Introduction
Medical professionals place over five million central venous catheters (CVC) in the United States each year1. CVCs give doctors the ability to provide nutrition, medication, hemodynamic support, and the venous access necessary to perform procedures on a wide variety of patients including those in critical condition. Despite being a common procedure, ultrasound guided CVC has a reported complications rate ranging from 5.0% to 21%1–3. These complications are wide ranging including hematoma, hemothorax, pneumothorax, accidental arterial puncture, and infection, all of which can increase the hospitalization time of the patient, increase medical costs, and increase the rate of mortality4–7. Most complications due to CVC occur during the process of needle insertion1,8. These mechanical complications are caused by a variety of factors including clinician inexperience, multiple catheterization attempts, and difficult patient anatomy such as large amounts of adipose neck tissue9–11. Therefore, improved training of mechanical needle insertion can help to reduce these needle insertion complications.
The process of inserting a CVC catheterization needle into the right internal jugular vein (IJ) begins with a surgeon using an ultrasound probe to identify the IJ vein at the apex of the sternal head of the sternocleidomastoid and the clavicle. A catheterization needle is then inserted between a 30 and 45 degree angle towards the ipsilateral nipple using the ultrasound as guidance. Minimizing the number of insertion attempts, avoiding the carotid artery, and aspirating the needle plunger are just a few of the important aspects of a successful insertion12. Catheterization of the right IJ vein at the apex of the sternocleidomastoid and clavicle is performed more often than femoral or subclavian CVC insertion due to its large vessel diameter, lower rate of infection compared to femoral insertion, and more superficial nature than the subclavian vein3,13.
Modern CVC training typically takes place using upper torso training manikins such as the CAE Healthcare (Sarasota, FL) Blue Phantom simulation manikin.14, 15. There is debate however over the long term effectiveness of these manikins. While one study concluded that simulation training is associated with improved in-hospital performance of CVC insertion5, another found that a CVC simulation course improves short term procedural skills, but these skills quickly degrade over time16. These manikin simulators have limited patient anatomy and unnatural tissue properties leaving residents unprepared to work with the wide variety of patients they will encounter in a clinical environment. Furthermore, manikin simulation relies on observation based qualitative feedback and assessment from an instructor which is vulnerable to bias such as “examiner burnout” due to busy work schedules17. More effective training solutions with a quantitative and qualitative feedback need to be developed to fulfill the training needs of surgical residents.
In recent years, haptic feedback and virtual reality (VR) medical simulators have become more sophisticated in their training capabilities18. A 2007 study by Morris et al. suggests that haptic feedback training in combination with visual feedback may be an effective training tool for skills where force sensitivity and fine motor skills are a crucial component19. A 2016 study by Mohamadipanah et al. indicated that the ability to better understand haptic sensations felt during a procedure could affect CVC performance20. In order to meet these new training recommendations, high and low fidelity haptic based patient simulators are being developed. Research has shown that high fidelity simulators such as the LAPSIM laparoscopic surgical simulator by Surgical Science (Gothenburg; Sweden) and PalpSim palpitation simulator by Coles et al. are able to differentiate between expert and novice trainees as well as provide complex haptic feedback to the user21,22. This type of high fidelity haptic simulator has yet to be tested and deployed for use in CVC training.
In light of this, the current paper presents the development and training efficacy testing of a novel VR and haptic feedback based training device for the right IJ vein CVC procedure. The VR haptic robotic CVC simulator shown in Figure 1 utilizes a unique combination of a navigable virtual ultrasound environment, as well as a haptic needle insertion force characterization developed in previous work23. The Material and Methods section of this paper gives an overview of the simulator hardware and the development of the haptic syringe, virtual ultrasound, graphical user interface, and details the experiments conducted to determine the effectiveness of the device. This is followed by the Results and Discussion sections about the experimental outcomes and the future of this training. Lastly the Conclusions section is presented.
Figure 1.
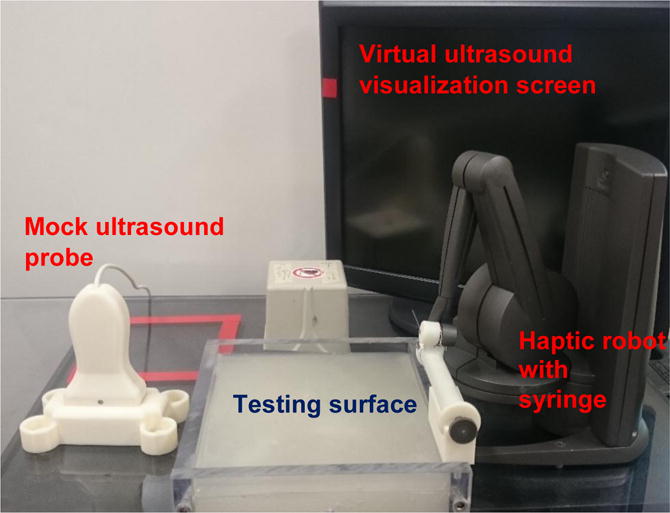
Virtual Reality Haptic Robotic Central Venous Catheterization Simulator
Materials and Methods
Development of the System Hardware and Software Overview
The simulator is controlled using a custom built personal computer running MathWorks (Natick, MA) MATLAB and Simulink software packages. Three degrees of haptic feedback and six degrees of freedom in needle position tracking are achieved using a 3D Systems Geomagic Touch X (Rock Hill, SC) haptic robotic arm and the Quanser (Markham, ON) Quarc software package. Also used is an Ascension 3D Guidance trakSTAR (Shelburne, VT) six degrees of freedom electromagnetic position tracking device. This probe provides position tracking for the ultrasound component of the device to be discussed in a later section. Virtual interaction between the devices is attained through the use of known starting locations and relative distance between the devices. The major components of the simulator can be divided into three parts: haptic robotic, virtual ultrasound imaging, and graphical user interface.
Haptic Robotic Component
The haptic robotic component of the simulator utilizes two devices: a mock syringe end effector and haptic robot arm. The custom built syringe end effector in Figure 2 was designed to attach to the Geomagic Touch X and fulfilled three main design requirements. First, the needle must retract into the syringe with minimal force. Minimal retraction force was necessary to ensure that all force feedback experienced by the user is caused by the haptic arm. Second, the syringe plunger must extend, or “aspirate”, and communicate this information to the program. During a CVC insertion, a surgeon must constantly aspirate the syringe in order to see the “flash” or blood entering the syringe whenever they puncture a vessel. Finally, the syringe must be similar in size to a real syringe used during CVC, so that it feels realistic to the user.
Figure 2.
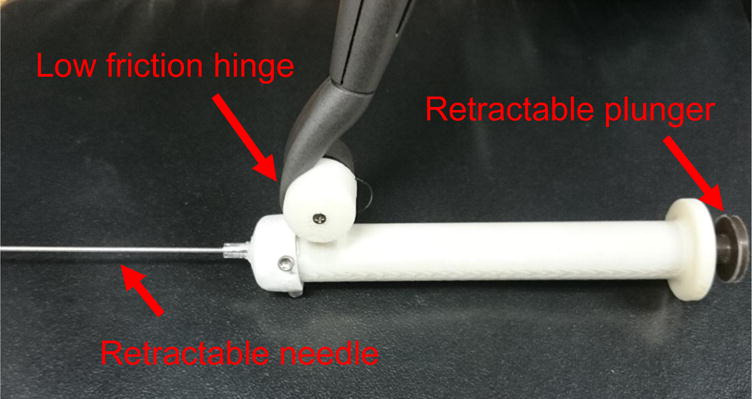
Custom built syringe end effector for Geomagic Touch X
The haptic force feedback provided by the Geomagic Touch X is calculated using a piecewise force characterization algorithm for linear needle insertion proposed by Gordon et al.23. Force data is inputted into the force characterization which outputs an equation for axial needle force verses needle depth. To create the most accurate haptic force characterization possible, an experiment using a fresh frozen unembalmed cadaver was conducted24. The cadaver obtained was that of an average sized middle aged male. Five needle insertions were attempted by an expert surgeon into the right IJ vein and the surrounding neck muscle tissue. Using these results, the linear needle force characterization in Figure 3 was created for this simulator.
Figure 3.
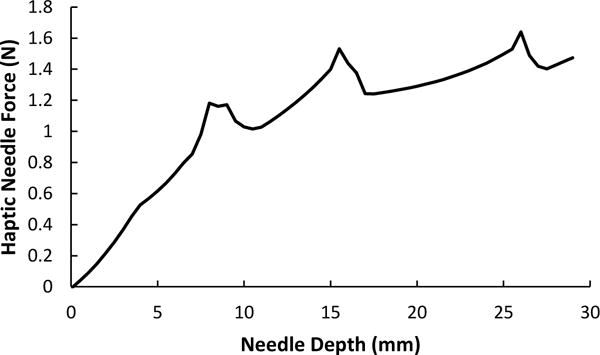
Haptic needle force characterization utilized in the virtual reality haptic robotic central venous catheterization simulator.
The needle insertion surface was created using polyvinyl chloride modified with plastisol. This soft material allows the user to compress the testing surface in a manner similar to human neck tissue. A flat sheet transparency is implanted approximately 5 mm from the surface of the phantom tissue. This allows the haptic needle to be pressed into the surface but not penetrate the tissue deeper than the sheet transparency layer. This very slight phantom tissue penetration also has the additional benefit of realistically preventing lateral movement of the needle during insertion.
Virtual Ultrasound Imaging
The Virtual Ultrasound Image generates visualization of the carotid artery, the right IJ vein, and the approaching needle as shown in Figure 4. Utilizing the 3D Guidance trakSTAR sensor, this image responds appropriately as the mock ultrasound probe is tilted, reversed, and scans across the area. The approaching needle deforms the tissue lines and the needle is visualized when it crosses the plane of the needle as shown in Figure 4. The vein and artery both also move appropriately. The artery slightly expands in size to resemble a pulse, the IJ vein image compresses when the mock probe is pushed into the soft scanning surface, and the IJ vein image deforms as the virtual needle is inserted. The screen will also indicate if flash appears, with a blue bar across the top of the screen, or if artery puncture occurs, with a red bar across the top of the screen. These colors were chosen because they are standard for simulated arterial and venous blood. This visualization provides a realistic platform for training the skills of manipulating an ultrasound probe and needle.
Figure 4.
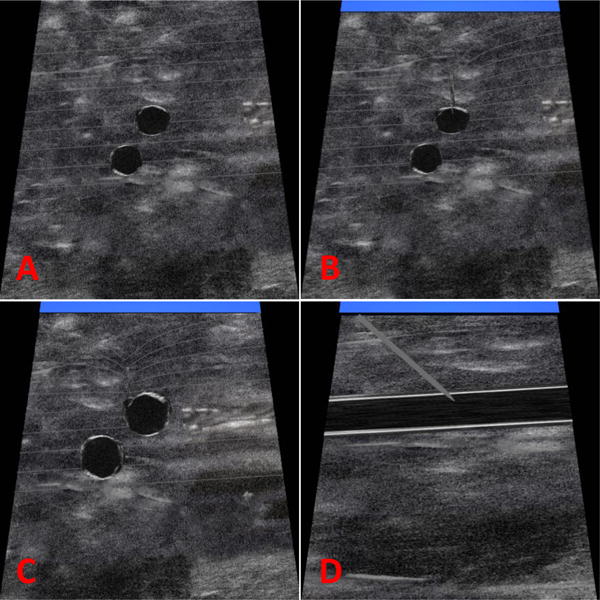
Virtual ultrasound images generated by the haptic robotic central venous catheterization simulator: (A) no vein compression, (B) needle tip entering vein and blue venous flash indicator, (C) offcenter needle deformation of vein and blue venous blood flash indicator, (D) longitudinal view of vein and needle and blue venous blood flash indicator.
Graphical User Interface
The graphical user interface (GUI) was created to step surgical residents through the procedure and allow them to practice on a wide variety of scenarios. When the system starts, a login screen welcomes the user and asks for credentials. The user is then sent to the home screen which include a list of tips, a plot of performance for their previous practice attempts, and a high score leaderboard to show the top 10 insertion scores among all users. The user then proceeds to a patient overview screen which reveals the patient information for the scenario to be practiced as well as what is necessary to achieve a perfect score for their insertion. The user then attempts a CVC insertion and a test results screen as shown in Figure 5 is generated. The test results screen provides the user with a performance score and letter grade, case difficulty, number of insertions, angle of insertion, accuracy of insertion to center of vein, percent of time spent aspirating, and high scores on the leaderboard. Each of the feedback factors also provides a score out of five stars. Star ratings are based on criteria given for a successful CVC procedure. Scores were calculated using the factors in Table 1 and equation 1.
| (1) |
All test information is saved to a central database location for future analysis. At this point the user can chose move on to another scenario, or return to the home screen. All scores and star ratings can be improved by more accurately following the procedure instructions given before the beginning of the simulation.
Figure 5.
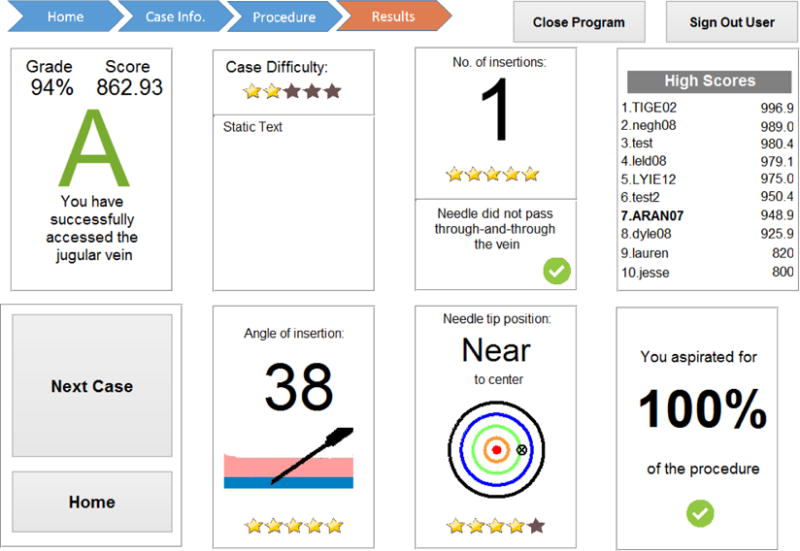
Example simulation results screen for the CVC simulator
Table 1.
Description of virtual reality haptic CVC simulator performace scoring variables
| Variables | Description |
|---|---|
| Is | 0 if needle ends in artery 0.5 if needle in neither vessel 1 if needle ends in vein |
| Θs | Average needle angle factor |
| Cs | Needle centering factor |
| as | Needle aspiration factor |
| Bs | Vein backwall puncture penalty |
| As | Multiple attempts penalty |
Surgical Resident Training Study
The surgical resident training study looked to answer three main research questions: how does resident performance using the simulator change over time, what factors most impact this change in performance, and what patient anatomical factors make a simulation scenario more or less challenging? To answer these questions, the VR haptic robotic CVC simulator was integrated into the curriculum of 13 first year surgical residents over three months. The participants were chosen randomly and began training during their first week of residency. They had no previous clinical experience in CVC. Institutional review board approval was obtained and identities of residents were made anonymous.
All 13 participants received identical training. The training regimen was as follows. During the introduction session, the residents were presented with the CVC procedure through a short lecture and video commonly used to teach CVC. The residents then performed a needle insertion into a traditional CVC training manikin to familiarize themselves with CVC patient anatomy and anatomical landmarks. A month later, the first training session introduced the residents to the VR haptic robotic CVC simulator and two needle insertions were attempted on the baseline training scenario. This baseline training scenario would be repeated to monitor the residents’ progress throughout the training program. The following month, training session two was conducted. This was a more extended session where residents were given the baseline training scenario on the simulator, followed by eight practice scenarios, and ending with the baseline training scenario. The final training session took place a month later and was identical to the second, but with different practice scenarios. By the end of testing, the residents completed 22 practice insertions on 17 different scenarios. For comparison, the 22 simulated insertions were also completed by an expert vascular surgeon with 20 years of experience and a third year surgical resident with approximately 25 clinical CVC insertions. This information allows a greater understanding about how clinical experience translates to haptic robot performance.
The 17 different patient scenarios were developed with the assistance of an experienced surgical resident who is responsible for teaching the CVC procedure. These scenarios were designed to test a wide variety of patient types including those who are morbidly obese, extremely thin, edematous, hypovolemic, hypotensive, hemodynamically unstable, and dehydrated. Simulator vessel depth’s range from 13 mm to 35 mm. Relative vessel position was based on known typical anatomical position of the right IJ vein and carotid artery and placed in positions that are typically considered more or less challenging for surgeons based on desired scenario difficulty (i.e. a more posterior artery in relation to the vein increases chance of arterial puncture). The sizes of the vessels ranged from 7 mm to 15 mm based on patient scenario and typical vessel size25–28. One of these scenarios was designated as the baseline training scenario. This represented a patient with average vessel depth, and vessel orientation. Over the course of training, the residents would repeat this baseline training scenario so their overall skill changes could be monitored.
Results
Comparing Baseline Training Scenario Tests
Figure 6 shows the average resident performance for the baseline training scenario as a percentage of the maximum possible score. The average user scores increased from 52% to 96% of the maximum possible score between the first overall and final overall test. The expert surgeon and experience surgical resident performed at a high level throughout the training with average scores of 98% and 96%, respectively. This indicates that the new surgical residents were able to achieve near expert proficiency with the robotic training after three training day sessions. These scores were based on the factors in Table 1.
Figure 6.
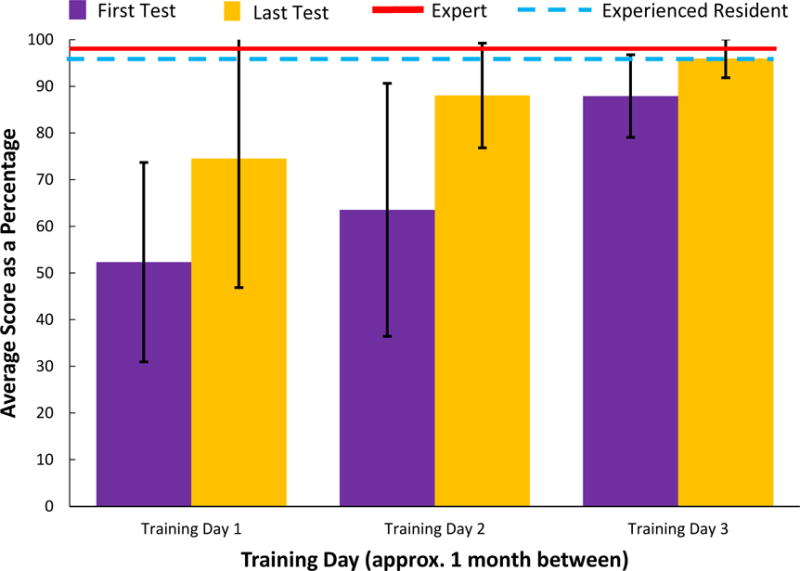
Performance of the surgical residents on the baseline training scenario across the three training days. The average score of an expert vascular surgeon and a third year surgical resident are show for reference.
Importantly, a two-way analysis of variance (ANOVA) test revealed statistically significant improvement (F(1,76) = 8.45, p = 0.00477) between the first and last test of each training day and a statistically significant improvement in performance (F(1,50) = 11.8, p = 0.00119) between the second and third training days. A decrease in performance is seen between baseline training scenario two and three. This decrease is typical of a learning curve when there is a large gap in time between learning sessions, which in this case was a month between baseline training scenario two and three. A Bartlett’s Test revealed there to be significant difference between the variance in scores between the six baseline training scenario insertions (χ2(5) = 42.4, p = 4.86*10−8). Using a Levene’s Test, the major statistical differences were found to be between the first and last baseline training scenarios on day two (F(1,22) = 4.30, p=8.3*10−5) and the first and last baseline training scenarios on day three (F(1,24) = 8.60 p = 0.00729).
On the final training day, all participants successfully inserted the needle into the vein when presented with the baseline training scenario: an improvement of 64%. Proper centering of the catheterization needle tip in the target vessel also is an important factor in CVC that leads to easier insertion of a guide wire. The mean distance between the needle tip and vein center decreased by 61% between the first and last baseline training scenario insertion. A Levene’s Test showed a significant decrease in the variance in distance between the needle tip and vein center across the baseline training scenarios (F(5,72) = 4.53, p = 0.00120).
In addition, throughout the course of training, the mean number of insertion attempts decreased from 1.92 to 1.23 attempts over the course of testing. Importantly, a Levene’s Test found an overall decrease (F(5,72) = 3.90, p = 0.00350) in the variance of the number of attempts over the six tests. Participants also aspirated their syringe 12% longer during insertion between the first and last baseline training scenarios. The fact that the residents are increasing their aspiration time shows that they are both improving their chosen grip and remembering to aspirate more often. Finally, while other data factors such as the number of artery punctures and number of times the rear wall of the vein was punctured were recorded, no meaningful trends in this data was found. Specifically, only one of the overall 78 baseline training scenario needle insertions resulted in an accidental artery puncture.
Comparing Simulated Patient Anatomical Factors
The different types of patient anatomy in which the surgical residents practiced could be divided into five groups: average, shallow vessels, deep vessels, contracted vessels, and abnormal (rare vessel positioning). The practice scores are plotted in Figure 7 and the mean scores of the different groups are listed in Table 2. Notably the contracted vessel scenarios had the lowest overall mean scores and the highest overall variance compared to other scenarios. Looking at the individual scoring factors in table 2, the reason for the lower scores for the contracted vessel scenarios is the fact that the trainees punctured the back wall of the vein more often (7.7% back wall puncture rate), punctured the artery more often (5.8% arterial puncture rate), and successfully inserted into the vein at a less often (85% successful insertion rate) when compared to the other scenarios. It is also notable that the average anatomy scenarios also a lower overall mean score of 529, but these scenarios as a whole took place earlier in the training, possibly biasing them towards lower scores.
Figure 7.
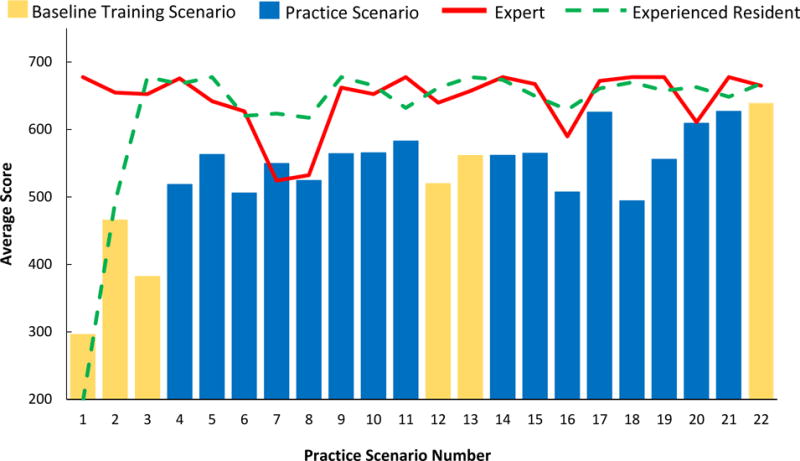
The average user, expert, and experienced resident scores for across each of the 22 simulated CVC insertions using the virtual reality haptic feedback CVC simulator. The repeated baseline training scenario is highlighted against the 16 unique practice scenarios.
Table 2.
Mean scores and values of scoring factors by anatomy type for the 16 practice scenarios (excluding baseline training scenario)
| Anatomy Types | |||||
|---|---|---|---|---|---|
| Average | Shallow vessel | Deep Vessels | contracted | Abnormal | |
| Mean score | 529 | 561 | 580 | 519 | 568 |
| Variance of Score | 19900 | 16500 | 12700 | 33200 | 16000 |
| Mean distance to vein center | 0.386 | 0.277 | 0.264 | 0.242 | 0.278 |
| Mean attempt | 1.49 | 1.67 | 1.28 | 1.40 | 1.35 |
| Mean angle (deg) | 40.9 | 39.9 | 41.2 | 41.1 | 42.3 |
| Mean in vessel | 87% | 94% | 96% | 85% | 96% |
| Mean in artery puncture rate | 0.0% | 1.9% | 1.3% | 5.8% | 3.9% |
| Mean back wall puncture rate | 0.0% | 5.8% | 0.0% | 7.7% | 0.0% |
Examining Table 2, the scenario type with the best overall scores was the deep vessel obese patients. There appears to be two main reasons. First, residents were able to successfully end the insertion with the needle in the vein 7.0% more often than the other scenarios combined. Second, the average number of insertion attempts during deep vessel patient scenarios was 1.29 compared to 1.51 for the other scenarios.
Discussion
The surgical resident training study resulted in findings for each of the three previously stated research questions. The first research question was how does resident performance using the simulator change over time? The results showed that resident performance on the simulator improves as more insertions are completed. The residents also increased their performance relative to an expert and experienced surgical resident. This suggests that they also are improving their CVC skills following the assumption that an expert performs well using the simulator because of their advanced CVC skills.
The second research question asked what factors most impact change in performance using the simulator. By far the greatest changes in performance occurred during the first four practice scenarios as seen in Figure 7. This suggests that it takes a surgical resident approximately three to four practice scenarios to familiarize themselves with the training device. The patient anatomy for a training scenario also had an effect on user performance. Overall, a combination of experience and the type of training scenario had the greatest impact on performance.
The final research question asked what patient anatomical factors make a simulation scenario more or less challenging. The anatomical feature that had the greatest impact on user performance was vessel size. Small contracted vessels proved difficult for residents throughout testing. This is understandable because small vessels require a higher degree of needle insertion accuracy and greater skill using the ultrasound guidance. It is also easier to puncture through the back wall of these small veins.
Setup time is approximately 10 minutes: turning on the computer and calibrating the system. In its current form, calibrating the device is best done by an individual, such as an instructor trained to setup the simulator. Future plans include distributing the device to several other institutions where an instructor will be taught how to operate the system. By expanding to more hospitals, the system will be able to collect large amounts of training data and create models of training performance. These models can then be utilized to automatically build dynamic individualized training programs for each surgical resident based on their performance. This type of individualized instruction has the potential to make a strong positive impact on CVC training.
The overall cost of the current VR haptic robotic simulation including the computer and software packages is $17,289. Over half of this cost is due to the haptic robotic arm ($10,870). This is more expensive than high end central line manikins, which range from $1,500 to $4,000. However, unlike manikins the VR haptic robotic simulation does not wear out, and can be configured to represent any body type or anatomy to provide a diverse training experience. Long term goals will seek to transform this system into an all-in-one training system for percutaneous procedures. This would replace multiple training manikins for different procedures. Future iterations will also convert the program to a more affordable software package and could include investigating lowering the quality of the haptic robotic arm to lower prices.
Conclusions
A new VR haptic robotic simulator for CVC insertions was developed, implementing a combination of haptic feedback needle insertion, a virtual ultrasound device, and user friendly GUI. The three research questions asking how does resident performance using the simulator change over time, what factors most impact this change in performance, and what patient anatomical factors make a simulation scenario more or less challenging were answered. Study participants were able to improve their performance on the system over time in several important CVC skills indicating that the system is successfully inducing a training effect. Furthermore, it was shown that participants were able to adapt to GUI feedback to improve their practice scores. Finally, the effects of patient anatomy on CVC difficulty has been explored. Factors such as vessel size and vessel depth may have an effect on the difficulty of practice needle insertions. Future work will seek to improve the overall training system by adding new features and expanding to more training locations. Further work will be conducted with the same surgical residents to observe their long term CVC patient outcomes to better understand the translation of skills gained through use of the simulation system.
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL127316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGee DC, Gould MK. Current concepts - Preventing complications of central venous catheterization. New Engl J Med. 2003;348:1123–1133. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 2.Sznajder J, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization: Failure and complication rates by three percutaneous approaches. Archives of Internal Medicine. 1986;146:259–261. doi: 10.1001/archinte.146.2.259. [DOI] [PubMed] [Google Scholar]
- 3.Ruesch S, Walder B, Tramer MR. Complications of central venous catheters: Internal jugular versus subclavian access - A systematic review. Crit Care Med. 2002;30:454–460. doi: 10.1097/00003246-200202000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Maecken T, Grau T. Ultrasound imaging in vascular access. Crit Care Med. 2007;35:S178–S185. doi: 10.1097/01.CCM.0000260629.86351.A5. [DOI] [PubMed] [Google Scholar]
- 5.Evans LV, Dodge KL, Shah TD, et al. Simulation Training in Central Venous Catheter Insertion: Improved Performance in Clinical Practice. Acad Med. 2010;85:1462–1469. doi: 10.1097/ACM.0b013e3181eac9a3. [DOI] [PubMed] [Google Scholar]
- 6.Yoon DY, Annambhotla S, Resnick SA, Eskandari MK, Rodriguez HE. Inadvertent Arterial Placement of Central Venous Catheters: Diagnostic and Therapeutic Strategies. Ann Vasc Surg. 2015;29:1567–1574. doi: 10.1016/j.avsg.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Mansfield PF, Gregurich MA. Complications and Failures of Subclavian-Vein Catheterization - Reply. New Engl J Med. 1995;332:1581–1581. doi: 10.1056/NEJM199412293312602. [DOI] [PubMed] [Google Scholar]
- 8.Tsotsolis N, Tsirgogianni K, Kioumis I, et al. Pneumothorax as a complication of central venous catheter insertion. Annals of Translational Medicine. 2015;3:40. doi: 10.3978/j.issn.2305-5839.2015.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisen LA, Narasimhan M, Berger J, Schneider RF, Mayo PH, Rosen MJ. Mechanical complications of central venous catheterization. Chest. 2004;126:746s–746s. doi: 10.1177/0885066605280884. [DOI] [PubMed] [Google Scholar]
- 10.Schummer W, Schummer C, Rose N, Niesen WD, Sakka SG. Mechanical complications and malpositions of central venous cannulations by experienced operators - A prospective study of 1794 catheterizations in critically ill patients. Intens Care Med. 2007;33:1055–1059. doi: 10.1007/s00134-007-0560-z. [DOI] [PubMed] [Google Scholar]
- 11.Blaivas M, Adhikari S. An unseen danger: Frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37:2345–2349. doi: 10.1097/CCM.0b013e3181a067d4. [DOI] [PubMed] [Google Scholar]
- 12.Graham AS, Ozment C, Tegtmeyer K, Lai S, Braner DAV. Central Venous Catheterization. New Engl J Med. 2007;356:e21. doi: 10.1056/NEJMvcm055053. [DOI] [PubMed] [Google Scholar]
- 13.Gordon AC, Saliken JC, Johns D, Owen R, Gray RR. US-guided puncture of the internal jugular vein: Complications and anatomic considerations. J Vasc Interv Radiol. 1998;9:333–338. doi: 10.1016/s1051-0443(98)70277-5. [DOI] [PubMed] [Google Scholar]
- 14.Kunkler K. The role of medical simulation: an overview. Int J Med Robot Comp. 2006;2:203–210. doi: 10.1002/rcs.101. [DOI] [PubMed] [Google Scholar]
- 15.Maran NJ, Glavin RJ. Low- to high-fidelity simulation - a continuum of medical education? Medical Education. 2003;37:22–28. doi: 10.1046/j.1365-2923.37.s1.9.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith CC, Huang GC, Newman LR, et al. Simulation Training and Its Effect on Long-Term Resident Performance in Central Venous Catheterization. Simulation in Healthcare. 2010;5:146–151. doi: 10.1097/SIH.0b013e3181dd9672. [DOI] [PubMed] [Google Scholar]
- 17.Wanzel KR, Ward M, Reznick RK. Teaching the surgical craft from selection to certification. Curr Prob Surg. 2002;39:577–659. doi: 10.1067/mog.2002.123481. [DOI] [PubMed] [Google Scholar]
- 18.Coles TR, Meglan D, John NW. The Role of Haptics in Medical Training Simulators: A Survey of the State of the Art. Ieee T Haptics. 2011;4:51–66. doi: 10.1109/TOH.2010.19. [DOI] [PubMed] [Google Scholar]
- 19.Morris D, Tan H, Barbagli F, Chang T, Salisbury K. Haptic feedback enhances force skill learning. World Haptics 2007: Second Joint EuroHaptics Conference and Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, Proceedings. 2007:21–26. [Google Scholar]
- 20.Mohamadipanah H, Parthiban C, Nathwani J, Rutherford D, DiMarco S, Pugh C. Can a virtual reality assessment of fine motor skill predict successful central line insertion? Am J Surg. 2016;212:573–+. doi: 10.1016/j.amjsurg.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Duffy AJ, Hogle NJ, McCarthy H, et al. Construct validity for the LAPSIM laparoscopic surgical simulator. Surg Endosc. 2005;19:401–405. doi: 10.1007/s00464-004-8202-9. [DOI] [PubMed] [Google Scholar]
- 22.R CT, W JN, G D, Caldwell CDG. Integrating Haptics with Augmented Reality in a Femoral Palpation and Needle Insertion Training Simulation. Ieee T Haptics. 2011;4:199–209. doi: 10.1109/TOH.2011.32. [DOI] [PubMed] [Google Scholar]
- 23.Gordon A, Kim I, B A, M J. Needle Insertion Force Model for Haptic Simulation. Proceedings of the ASME International Manufacturing Science and Engineering Conference. 2015 [Google Scholar]
- 24.Pepley D, Yovanoff M, Mirkin K, Miller S, Han D, Moore J. Measurement of Syringe Needle Forces for a Haptic Robotic Training Device. Journal of Medical Devices. 2017 [Google Scholar]
- 25.Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transpl. 1998;13:134–138. doi: 10.1093/ndt/13.1.134. [DOI] [PubMed] [Google Scholar]
- 26.Bannon MP, Heller SF, Rivera M. Anatomic considerations for central venous cannulation. Risk Management and Healthcare Policy. 2011;4:27–39. doi: 10.2147/RMHP.S10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parmar S, Parikh S, Mehta H. Anatomical variations of the internal jugular vein in relation to carotid artery : an ultrasound study. Int J Med Sci Public Health. 2013;2:223–228. [Google Scholar]
- 28.DENYS BG, URETSKY BF. Anatomical variations of internal jugular vein location: Impact on central venous access. Crit Care Med. 1991;19:1516–1519. doi: 10.1097/00003246-199112000-00013. [DOI] [PubMed] [Google Scholar]


