Abstract
Aspergillus fumigatus is the most common mold species to cause disease in immunocompromised patients. Infection usually begins when its spores (conidia) are inhaled into the airways, where they germinate, forming hyphae that penetrate and destroy the lungs and disseminate to other organs, leading to high mortality. The ability of hyphae to penetrate the pulmonary epithelium is a key step in the infectious process. A. fumigatus produces extracellular proteases that are thought to enhance penetration by degrading host structural barriers. This study explores the role of the A. fumigatus transcription factor XprG in controlling secreted proteolytic activity and fungal virulence. We deleted xprG, alone and in combination with prtT, a transcription factor previously shown to regulate extracellular proteolysis. xprG deletion resulted in abnormal conidiogenesis and formation of lighter colored, more fragile conidia and a moderate reduction in the ability of culture filtrates (CFs) to degrade substrate proteins. Deletion of both xprG and prtT resulted in an additive reduction, generating a mutant strain producing CF with almost no ability to degrade substrate proteins. Detailed proteomic analysis identified numerous secreted proteases regulated by XprG and PrtT, alone and in combination. Interestingly, proteomics also identified reduced levels of secreted cell wall modifying enzymes (glucanases, chitinases) and allergens following deletion of these genes, suggesting they target additional cellular processes. Surprisingly, despite the major alteration in the secretome of the xprG/prtT null mutant, including two to fivefold reductions in the level of 24 proteases, 18 glucanases, 6 chitinases, and 19 allergens, it retained wild-type virulence in murine systemic and pulmonary models of infection. This study highlights the extreme adaptability of A. fumigatus during infection based on extensive gene redundancy.
Keywords: Aspergillus fumigatus, protease secretion, transcription factor, proteomics, virulence
Introduction
Aspergillus fumigatus is a common saprophytic mold which produces abundant microscopic conidia (2–4 micrometers) that can be inhaled into the pulmonary alveoli to cause a variety of pathological conditions (Kwon-Chung and Sugui, 2013). In the context of the immunocompromised patient, the infection, termed Invasive Pulmonary Aspergillosis (IPA), is life-threatening and severe (Kosmidis and Denning, 2015). The neutropenic status of these patients culminates in their inability to destroy inhaled conidia resulting in fungal growth and penetration through the pulmonary epithelium into the blood stream (Latge, 2001).
The success of A. fumigatus as a pathogen is not a result of direct adaptation to the host. Rather it should be viewed as an accidental interaction between a common and hardy environmental mold and a weakened host. A. fumigatus survives in the compromised host due to a chance combination of pre-existing capabilities. They include the abundant release of small conidia protected by a non-immunogenic layer of hydrophobins and oxygen-radical quenching pigments. Growing hyphae effectively endure oxidative stress and hypoxia, efficiently collect scarce iron and secrete toxins that further depress host immune function and proteases that degrade host tissue (Abad et al., 2010; Kwon-Chung and Sugui, 2013). In human A549 alveolar epithelial cells, culture filtrates (CFs) of A. fumigatus can disrupt the actin cytoskeleton, activate NFκB signaling and induce the production of proinflammatory cytokines. These cellular events can be prevented by addition of serine protease inhibitors to the secreted CF, implying that they are directly dependent on secreted fungal proteases (Kogan et al., 2004; Sharon et al., 2011). A. fumigatus-secreted proteases and gliotoxin also induce platelet activation that may serve as a mechanism for activating the immune defenses and inducing inflammation (Speth et al., 2013). Additionally, the secreted A. fumigatus alkaline protease Alp1 cleaves the complement components C3, C4, and C5 that contribute to evasion from the host immune response (Behnsen et al., 2010). Alp1 is also a major allergen (Aspf 13) and promotes airway hyper-responsiveness and bronchoconstriction in asthma (Balenga et al., 2015).
In previous work we have identified the transcription factor PrtT, a positive regulator of secreted proteases in A. fumigatus. Deletion of prtT results in greatly reduced secreted protease activity and a reduction in the transcription of secreted proteases. ΔprtT CF showed reduced killing of A549 lung alveolar cells and erythrocyte lysis (Bergmann et al., 2009; Sharon et al., 2009, 2011). However, the ΔprtT strain showed wild-type virulence in infected neutropenic mice suggesting that perhaps residual protease activity was sufficient to enable virulence in this setting (Bergmann et al., 2009; Sharon et al., 2009).
In the related mold A. nidulans, that lacks a prtT homolog, the transcription factor XprG regulates extracellular protease production in response to nutrient stress (Katz et al., 2013, 2015; Katz and Cooper, 2015). Deletion of A. nidulans xprG resulted in complete loss of halo formation on skimmed milk (SM) agar plates and an inability to grow on medium containing BSA as sole carbon or nitrogen source. XprG is a member of the p53-like transcription factors, also known as the NDT80/PhoG-like family. NDT80 transcription factors are found in animals, fungi, and amoeba. Fungal NDT80 genes were studied in detail in Saccharomyces cerevisiae (Pak and Segall, 2002), Neurospora crassa (Hutchison and Glass, 2010) Candida albicans (Chen et al., 2004; Sellam et al., 2009, 2010) and A. nidulans (Katz et al., 2013, 2015; Katz and Cooper, 2015). The consensus is that in response to nutrient stress and deprivation they activate specific target genes such as proteases, phosphatases, secondary metabolites, and genes involved in meiosis (itself a stress response initiated by starvation) and autolysis.
We hypothesized that XprG also co-regulates protease production in A. fumigatus and that deletion of both prtT and xprG would completely abolish secreted protease activity, reducing fungal virulence. To test this hypothesis we prepared A. fumigatus strains deleted in prtT, xprG alone and in combination. The effects of these mutations in vitro and during infection in vivo are described.
Materials and Methods
Strains and Culture Conditions
The strains used in this study are detailed in Table 1. A. fumigatus conidia were harvested in 0.2% (vol/vol) Tween 20, resuspended in double-distilled water (DDW) and counted with a hemocytometer. For continuous growth, A. fumigatus strains were grown on YAG medium, that consists of 0.5% (wt/vol) yeast extract, 1% (wt/vol) glucose, and 10 mM MgCl2, supplemented with trace elements, vitamins, and 1.5% (wt/vol) agar when needed (Bainbridge, 1971). SM medium consisted of 1% (wt/vol) glucose, 1% (wt/vol) SM (Difco, Livonia, MI, United States), 0.1% (wt/vol) Casamino Acids (Difco), 7 mM KCl, 2 mM MgSO4 and 50 mM Na2HPO4-NaH2PO4 buffer (pH 5.3), supplemented with vitamins, trace elements and 1.5% agar when needed. NaNO3-depleted Aspergillus minimal medium (Weidner et al., 1998) was used for collagen medium with 0.1% (wt/vol) yeast extract, 0.5% (wt/vol) glucose and collagen as sole carbon and nitrogen source. Peptone medium contained 1% (wt/vol) glucose, 0.4% peptone (Difco), 7 mM KCl, 2 mM MgSO4 and 50 mM Na2HPO4-NaH2PO4 buffer (pH 5.3), supplemented with vitamins and trace elements. Genetically modified organisms and pathogens used in this study were maintained in accordance with TAU Institutional Policies.
Table 1.
Strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| KU80 (ΔakuB) | CEA17, AFUA_2G02620::pyrG | da Silva Ferreira et al., 2006 |
| ΔPrtT | AFUA_4G10120::hph | This work |
| ΔXprG | AFUA_8G04050:: ptrA | This work |
| ΔXprG/ΔPrtT | AFUA_8G04050:: ptrA; | This work |
| AFUA_4G10120::hph | ||
| PrtT KI | AFUA_4G10120::hph; | This work |
| AFUA_4G10120-phl | ||
| XprG KI | AFUA_8G04050:: ptrA | This work |
| AFUA_8G04050-hph | ||
| ΔXprG/PrtT KI | AFUA_8G04050:: ptrA; | This work |
| AFUA_4G10120::hph | ||
| AFUA_4G10120-phl | ||
Generation and Verification of A. fumigatus Mutant Strains
All strains were prepared in the Ku80 null background, strain AkuBKU80 (da Silva Ferreira et al., 2006). Full details of the construction and verification of the strains shown in Table 1, including a list of the primers used (Supplementary Table S1), are provided in the Supplementary Data Section.
Conidial Stability in Detergent Storage
Freshly harvested A. fumigatus conidia (107/ml) were suspended in DDW +0.5% (vol/vol) Tween 20 at 37°C. At different time-points, aliquots were diluted, plated on YAG plates and the number of colonies counted.
Conidial Disruption by Glass Beads
Freshly harvested A. fumigatus conidia (5 × 107/ml) were suspended in 0.5 ml DDW +0.1% Tween 20 and mixed with 0.5 ml (packed volume) of acid washed glass beads, 150–212 μm (Sigma–Aldrich Corp., St. Louis, MO, United States). They were then vortexed on medium strength for up to 10 min. At each time point a sample was taken, diluted and plated on YAG plates. The plates were incubated at 37°C for 24–36 h, colonies were counted and survival rates were calculated as the percentage of viable spores.
Analysis of Fungal Enzymatic Activity
Proteolytic activity on solid medium was assessed by spotting conidia on SM plates containing 0.1% Tween 20. The colonies were grown for 48 h at 37°C, then transferred to room temperature for another 48 h and subsequently photographed. Supernatants were collected from Aspergillus cultures grown in liquid SM for 48 h at 37°C. Azocasein (Sigma) was dissolved at a concentration of 5 mg/ml in assay buffer containing 50 mM Tris (pH 7.5), 0.2 M NaCl, 5 mM CaCl2 and 0.05% Triton X-100 as previously described (Kogan et al., 2004). The azocasein solution (400 μl) was mixed with 100 μl portions of supernatants from Aspergillus cultures and incubated by shaking for 90 min at 37°C. The reactions were stopped by adding of 150 μl 12% (vol/vol) trichloroacetic acid, and the reaction mixtures were allowed to stand at room temperature for 30 min. Tubes were then centrifuged for 3 min at 8,000 g, and 100 μl of each supernatant was added to 100 μl of 1 M NaOH. The absorbance of released azo dye at 436 nm was determined with a spectrophotometer. Proteolytic activity on bovine serum albumin (BSA) was measured by growing A. fumigatus in 24-well plates with 1 ml/well liquid peptone medium containing 0.1% BSA, respectively, for 24–72 h at 37°C. Supernatants were boiled in sample buffer and run on an 8% SDS-PAGE gel followed by Coomassie staining to visualize the proteins.
Scanning Electron Microscopy (SEM) Analysis
Aspergillus fumigatus wild-type and mutant strains were grown for 72 h at 37°C on YAG agar plates. Fixation and processing of samples for SEM was performed as described earlier (Fichtman et al., 2014; Hover et al., 2016). Briefly, small areas of conidiating mycelium were carefully excised from the zone of interaction and vapor fixed with 8% (vol/vol) paraformaldehyde and 4% (vol/vol) glutaraldehyde dissolved in water for 1 h in a closed chamber. Secondary vapor fixation was then carried out in an aqueous solution of 2% (wt/vol) osmium tetroxide for 1 h. The samples were submerged for 10 min in DDW and then dehydrated (10 min, twice for each step) under a series of ethanol concentrations (7.5, 15, 30, 50, 70, 90, 95, and 100%). Next, samples underwent critical point drying (CPD) using a K850 CPD dryer (Quorum Technologies, United Kingdom). Coating was done with 3 nm iridium using a Q150T coater (Quorum Technologies, United Kingdom). Samples were imaged with a Merlin scanning electron microscope (Zeiss, Germany).
LC-MS/MS Analysis and Identification of Secreted Proteins
Aspergillus fumigatus strains at a concentration of 106 condia/ml were grown in liquid collagen medium for 72 h at 37°C. Total protein was TCA-precipitated from the CFs, digested with trypsin, iTRAQ-labeled and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as detailed in the Supplementary Data.
Murine Models for Invasive Aspergillosis
For the cyclophosphamide/cortisone acetate neutropenic model (Ejzykowicz et al., 2009), 6-week-old female ICR mice were injected intraperitoneally with cyclophosphamide (150 mg/kg in PBS) at 3 days prior to infection, on the day of infection and at 3 days post-infection. Cortisone acetate (150 mg/kg PBS with 0.1% Tween 20) was injected subcutaneously at 3 days prior to conidial infection.
For intranasal infection, mice were anesthetized by intraperitoneal injection of a solution of 250 μl xylazine (VMD, Arendonk, Belgium) and ketamine (Imalgene, Fort Dodge, IA, United States) at a concentration of 1.0 and 10 mg/ml, respectively (dissolved in PBS). Following anesthesia, the mice were inoculated intranasally with 2.5 × 105 (intranasal model) or intravenously (IV) through the tail vein (IV model) with freshly harvested conidia of AkuBKU80, ΔXprG, or ΔXprG/ΔPrtT in PBS +0.1% (vol/vol) Tween 20. The inoculum was verified by quantitative culture. The animals were monitored for survival for up to 18 days. For infection of immunocompetent mice, 6-week-old female ICR mice were inoculated intranasally with 1 × 107 conidia and sacrificed 24 h or 48 h later. Excised lungs were ground and plated on YAG agar plates for CFU enumeration. Histological analysis was performed with Gomori methenamine silver stain (GMS, stains fungal elements black) or haematoxylin and eosin stain (H&E, stains host-cell nuclei purple, cytosol pink). Statistical analysis of mouse survival was performed with GraphPad Prism 4 software (GraphPad Software, San Diego, CA, United States). Animal studies were authorized by the Tel Aviv University Animal Welfare Committee according to the Israel Ministry of Health guidelines and carried out in accordance with Tel Aviv University institutional policies.
Results
Generation of A. fumigatus prtT, xprG, xprG/prtT Null Mutants and Reconstituted Strains
A single A. fumigatus xprG ortholog (AFUA_8G04050) was identified by amino-acid similarity to A. nidulans XprG (70% identity). It is predicted to be 1752 nucleotides long and to contain one intron. A. fumigatus XprG encodes a protein 583 amino acids in length, containing a predicted NDT80/PhoG like DNA-binding domain (amino-acid residues 156–327) and a MAP65/ASE1 domain (amino acid residues 336–505) shared by microtubule-binding proteins. The similarity to NDT80 family genes vib-1 from N. crassa and S. cerevisiae ndt80 is limited to the PhoG like DNA-binding domain. To analyze the function of XprG and its interaction with PrtT in A. fumigatus, we prepared ΔPrtT, ΔXprG, ΔXprG/ΔPrtT null mutants and corresponding reconstituted strains PrtT-KI, XprG-KI, and PrtT-KI/ΔXprG by transformation with a circular plasmid containing the PrtT gene and the phleomycin resistance cassete for selection (see Supplementary Data for full details about strain construction and verification).
Abnormal Conidiogenesis in the ΔXprg and ΔXprG/ΔPrtT A. fumigatus Mutants
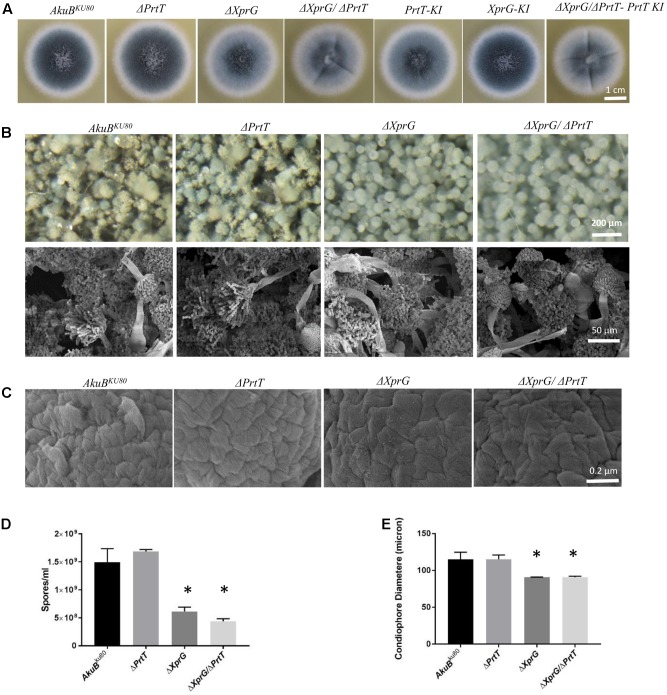
The aforementioned null and reconstituted strains were point inoculated on YAG agar plates and examined for differences in morphology after growth for 72 h at 37°C. Results show that the ΔXprG, ΔXprG/ΔPrtT null strains produced lighter colored conidia as compared to the AkuBKU80, ΔPrtT, and XprG-KI strains (Figure 1A). Examination of the conidiophore structure by light microscopy and SEM revealed that the ΔXprG and ΔXprG/ΔPrtT strains produced significantly smaller, more compact conidiophores (P < 0.02) with shorter conidial chains (Figures 1B,E). The conidial hydrophobin outer rodlet layer remained intact and unchanged (Figure 1C) and the surface hydrophobicity of the mutant colonies, based on their ability to exclude water, was unchanged (not shown). Reflecting the shorter conidial chains, the number of conidia produced by the ΔXprG and ΔXprG/ΔPrtT strains was significantly reduced three–fourfold per plate (P < 0.05) compared to AkuBKU80 and ΔPrtT (Figure 1D). Radial growth rates of the ΔPrtT, ΔXprG, ΔXprG/ΔPrtT strains were similar to that of AkuBKU80 at both 37 and 48°C (Data not shown). The lighter conidial color of the PrtT-KI complemented strain compared to the deleted strain (Figure 1A) is unexpected and may be due to multiple integration of the complementing plasmid.
FIGURE 1.
Deletion of xprG and xprG/prtT leads to abnormal conidiogenesis. Phenotypic analysis of the ΔXprG and ΔXprG/ΔPrtT strains compared to the wild-type AkuBKU80 and ΔPrtT strains shows that they produce (A) lighter colored conidia when growing on YAG agar plates (B) conidiophores with reduced diameter and shorter spore chains (Light microscopy and SEM) (C) conidia with a normal rodlet outer layer (SEM, high magnification), (D) a significantly reduced number of conidia (∗P < 0.005). (E) Quantification of reduction in conidiophore diameter (based on the diameter of 50 conidiophores per strain) (∗P < 0.02).
The Conidia Produced by the ΔXprg and ΔXprG/ΔPrtT A. fumigatus Mutants Are More Fragile
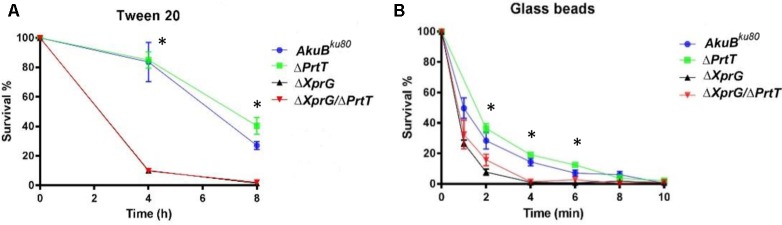
We evaluated the ability of the mutant conidia to withstand osmotic or detergent disruption by measuring their survival in water or in water containing Tween 20 detergent. Conidia from the ΔXprG and ΔXprG/ΔPrtT strains exhibited similar increased susceptibility to the detergent, losing >90% viability after 4 h as compared to loss of only <20% viability in the AkuBKU80 and ΔPrtT strains (Figure 2A). There were no differences in conidial stability during storage in DDW, suggesting the mutants are not osmotically sensitive (data not shown). The ability of the mutant conidia to withstand physical disruption was assessed by subjecting them to agitation in the presence of glass beads. Conidia from the ΔXprG and ΔXprG/ΔPrtT strains exhibited increased susceptibility to glass-bead agitation, losing all viability after 4 min agitation, compared to 10 min for the AkuBKU80 and ΔPrtT strains (Figure 2B).
FIGURE 2.
The ΔXprG and ΔXprG/ΔPrtT mutants produce conidia that are more sensitive to chemical and mechanical stress. (A) 103 conidia/ml were suspended in 0.5% Tween 20 for the time indicated, plated and the number of colonies counted in comparison to controls suspended in DDW alone. ∗P < 0.001 AkuBku80 vs. ΔXprG and ΔXprG/ΔPrtT mutants. (B) 5 × 107 conidia/ml were agitated in the presence of acid-washed glass beads for the time indicated, plated and the number of colonies counted in comparison to untreated controls. ∗P < 0.05 AkuBku80 vs. ΔXprG and ΔXprG/ΔPrtT mutants.
Taken together these results show that deletion of xprG in the AkuBKU80 and ΔPrtT strains resulted in the production of smaller conidiophores containing less and more fragile spores.
Deletion of xprG and prtT in A. fumigatus Results in an Additive Reduction in the Ability to Degrade Casein and Albumin
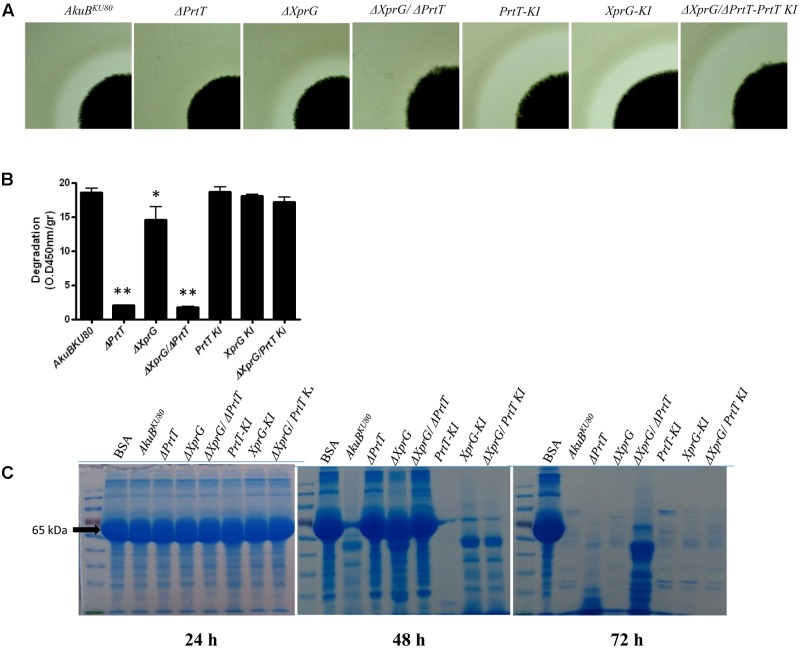
To investigate the effect of xprG deletion on secreted protease activity, we point inoculated the strains on SM agar plates and visually determined the formation of a proteolytic halo due to casein degradation around the fungal colony. As we have previously shown (Sharon et al., 2009), deletion of prtT resulted in the formation of a very weak halo compared to the control strain AkuBKU80, whereas deletion of xprG (ΔXprG) led to a partially reduced halo. Deletion of both xprG and prtT (ΔXprG/ΔPrtT) completely abolished the formation of a proteolytic halo (Figure 3A) and reduced azocasein degradation by over 90% (Figure 3B). Halo formation was restored in the reconstituted strain XprG-KI and slightly increased in the PrtT-KI and PrtT-KI/ΔXprG strains. This increase, however, was not seen in the azocasein or BSA degradation assays described below. We used a more sensitive SDS-PAGE-based BSA degradation assay to better differentiate between the proteolytic activities of the ΔXprG,ΔPrtT and ΔXprG/ΔPrtT mutants (Figure 3C). Strains were grown for 24–72 h on peptone medium containing 0.1% BSA. The supernatant was separated on an SDS-PAGE gel followed by Coomassie staining to visualize the degradation of the BSA protein. The control AkuBKU80 and reconstituted strains substantially degraded the BSA substrate protein after 48 h of incubation, whereas the ΔPrtT, ΔXprG, and ΔXprG/ΔPrtT strains did not (Figure 3C). After 72 h of incubation, all strains except ΔXprG/ΔPrtT had completely degraded the BSA. Taken together, our findings indicate that deletion of both prtT and xprG results in an additive reduction in the ability to degrade casein or albumin. Previous analyses using protease inhibitors and deletion mutants have demonstrated that secreted A. fumigatus serine proteases, and in particular Alp1, are primarily responsible for the degradation of these substrates (Jaton-Ogay et al., 1994; Tomee et al., 1997; Kogan et al., 2004).
FIGURE 3.
Proteolytic activity following deletion of prtT and xprG. Proteolytic activity of the fungal strains was assessed by (A) Halo formation on SM agar plates containing 0.1% Tween 20 following 48 h of growth at 37°C followed by another 48 h at room temperature. (B) Degradation of azocasein by A. fumigatus culture filtrates (CFs). The absorbance of the released azo dye was measured spectrophotometrically at 450 nm. (∗P < 0.05, ∗∗P < 0.0001). (C) BSA proteolysis by A. fumigatus CFs followed by SDS-PAGE analysis of the cleaved substrate. BSA (65 kDa, arrow) and other proteins were stained by Coomassie.
Proteomic Analysis of the ΔPrtT, ΔXprG, and ΔXprG/ΔPrtT Secretomes
To identify the repertoire of proteins secreted by the ΔPrtT, ΔXprG, and ΔXprG/ΔPrtT mutants compared to the control AkuBKU80 strain, conidia were cultured in liquid MM containing collagen for 72 h at 37°C with shaking. Supernatants were TCA-precipitated, treated after Yeung and Stanley (2009), followed by a Wessel-Flügge precipitation, labeled with iTRAQ 4-plex isobaric labeling for quantitative proteomics approach and analyzed by LC-MS/MS. A total of 938 proteins were identified in the fungal supernatants, 274 of which were predicted to be secreted by in silico analysis (secretion signal found by signalP for 236 proteins and targetP for 274 proteins) (Supplementary Table S2).
Cell lysis cannot be completely excluded during cultivation and sample preparation. It is therefore possible that a proportion of the secretome consisted of proteins without a secretion signal, which had been released by cell lysis. However, the comparison of the abundance (based on normalized PSM values) of typical intracellular, non-secreted proteins like actin (Afu6g04740) or tubulin (Afu1g02550) with the abundance of a secreted protein like lap2 (Afu3g00650) revealed a depletion by a factor between 12 and 106. In addition, fungi are able to release typical intracellular proteins via extracellular vesicles to the extracellular space (Joffe et al., 2016). It can be therefore assumed that the contamination of the secretome by cell lysis was relatively low.
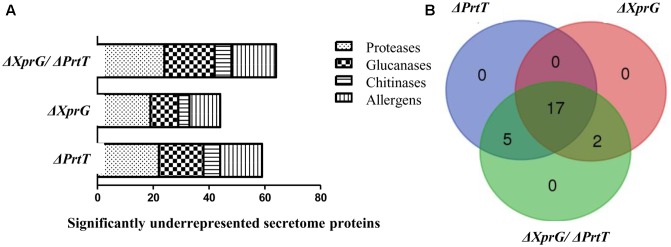
Deletion of prtT resulted in strongly reduced secretion of 22 proteases, including Lap1, Lap2, Cp1, Cp3 Alp1, Alp2, and Mep (Figure 4 and Supplementary Table S3). Deletion of xprG resulted in reductions in the secretion of 19 proteases, including most strongly Lap1, Sxa2, DppV, DppIV, Mep, and SedC. Notably, several proteases that were strongly reduced in the ΔPrtT mutant were only slightly reduced (Pep2, DapB, Cps1, Cp1, Cp3) or not reduced (CtsD, Ape3, Lap2, Alp1, Alp2) in the ΔXprG strain (Figure 4 and Supplementary Table S3). This provides a good explanation for the higher secreted protease activity of the ΔXprG vs. the ΔPrtT mutant, especially the lack of reduction in the major neutral serine protease Alp1 (Behnsen et al., 2010). Deletion of both prtT and xprG resulted in the strongest reductions in the secretion of 24 proteases comprising the entire joint dataset of the ΔPrtT and ΔXprG mutants, including 14 serine proteases, 7 metalloproteases and 3 aspartic proteases (Figure 4 and Supplementary Table S3). Interestingly, in all three mutant strains, the secretion of numerous glucanases, chitinases, and allergens was also reduced, indicating that these transcription factors not only regulate the expression of secreted proteases but also of cell wall enzymes and antigenic proteins (Supplementary Table S3). The significance of these results will be explained in the discussion. No proteins showed increased secretion in the mutants vs. the control AkuBKU80 strain.
FIGURE 4.
Effect of the deletion of prtT, xprG and xprG/prtT on the secretome of A. fumigatus. Deletion of prtT, xprG and xprG/prtT (A) reduces the secretion not only of proteases but also of glucanases and chitinases participating in cell wall biosynthesis and of numerous allergenic proteins. (B) Venn diagram illustrating the degree of overlap in the secretome datasets of the mutant strains.
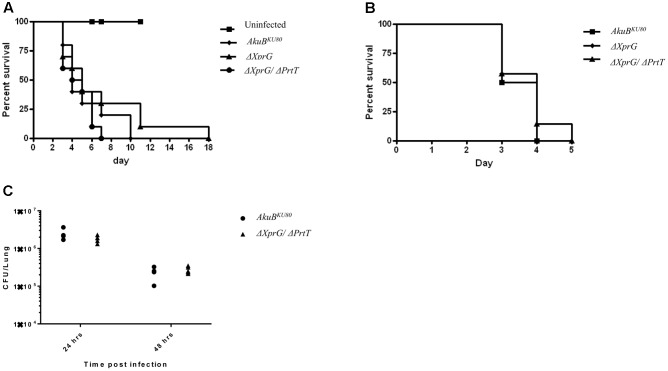
The ΔXprG and ΔXprG/ΔPrtT Strains Exhibit Normal Virulence in Infected Mice
Previous studies have shown that deletion of prtT in A. fumigatus does not affect virulence in lung-infected neutropenic mice (Bergmann et al., 2009; Sharon et al., 2009). We hypothesized that the almost complete lack of secreted protease activity in the ΔXprG/ΔPrtT strain would result in a noticeable reduction in virulence. To test this, we compared the virulence of the control AkuBKU80, ΔXprG and ΔXprG/ΔPrtT strains in two mouse models of invasive aspergillosis. Both models reproduce profound neutropenia (as found, for example following chemotherapy in leukemic patients), by immunosuppressing the mice with a combination of cortisone acetate and cyclophosphamide. In the first model, the mice were infected intranasally with freshly harvested conidia. In the second model reproducing disseminated infection, mice were infected IV. The number of live mice in each group was recorded daily throughout the experiment. The ΔPrtT strain was not included as we have previously shown it to be fully virulent in these models (Sharon et al., 2009). Figure 5 shows survival curves obtained during the course of the experiment for the lung (Figure 5A) and disseminated (Figure 5B) models. No significant differences in virulence were found for the ΔXprG and ΔXprG/ΔPrtT mutants compared to the control AkuBKU80 strain (P-value > 0.3). Next, we analyzed the response of immunocompetent mice to intranasal pulmonary infection (107 conidia/mouse) with ΔXprG/ΔPrtT and control AkuBKU80 strains. Outputs included lung CFU (Figure 5C) and histology (Supplementary Figure S6), 24 and 48 h following infection. There was no significant difference in lung fungal load (P-value > 0.2) between the ΔXprG/ΔPrtT and control AkuBKU80 strains (Figure 5C). Histology revealed abundant conidia in the bronchi of both mutant and control strains 24 h after infection (CMS stain, right panel, arrows) accompanied by a considerable influx of red blood cells and lymphocytes (H&E stain, left panel) (Supplementary Figure S6). As expected in immunocompetent mice, the number of conidia strongly decreased 48 h after infection, as did the number of red blood cells and lymphocytes (Supplementary Figure S6). Taken together, these results suggest that immunocompetent mice mount a similar and effective immune response to both ΔXprG/ΔPrtT and control AkuBKU80 infection.
FIGURE 5.
Deletion of xprG and xprG/prtT, does not affect virulence in a neutropenic murine model of (A) pulmonary aspergillosis, (B) disseminated aspergillosis and (C) pulmonary fungal load in immunocompetent mice. Cortisone acetate plus cyclophosphamide (neutropenia model) were used to immunocompromise the infected mice (A,B). Survival curves are shown for mice infected (A) intranasally with an inoculum of 2.5 × 105 conidia per mouse (n = 10 mice/group) and (B) intravenously via the lateral tail vein with an inoculum of 2.5 × 105 conidia per mouse (n = 7 mice/group). These experiments were repeated twice with similar results. (C) Immunocompetent mice were infected intranasally with an inoculum of 1 × 107 conidia per mouse (n = 5 mice/group) and total lung fungal load (CFU/lung) assessed 24 and 48 h post-infection. (Additional histological analysis is presented in the Supplements- Supplementary Figure S6).
Discussion
This study describes the disruption of the putative transcriptional activator XprG in A. fumigatus, alone and in combination with the previously described transcription factor PrtT. We chose to study XprG in A. fumigatus because (i) it regulates protease expression in A. nidulans and we reasoned it may also do so, in combination with PrtT, in A. fumigatus, (ii) regulation of protease expression by combinations of transcription factors in pathogenic filamentous fungi is poorly understood and (iii) we hypothesized that deletion of two transcriptional regulators controlling the expression of multiple proteases could shed more light on the involvement of secreted proteases in the pathogenesis of A. fumigatus.
We gained several important insights from this study. First, we found considerable divergence in the role of XprG in A. nidulans and A. fumigatus. In A. nidulans that lacks a PrtT homolog XprG is the major activator of secreted protease activity under starvation. In A. fumigatus, this function is carried out by PrtT, with XprG performing a secondary role. Increasing functional divergence is found in the N. crassa XprG homolog vib-1 – in addition to controlling conidial pigmentation and increasing extracellular protease activity it also controls protoperithecial sexual development (Hutchison and Glass, 2010). Further functional separation has occurred in S. cerevisiae ndt80 (Pak and Segall, 2002) that controls meiosis and C. albicans CaNdt80 (Chen et al., 2004; Sellam et al., 2009, 2010), that regulates azole resistance, hyphal growth and virulence. The basis for this diversity likely stems from the extreme sequence divergence outside of the conserved NDT80/PhoG like DNA-binding domain.
We also found evidence for overlap in ndt80/vib-1/XprG function. As previously shown in A. nidulans xprG and N. crassa vib-1 null mutants, deletion of A. fumigatus xprG resulted in the formation of pale conidia, suggesting a role in melanin biosynthesis. We further showed that in A. fumigatus, conidiogenesis is impaired and the resulting conidia are weaker and more prone to chemical and physical disruption. Most interestingly, we show here that A. fumigatus XprG and PrtT activate expression of glucanases, chitinases and numerous allergens. C. albicans CaNdt80 also activates genes encoding cell wall components including expression of chitinase Cht3p and cell wall glucosidase Sun41p that are essential for the completion of cell separation (Sellam et al., 2010). However, despite reduced levels of numerous secreted glucanases and chitinases in A. fumigatus ΔXprG, we found no obvious evidence for cell wall alterations by either SEM, transmission electron microscopy (TEM) or cell wall inhibitor analysis (data not shown). CaNdt80 also activates CDR1 efflux pump and ergosterol biosynthesis and its deletion results in azole sensitivity. Nevertheless, deletion of A. fumigatus xprG did not increase azole sensitivity (data not shown) suggesting that it does not affect these targets.
Our analysis of XprG and PrtT showed that both genes are not essential for maintaining virulence. Considering that the double-mutant has almost no detectable secreted protease activity in vitro and exhibits strong (two–fivefold) reductions in 24 secreted proteases [including all 5 proteases that are activated during in vivo infection (McDonagh et al., 2008)] as well as 18 glucanases and 6 chitinases, the unaffected virulence in the mouse model is unexpected. The A. fumigatus genome encodes approximately 50 putative secreted proteases, and some may be alternatively activated in vivo, compensating for the reductions seen in ΔPrtT/ΔXprG. Another possible explanation is that under in vivo stress, ΔXprG/ΔPrtT activates alternative virulence determinants that are not activated in the wild type. For example, we have previously shown that deletion of prtT resulted in the upregulation of four secondary metabolite clusters, including genes for the biosynthesis of toxic pseurotin A (Hagag et al., 2012). These factors could increase its virulence to wild type levels, despite its inability to produce secreted proteases. Functional gene redundancy could also account for our findings. Such redundancy has been repeatedly shown in A. fumigatus, following multiple deletions in large gene families encoding chitin synthases (Muszkieta et al., 2014), α-1,3-glucan synthases (Henry et al., 2012), and oligopeptide transporters (Hartmann et al., 2011).
Interestingly, the A. fumigatus strains generated here showed reductions in the expression of 21 of the 23 allergens identified in A. fumigatus, including those encoding the proteases Alp1/Aspf13, Alp2/Aspf18, Pep1/Aspf10, and Mep/Aspf5. Allergens encoded by fungal proteases activate an allergic Th-2 response in vivo by cleaving airway fibrinogen and generating fragments that act as TLR4 ligands on alveolar macrophages and airway epithelium (Millien et al., 2013). While secreted A. fumigatus proteases are not critical for infection of the immunocompromised host, our findings propose that they could be highly important in determining the response in fungal allergies. This possibility can be further explored with the A. fumigatus strains generated in this study.
Author Contributions
ES, BH, SH, SA, YS, BF, and TK performed the experiments. AH, AB, OK, and NO conceived and planned the project and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Till Kindel for excellent technical assistance.
Footnotes
Funding. This study was provided by the Israel Ministry of Health Infect-ERA (Grant 11080) to NO the Israel Science Foundation (985/15) to AH and by the Deutsche Forschungsgemeinschaft within the Collaborative Research Center TR124 FungiNet (project Z2) to TK and OK.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2017.02490/full#supplementary-material
References
- Abad A., Fernandez-Molina J. V., Bikandi J., Ramirez A., Margareto J., Sendino J., et al. (2010). What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 27 155–182. 10.1016/j.riam.2010.10.003 [DOI] [PubMed] [Google Scholar]
- Bainbridge B. W. (1971). Macromolecular composition and nuclear division during spore germination in Aspergillus nidulans. J. Gen. Microbiol. 66 319–325. 10.1099/00221287-66-3-319 [DOI] [PubMed] [Google Scholar]
- Balenga N. A., Klichinsky M., Xie Z., Chan E. C., Zhao M., Jude J., et al. (2015). A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 6:6763. 10.1038/ncomms7763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnsen J., Lessing F., Schindler S., Wartenberg D., Jacobsen I. D., Thoen M., et al. (2010). Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect. Immun. 78 3585–3594. 10.1128/IAI.01353-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann A., Hartmann T., Cairns T., Bignell E. M., Krappmann S. (2009). A regulator of Aspergillus fumigatus extracellular proteolytic activity is dispensable for virulence. Infect. Immun. 77 4041–4050. 10.1128/IAI.00425-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. G., Yang Y. L., Shih H. I., Su C. L., Lo H. J. (2004). CaNdt80 is involved in drug resistance in Candida albicans by regulating CDR1. Antimicrob. Agents Chemother. 48 4505–4512. 10.1128/AAC.48.12.4505-4512.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Ferreira M. E., Kress M. R., Savoldi M., Goldman M. H., Hartl A., Heinekamp T., et al. (2006). The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5 207–211. 10.1128/EC.5.1.207-211.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejzykowicz D. E., Cunha M. M., Rozental S., Solis N. V., Gravelat F. N., Sheppard D. C., et al. (2009). The Aspergillus fumigatus transcription factor Ace2 governs pigment production, conidiation and virulence. Mol. Microbiol. 72 155–169. 10.1111/j.1365-2958.2009.06631.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtman B., Shaulov L., Harel A. (2014). Imaging metazoan nuclear pore complexes by field emission scanning electron microscopy. Methods Cell Biol. 122 41–58. 10.1016/B978-0-12-417160-2.00002-3 [DOI] [PubMed] [Google Scholar]
- Hagag S., Kubitschek-Barreira P., Neves G. W., Amar D., Nierman W., Shalit I., et al. (2012). Transcriptional and proteomic analysis of the Aspergillus fumigatus DeltaprtT protease-deficient mutant. PLOS ONE 7:e33604. 10.1371/journal.pone.0033604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann T., Cairns T. C., Olbermann P., Morschhauser J., Bignell E. M., Krappmann S. (2011). Oligopeptide transport and regulation of extracellular proteolysis are required for growth of Aspergillus fumigatus on complex substrates but not for virulence. Mol. Microbiol. 82 917–935. 10.1111/j.1365-2958.2011.07868.x [DOI] [PubMed] [Google Scholar]
- Henry C., Latge J. P., Beauvais A. (2012). alpha1,3 glucans are dispensable in Aspergillus fumigatus. Eukaryot. Cell 11 26–29. 10.1128/EC.05270-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hover T., Maya T., Ron S., Sandovsky H., Shadkchan Y., Kijner N., et al. (2016). Mechanisms of bacterial (Serratia marcescens) attachment to, migration along, and killing of fungal hyphae. Appl. Environ. Microbiol. 82 2585–2594. 10.1128/AEM.04070-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison E. A., Glass N. L. (2010). Meiotic regulators Ndt80 and ime2 have different roles in Saccharomyces and Neurospora. Genetics 185 1271–1282. 10.1534/genetics.110.117184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaton-Ogay K., Paris S., Huerre M., Quadroni M., Falchetto R., Togni G., et al. (1994). Cloning and disruption of the gene encoding an extracellular metalloprotease of Aspergillus fumigatus. Mol. Microbiol. 14 917–928. 10.1111/j.1365-2958.1994.tb01327.x [DOI] [PubMed] [Google Scholar]
- Joffe L. S., Nimrichter L., Rodrigues M. L., Del Poeta M. (2016). Potential roles of fungal extracellular vesicles during infection. mSphere 1:e00099–16. 10.1128/mSphere.00099-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. E., Braunberger K., Yi G., Cooper S., Nonhebel H. M., Gondro C. (2013). A p53-like transcription factor similar to Ndt80 controls the response to nutrient stress in the filamentous fungus, Aspergillus nidulans. F1000Res. 2:72. 10.12688/f1000research.2-72.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. E., Buckland R., Hunter C. C., Todd R. B. (2015). Distinct roles for the p53-like transcription factor XprG and autophagy genes in the response to starvation. Fungal Genet. Biol. 83 10–18. 10.1016/j.fgb.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Katz M. E., Cooper S. (2015). Extreme diversity in the regulation of Ndt80-Like transcription factors in fungi. G3 5 2783–2792. 10.1534/g3.115.021378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan T. V., Jadoun J., Mittelman L., Hirschberg K., Osherov N. (2004). Involvement of secreted Aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J. Infect. Dis. 189 1965–1973. 10.1086/420850 [DOI] [PubMed] [Google Scholar]
- Kosmidis C., Denning D. W. (2015). The clinical spectrum of pulmonary aspergillosis. Thorax 70 270–277. 10.1136/thoraxjnl-2014-206291 [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Sugui J. A. (2013). Aspergillus fumigatus–what makes the species a ubiquitous human fungal pathogen? PLOS Pathog. 9:e1003743. 10.1371/journal.ppat.1003743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latge J. P. (2001). The pathobiology of Aspergillus fumigatus. Trends Microbiol. 9 382–389. 10.1016/S0966-842X(01)02104-7 [DOI] [PubMed] [Google Scholar]
- McDonagh A., Fedorova N. D., Crabtree J., Yu Y., Kim S., Chen D., et al. (2008). Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLOS Pathog. 4:e1000154. 10.1371/journal.ppat.1000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millien V. O., Lu W., Shaw J., Yuan X., Mak G., Roberts L., et al. (2013). Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 341 792–796. 10.1126/science.1240342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszkieta L., Aimanianda V., Mellado E., Gribaldo S., Alcazar-Fuoli L., Szewczyk E., et al. (2014). Deciphering the role of the chitin synthase families 1 and 2 in the in vivo and in vitro growth of Aspergillus fumigatus by multiple gene targeting deletion. Cell. Microbiol. 16 1784–1805. 10.1111/cmi.12326 [DOI] [PubMed] [Google Scholar]
- Pak J., Segall J. (2002). Role of Ndt80, Sum1, and Swe1 as targets of the meiotic recombination checkpoint that control exit from pachytene and spore formation in Saccharomyces cerevisiae. Mol. Cell. Biol. 22 6430–6440. 10.1128/MCB.22.18.6430-6440.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam A., Askew C., Epp E., Tebbji F., Mullick A., Whiteway M., et al. (2010). Role of transcription factor CaNdt80p in cell separation, hyphal growth, and virulence in Candida albicans. Eukaryot. Cell 9 634–644. 10.1128/EC.00325-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam A., Tebbji F., Nantel A. (2009). Role of Ndt80p in sterol metabolism regulation and azole resistance in Candida albicans. Eukaryot. Cell 8 1174–1183. 10.1128/EC.00074-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon H., Amar D., Levdansky E., Mircus G., Shadkchan Y., Shamir R., et al. (2011). PrtT-regulated proteins secreted by Aspergillus fumigatus activate MAPK signaling in exposed A549 lung cells leading to necrotic cell death. PLOS ONE 6:e17509. 10.1371/journal.pone.0017509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon H., Hagag S., Osherov N. (2009). Transcription factor PrtT controls expression of multiple secreted proteases in the human pathogenic mold Aspergillus fumigatus. Infect. Immun. 77 4051–4060. 10.1128/IAI.00426-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth C., Hagleitner M., Ott H. W., Wurzner R., Lass-Florl C., Rambach G. (2013). Aspergillus fumigatus activates thrombocytes by secretion of soluble compounds. J. Infect. Dis. 207 823–833. 10.1093/infdis/jis743 [DOI] [PubMed] [Google Scholar]
- Tomee J. F., Wierenga A. T., Hiemstra P. S., Kauffman H. K. (1997). Proteases from Aspergillus fumigatus induce release of proinflammatory cytokines and cell detachment in airway epithelial cell lines. J. Infect. Dis. 176 300–303. 10.1086/517272 [DOI] [PubMed] [Google Scholar]
- Weidner G., d’Enfert C., Koch A., Mol P. C., Brakhage A. A. (1998). Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5’-monophosphate decarboxylase. Curr. Genet. 33 378–385. 10.1007/s002940050350 [DOI] [PubMed] [Google Scholar]
- Yeung Y. G., Stanley E. R. (2009). A solution for stripping antibodies from polyvinylidene fluoride immunoblots for multiple reprobing. Anal. Biochem. 389 89–91. 10.1016/j.ab.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.