Abstract
Objective
To compare the rates of shared decision making (SDM) reported by parents of children with medical complexity (CMC) with the rates of SDM reported by parents of noncomplex children with special health care needs (CSHCN).
Study design
We examined the 2009–2010 National Survey of Children with Special Health Care Needs, a representative survey of 40,242 parents of CSHCN. CMC was defined as needing or using more medical care than usual, seeing 2 or more subspecialists, and positive response on at least 3 other items on the CSHCN Screener. We identified three subgroups each of CMC and noncomplex CSHCN by sentinel diagnoses: asthma, seizures, other diagnoses. SDM was defined as a binary composite variable, derived from 4 discrete items. We constructed four step-wise multivariable models to assess the relative odds of SDM, adjusted for sociodemographic characteristics (age, income, language, race, ethnicity, marital status), behavioral comorbidity, family centered care, and patient centered medical home.
Results
The study population included 39,876 respondents. Compared with noncomplex CSHCN, CMC had a lower likelihood of SDM (adjusted odds ratio (aOR) 0.76, 0.64–0.91), which persisted in diagnostic subgroups: CMC with asthma (aOR 0.67, 0.49–0.92), CMC with other diagnoses (aOR 0.74, 0.58–0.94); but not CMC with seizures (aOR 0.95, 0.59–1.51).
Conclusions
Shared decision making is less common for CSHCN with complex needs than those without complex needs. Health-system interventions targeting future-oriented care planning may improve SDM for CMC.
Keywords: clinical decision support, children with special health care needs
Shared decision making (SDM) is a central component of quality care for patients with chronic illnesses.1 SDM uses bidirectional exchange of information between providers, patients, and caregivers to reach treatment plan agreement.2–4 Essential elements of SDM include defining the problem, presenting options, elucidating patient and provider preferences, and assessing patient self-efficacy.5 SDM facilitates productive interactions between the medical team, patient, and caregivers particularly in situations of clinical uncertainty without a best treatment option.6, 7
SDM is thought to be important for preventing ambulatory care sensitive hospitalizations.8 Prior studies suggest that SDM improves care quality for adults with heart failure and type 2 diabetes and may reduce health inequalities for populations with lower literacy and lower self-efficacy.9 In children, SDM is associated with decreased disease severity in asthma, Attention Deficit Hyperactivity Disorder (ADHD), and type 1 diabetes.10–13 Among all children with special health care needs (CSHCN), parent report of low SDM is associated with increased functional disability, public insurance, and higher out-of-pocket expenses.14, 15
Children with medical complexity (CMC) are a subset of CSHCN with high service needs, high resource utilization, and often, severe functional disability.16 CMC comprise less than 5% of the US child population, receive poorer quality care, but consume over 30% of child healthcare expenses.16–19 According to the American Academy of Pediatrics, SDM is crucial for improving the health and satisfaction of children with disabilities and is the basis for patient-centered care.4 Care coordination across clinical settings and subspecialties may improve care quality for CMC, but providers’ poor communication skills sometimes hinder effective care coordination.18, 20, 21 Tools that promote SDM may improve parent-provider communication through integration of parent’s understanding, values, and self-efficacy.5, 22 SDM is particularly crucial for CMC as it enables alignment of care decisions longitudinally in a patient population whose multiple providers, complex illnesses, and frequent hospitalizations put them at high risk for fragmented, episodic care that can precipitate health crises. Little is known, however, about the prevalence and impact of SDM for CMC.10, 14, 20
The purpose of this study is to compare parent-reported rates of SDM for CMC and noncomplex CSHCN (NC). We hypothesized that CMC would report lower SDM than NC and that this difference would persist when comparing children with similar sentinel diagnoses.
Methods
We conducted a cross-sectional analysis of the 2009–2010 National Survey of Children with Special Healthcare Needs (NS-CSHCN), a population-based evaluation of care quality for CSHCN representative of CSHCN nationally and by state.23–25 This version of the survey included a revised set of SDM questions felt to capture key elements of SDM with more reliability and validity as reflected by thought leaders and parents of CSHCN through multiple cognitive interviews.14 Conducted by the National Center for Health Statistics through a random-digit dialing system of landlines and cell-phone numbers, parents with a positive response to at least one domain of the CSHCN screener screen into the survey.26 If multiple children are eligible, one child is randomly selected as the focus of the survey.
We examined all 40,242 survey responses from the 2009–2010 NS-CSHCN data set. After excluding responses missing the SDM composite score (described below), 39,876 (99.1%) of all surveys were available for analysis.
The primary outcome variable was shared decision making (SDM) as assessed by 4 survey items.27 On a 4-point Likert scale (never, sometimes, usually, or always), each parent reported their frequency for receiving each of the following from their child’s medical providers: discussed a range of treatment options, encouraged to ask questions or raise concerns, made it easy to ask questions or raise concerns, and considered and respected family’s treatment choices. Positive SDM was defined as parent report of “usually” or “always” on all 4 items.27
The main predictor variable was child with medical complexity (CMC), defined in prior studies of the NS-CSHCN as a child meeting all of the following three criteria: (1) need for more medical care than usual; (2) positive responses to at least three of four remaining screener items (increased functional limitations, need for: prescription medications, special therapies, or developmental and behavioral treatment), and (3) visits to two or more subspecialists in the last 12 months.16, 19 All children not classified as CMC were classified as non-complex children with special health care needs.
Three subgroups of both CMC and NC were defined based on the presence of the following sentinel diagnoses: (1) asthma, (2) seizure disorder, or (3) other diagnoses (ie, neither asthma nor seizure disorder) with children with both asthma and seizures represented in both subgroups. These subgroups were chosen for comparison due to the known positive effects of SDM on health outcomes in NC with asthma and the relatively high prevalence of childhood seizure disorder and asthma.12, 28, 29 Children with seizure disorder account for over 50% of hospitalizations in children with neurologic impairment, a subgroup of CSHCN known for high health resource use.30 High hospital resource use could signal poor care quality including low SDM. Other chronic conditions with known positive outcomes associated with SDM, such as Type 1 diabetes, were not prevalent enough in the cohort to warrant separate comparisons and were combined into the “other diagnoses” (ODx) category.13
For unadjusted and adjusted analyses, we examined any measureable child characteristics that have been shown in prior studies to be associated with likelihood of receiving SDM, identification as CMC, or diagnoses of asthma or seizures. Child- and family-level demographic and clinical characteristics assessed included child age, race/ethnicity, maternal education, household language, household income, insurance type, functional limitation, and behavioral comorbidity. Functional limitation was divided into 3 strata (never affected, moderately affected, a great deal affected) as described in prior studies.14 Children with epilepsy have increased behavioral comorbidities compared with children without epilepsy.31–33 Using previously described patterns of behavioral comorbidities in children with epilepsy, behavioral comorbidity was defined as having one or more of: ADHD, depression, anxiety, or behavioral disorders such as conduct disorder or oppositional defiant disorder.31, 32 Health-system-level characteristics assessed for each child included family-centered care (FCC) and patient-centered medical home (PCMH), composite measures with detailed methodology presented elsewhere.34, 35
Statistical Analyses
Descriptive statistics for sample characteristics were weighted to reflect US population-level estimates using sampling weights provided by the NS-CSHCN.27 Weighted categorical percentages were compared across all children (CMC vs NC) and across subgroups stratified by sentinel diagnoses (asthma, seizure disorder, other). Bivariate comparisons were assessed with the Student t-test for continuous data and with Rao-Scott Chi-squared test for categorical data.
Multiple logistic regression models were generated to assess the relationship between SDM and complexity (CMC vs NC) for each sentinel diagnosis. Covariates were selected based on a relative difference of at least 10% in the OR for SDM and complexity (CMC vs NC) before versus after adjusting for the covariate.36 Modeling applied covariates in a stepwise fashion. This included an unadjusted model (Model 1) and models adjusted for: sociodemographic characteristics (Model 2: child age, ethnicity, insurance and parent income, language, education, marital status), behavioral comorbidities (Model 3), health provider factors (Model 4: FCC), and health system factors (Model 5: PCMH). FCC was evaluated before PCMH because FCC is a nested subdomain of PCMH, but is not an anchoring subcomponent of the composite PCMH measure.27, 37 Due to previously well-described rates of SDM in NC with asthma, and associations between SDM and better asthma outcomes in NC with asthma after using an asthma web portal, additional models compared the SDM of each subgroup against NC with asthma.10, 12
All analyses were performed using SAS, Version 9.4 (SAS Institute Inc, Cary, North Carolina) survey procedures.27 The study was reviewed by the Stanford University Institutional Review Board and deemed exempt.
Results
The sociodemographic and health characteristics of the study population are described in Table I. Compared with non-complex children (NC), CMC were slightly younger, more likely to be boys, more likely to have great functional limitations, more likely to have a behavioral comorbidity, and more likely to use health care services. Compared with NC families, CMC families were less likely to report receiving family-centered care and were less likely to report having a patient-centered medical home. These differences in sociodemographic and health characteristics persisted for subgroups of all sentinel diagnoses except for sex in the seizure subgroups and FCC in the seizure and other diagnoses subgroups.
Table 1. Characteristics of Non-Complex CSHCN (NC) versus Children with Medical Complexity (CMC)a.
All data represent the % of children in the group with that characteristic (i.e. “column %”) unless otherwise noted
| NC overall | CMC overall | |
|---|---|---|
|
|
||
| n=37,377 | n=2499 | |
|
|
||
| Characteristic | Weighted n=10,291,328 | Weighted n=711742 |
| Child age – mean (SE) in years** | 10.0 (0.04) | 9.0 (0.17) |
| Male* | 58.9 | 63.9 |
|
| ||
| Special Health Care Needs Criteria | ||
|
| ||
| Needs/uses prescription medicines** | 75.7 | 84.5 |
| Needs/uses more medical care than usual** | 38.1 | 100.0 |
| Functional limitations more than usual** | 18.7 | 91.2 |
| Needs/uses special therapies** | 17.2 | 84.3 |
| Needs/uses treatment for emotional/developmental/behavioral issues** | 28.3 | 80.3 |
|
| ||
| Functional Limitations to Daily Activities** | ||
|
| ||
| Never Affected | 36.8 | 0.9 |
| Moderately Affected | 40.1 | 16.8 |
| A Great Deal Affected | 23.1 | 82.3 |
|
| ||
| Highest Household Education Level | ||
|
| ||
| Less than HS | 11.3 | 8.4 |
| HS Graduate | 20.0 | 18.1 |
| More than HS | 68.7 | 73.5 |
|
| ||
| Household Income, by % federal poverty level | ||
|
| ||
| 0–99% | 22.1 | 22.5 |
| 100–199% | 21.8 | 22.9 |
| 200–399% | 28.5 | 28.5 |
| 400+% | 27.6 | 26.1 |
|
| ||
| Household Characteristics | ||
|
| ||
| Single Mother Household | 25.3 | 25.1 |
| Household Language Spanish | 5.7 | 5.9 |
|
| ||
| Child Race/Ethnicity | ||
|
| ||
| Hispanic | 16.7 | 16.6 |
| Non-Hispanic, White | 59.2 | 63.0 |
| Non-Hispanic, Black | 16.3 | 12.2 |
| Other | 7.8 | 8.2 |
|
| ||
| Child Diagnosisb | ||
|
| ||
| Diabetes | 1.7 | 2.0 |
| Asthma** | 35.6 | 29.1 |
| ADHD** | 27.9 | 40.3 |
| Depression** | 9.2 | 20.0 |
| Anxiety** | 14.7 | 40.0 |
| Behavioral Disorder** | 11.8 | 28.9 |
| Autism Spectrum Disorder** | 6.0 | 30.2 |
| Developmental Delay** | 13.6 | 62.9 |
| Intellectual Disability** | 4.0 | 28.3 |
| Seizures** | 2.1 | 17.3 |
| Cerebral Palsy** | 0.9 | 11.4 |
| Muscular Dystrophy** | 0.2 | 1.5 |
| Down Syndrome** | 0.8 | 4.8 |
| Joint Problems** | 2.4 | 11.0 |
| Allergies** | 48.2 | 52.4 |
| Behavioral Comorbidity**,c | 37.6 | 59.9 |
|
| ||
| Child Health Care Characteristics | ||
|
| ||
| Family Centered Care**,d | 65.2 | 57.1 |
| Patient Centered Medical Home**,e | 44.4 | 23.4 |
|
| ||
| Insurance** | ||
|
| ||
| Private | 53.6 | 36.4 |
| Public | 35.4 | 42.8 |
| Both | 7.5 | 19.2 |
| Uninsured | 3.6 | 1.7 |
|
| ||
| Health Care Utilization (Last 12 months) | ||
|
| ||
| Number of ER visits – mean (SE)** | 0.9 (0.02) | 2.0 (0.12) |
| Number of well child care – mean (SE)** | 1.4 (0.01) | 1.9 (0.08) |
| Number of specialty visits – mean (SE)** | 0.7 (0.01) | 3.4 (0.07) |
| Received All Specialty Care Needed**,f | 96.1 | 90.2 |
Results are weighted based on standard methods for the NS-CSHCN to reflect national estimates of the US population.26
All comparisons by Student’s t-test for continuous data and by Rao-Scott Chi-squared test for categorical data:
p<0.05;
p<0.001.
Percentages of diagnoses will add up to more than 100% as patients may have more than one diagnosis.
Behavioral comorbidity defined as having one or more of: ADHD, depression, anxiety, behavioral disorder (i.e. oppositional defiant disorder).
Family centered care: “usually” or “always” responses to all five descriptions of the child’s medical provider: (1) spends enough time with them, (2) listens carefully, (3) is sensitive to the family culture/values, (4) provides enough information, and (5) makes the family feel like partners.26
Patient centered medical home: “usually” or “always” responses to all five of the following: (1) having a usual source of care, (2) having a personal doctor or nurse, (3) obtaining all needed referrals for specialty care, (4) receiving help in coordinating health-related care, and (5) receiving FCC.26
Specialty care needed: Answered “no” to needing care from a specialty doctor in the past 12 months or answered “yes” to needing care from a specialty doctor in the past 12 months and “yes” to receiving all the care from a specialty doctor that they needed.
Table 2 shows the unadjusted rates of SDM among families of CMC compared with families of NC. SDM was less common among families with CMC than among families with NC (60.5% vs 71%, P < .001). This held true within subgroups of CMC and NC by diagnoses: asthma (55.8% vs 72.1%, p<0.001) and other diagnoses (60.7% vs 70.3%, p<0.001) but not seizures (68.5% vs 69.1%, p=0.89).
Table 2.
Rates of SDM by population
| Rate of SDM (%)
|
||
|---|---|---|
| Sentinel Diagnosis | Noncomplex Children (NC) | Children with Medical Complexity (CMC) |
| All diagnoses* | 71.0 (69.8–72.1) | 60.5 (55.1–66.0) |
| Asthma* | 72.2 (70.1–74.3) | 55.7 (46.1–65.3) |
| Seizures | 69.0 (60.8–77.3) | 68.5 (57.3–79.7) |
| Other diagnoses* | 70.3 (68.9–71.7) | 60.7 (53.2–68.1) |
Chi-squared test applied to all categorical data.
P-value<0.001
Table 3 shows the unadjusted and adjusted odds ratios (OR) for SDM across each subgroup by sentinel condition. CMC were less likely to report SDM when compared with their NC counterparts (OR 0.63, 95% CI 0.53–0.74). This finding persisted for all CMC subgroups when compared with their respective NC subgroups except for CMC with seizures. CMC with asthma were less likely to report SDM than NC with asthma (OR 0.49, 95% CI 0.36–0.65) as were CMC with other diagnoses (OR 0.65, 95% CI 0.53–0.81). However CMC with seizures had similar reported SDM compared with NC with seizures (OR 0.98, 95% CI 0.57–1.67). These patterns in likelihood of SDM for CMC persisted after a series of adjustments for sociodemographic characteristics (Model 2), behavioral comorbidities (Model 3), FCC (Model 4), and PCMH (Model 5).
Table 3.
Unadjusted and adjusted OR for SDM for children with sentinel diagnoses by complexity (weighted)
| Unadjusted OR for SDM |
Adjusted OR for SDM | ||||
|---|---|---|---|---|---|
|
|
|||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| All Noncomplex | Ref | Ref | Ref | Ref | Ref |
| All Complex | 0.63 (0.53–0.74) | 0.62 (0.53–0.73) | 0.67 (0.57–0.79) | 0.71 (0.60–0.85) | 0.76 (0.64–0.91) |
|
| |||||
| Noncomplex with asthma | Ref | Ref | Ref | Ref | Ref |
| Complex with asthma | 0.49 (0.36–0.65) | 0.50 (0.37–0.67) | 0.57 (0.42–0.77) | 0.63 (0.46–0.86) | 0.67 (0.49–0.92) |
|
| |||||
| Noncomplex with seizures | Ref | Ref | Ref | Ref | Ref |
| Complex with seizures | 0.98 (0.57–1.67) | 0.88 (0.56–1.38) | 0.87 (0.57–1.32) | 0.91 (0.57–1.44) | 0.95 (0.59–1.51) |
|
| |||||
| Noncomplex with ODx | Ref | Ref | Ref | Ref | Ref |
| Complex with ODx | 0.65 (0.53–0.81) | 0.63 (0.50–0.78) | 0.66 (0.53–0.82) | 0.69 (0.54–0.87) | 0.74 (0.58–0.94) |
SDM=shared decision making
Model 1: unadjusted
Model 2: age, sex, ethnicity, income, insurance, language, education, marital status
Model 3: age, sex, ethnicity, income, insurance, language, education, marital status, behavioral comorbidity
Model 4: age, sex, ethnicity, income, insurance, language, education, marital status, behavioral comorbidity, family centered care
Model 5 (full model): age, sex, ethnicity, income, insurance, language, education, marital status, behavioral comorbidity, family centered care, patient centered medical home
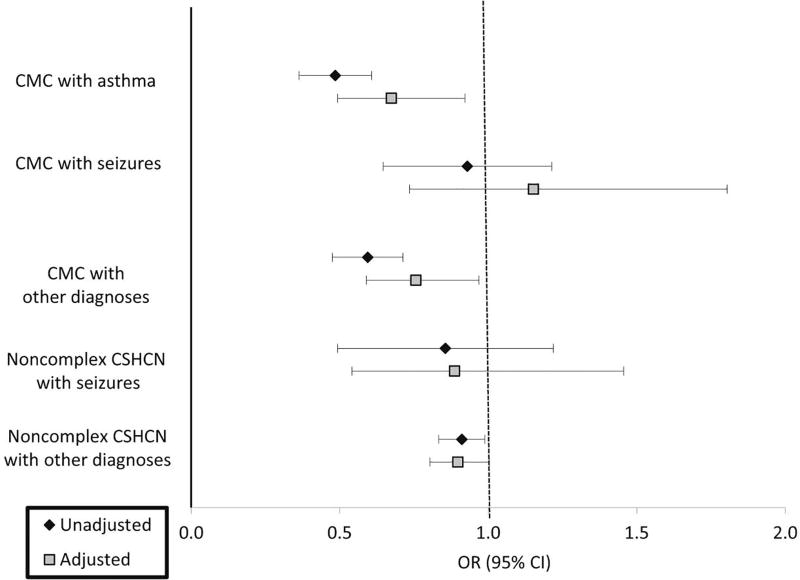
Additional models compared SDM of each CMC subgroup against NC with asthma with the same covariates. For each of the CMC and NC subgroups, the Figure shows the unadjusted and adjusted OR for SDM, which closely mirror the observed patterns described above.
Figure.
Unadjusted and adjusted* OR for achieving SDM based on sentinel diagnosescompared to noncomplex children with asthma (weighted)
ODx = other diagnoses
*Model 5: Adjusted for age, sex, ethnicity, income, insurance, language, education, single mother, behavioral, family centered care, patient centered medical home
Discussion
In this study of a large nationally representative sample, we found that families of CMC experienced lower rates of SDM with medical providers when compared with families of CSHCN without medical complexity. These differences persisted after adjusting for sociodemographic, behavioral, provider, and health-system-level characteristics.
Families of CMC may experience less SDM for a number of reasons. Lower rates of SDM may be explained in part by multiple outpatient subspecialty visits and hospitalizations that often give priority to urgent, provider-led decisions over collaborative, long-term planning. Frequent encounters but with different subspecialists and different health systems may make it difficult to align care decisions for CMC. SDM particularly around future-oriented planning is difficult in the context of clinical uncertainty and social complexity, which are both common among CMC. Although SDM has demonstrated promising positive effects on care quality and reduced health care redundancy, few interventions exist to help support patient and provider discussions about these areas of clinical uncertainty.7, 38 Many decision aids in pediatrics focus on treatment adherence rather than clinical decisions with no clear best option. CMC also experience greater unmet health needs, which may result in parents who are more dissatisfied or disillusioned with the health care system and therefore less likely to engage in SDM.19 CMC account for a disproportionate amount of hospitalizations, whereas most SDM interventions have been centered in the outpatient environment.39, 40 Provider time constraints and a system structured for episodic care may amplify the lack of SDM for CMC by perpetuating acute care management with an episodic approach rather than an approach that contextualizes acute care decisions within a child’s longitudinal, future-minded care plan, as desired by parents of CMC.38
We also found disproportionately lower SDM rates for CMC with asthma. One hypothesis for this finding may be the focused efforts to improve quality of care for NC with asthma, whose medical homes typically reside in primary care or a single subspecialist, compared with CMC with asthma, who may rotate through multiple subspecialists without a clear primary care provider.10, 12, 41, 42 In contrast, parents of CMC with seizure disorder reported similar rates of SDM as parents of NC with seizure disorder. This finding was contrary to our a priori hypothesis, based on high hospital resource use among CMC with seizure disorder, that CMC with seizure disorder would have lower rates of SDM.19 Our findings may be different than our hypothesis due to the relative heterogeneity of seizure disorders. The many causes of seizures (genetic v. febrile) and varied seizure morphologies (absence v. refractory epilepsy) may result in varied severity of seizures and overall health that are not discerned in this survey. Alternatively, all children with a persistent seizure disorder are cared for by a neurologist, and having a consistent provider to manage a single disease may enable more consistent use of standardized approaches in decision making unlike a child with asthma who may be cared for by a primary care provider, pulmonologist, allergist, or complex care specialist.
These findings build on prior studies that suggest CMC have elevated care needs yet receive poorer quality care.14, 24 SDM has proven effective during acute care management such as in reducing antibiotic use in acute otitis media without prolonging visit duration.7, 24, 43 Most prior work on SDM has focused on single disease or procedure-specific decision aids and may not take into account the heterogeneity of medical illnesses seen in CMC.44, 45 Development of SDM interventions aimed at acute care management of CMC that ensure greater continuity of treatment plans during acute care encounters should be further explored. For children with asthma there are established processes around self-management including the asthma action plan to facilitate SDM.44, 45 Similarly, improving SDM for CMC may require greater focus on contingency plans for high-risk situations, such as hospital discharge and health emergencies.46
There are several important limitations to this study. The first is in the use of survey data based on parent self-report. Recall bias may affect the accuracy of information captured in self-report of child health utilization and diagnoses, which may be reflected in the high prevalence of reported ADHD. This could result in distortion of the associations of complexity and SDM. The survey data also does not include child-reported SDM, an essential consideration particularly for adolescents with special health care needs, although adolescents accounted for a small portion of the surveyed population. Sampling bias may have produced higher response rates from parents who had more positive experiences with the health care system, which may attribute to the overall high rates of SDM in the study population. Measurement bias may affect the definition of SDM, which relies on parent report of overall decision making rather than of specific encounters. Some aspects of clinical care that affect SDM such as self-efficacy, decision follow-up, and provider preferences and recommendations were not measured, which may reflect an overall lack of a consensus definition of SDM that hinders the creation of reliable, valid measures. Measurement bias may also apply to our definition of CMC; for instance, technology-assisted care is not captured in this version of the survey. Additionally, we were unable to assess geographic factors such as rural residence that may affect SDM. The cross-sectional nature of the survey data also limits any ability to draw causal inference between SDM and patient complexity.
The observation of lower rates of SDM among CMC is concerning because families of CMC often face clinical uncertainty, and SDM is an evidence-based tool for addressing medical treatment options with no clear best option. SDM remains an important component of quality care guidelines for CMC, including effective care coordination and goal-centered care plans. Future studies that explore SDM in CMC subgroups such as CMC with asthma may help identify unique barriers faced by CMC that result in low SDM in spite of existing efficacious SDM interventions in asthma care. Our findings also suggest a need for future studies to explore family preferences for SDM and to identify the circumstances under which increased SDM may be associated with improved health outcomes for CMC.
Acknowledgments
The authors thank Suzan Carmichael, PhD, Rita Popat, PhD, and Peiyi (Peggy) Kan, MS for biostatistical support and Alan Schroeder, MD, Lauren Destino, MD, and Brett Palama, MD for discussions regarding key findings.
Supported by the Stanford Center for Clinical and Translational Research and Education (NIH KL2 TR 001083 [to J.L.L.]) and (NIH UL1 TR 001085 [to J.L.L.]) and the Clinical Excellence Research Center [to J.L.L.].
Abbreviations
- CMC
children with medical complexity
- CSHCN
children with special health care needs
- NS-CSHCN
National Survey of Children with Special Health Care Needs
- NC
noncomplex children with special health care needs
- FCC
family centered care
- PCMH
patient centered medical home
- ODx
other diagnoses (not asthma or seizures)
- OR
odds ratio
- aOR
adjusted odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of this study were presented as a poster during the Pediatric Academic Societies Meeting, Baltimore, Maryland, April 29-May 3, 2016.
References
- 1.Institute of Medicine. Initial national priorities for comparative effectiveness research. National Academies Press; Washington, DC: 2009. [Google Scholar]
- 2.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Social science & medicine (1982) 1997;44:681–92. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 3.Gabe J, Olumide G, Bury M. 'It takes three to tango': a framework for understanding patient partnership in paediatric clinics. Social science & medicine (1982) 2004;59:1071–9. doi: 10.1016/j.socscimed.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 4.Adams RC, Levy SE. Shared Decision-Making and Children With Disabilities: Pathways to Consensus. Pediatrics. 2017;139 doi: 10.1542/peds.2017-0956. [DOI] [PubMed] [Google Scholar]
- 5.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient education and counseling. 2006;60:301–12. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 6.McAllister JW. Achieving a Shared Plan of Care with Children and Youth with Special Health Care Needs. Lucille Packard Foundation for Children's Health. 2014:13. [Google Scholar]
- 7.Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. The Cochrane database of systematic reviews. 2011:Cd001431. doi: 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. The Milbank quarterly. 1996;74:511–44. [PubMed] [Google Scholar]
- 9.Durand MA, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PloS one. 2014;9:e94670. doi: 10.1371/journal.pone.0094670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiks AG, Localio AR, Alessandrini EA, Asch DA, Guevara JP. Shared decision-making in pediatrics: a national perspective. Pediatrics. 2010;126:306–14. doi: 10.1542/peds.2010-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. American journal of respiratory and critical care medicine. 2010;181:566–77. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiks AG, Mayne SL, Karavite DJ, Suh A, O'Hara R, Localio AR, et al. Parent-reported outcomes of a shared decision-making portal in asthma: a practice-based RCT. Pediatrics. 2015;135:e965–73. doi: 10.1542/peds.2014-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valenzuela JM, Smith LB, Stafford JM, D'Agostino RB, Jr, Lawrence JM, Yi-Frazier JP, et al. Shared decision-making among caregivers and health care providers of youth with type 1 diabetes. Journal of clinical psychology in medical settings. 2014;21:234–43. doi: 10.1007/s10880-014-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smalley LP, Kenney MK, Denboba D, Strickland B. Family perceptions of shared decision-making with health care providers: results of the National Survey of Children With Special Health Care Needs, 2009–2010. Maternal and child health journal. 2014;18:1316–27. doi: 10.1007/s10995-013-1365-z. [DOI] [PubMed] [Google Scholar]
- 15.Fiks AG, Mayne S, Localio AR, Alessandrini EA, Guevara JP. Shared decision-making and health care expenditures among children with special health care needs. Pediatrics. 2012;129:99–107. doi: 10.1542/peds.2011-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SKM, Simon TD, et al. Children With Medical Complexity: An Emerging Population for Clinical and Research Initiatives. Pediatrics. 2011;127:529–38. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon TD, Berry J, Feudtner C, Stone BL, Sheng X, Bratton SL, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126:647–55. doi: 10.1542/peds.2009-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry JG, Hall M, Neff J, Goodman D, Cohen E, Agrawal R, et al. Children with medical complexity and Medicaid: spending and cost savings. Health affairs (Project Hope) 2014;33:2199–206. doi: 10.1377/hlthaff.2014.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo DZ, Goudie A, Cohen E, Houtrow A, Agrawal R, Carle AC, et al. Inequities In Health Care Needs For Children With Medical Complexity. Health Affairs. 2014;33:2190–8. doi: 10.1377/hlthaff.2014.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethell C, Zuckerman K, Stumbo S, Robertson J, Lindly O, Brown C, et al. Children with Special Health Care Needs in California: A Profile of Key Issues. Lucille Packard Foundation for Children's Health. 2013:35. [Google Scholar]
- 21.Tschudy MM, Raphael JL, Nehal US, O'Connor KG, Kowalkowski M, Stille CJ. Barriers to Care Coordination and Medical Home Implementation. Pediatrics. 2016;138 doi: 10.1542/peds.2015-3458. [DOI] [PubMed] [Google Scholar]
- 22.Stille CJ, Fischer SH, La Pelle N, Dworetzky B, Mazor KM, Cooley WC. Parent partnerships in communication and decision making about subspecialty referrals for children with special needs. Academic pediatrics. 2013;13:122–32. doi: 10.1016/j.acap.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Bethell CD, Blumberg SJ, Stein RE, Strickland B, Robertson J, Newacheck PW. Taking Stock of the CSHCN Screener: A Review of Common Questions and Current Reflections. Academic pediatrics. 2015;15:165–76. doi: 10.1016/j.acap.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strickland BB, Jones JR, Newacheck PW, Bethell CD, Blumberg SJ, Kogan MD. Assessing systems quality in a changing health care environment: the 2009–10 national survey of children with special health care needs. Maternal and child health journal. 2015;19:353–61. doi: 10.1007/s10995-014-1517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Survey of Children with Special Health Care Needs. Data Resource Center for Child and Adolescent Health. 2009–2010. [Google Scholar]
- 26.Bethell CD, Read D, Stein RE, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambulatory pediatrics : the official journal of the Ambulatory Pediatric Association. 2002;2:38–48. doi: 10.1367/1539-4409(2002)002<0038:icwshc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.CAHMI. Data Resource Center for Child and Adolescent Health. 2012. 2009–2010 NS-CSHCN Indicator and Outcome Variables SAS Codebook, Version 1. [Google Scholar]
- 28.Waaler PE, Blom BH, Skeidsvoll H, Mykletun A. Prevalence, classification, and severity of epilepsy in children in western Norway. Epilepsia. 2000;41:802–10. doi: 10.1111/j.1528-1157.2000.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 29.Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia. 1996;37:19–23. doi: 10.1111/j.1528-1157.1996.tb00506.x. [DOI] [PubMed] [Google Scholar]
- 30.Berry JG, Poduri A, Bonkowsky JL, Zhou J, Graham DA, Welch C, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS medicine. 2012;9:e1001158. doi: 10.1371/journal.pmed.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones JE, Austin JK, Caplan R, Dunn D, Plioplys S, Salpekar JA. Psychiatric disorders in children and adolescents who have epilepsy. Pediatrics in review / American Academy of Pediatrics. 2008;29:e9–14. doi: 10.1542/pir.29-2-e9. [DOI] [PubMed] [Google Scholar]
- 32.Pellock JM. Defining the problem: psychiatric and behavioral comorbidity in children and adolescents with epilepsy. Epilepsy & behavior : E&B. 2004;5(Suppl 3):S3–9. doi: 10.1016/j.yebeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Butler AM, Elkins S, Kowalkowski M, Raphael JL. Shared decision making among parents of children with mental health conditions compared to children with chronic physical conditions. Maternal and child health journal. 2015;19:410–8. doi: 10.1007/s10995-014-1523-y. [DOI] [PubMed] [Google Scholar]
- 34.Blumberg SJ, Welch EM, Chowdhury SR, Upchurch HL, Parker EK, Skalland BJ. Design and operation of the National Survey of Children with Special Health Care Needs, 2005–2006. Vital and health statistics Ser 1, Programs and collection procedures. 2008:1–188. [PubMed] [Google Scholar]
- 35.National Survey of Children with Special Health Care Needs. Center for Disease Control and Prevention. [Google Scholar]
- 36.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American journal of epidemiology. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 37.CAHMI. Measuring medical home for children and youth: methods and findings from the National Survey of Children with Special Health Care Needs and the National Survey of Children's Health. US Department of Health and Human Services: 2009. [Google Scholar]
- 38.Hummelinck A, Pollock K. Parents' information needs about the treatment of their chronically ill child: a qualitative study. Patient education and counseling. 2006;62:228–34. doi: 10.1016/j.pec.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Berry JG, Hall M, Hall DE, Kuo DZ, Cohen E, Agrawal R, et al. Inpatient growth and resource use in 28 children's hospitals: a longitudinal, multi-institutional study. JAMA pediatrics. 2013;167:170–7. doi: 10.1001/jamapediatrics.2013.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130:e1463–70. doi: 10.1542/peds.2012-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiks AG, Mayne S, Localio AR, Feudtner C, Alessandrini EA, Guevara JP. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC pediatrics. 2012;12:153. doi: 10.1186/1471-2431-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Academy of Allergy, Asthma, and Immunology. Asthma Treatment and Management. Milwaukee: The Academy; c2017. [cited 2017 May 13]. Available from: External link: http://www.aaaai.org/conditions-and-treatments/asthma. [Google Scholar]
- 43.Siegel RM. Acute Otitis Media Guidelines, Antibiotic Use, and Shared Medical Decision-Making. Pediatrics. 2010;125:384–6. doi: 10.1542/peds.2009-3208. [DOI] [PubMed] [Google Scholar]
- 44.Global Initiative for Asthma. [cited 2017 May 13];Global strategy for asthma management and prevention. 2016 Available from: www.ginasthma.org.
- 45.Stiggelbout AM, Van der Weijden T, De Wit MP, Frosch D, Legare F, Montori VM, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ (Clinical research ed) 2012;344:e256. doi: 10.1136/bmj.e256. [DOI] [PubMed] [Google Scholar]
- 46.Adams S, Cohen E, Mahant S, Friedman JN, Macculloch R, Nicholas DB. Exploring the usefulness of comprehensive care plans for children with medical complexity (CMC): a qualitative study. BMC pediatrics. 2013;13:10. doi: 10.1186/1471-2431-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]