,32 This raises the possibility that the effects of MI may be stronger if delivered over a longer period of time or take longer to manifest.
Although LAUNCH resulted in a statistical significantly greater reduction in BMIz than STC and MI, it was surprising that the STC group showed a slight decrease in BMIz across the 6-month period. In our pilot study of LAUNCH, the STC group demonstrated an increase in BMIz12 while LAUNCH showed a decrease. This difference in the direction of change in the STC group between the two studies could be a reflection of the societal emphasis on reducing obesity, which may also reflect why some families sought outside guidance on weight management during the course of the study.
Changing the diet and activity of preschool-age children to reduce obesity requires child behavior management skills training for parents. Targeting increasing parent motivation or overcoming ambivalence about dietary changes is not sufficient. Home visits appear to be an especially effective way to assist parents in the acquisition and generalization of these skills as they provide an opportunity for in-vivo practice of these skills with supervision and modeling by interventionists in their home. Future research needs to test the intervention with a broader population sample and test models for dissemination.
Supplementary Material
Acknowledgments
We thank the 27 pediatric practices in Greater Cincinnati/Northern Kentucky for their enthusiastic and continuous collaboration. None of them received any compensation. We also thank the families who so generously gave of their time to participate in the study. We are grateful to our research personnel including Angela Combs, Jared Connor, and Megan Richardson.
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01DK091251), the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) (UL1 TR001425), and the NIH (T32 DK063929). An independent Data Safety and Monitoring Board participated in protocol review and provided study oversight.
Abbreviations
- LAUNCH
Learning about Activity and Understanding Nutrition for Child Health
- MI
Motivational Interviewing
- STC
Standard Care
- BMI
Body mass index
- BMIz
Body mass index z-score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Trial Registration. Clinicaltrials.gov NCT01546727.
References
- 1.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster BA, Farragher J, Parker P, Sosa ET. Treatment Interventions for Early Childhood Obesity: A Systematic Review. Acad Pediatr. 2015;15:353–361. doi: 10.1016/j.acap.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370:403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 5.Nader PR, O'Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006;118:e594–601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 6.He Q, Ding ZY, Fong DY, Karlberg J. Blood pressure is associated with body mass index in both normal and obese children. Hypertension. 2000;36:165–170. doi: 10.1161/01.hyp.36.2.165. [DOI] [PubMed] [Google Scholar]
- 7.Williams CL, Strobino B, Bollella M, Brotanek J. Body size and cardiovascular risk factors in a preschool population. Prev Cardiol. 2004;7:116–121. doi: 10.1111/j.1520-037x.2004.03224.x. [DOI] [PubMed] [Google Scholar]
- 8.Al Mamun A, Cramb SM, O'Callaghan MJ, Williams GM, Najman JM. Childhood overweight status predicts diabetes at age 21 years: a follow-up study. Obesity (Silver Spring) 2009;17:1255–1261. doi: 10.1038/oby.2008.660. [DOI] [PubMed] [Google Scholar]
- 9.Mannino DM, Mott J, Ferdinands JM, Camargo CA, Friedman M, Greves HM, et al. Boys with high body masses have an increased risk of developing asthma: findings from the National Longitudinal Survey of Youth (NLSY) Int J Obes (Lond) 2006;30:6–13. doi: 10.1038/sj.ijo.0803145. [DOI] [PubMed] [Google Scholar]
- 10.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories A Systematic Review and Meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark LJ, Spear S, Boles R, Kuhl E, Ratcliff M, Scharf C, et al. A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers. Obesity (Silver Spring) 2011;19:134–141. doi: 10.1038/oby.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolling C, Crosby L, Boles R, Stark L. How pediatricians can improve diet and activity for overweight preschoolers: a qualitative study of parental attitudes. Acad Pediatr. 2009;9:172–178. doi: 10.1016/j.acap.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quattrin T, Roemmich JN, Paluch R, Yu J, Epstein LH, Ecker MA. Efficacy of family-based weight control program for preschool children in primary care. Pediatrics. 2012;130:660–666. doi: 10.1542/peds.2012-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taveras EM, Gortmaker SL, Hohman KH, Horan CM, Kleinman KP, Mitchell K, et al. Randomized controlled trial to improve primary care to prevent and manage childhood obesity: the High Five for Kids study. Arch Pediatr Adolesc Med. 2011;165:714–722. doi: 10.1001/archpediatrics.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlow SE, Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 17.Duncan DT, Hansen AR, Wang W, Yan F, Zhang J. Change in Misperception of Child's Body Weight among Parents of American Preschool Children. Child Obes. 2015;11:384–393. doi: 10.1089/chi.2014.0104. [DOI] [PubMed] [Google Scholar]
- 18.Garrett-Wright D. Parental perception of preschool child body weight. J Pediatr Nurs. 2011;26:435–445. doi: 10.1016/j.pedn.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Pagnini DL, Wilkenfeld RL, King LA, Booth ML, Booth SL. Mothers of pre-school children talk about childhood overweight and obesity: The Weight Of Opinion Study. J Paediatr Child Health. 2007;43:806–810. doi: 10.1111/j.1440-1754.2007.01199.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States advance data from vital and health statistics. 314. Hyattsville, MD: National Center for Health Statistics; 2000. [PubMed] [Google Scholar]
- 21.Stark LJ, Filigno SS, Bolling C, Ratcliff MB, Kichler JC, Robson SL, et al. Learning about Activity and Understanding Nutrition for Child Health (LAUNCH): Rationale, design, and implementation of a randomized clinical trial of a family-based pediatric weight management program for preschoolers. Contemp Clin Trials. 2017;52:10–19. doi: 10.1016/j.cct.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brotman LM, Klein RG, Kamboukos D, Brown EJ, Coard SI, Sosinsky LS. Preventive intervention for urban, low-income preschoolers at familial risk for conduct problems: a randomized pilot study. J Clin Child Adolesc Psychol. 2003;32:246–257. doi: 10.1207/S15374424JCCP3202_10. [DOI] [PubMed] [Google Scholar]
- 23.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 24.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 25.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- 26.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ (Clinical research ed) 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paluch RA, Epstein LH, Roemmich JN. Comparison of methods to evaluate changes in relative body mass index in pediatric weight control. Am J Hum Biol. 2007;19:487–494. doi: 10.1002/ajhb.20608. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for Obesity and Intervention for Weight Management in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317:2427–2444. doi: 10.1001/jama.2017.0332. [DOI] [PubMed] [Google Scholar]
- 29.Foster BA, Aquino CA, Gil M, Gelfond JA, Hale DE. A Pilot Study of Parent Mentors for Early Childhood Obesity. J Obes. 2016;2016:2609504. doi: 10.1155/2016/2609504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvy SJ, de la Haye K, Galama T, Goran MI. Home visitation programs: an untapped opportunity for the delivery of early childhood obesity prevention. Obes Rev. 2017;18:149–163. doi: 10.1111/obr.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz RP, Hamre R, Dietz WH, Wasserman RC, Slora EJ, Myers EF, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med. 2007;161:495–501. doi: 10.1001/archpedi.161.5.495. [DOI] [PubMed] [Google Scholar]
- 32.Resnicow K, McMaster F, Bocian A, Harris D, Zhou Y, Snetselaar L, et al. Motivational interviewing and dietary counseling for obesity in primary care: an RCT. Pediatrics. 2015;135:649–657. doi: 10.1542/peds.2014-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.