Abstract
During the initial stages of innervation of developing skeletal muscles, the terminal branches of axons from multiple motor neurons form neuromuscular junctions (NMJs) on a small region of each muscle fiber, the motor endplate. Subsequently, the number of axonal inputs at the endplate region is reduced so that, at maturity, each muscle fiber is innervated by the terminals of a single motor neuron. The Schwann cells associated with the axon terminals are involved in the removal of these synapses but do not select the axon that is ultimately retained on each fiber. Schwann cells perform this function by disconnecting terminal branches from the myofiber surface and by attacking them phagocytically. Here we discuss how this behavior is regulated and argue that such regulation is not unique to development of neuromuscular innervation but is also expressed in the response of the mature NMJ to various manipulations and pathologies.
Introduction
The neuromuscular junction (NMJ) was the first synapse shown to undergo dramatic remodeling during early development. This remodeling is now termed “synapse elimination”. Because it was widely used as a model synapse to study synaptic transmission, it was known that each muscle fiber in most mammalian muscles is innervated by a branch of a single motor axon at a site near the center of each fiber (the “endplate”). Therefore, the discovery that each endplate is innervated by multiple axons during early postnatal development in rodent muscles came as a surprise [1]. This discovery was in fact a re-discovery of the multiple innervation described anatomically in early immature muscles by a student of Ramón y Cajal [2]. Very quickly it became clear that this multiple innervation was created by a constant pool of motor neurons innervating each muscle. Each motor neuron branched profusely and came to innervate an excessive number of endplates [3]. During early postnatal development, a competition among convergent axons for the sole occupancy of each motor endplate occurs. This competition ensured that only one, but in all cases at least one, of the initial inputs remained. The excess inputs were removed and the branching of each motor neuron reduced. As a result, the sizes of the motor units (the number of fibers innervated by each motor neuron) declined several-fold to the smaller adult level. Much of the initial work was accomplished through physiological analysis of muscle innervation and motor unit contractions. However, with the discovery of fluorescent proteins, their transgenic expression in motor neurons, and the production of fluorescent alpha-bungarotoxin (a snake toxin that functions as selective ligand for the nicotinic acetylcholine receptors (AChR) concentrated in the postsynaptic membrane at the endplate), elegant imaging of the elimination as it occurs at individual, identified endplates in living mouse muscles became possible [4]. By this repeated imaging of the same NMJs in living animals (vital imaging), the fate of individual axons competing for the same postsynaptic receptors could be examined. This made it possible to determine that., at least in its final stages, the motor axons compete for the same synaptic space on each muscle fiber. The winning axon displaces the losing axon(s) and occupies the territory of the losers [4]. The winner is not preordained as ablation of the axon of the apparent winning axon allows the apparent losing axon to win [5]. A number of experiments motivated by studies showing the importance of experience in development of the visual system were then conducted to demonstrate that the course of neuromuscular synapse elimination is heavily influenced by neural activity. Elimination could be slowed by inhibiting the transmission of impulses to NMJs and it could be sped up by increasing the impulse activity [6]. Some technically impressive experiments also demonstrated that the more active axon in the multiple innervation is favored in the competition at each endplate [7,8].
Terminal Schwann cells
Glia (Schwann cells) are present at NMJs and could play a role in this synapse elimination. Several non-myelinating “terminal Schwann cells” (tSCs, also known as “perisynaptic Schwann cells”) are present at the NMJ. As shown by electron and light microscopy, their processes cap the branches of the adult nerve terminals that occupy the synaptic “gutters,” the depressions in the muscle surface where AChR are highly concentrated. The tSCs come into close apposition with the muscle fiber only at the edges of these gutters. Therefore, the tSCs mirror in the placement of their processes the distribution the nerve terminals themselves. Like astrocytes in the brain, tSCs “tile” the nerve terminals [9] (Fig. 1A). That is, each tSC covers its own contiguous territory of the nerve terminal within the NMJ with very little overlap with its neighbors. This territorial behavior is absent during early postnatal development and in adults following denervation and the consequent degeneration of the nerve terminal, suggesting it results from competition among the tSCs for a contiguous contact with nerve. Exactly how tSCs differ from the myelinating SCs that wrap the motor axon right up to the nerve terminal and from other non-myelinating SCs that wrap small caliber axons in peripheral nerves is not entirely clear. However, given the plasticity of SCs in development and in injured nerves [10], it seems likely the cells are all derivatives of common neural crest progenitors. Indeed, there is evidence of plasticity in the differentiation of tSCs. Non-myelinating tSCs at the site of the nerve entry into the endplate at some NMJs appear to trans-differentiate and myelinate a segment of the nerve that was once synaptic. This is accompanied by a corresponding loss of AChRs at this site within the endplate [11].
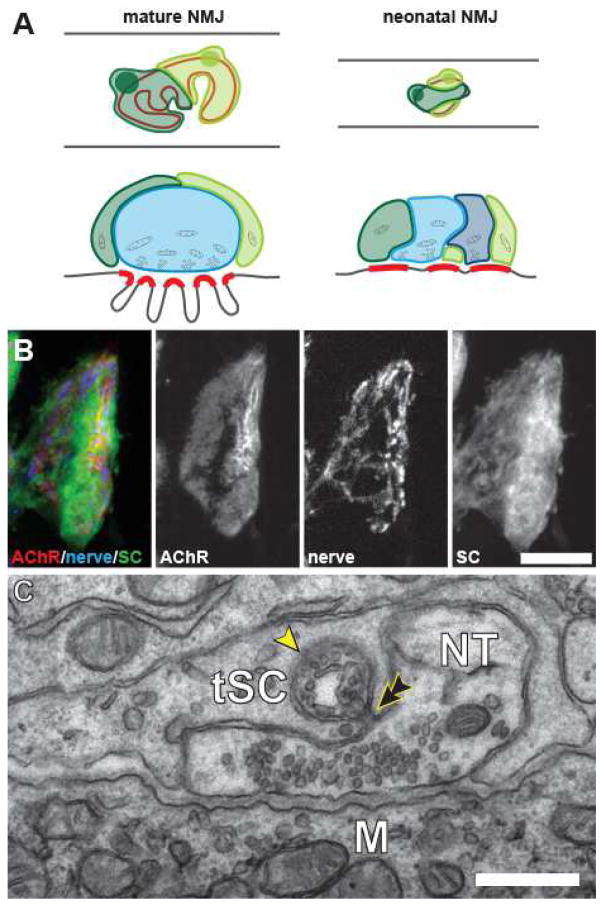
Figure 1. The arrangement of the cellular components at mouse neuromuscular junction.
(A) At mature NMJs, each tSCs (shown in shades of green) covers a non-overlapping portion of the nerve terminal (blue) that in turn covers the postsynaptic AChRs (indicated in red). At neonatal NMJs, this partitioning of terminals by tSCs has not yet been established and these cells interdigitate. Processes of the neonatal tSCs (in shades of green) make synapse-like apposition to the portions of the postsynaptic AChR aggregates unoccupied by the immature nerve terminals (in shades of blue). (B) Confocal images of the postsynaptic AChR aggregate (AChR), the motor axon terminals (nerve) and Schwann cells (SC) at a neonatal NMJ. As illustrated in (A), a significant portion of the oval-shaped AChR aggregate is not apposed by the innervating motor axon terminals, but rather by the processes of tSCs. (B) Electron micrograph of a single section in a serial series showing a tSCs consuming a portion of the presynaptic motor axon terminal (NT) that is still in contact with the target muscle fiber (M). A phagocytic vesicle containing components of the nerve terminal (arrowhead) is situated within tSC cytoplasm and has almost completely pinched off (double arrowhead) from this nerve terminal. Scale bars: 10 μm in (B) and 500 nm in (C).
tSCs respond to transmitter release and have a role in reinnervation upon nerve injury
Terminal SCs sense the presence of the innervating nerve terminal and probably the postsynaptic muscle fiber [12]. Upon denervation of the NMJ, the tSCs remain behind at the synapse but their behavior and their biochemistry change dramatically. The loss of the nerve is known to alter the expression of a host of genes, both positively and negatively. For instance, denervation leads to upregulation or downregulation of the intermediate filament proteins nestin and glial fibrillary acidic protein, respectively [13,14]. Interestingly, initial experiments by Katz et al. in frog discovered that, with a delay following denervation of muscles, tSCs produce and release quanta of ACh [15]. This release differs from that from nerve terminals [16] and may be explained by expression within SCs at denervated junctions of the synthetic enzyme for ACh [17]; it is known that cells that are artificially loaded with ACh can perform quantal release [18].
A prominent tSC behavior initiated by removal of the nerve is growth of long processes that radiate away from the endplate through the spaces between the muscle fibers [19]. This behavior may be initiated by the removal of signals normally provided to tSCs via transmitter release from the nerve terminal. ATP and acetylcholine activate purinergic and muscarinic receptors, respectively, and subsequently produce cytoplasmic Ca2+ elevations within tSCs [12]. The growth of these processes following denervation can be so extensive that tSCs begin to abandon portions of the AChR sites within the endplates [20]. This growth is not only initiated by denervation; it can be induced by blockade of impulse traffic to the synapse or by blocking transmitter release from the nerve terminals (via botulinum toxin) [21].
SCs in the peripheral nerves persist within the basal lamina (endoneurial) tubes and extend processes – just like tSCs at NMJs – upon axon degeneration [22]. The SC growth is usually confined to the tubes except where interruptions in these tubes occur [22]. These processes support nerve regeneration and the extension of new nerve branches. The SC-filled tubes account for the rapid regeneration of the innervation of muscles and, following simple, crush injuries to the nerves, promote the regeneration of the same axons to the endplates to which they were initially connected [23]. Following more extensive injuries (such as in the case of severed nerves), regeneration is delayed due to the gap formed in these tubes and the precision at which axons cross the gap and enter the corresponding tube distal to the lesion is markedly less [23].
The processes extended from the tSCs are important for guiding nerve regeneration [21]. Once axons arrive back at endplates following denervation, they grow terminal sprouts along tSC processes that have extended from these sites. This growth can lead to re-innervation of adjacent endplates [21]. Terminal SCs also appear to account for the sprouting that explains reinnervation of muscle fibers after partial denervation, i.e. where only a portion of the NMJs in a muscle become denervated. The growth of tSCs processes from individual denervated endplates forms links with adjacent innervated endplates and guides the growth of axons to the denervated endplates [24].
That SCs have the ability to stimulate nerve growth like this is clearly shown by experiments in which pieces of nerve previously denervated so that they contain no axons are implanted onto the surface of an innervated muscle. When SC processes extended from this nerve piece grow into the zone of endplates in the host muscle, they induce extensive sprouts from the nerves there. This growth extends along the processes of the growing glial cells [24]. The ability of SCs to support nerve growth may change with the time they are deprived of axon contact. For example, there is a deficiency in the growth of axons into peripheral nerves after long periods of nerve absence and this deficiency is correlated with the atrophied status of the SCs in the long-term denervated nerves [25].
tSCs are active during developmental synapse elimination
Given the ability of tSCs to influence nerve growth and their location at the endplates of developing muscles, investigators have been interested in whether tSCs might play a role in synapse elimination [12,26,27]. Smith et al. [27] conducted a detailed light and electron microscopic study of this issue. They used fluorescence labeling of junctions in the sternomastoid muscle of mice near the time of birth to show that several tSCs are present at each endplate. At this stage the AChR are arranged in a plaque rather than the pretzel shape of synaptic gutters present at the conclusion of synapse elimination approximately 2 weeks after birth. Light microscopy suggested that nerve terminal coverage of these AChR is incomplete and that tSCs (and their processes) come in close contact with a large fraction of these receptors (Fig. 1A). An electron microscopic (EM) study, in which serial cross-sections of NMJs were prepared and the entirety of NMJs digitally reconstructed, confirmed that processes of the tSCs came into synapse-like apposition of the muscle cell membrane (Fig. 1B). Nerve terminals could be identified by tracing processes containing synaptic vesicles at the muscle surface through the serial sections. At postnatal day 3, up to 4–5 separate motor axons contact the muscle surface. These nerve processes came within 50 nm of the muscle surface, a typical synaptic distance at mature NMJs. Accumulations of vesicles and dense material suggested the presence of active zones. Thus, these junctions were clearly polyneuronally innervated and this anatomical polyneuronal innervation was found to disappear, as expected, over postnatal development. The major departure of this study from a previous serial EM study, in which up to 10 inputs to young NMJs were shown to be gradually eliminated from each junction [28], was the analysis of the distribution of tSCs and their processes. These cells could be identified by their cell bodies located above the junction. Their processes could be followed over the synapse and reconstructed. Up to 5 such tSCs were present at junctions in 3 day old sternomastoid muscle. The processes of these tSCs were quite complex. They branched extensively, and confirming results from light microscopy [9], interdigitated extensively and had not yet taken up territories as at mature junctions. Taken together with the light microscopic observations of AChR and SC processes, these findings strongly suggested that the tSCs appose AChRs in the developing endplate. Measuring the total contact areas of tSCs and nerve terminals over the course of the first few days after birth showed that tSCs were expanding their contacts at the expense of the nerve terminals, suggesting displacement of the nerve contacts by SCs. Evidence of such displacement was found in the occurrence of fingers of SC processes penetrating into the synaptic cleft between nerve terminals and the muscle fiber (Fig. 1B). Terminal SCs were found to be phagocytosing bits of nerve terminals that were still in synaptic contact with the muscle fiber. Most tSCs contained numerous examples of what appeared to be nerve terminal debris. Analysis of the images showed that these tSC activities were directed against all converging immature nerve terminals at each synapse. This argues strongly that the tSCs themselves [27] are not selecting the winner in the competition at each endplate. Nonetheless, the tSCs “attack” on all the nerve terminals appears to create vacated synaptic sites that then can be competed for by the re-growth of nearby nerve terminal branches. Indeed, such a model of random removal of nerve inputs over small portions of the postsynaptic membrane and their stochastic re-occupation by nearby nerve terminals can explain many of the features of neuromuscular synapse elimination [5].
Control of tSC activities
These observations raise the issue of why tSCs should behave in this manner at developing NMJs but have a stable relationship with nerve terminals at adult junctions. Some of the neonatal behaviors of tSCs reappear during repair/reinnervation of mature synapses. Why do tSCs switch from a “maintenance” mode to a phagocytic or “repair” mode and vice versa [10,12]? There are clearly a number of cytokines and trophic factors that likely play a role. The expression of one of these factors, a motor axon-tethered isoform of neuregulin1 (NRG1-III), has a temporal peak that coincides with the period of synapse elimination [29]. Analogous NRG1-driven SCs behaviors also aid in the sorting and myelination of developing axons as well as phagocytosis of axonal debris upon injury [30,31]. At developing NMJs, overexpression of NRG1-III by motor neurons exaggerates the neonatal tSC behaviors and accelerates synapse elimination. Conversely, genetic perturbations that reduce NRG1 signaling delay synapse elimination. Since a number of manipulations in addition to expression of NRG1-III can be shown to influence the rate of synapse elimination, control of synapse elimination is clearly multifactorial. Neuronal activity, as mentioned above, is clearly important [32–35]. There is evidence that NRG1 expression is itself influenced by the level of activity of neurons [36,37], suggesting a mechanism by which activity may lie upstream of NRG1-III regulation of tSCs.
Adult NMJs that have a highly stable pretzel of AChR can also be induced to undergo remodeling by increasing the expression of NRG1-III in motor axons [29]. The AChR become fragmented into small islands, the nerve terminals become varicose, and the number of tSCs is increased (Fig. 2). Vital imaging of NMJs in these animals shows a continuous turnover of some of these islands of AChR and continued growth of the motor axon terminals. Ultrastructural examinations of these junctions show the tSC behaviors seen during synapse elimination: intrusion of tSCs into the synaptic cleft and their phagocytosis of nerve terminal processes. These observations suggest that the level of expression of NRG1-III by motor neurons might be a mechanism for controlling the behavior of tSCs even in the adult. Since the same fragmented morphology seen in these adult junctions occurs in a variety of experimental and pathological conditions, including alterations in transcription factors, cell adhesion molecules, and in various dystrophies and myopathies [38–40], it is tempting to speculate that tSC behaviors could be responsible for the remodeling of NMJs in various pathologies.
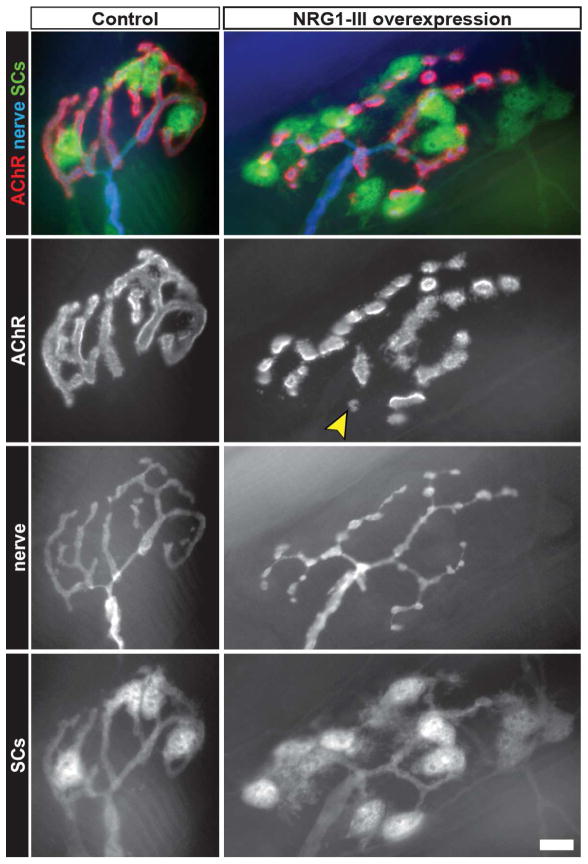
Figure 2. Changes to morphology of adult NMJs in response to NRG1-III overexpression by motor neurons.
Unlike the mature NMJs of control mice, those in animals that overexpress NRG1-III show signs of remodeling. The postsynaptic AChR aggregates are no longer found in “pretzel-like” continuous gutters, but in fragmented islands that are innervated by varicose presynaptic terminal branches. Some AChR-islands are abandoned by the motor terminal branches (arrowhead) and appear to be in the process of elimination. In addition, there is a significant increase in the number of tSCs, some of which extend long processes within and beyond the synaptic area. Scale bar: 10 μm.
The phagocytic and intrusive properties of SCs at the developing or adult NMJs do not represent special behaviors of these cells. Firstly, during the development of peripheral nerves, SCs migrate along nerves, proliferate and invade the nerve bundles with their processes, and separate axons [41], just as tSCs do at developing junctions. Following nerve damage, the SCs distal to the injury site help phagocytose the debris and tSCs at endplates intrude between the degenerating nerve terminal and the muscle fiber [42]. Terminal SCs have also been shown to penetrate into and phagocytose withdrawing axon branches during synapse elimination [43]. In myasthenia gravis, tSCs send phagocytic processes to invade nerve terminals [44]; similarly, after the injection of myotoxins, tSCs send phagocytic processes to separate the nerve and muscle endplate [45,46]. Cellular damage, either from denervation or disease, can lead to the release of damage-associated molecular pattern molecules (DAMPs) [47–49]. In addition to being strong activators of the innate immune response, recent evidence supports a role of DAMPs in the activation of SCs through molecular pathways found in both immune cells and SCs, including pathways for toll-like receptors and cytokines [50,51]. Indeed, invasion of damage sites by immune cells, in addition to the activation of SC phagocytic and intrusive behaviors, is common in all these conditions. Importantly, the changes in SC morphology after injury do not appear appreciably different from those during synapse elimination. It is thus likely that the SC behaviors capable of producing presynaptic changes seen during early postnatal development are reactivated to repair injured synaptic sites formed by regenerating axon terminals.
Conclusion
Schwann cells engage in activities that remodel synapses and nerve terminals under many circumstances throughout the life of the animal. These activities include intrusion into synapses and phagocytosis. Such activities must be carefully regulated to allow synaptic maintenance. Observations suggest that tSCs may promote synapse elimination by creating vacant synaptic sites that then can be reoccupied by the competing axon terminals. However, there is no evidence at present that these cells select the victor in this competition.
Highlights.
Terminal Schwann cells actively participate in neuromuscular synapse elimination
Neuregulin1 type-III is a key regulator of neonatal terminal Schwann cell behaviors
Neonatal Schwann cell-like behaviors cause alteration of normally stable adult nmjs
Neonatal Schwann cell behaviors are reactivated in various nmj pathologies
Acknowledgments
Supported by NIH grant NS20480 and by startup funds from Texas A&M University (to WT and MH). We thank U.J. McMahan for comments on the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Redfern PA. Neuromuscular transmission in new-born rats. J Physiol. 1970;209:701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tello F. Degeneration et regeneration des plagues motrices. Travaux du Laboratorire de Recherches Biologiques de l’Universit’e de Madrid. 1907;5:117–149. [Google Scholar]
- 3.Brown MC, Jansen JKS, Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976;261:387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **4.Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. The authors utilized transgenic expression of two distinct fluorochromes that by variegation mark separate pool of axons innervating muscle fibers and examine vitally the competition between convergent axons during the last stages of synapse elimination. [DOI] [PubMed] [Google Scholar]
- *5.Turney SG, Lichtman JW. Reversing the outcome of synapse elimination at developing neuromuscular junctions in vivo: evidence for synaptic competition and its mechanism. PLoS Biol. 2012;10:e1001352. doi: 10.1371/journal.pbio.1001352. The investigators utilized laser ablation of axons to manipulate the innervation of individual NMJs during the synapse elimination and demonstrated that the winner of the interneuronal competition at individual junction is not predetermined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson WJ. Activity and synapse elimination at the neuromuscular junction. Cellular Molecular Neurobiol. 1985;5:167–182. doi: 10.1007/BF00711091. [DOI] [PubMed] [Google Scholar]
- 7.Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- *8.Favero M, Busetto G, Cangiano A. Spike timing plays a key role in synapse elimination at the neuromuscular junction. Proc Natl Acad Sci U S A. 2012;109:E1667–1675. doi: 10.1073/pnas.1201147109. The investigators stimulated subsets of axons innervating the rat soleus muscles to control the timing and amount of their activity and investigate the consequences for synapse elimination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brill MS, Lichtman JW, Thompson W, Zuo Y, Misgeld T. Spatial constraints dictate glial territories at murine neuromuscular junctions. J Cell Biol. 2011;195:293–305. doi: 10.1083/jcb.201108005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balice-Gordon RJ, Lichtman JW. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J Neurosci. 1993;13:834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Ko CP, Robitaille R. Perisynaptic Schwann Cells at the Neuromuscular Synapse: Adaptable, Multitasking Glial Cells. Cold Spring Harb Perspect Biol. 2015;7:a020503. doi: 10.1101/cshperspect.a020503. A review of experiments that show communication between SCs and motor axons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgiou J, Robitaille R, Trimble WS, Charlton MP. Synaptic regulation of glial protein expression in vivo. Neuron. 1994;12:443–455. doi: 10.1016/0896-6273(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 14.Kang H, Tian L, Son YJ, Zuo Y, Procaccino D, Love F, Hayworth C, Trachtenberg J, Mikesh M, Sutton L, et al. Regulation of the intermediate filament protein nestin at rodent neuromuscular junctions by innervation and activity. J Neurosci. 2007;27:5948–5957. doi: 10.1523/JNEUROSCI.0621-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birks R, Katz B, Miledi R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dennis MJ, Miledi R. Electrically induced release of acetylcholine from denervated Schwann cells. J Physiol. 1974;237:431–452. doi: 10.1113/jphysiol.1974.sp010490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockes JP. Assays for cholinergic properties in cultured rat Schwann cells. Proc Roy Soc Lond B. 1984;222:121–134. doi: 10.1098/rspb.1984.0053. [DOI] [PubMed] [Google Scholar]
- 18.Dan Y, Poo MM. Quantal transmitter secretion from myocytes loaded with acetylcholine. Nature. 1992;359:733–736. doi: 10.1038/359733a0. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds ML, Woolf CJ. Terminal Schwann cells elaborate extensive processes following denervation of the motor endplate. J Neurocytol. 1992;21:50–66. doi: 10.1007/BF01206897. [DOI] [PubMed] [Google Scholar]
- *20.Kang H, Tian L, Mikesh M, Lichtman JW, Thompson WJ. Terminal Schwann cells participate in neuromuscular synapse remodeling during reinnervation following nerve injury. J Neurosci. 2014;34:6323–6333. doi: 10.1523/JNEUROSCI.4673-13.2014. Authors investigate the delay in reinnervation by different methods of denervation and its consequences for the disposition of tSCs and their processes as well as the subsequent reoccupation of endplates by returning motor axon terminals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son Y-J, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 22.Scherer SS, Easter SS., Jr Degenerative and regenerative changes in the trochlear nerve of goldfish. J Neurocytol. 1984;13:519–565. doi: 10.1007/BF01148079. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nat Neurosci. 2002;5:861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- 24.Son Y-J, Thompson WJ. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron. 1995;14:133–141. doi: 10.1016/0896-6273(95)90247-3. [DOI] [PubMed] [Google Scholar]
- 25.Sulaiman OA, Gordon T. Role of chronic Schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery. 2009;65:A105–114. doi: 10.1227/01.NEU.0000358537.30354.63. [DOI] [PubMed] [Google Scholar]
- *26.Barik A, Li L, Sathyamurthy A, Xiong WC, Mei L. Schwann Cells in Neuromuscular Junction Formation and Maintenance. J Neurosci. 2016;36:9770–9781. doi: 10.1523/JNEUROSCI.0174-16.2016. Authors conducted an inducible genetic ablation of SCs at various points during embryonic and postnatal development to examine the consequences for formation and maintenance of NMJs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Smith IW, Mikesh M, Lee YI, Thompson WJ. Terminal schwann cells participate in the competition underlying neuromuscular synapse elimination. J Neurosci. 2013;33:17724–17736. doi: 10.1523/JNEUROSCI.3339-13.2013. A detailed electron microscopic analysis of the disposition of nerve terminals and tSCs at mouse NMJs demonstrated active glial participation during neuromuscular synapse elimination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Tapia JC, Wylie JD, Kasthuri N, Hayworth KJ, Schalek R, Berger DR, Guatimosim C, Seung HS, Lichtman JW. Pervasive synaptic branch removal in the Mammalian neuromuscular system at birth. Neuron. 2012;74:816–829. doi: 10.1016/j.neuron.2012.04.017. A detailed examination of innervation in embryonic and early postnatal muscles show that individual NMJs may each be innervated by up to ~10 separate motor axons, a larger convergence than previously thought. [DOI] [PubMed] [Google Scholar]
- *29.Lee YI, Li Y, Mikesh M, Smith I, Nave KA, Schwab MH, Thompson WJ. Neuregulin1 displayed on motor axons regulates terminal Schwann cell-mediated synapse elimination at developing neuromuscular junctions. Proc Natl Acad Sci U S A. 2016;113:E479–487. doi: 10.1073/pnas.1519156113. The study shows the consequences of manipulating neuregulin1 expression for the innervation of motor endplates and provides additional evidence for the influence tSCs exert on developmental neuromuscular synapse elimination and in shaping the morphology of NMJs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 31.Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16:48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- *32.Je HS, Yang F, Ji Y, Potluri S, Fu XQ, Luo ZG, Nagappan G, Chan JP, Hempstead B, Son YJ, et al. ProBDNF and mature BDNF as punishment and reward signals for synapse elimination at mouse neuromuscular junctions. J Neurosci. 2013;33:9957–9962. doi: 10.1523/JNEUROSCI.0163-13.2013. The study suggests that processing of BDNF differentially affects converging motor axons depending on their activity levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- 34.Rafuse VF, Polo-Parada L, Landmesser LT. Structural and functional alterations of neuromuscular junctions in NCAM-deficient mice. J Neurosci. 2000;20:6529–6539. doi: 10.1523/JNEUROSCI.20-17-06529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Tetruashvily MM, McDonald MA, Frietze KK, Boulanger LM. MHCI promotes developmental synapse elimination and aging-related synapse loss at the vertebrate neuromuscular junction. Brain Behav Immun. 2016;56:197–208. doi: 10.1016/j.bbi.2016.01.008. Manipulation of MHC class1 proteins altered the rate of neuromuscular synapse elimination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Bates R, Yin DM, Shen C, Wang F, Su N, Kirov SA, Luo Y, Wang JZ, Xiong WC, et al. Specific regulation of NRG1 isoform expression by neuronal activity. J Neurosci. 2011;31:8491–8501. doi: 10.1523/JNEUROSCI.5317-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loeb JA, Hmadcha A, Fischbach GD, Land SJ, Zakarian VL. Neuregulin expression at neuromuscular synapses is modulated by synaptic activity and neurotrophic factors. J Neurosci. 2002;22:2206–2214. doi: 10.1523/JNEUROSCI.22-06-02206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulakowski SA, Parker SD, Personius KE. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission, and muscle function. Journal of applied physiology. 2011;111:844–852. doi: 10.1152/japplphysiol.00070.2011. [DOI] [PubMed] [Google Scholar]
- 39.Lyons PR, Slater CR. Structure and function of the neuromuscular junction in young adult mdx mice. J Neurocytol. 1991;20:969–981. doi: 10.1007/BF01187915. [DOI] [PubMed] [Google Scholar]
- *40.Seaberg B, Henslee G, Wang S, Paez-Colasante X, Landreth GE, Rimer M. Muscle-derived extracellular signal-regulated kinases 1 and 2 are required for the maintenance of adult myofibers and their neuromuscular junctions. Mol Cell Biol. 2015;35:1238–1253. doi: 10.1128/MCB.01071-14. The authors show that manipulations of erk signaling can cause dramatic changes in NMJ morphology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters A, Muir AR. The relationship between axons and Schwann cells during development of peripheral nerves in the rat. J Exp Physiol. 1959;44:117–130. doi: 10.1113/expphysiol.1959.sp001366. [DOI] [PubMed] [Google Scholar]
- 42.Miledi R, Slater CR. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970;207:507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Maselli RA, Ng JJ, Anderson JA, Cagney O, Arredondo J, Williams C, Wessel HB, Abdel-Hamid H, Wollmann RL. Mutations in LAMB2 causing a severe form of synaptic congenital myasthenic syndrome. J Med Genet. 2009;46:203–208. doi: 10.1136/jmg.2008.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Couteaux R, Mira JC, d’Albis A. Regeneration of muscles after cardiotoxin injury. I. Cytological aspects. Biol Cell. 1988;62:171–182. [PubMed] [Google Scholar]
- 46.Jirmanová I, Thesleff S. Ultrastructural study of experimental muscle degeneration and regeneration in the adult rat. Z Zellforsch Mikrosk Anat. 1972;131:77–97. doi: 10.1007/BF00307202. [DOI] [PubMed] [Google Scholar]
- 47.Chernov AV, Dolkas J, Hoang K, Angert M, Srikrishna G, Vogl T, Baranovskaya S, Strongin AY, Shubayev VI. The calcium-binding proteins S100A8 and S100A9 initiate the early inflammatory program in injured peripheral nerves. J Biol Chem. 2015;290:11771–11784. doi: 10.1074/jbc.M114.622316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Man LL, Liu F, Wang YJ, Song HH, Xu HB, Zhu ZW, Zhang Q, Wang YJ. The HMGB1 signaling pathway activates the inflammatory response in Schwann cells. Neural Regen Res. 2015;10:1706–1712. doi: 10.4103/1673-5374.167773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol. 2017;17:165–178. doi: 10.1038/nri.2016.150. A thorough review of the role of the immune system in muscle injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goethals S, Ydens E, Timmerman V, Janssens S. Toll-like receptor expression in the peripheral nerve. Glia. 2010;58:1701–1709. doi: 10.1002/glia.21041. [DOI] [PubMed] [Google Scholar]
- 51.Tzekova N, Heinen A, Kury P. Molecules involved in the crosstalk between immune- and peripheral nerve Schwann cells. J Clin Immunol. 2014;34(Suppl 1):S86–104. doi: 10.1007/s10875-014-0015-6. [DOI] [PubMed] [Google Scholar]