Abstract
An age-related decline in face processing, even under conditions in which learning and memory are not implicated, has been well documented, but the mechanism underlying this perceptual alteration remains unknown. Here, we examine whether this behavioral change may be accounted for by a reduction in white matter connectivity with age. To this end, we acquired diffusion tensor imaging data from 28 individuals aged 18 to 86 years and quantified the number of fibers, voxels, and fractional anisotropy of the two major tracts that pass through the fusiform gyrus, the pre-eminent face processing region in the ventral temporal cortex. We also measured the ability of a subset of these individuals to make fine-grained discriminations between pairs of faces and between pairs of cars. There was a significant reduction in the structural integrity of the inferior fronto-occipital fasciculus (IFOF) in the right hemisphere as a function of age on all dependent measures and there were also some changes in the left hemisphere, albeit to a lesser extent. There was also a clear age-related decrement in accuracy of perceptual discrimination, especially for more challenging perceptual discriminations, and this held to a greater degree for faces than for cars. Of greatest relevance, there was a robust association between the reduction of IFOF integrity in the right hemisphere and the decline in face perception, suggesting that the alteration in structural connectivity between the right ventral temporal and frontal cortices may account for the age-related difficulties in face processing.
INTRODUCTION
The discrimination and recognition of faces may be among the most taxing of all perceptual challenges confronted by observers in their day-to-day life. Not only does the observer need to derive precise information about gaze position, gender, and affect of the face, but the face must also be perceptually individuated from all other faces so that identity can be assigned. Moreover, all of these processes must be executed accurately and rapidly, notwithstanding the ambiguity of the input arising from the commonality of input features (all faces have two eyes, a nose, and a mouth in the same spatial arrangement). Although it is well established that there is an age-related decline in face recognition (Salthouse, 2004; Maylor & Valentine, 1992; Shapiro & Penrod, 1986), evident even in individuals from age 50, but with acceleration after 70 years (Chaby, Jemel, George, Renault, & Fiori, 2001; Crook & Larrabee, 1992), this decline has often been associated with the concomitant decrement in memory and learning (Lamont, Stewart-Williams, & Podd, 2005; Parkin, 1993).
The reduction in face processing proficiency, however, may not be solely a function of memory or learning changes, as older observers perform more poorly than their younger counterparts even when the need for memory or learning of faces is minimized (Boutet & Faubert, 2006). The age-related decline is also apparent when the task is purely perceptual and is based solely on shape and facial geometry; for example, in one recent study, faces shown from the same vantage point were equally well discriminated by observers, independent of age, but faces shown from different viewpoints were more poorly discriminated by older observers and there was no improvement in performance even with prolonged exposure duration (Habak, Wilkinson, & Wilson, in press). The age-related reduction in face discrimination is also revealed when faces are degraded but is also apparent, albeit to a lesser extent, even with nondegraded faces (Grady, McIntosh, Horwitz, & Rapoport, 2000).
There is a diverse array of age-related changes in the brain (and in the eye and in the visual pathways; see for example, Owsley, Sekuler, & Boldt, 1981) that may account for the decline in perceptual face processing (as well as in other aspects of perception and cognition), including a decrease in total brain volume, increased cortical thinning, and increased gyral atrophy. Evidence to support these age-dependent neural changes has generally been gleaned from postmortem studies, but the recent advent of high-resolution imaging has yielded considerable data from both structural and functional magnetic resonance imaging (fMRI) investigations elucidating age-related cortical alterations (for recent reviews, see Raz & Rodrigue, 2006; Wozniak & Lim, 2006).
Aside from the documented changes in brain volume and the modifications in sulcal morphology with age, one possible neural explanation for the alterations in cognitive function that is receiving recent attention is a breakdown in cortical connectivity. Evidence supporting the idea that a reduction in intracortical connectivity underlies age-related changes includes the loss of myelinated fibers as a function of age (Marner, Nyengaard, Tang, & Pakkenberg, 2003) and loss and deformation of the myelin sheath with aging (Peters, 2002a). As an example, one recent study reported that, across individuals aged 18 to 93 years, there is a 10% decrease in myelinated fiber length per decade (roughly 45% from 20 to 40 years of age) (Marner et al., 2003). Recent diffusion tensor imaging (DTI) and diffusion-weighted imaging have also begun to uncover age-related microstructural changes in the white matter (Jones et al., 2006; Sullivan & Pfefferbaum, 2006; Pfefferbaum & Sullivan, 2003), reflected in an increase in the average diffusion coefficient (Sullivan, Pfefferbaum, Adalsteinsson, Swan, & Carmelli, 2002) and a decrease in anisotropy (Salat et al., 2005), both of which indicate a reduction in the structural integrity of axonal fibers. These white matter changes also appear to bear a direct relationship to cognitive decline with documented correlations between white matter integrity and various neurocognitive functions, including executive functions, attention span, and processing speed and reading (e.g., Madden et al., 2004; Klingberg et al., 2000; Filley, 1998).
The critical question to be addressed here is whether changes in white matter connectivity might be associated with, and possibly even account for, the age-related decline in face processing. Although there are well-demarcated focal cortical regions associated with face processing such as the fusiform face area, the occipital face area, and the superior temporal sulcus (Grill-Spector, 2003; Kanwisher, McDermott, & Chun, 1997), there is growing documentation of reliance on a more widespread cortical network which includes the “core” areas mentioned above but also the amygdala and insula, where emotional expression is processed (Breiter et al., 1996); the inferior frontal gyrus, where semantic information is accessed (Leveroni et al., 2000); and regions of the reward circuitry, where information about facial beauty may be represented (Aharon et al., 2001). Given that face processing is mediated by a distributed network involving multiple regions of the ventral visual cortex and frontal cortex, and extensive interactions between them (Fairhall & Ishai, 2007; Gobbini & Haxby, 2006; Ishai, Schmidt, & Boesiger, 2005; McIntosh et al., 1994), a reduction in connectivity will have clear adverse consequences in face processing.
Some supporting evidence for the hypothesis that changes in connectivity or neural circuitry may be responsible for the cognitive decline in face processing comes from a small set of studies that have examined the neural substrate underlying the perception of faces as a function of age. In one such study, notwithstanding the finding that comparable fusiform face activation in young and old adults was demonstrated during the perception of faces, as revealed by positron emission tomography (PET) imaging (Grady et al., 1994), the distribution of activation across other cortical regions differed. Of particular interest, older individuals showed decreased medial temporal cortex activation and increased prefrontal activation, relative to the younger individuals. These results led the authors to surmise that an increased need for cognitive resources during the performance of complex tasks and an age-related change in functional connectivity was likely responsible for the altered cortical profile in the older individuals. In a follow-up PET study comparing the activation of face-responsive cortical networks between young and old observers, there was age-mediated deterioration of face-responsive populations (Grady et al., 2000) but also consequent recruitment of additional regions such as prefrontal cortices (see also Cabeza, Anderson, Locantore, & McIntosh, 2002), more evident in the right than in the left hemisphere. Whereas these studies document changes in the functional connectivity of regions mediating face processing, it remains an open question as to whether disruptions in white matter circuitry might be responsible for the apparent functional alterations.
To examine this exact issue, we first explore, using DTI and tractography, whether there are age-related alterations in white matter connectivity in those cortical areas subserving face processing in a group of 28 individuals aged 18 to 86. We then investigate whether these individuals exhibit a behavioral decrement in face processing and, finally, we directly evaluate the relationship between the various DTI measures and the observed cognitive profile. In this investigation, we focus our efforts on the two long-range fiber tracts that pass through the fusiform gyrus. (I) the inferior longitudinal fasciculus (ILF), which passes through the fusiform and lingual gyri and the cuneus and traverses the superior, inferior, and middle temporal gyri as well as the hippocampus and parahippocampus and (ii) the inferior fronto-occipital fasciculus (IFOF), which projects between the lingual, fusiform, and inferior temporal gyri and the inferolateral and dorsolateral frontal cortex (Catani, Jones, Donato, & Ffytche, 2003) (see Figure 1).
Figure 1.
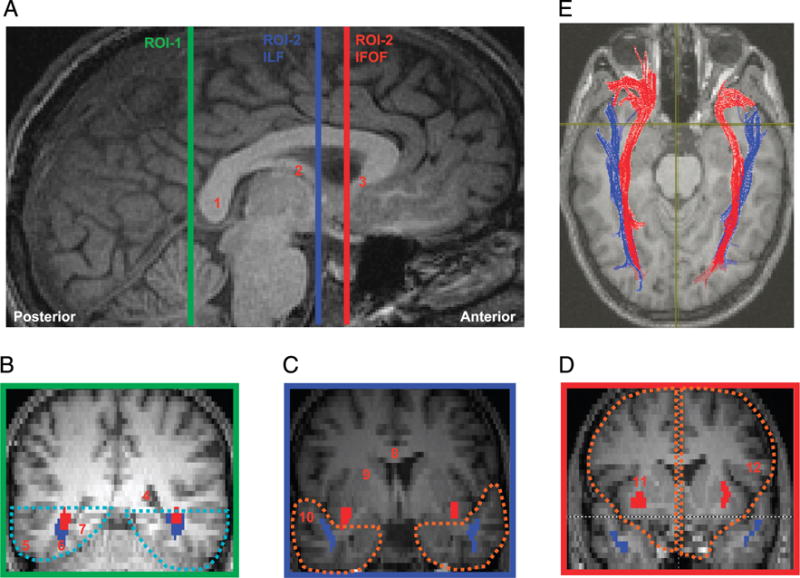
(A) Sagittal slice showing delineation of location for defining ROIs used for extracting the ILF and the IFOF. All ROIs were defined on each individual’s native space along the mid-sagittal plane. (B–D) The coronal slices are color coordinated to indicate their position on the mid-sagittal plane. (B) ROI-1, which encompasses the ventral occipito-temporal region is marked in cyan dotted lines in the coronal slice demarcated by the green line in (A). (C) ROI-2 for extracting ILF (blue fibers), which encompasses the anterior temporal lobe in each hemisphere, is marked in orange dotted lines in the coronal slice demarcated by the blue line in (A). Note that for extraction of the ILF fibers, the IFOF fibers (shown in red) were removed to avoid inclusion with the ILF fibers. (D) ROI-2 for extracting the IFOF (red fibers), which encompasses the frontal lobe in each hemisphere, is marked in orange dotted lines in the coronal slice demarcated by the red line in (A). Note that between the coronal slices corresponding to the blue and red lines marked on the sagittal plane in (A), the IFOF diverges toward the frontal cortex through the floor of the external capsule. 1 = Splenium of the callosum; 2 = Fornix; 3 = Rostrum of the callosum; 4 = Posterior horn of the lateral ventricle; 5 = Inferior temporal gyrus; 6 = Posterior fusiform gyrus; 7 = Inferior lingual gyrus; 8 = Body of the callosum; 9 = Internal capsule; 10 = Superior temporal gyrus; 11 = External capsule; 12 = Inferior frontal gyrus.
The rationale for this approach was supported by two main motivations. The first motivation is that, as mentioned above, the FFA and its connections form part of the “core” of the distributed neural network subserving the perception of faces (Fairhall & Ishai, 2007; Gobbini & Haxby, 2006; Haxby, Hoffman, & Gobbini, 2002) and, as such, serve as a good starting point for investigating the neural correlate of age-related face processing decline. The second motivating factor is that previous research has already demonstrated that a breakdown in white matter integrity in this very region can adversely affect face processing: A recent study examining volumetric changes in the ventral temporal cortex in individuals with congenital prosopagnosia has documented a reduction in the cortical volume of anterior regions of the temporal cortex (Behrmann, Avidan, Gao, & Black, 2007). Additionally, a related study in the same individuals has shown that there is a reduction in fiber integrity between the fusiform gyrus and more anterior regions of the temporal cortex and that this disruption in structural integrity is correlated with, and may even account for, the face processing impairment in these individuals (Thomas, Avidan, Jung, & Behrmann, submitted). Adopting a similar approach here, we examine the relationship between white matter structures and behavioral processing in the domain of face perception in individuals across a large age span.
METHODS
Participants
A group of 28 individuals (16 men, 12 women), aged between 18 and 86 years (mean = 42.2. years, SD = 22.2), with no major health problems and no neurological or psychiatric history, was recruited for the DTI portion of this project. A subset of these individuals (n = 22) roughly evenly distributed into four disparate decades, spanning the age range of the larger sample (aged 20–30: n = 6; 40–50: n = 5; 60–70: n = 6; 80–90: n = 5), also completed the behavioral experiments. All participants were right-handed (with the exception of 2), were native English speakers, and all consented to participate. The protocol for this study was approved by the Institutional Review Boards of Carnegie Mellon University and the University of Pittsburgh. All individuals had visual acuity at least as good as 20/40 normally or with correction.
MRI and DTI Acquisition Protocol
All participants were scanned at the Brain Imaging Research Center in Pittsburgh on a 3-T Siemens Allegra scanner equipped with a standard quadrature birdcage head coil. The DTI sequence was based on a single-shot spin-echo, echo-planar imaging (EPI) sequence with diffusion sensitizing gradients applied on either side of the 180° refocusing pulse (Basser, Mattiello, & LeBihan, 1994). Diffusion-weighted images were acquired along the horizontal plane along six noncollinear directions: XY, XZ, YZ, −XY, −XZ, and −YZ. In addition, images were acquired with b = 0. The scan was prescribed so as to get maximum coverage of the temporal lobe and the frontal lobe. Specific DTI parameters: TR = 4900 msec, TE = 82 msec, flip angle = 90°, FOV = 210 × 210 mm2, acquisition matrix size = 80 × 128, 34 axial slices, 3 mm thickness (no gap), pixel size = 1.64 × 1.64 mm2, diffusion weighting b = 850 sec/mm2. Twelve repetitions of the complete set were collected and averaged to increase signal-to-noise without introducing motion artifacts. The duration of the DTI scan was approximately 7 min. During each scanning session, along with the DTI acquisition, high-resolution anatomical scans and blood oxygenation level-dependent contrast images were also acquired as part of another study. The high-resolution anatomical scan (T1-weighted 3-D MPRAGE) was used for co-registration with the b = 0 image. Specific scanning parameters: inversion time = 800 msec, TE = 3.04 msec, flip angle = 8°, FOV = 256 × 256 mm2, matrix size = 256 × 256, slice thickness = 1 mm, number of slices = 192, orientation of slices was sagittal. The entire session lasted approximately 1.5 hr (additional functional data for a separate project were acquired at the same time) and the DTI acquisition was performed after the fMRI acquisition without repositioning the subject.
Diffusion Tensor Computation
Computation of the diffusion tensor and fiber tracking was performed using DTIstudio, a software package developed using C++, designed to operate on a Microsoft Windows platform (Jiang, van Zijl, Kim, Pearlson, & Mori, 2006). DTIstudio calculates the diffusion tensors by solving an overdetermined linear equation system using least square fitting. By diagonalizing the diffusion tensor for each voxel, the program generates as output the six components of a diffusion tensor (Dxx, Dyy, Dzz, Dxy, Dxz, and Dyz), three eigenvectors that characterize the direction of diffusion and three eigenvalues that characterize the magnitude of the diffusion in the corresponding eigenvector. Because long-range fibers can be prematurely terminated (increase in noise as fiber propagation gets longer) (Lori et al., 2002), a tensor smoothing algorithm (Westin et al., 2002) was employed before fiber tracking as this is known to reduce residual errors and increase group differences (Jones, Symms, Cercignani, & Howard, 2005). From the diffusion tensor, the program also generates fractional anisotropy (FA) values for each pixel. The FA ranges from a scale of 0 to 1, where 1 reflects extremely anisotropic, that is, linear diffusion and 0 reflects isotropic diffusion. Gray matter and white matter are known to have distinctly different diffusion characteristics, with the former having a maximum FA <0.20 and the latter, a maximum FA <0.80. An FA threshold of 0.20 has been proposed as appropriate for segmenting gray from white matter (Cercignania, Bozzalia, Iannuccia, Comib, & Filippia, 2001).
Fiber Tracking Computation
Fiber tracking was initiated by specifying three parameters: the minimum FA threshold for starting tracking, the minimum FA for stopping tracking, and the critical angle threshold for stopping tracking in case the algorithm encounters a sharp turn in the fiber direction. DTIstudio uses these parameters to generate three-dimensional fiber tracts using the Fiber Assignment by Continuous Tracking (FACT) algorithm and a brute-force reconstruction approach, which uses all the pixels in the entire volume as “seed” pixels to generate the fibers (Mori, Crain, Chacko, & van Zijl, 1999; Xue, van Zijl, Crain, Solaiyappan, & Mori, 1999). The coordinates of all the fibers generated in an individual brain are stored in an index matrix. By drawing a region of interest (ROI) in a user-defined region of the brain, the fibers in the region are regenerated from the index matrix and quantitative measures of the extracted fibers, such as the mean FA of the tracts, are made available.
Definition of ROIs
A multiple ROI approach was employed to visualize and measure the fibers within the fasciculi of interest as this approach has been known to ensure robust recovery of the major fiber tracts in the human brain (Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori, 2004). The multiple ROI approach essentially consists of three ROI-based Boolean operations: OR (union), AND (intersection), and NOT (exclusion). Between two ROIs (ROI-1 and ROI-2), the OR operation generates fibers that pass through either ROI-1 or ROI-2; the AND operation includes fibers that are common to ROI-1 and ROI-2; and the NOT operation retains the fibers passing through ROI-1 while removing fibers penetrating ROI-2 (Mori & van Zijl, 2002). The multiple ROI approach, in conjunction with brute-force fiber reconstruction, is less susceptible to noise and partial volume effects, and has been found to increase the reliability of DTI-based fiber tracking (Huang, Zhang, van Zijl, & Mori, 2004).
As shown in Figure 1, we demarcated three ROIs along the mid-sagittal plane in native space for each individual. The fiber tracts in the ILF and IFOF were extracted by specifying two ROIs: a source ROI (ROI-1) and a target ROI (ROI-2), and the two ROIs were also reversed such that ROI-2 was considered as the source ROI and ROI-1 as the target ROI in order to ensure maximum recovery of the fibers within each fasciculus. To delineate these ROIs accurately, we coregistered and resliced the high-resolution T1-weighted images with the b = 0 images of the diffusion-weighted images using SPM2 (Wellcome Department of Cognitive Neurology, London, UK) and an in-house Matlab program. The coregistered dicom images were imported to the DTI studio image viewer and demarcation of the user-defined ROIs was performed on these images. All fiber tracking was performed in native space. Specific fiber-tracking protocols were created to ensure consistency in drawing the source and target ROIs across subjects. The two fasciculi of interest, the ILF and the IFOF, were extracted by one of the authors (C.T.) who has had considerable experience and training in tractography using the prescribed protocol (see Figure 1).
Inferior fronto-occipito fasciculus (IFOF)
For each hemisphere, ROI-1 was defined as the ventral aspect of the occipito-temporal cortex (VOTC) inferior to the floor of the posterior horn of the lateral ventricles on the first coronal slice posterior to the splenium of the corpus callosum. Because the IFOF shares a similar fiber trajectory with the ILF until the floor of the external capsule, to avoid inclusion of the ILF fibers, ROI-2 was defined as the region of the frontal cortex on the first coronal slice posterior to the tip of the rostrum of the corpus callosum in each hemisphere. Thus, the IFOF was defined as the set of long-range fibers between the posterior VOTC region and the entire frontal cortex of each hemisphere. After the IFOF fibers were extracted, they were removed from the analysis using the NOT operation to prevent inclusion of the fibers during extraction of the ILF fibers, given that the two tracts share similar trajectories at the level of the ventral occipito-temporal cortex.
Inferior longitudinal fasciculus (ILF)
The ROI-1 was the same for extraction of ILF fibers. To avoid including the optic radiation from the lateral geniculate nucleus of the thalamus, and, considering the fact that the ILF extends to the anterior temporal cortex with lateral and medial branches, ROI-2 was defined as the anterior region of the temporal cortex on the coronal slice coinciding with the point where the fornix descends towards the mammillary bodies. In other words, the ILF for each hemisphere was defined as the set of longrange fibers between the VOTC region and the entire anterior temporal cortex.
Dependent Measures
To explore the status of the white matter tracts as a function of age, we obtained measurements of the number of fibers,1 number of voxels through which the fibers pass (index of volume), and the average FA of the ILF and IFOF. Given that we expected a decrease in the number of fibers and voxels across the entire brain as a function of age, we normalized the values obtained from the specific fiber tracts by dividing them by the whole-brain measures and expressing them as percentages. Measurements were obtained from each hemisphere separately. Whereas the number of fibers and the number of voxels reflect gross measurements of a tract, FA reflects the microstructural properties of the axons within a voxel. Because one of the parameters required for extraction of a tract is FA in a voxel, density and volume of tracts are likely to be correlated with decrease in FA value. However, FA need not always correlate with gross fiber measures. For this and other reasons, the exact dependent measures one should use remain controversial and so we report both number and volume of fibers, as well as FA. Such measures, as well as measures of anisotropy, are all potentially revealing of the integrity of the fiber tracts and, in particular, when used in tandem, are likely to be more reliable and illuminating (Alexander & Lobaugh, 2007). Indeed, if by all three measures the pattern in the reduction of the macro- and microstructural properties of a tract is consistent, this further attests to the reliability of the finding.
Statistical Analysis
A stepwise regression analysis was performed on each fasciculus (ILF and IFOF) separately for each hemisphere with percentage fibers, percentage voxels, and FA value as the dependent measures and age, gender, and handedness as possible factors. The criterion for entry into the stepwise regression was set to p < .05. Age, gender, and handedness were all entered as factors in a stepwise regression for each dependent measure separately and the results are considered first for whole-brain measures and then for percent fibers, percent voxels, and then average anisotropy, in turn, for each of the two tracts in each hemisphere. As will be evident from the results, handedness does not play a significant role in any analyses and gender only plays a rather minimal role.
Behavioral Paradigm
To explore the presence of an age-related decline in perceptual performance, participants viewed two face images, presented side-by-side on a computer screen simultaneously for same/different discrimination. In order to determine whether any age-related changes were specific to faces, in a blocked design, faces and cars were both presented (see Figure 2). The faces could be either the same (25% of trials; n = 55) or different (75%; n = 165). The different trials could be from easy, medium, or difficult conditions (n = 55 in each of these levels of difficulty). The easy condition consisted of a picture of two different faces (say Face A and Face B). For the medium and difficult trials, the two faces (say Face A and Face B) were morphed together using the MorphMan 4.0 software. For the medium condition, Face A was presented with a morph that comprised 33% of Face A and 66% of Face B, whereas in the difficult condition, Face A was presented with a morph that comprised 66% of Face A and 33% of Face B. Each stimulus was roughly 2 × 3 in. (aspect ratio switched for cars and faces as faces vertically longer and cars horizontally longer). The midpoint of each stimulus was located 5.2 in. from the fixation point and subjects viewed the display at approximately 50 cm. The identical experiment was run using cars as the stimuli. The order of experiment (car, faces) was counterbalanced across participants.
Figure 2.
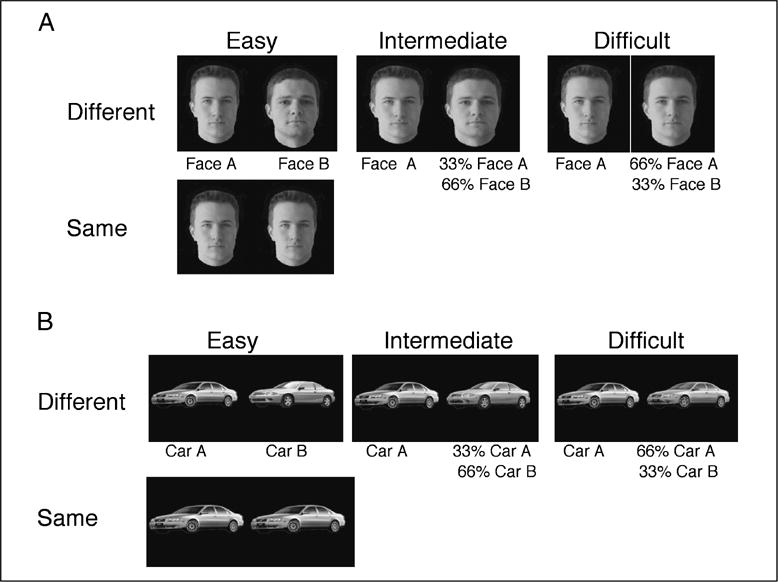
Examples of stimuli used in the (A) face and (B) car behavioral experiments. For each stimulus set, one quarter of the pairs of images were identical and required a “same” response, whereas the remaining three-quarters were different and required a “different” response. The different pairs could be easily discriminable (two entirely different faces or cars), intermediate in difficulty of discrimination (one face or car morphed with 66% of an entirely different image) or difficult to discriminate (one face or car morphed with an image containing 66% of itself and 33% of a different image).
Procedure and Analysis
Participants were informed that, on each trial, two faces or two cars would appear simultaneously on either side of a red fixation dot, which occupied the center of the screen. Participants were told to fixate on the dot and to decide whether the two faces were exactly the same or different in any way. If the pictures were different, participants were to press the “D” key on the keyboard, and if they were the same, they were to press the “S” key. The order of the trials was randomized within a block. Faces and cars were presented in separate blocks and the order of the blocks was counterbalanced across participants. Each display was presented for an unlimited exposure duration so as not to penalize the older participants with brief exposure duration. Also, given that reaction time scales with age, we chose to examine accuracy of performance rather than reaction time, and participants were given unlimited time to make their response. Participants were told to respond as accurately as possible.
RESULTS
White Matter Connectivity in the Ventral Visual Cortex and Aging
The question to be addressed at the outset is whether the documented age-related changes in white matter as a function of age are apparent in the whole brain. As evident from Figure 3, there is a statistically significant decline in the number of fibers and the number of voxels through which these fibers pass in the whole brain as a function of age. Age accounted for 39.6% and 37.1% of the variance for each measure, respectively, and gender contributed an additional 10% to 12% with men having both more fibers and more voxels (58,463 fibers and 82,826 voxels) than women (47,108 fibers and 67,090 voxels), with the difference likely attributable to that of greater head and brain size in men (not corrected for body size). For neither measure (nor for any subsequent measure) was handedness a statistically relevant factor and it is not included in these or any subsequent regression analyses (this is not at all surprising as only two individuals are left-handed). These findings replicate the previous data revealing a reduction in white matter volume with age (Marner et al., 2003).
Figure 3.
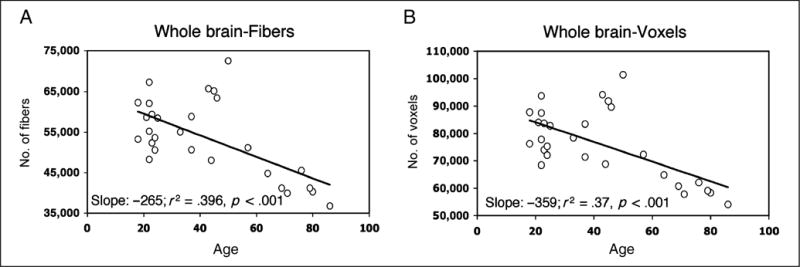
Number of (A) fibers and (B) voxels through which fibers pass, calculated across the whole brain for each participant, plotted as a function of age. The slope, variance accounted for, and significance levels are shown for each graph.
Of greater interest for the current study is whether there are specific age-related differences in the fiber tracts passing through the fusiform gyrus. To examine this, we assessed the effects of age on the percentage of fibers, percentage of voxels, and mean FA for the IFOF and ILF in the right and left hemispheres, respectively. We also included gender as a factor in the regression so as to parcel out its contribution but the major factor of interest is age. Figure 4 displays the percentage of fibers (normalized across all the number of fibers in the whole brain) for the IFOF and ILF in each hemisphere. As is evident from this figure, age accounts for a significant amount of the variance (25.5%; p < .02) in the percentage of fibers in the right IFOF and gender does not account for any additional variance. Neither age nor gender account for a significant amount of the variance in the percentage of fibers in the left IFOF or in the ILF in either hemisphere. To examine whether the slope describing age versus percentage fibers in the right IFOF differs from the slopes for the left IFOF and the right and left ILF, we computed a confidence interval around the right IFOF slope (with alpha .05; .00776 and −.00456 for upper and lower limits), adopting a procedure outlined to compare multiple slope values using the standard error of estimation from the regression equation (Chang, Amesur, Klatzky, Zajko, & Stetten, 2006). The other three slopes all fall outside of the confidence interval (left ILF is just marginal), attesting to the specificity of the relationship between the decrease in fibers in the right IFOF and aging.
Figure 4.
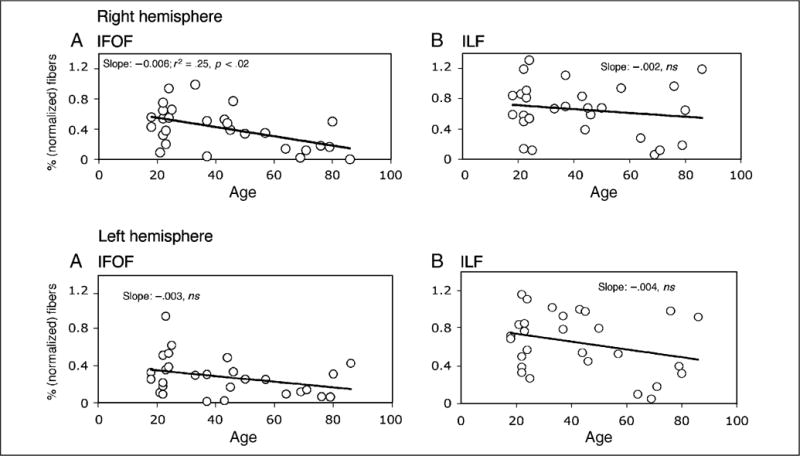
The normalized percentage (as a function of whole brain) of fibers extracted from the right (upper panels) and left (lower panels) hemisphere for the IFOF (left) and ILF (right) as a function of age. As shown by the slope, it is only in the right IFOF where age accounts for a significant amount of the variance.
The pattern of decreasing fibers in the right hemisphere IFOF as a function of age is clearly apparent in Figure 5, which shows an axial and coronal slice of the fiber tracings of the right and left IFOF for four participants selected to represent a range of ages. As evident from the figure, there is an obvious decrement in the number of fibers in the oldest of the four individuals (aged 69 years old) compared with the intermediate participants (aged 33 and 44 years old). These intermediate aged individuals also show a decrement relative to the younger participant (aged 24 years old). The age-related decrement across all four individuals is disproportionately apparent in the right hemisphere (see also the fibers shown in Figure 1 for a 22-year-old participant).
Figure 5.
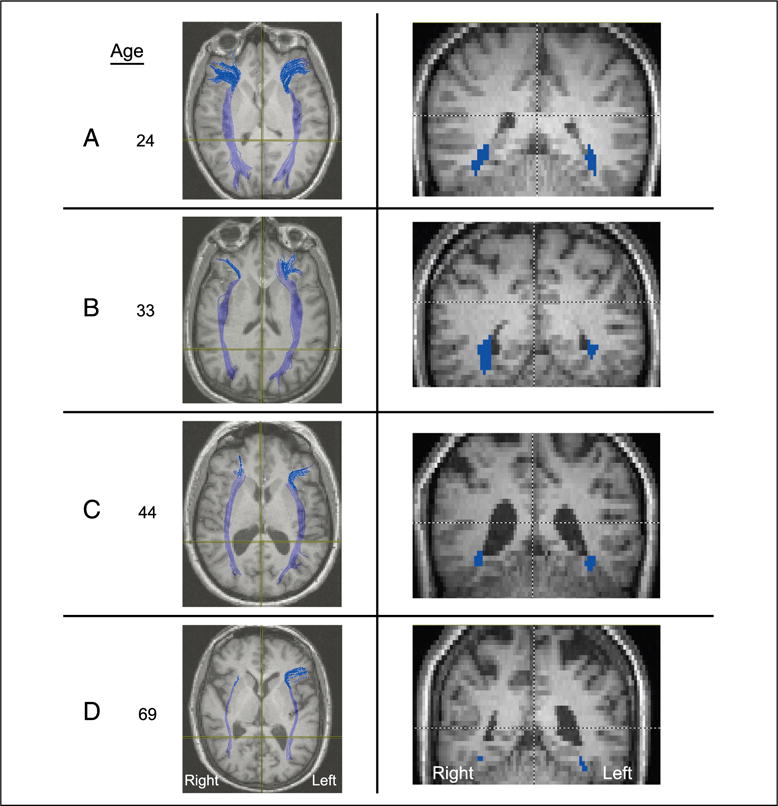
Representative axial (left) and coronal (right) slices taken from four participants, aged 24, 33, 44, and 69 years, revealing the loss of fibers as a function of increasing age, especially in the right hemisphere. Note that radiological convention is adopted with the right hemisphere depicted on the left of the image and vice versa.
As expected, the same analysis but now using the percentage of voxels through which the fibers pass as the dependent measure (see Figure 6) reveals similar findings with respect to age. Only in the right IFOF (21.9%) and in no other tract does age account for a significant amount of the variance (see slopes and significance value on the figure). It is also the case that no other slope falls within the 95% confidence intervals set around the right IFOF slope (with alpha .05; −.01125 and −.01155 for upper and lower limits). These findings provide a replication and additional support for the observation of age-related decline in the right IFOF, as obtained above from measuring the number of fibers.
Figure 6.
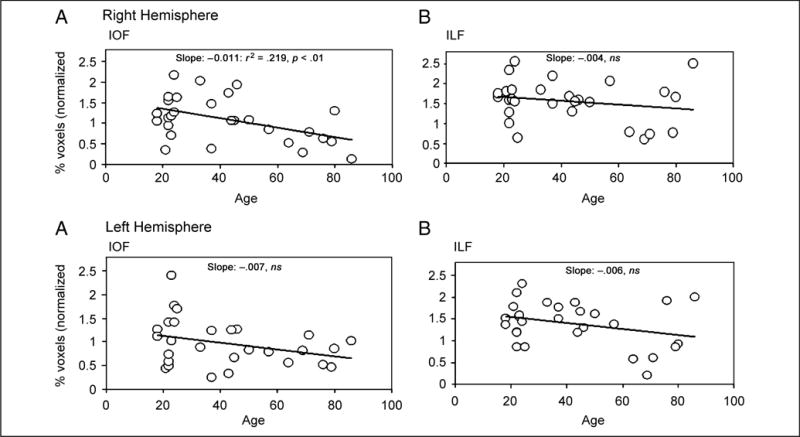
The normalized percentage (as a function of whole brain) of voxels extracted from the right (upper panels) and left (lower panels) hemisphere for the IFOF (left) and ILF (right) as a function of age. As shown by the slope, it is only the right IFOF for which age accounts for a significant amount of the variance.
With regard to FA (see Figure 7), all tracts show significant age-related decrease in anisotropy, with the exception of the right ILF. For the right ILF, the amount of variance accounted for by the conjunction of age and gender is less than 10% and neither of these factors in isolation nor pairwise is significant. In contrast, age alone accounts for 33% of the variance (p < .0001) in the right IFOF and gender does not contribute additionally. Age also accounts for a significant amount of the variance in the anisotropy of the left IFOF (30.1%; p < .001) and of the left ILF (48.7%; p < .0001), whereas gender does not contribute significantly in either case. There are no differences in the FA slopes between that of the left IFOF and of the left ILF (as revealed by projecting confidence intervals around the left IFOF slope). It is the case, however, that neither of these left tract slopes nor the right ILF slope falls within the confidence interval of the right IFOF.
Figure 7.
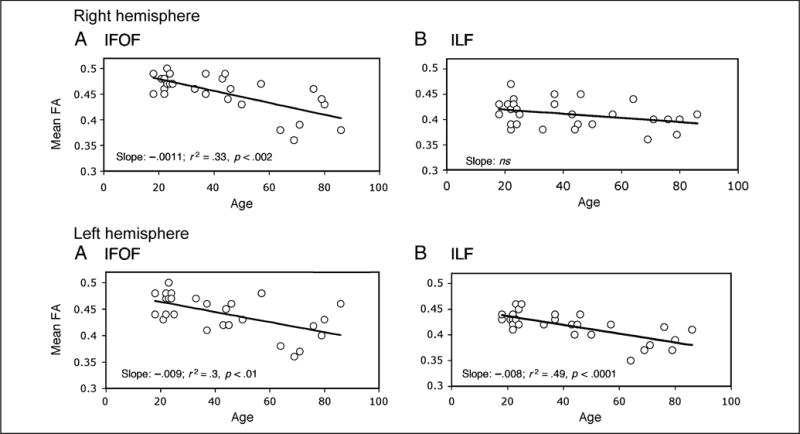
The fractional anisotropy (FA) values extracted from the right (upper panels) and left (lower panels) hemisphere for the IFOF (left) and ILF (right) as a function of age. As shown by the slope, age accounts for a significant amount of the variance for the right IFOF and left ILF and IFOF.
These findings suggest that, notwithstanding the generalized age-related microstructural changes in brain morphology, as revealed through the significant reduction in connectivity in the whole brain, the pattern of white matter connectivity in the ventral temporal cortex shows a more specific profile of alteration. It is not the case that all tracts are equally affected and that both hemispheres are equally affected by age. The tracts in the left hemisphere show minimal, if any, age-related reduction in the number of fibers or their volume, but there is an alteration in the microstructural integrity of the tracts, as revealed by the decrement in FA. Note also that the slopes that describe the relationship between FA values in the two left hemisphere tracts are not different from one another. In the right hemisphere, the IFOF shows a reduction in the percentage of fibers and the percentage of volume of fibers as well as a significant decrease in FA, as a function of age, whereas the ILF on the right shows no obvious age-related alteration on any of the dependent measures in excess of that predicted by whole-brain changes. Importantly, too, when one sets confidence intervals around the slope derived for the right IFOF, there is no other tract that shares the same relationship between reduction in fibers, voxels, or FA and aging. The major finding, then, is that it is the IFOF in the right hemisphere that shows particular age-related vulnerability, although there is a tendency for the tracts in the left hemisphere to show some reduction in microstructural integrity, as revealed in FA values (albeit not to quite the same extent) but not in the volume or number of fibers in the tract.
The hemispheric asymmetry in tract integrity is further confirmed by a paired t test directly comparing the right and left IFOF using the percentage of fibers first, then using the percentage of voxels and then using FA value. In both cases, there are significantly fewer fibers and less volume in the right than left IFOF (% fibers: t = 2.4, p < .02; % voxels: t = 1.9, marginal p < .06; FA: t = 2.04, p = .05). The asymmetry between the hemispheres is of great interest in that the right hemisphere is considered to play a more prominent role in face processing than the left (Kanwisher et al., 1997; Sergent, Ohta, & MacDonald, 1992; Meadows, 1974). That we see a greater structural change in the right hemisphere is consistent with the possibility that the white matter changes are associated with an age-related decline in face processing. Before considering the exact contribution of the right IFOF to face processing, we need to examine the functional consequences of the apparent white matter reduction. To do so, in the next section, we examine the behavioral profile as a function of age and then explore whether there is any correlation between the DTI findings and task performance.
Behavioral Analysis of Face Processing
To examine the cognitive profile of face (and car) discrimination as a function of age, we collected behavioral data from a subset of our participants, roughly drawn from four distinct decades spanning the age range of the larger sample. The error data from this experiment were subjected to a repeated measures analysis of variance with age as a between-subjects variable and stimulus type (faces, cars) and level of difficulty (easy, medium, difficult) as within-subjects factors. “Same” trials were excluded from the analysis as performance was uniformly accurate for all age groups in the “same” trials. As shown in Figure 8, there is a significant main effect of age [between-subjects factor: F(3, 18) = 3.88, p < .05], with poorer performance emerging with increasing age. There is also a main effect of difficulty [F(2, 6) = 255.4, p < .0001], with best performance on easy then medium and then difficult trials but no main effect of stimulus type [F(1, 3) = .54, p > .5]. There is also an interaction of Stimulus × Difficulty [F(2, 6) = 5.85, p < .01], with more errors on the difficult condition for faces than for cars. Of particular interest, however, is the three-way interaction between age, level of difficulty, and stimulus type [F(6, 36) = 3.01, p = .057]. No other effects were significant. Post hoc t tests (with Bonferroni correction) revealed a significant difference between easy and medium car trials, but not face trials, for the 60-year-olds.
Figure 8.
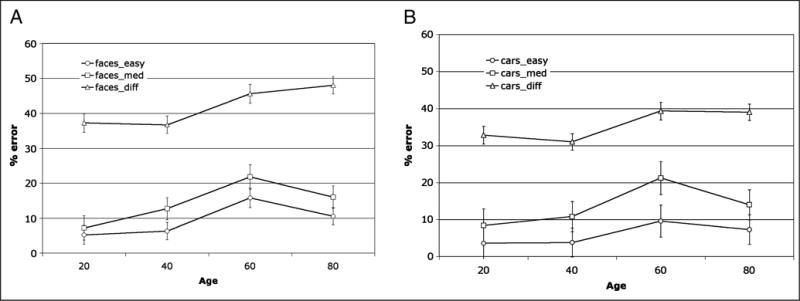
Mean percent error for performance on (A) faces and (B) car discrimination experiment for the different trials only (shown for easy, medium, and difficult trials) as a function of age. Note the three-way interaction between level of difficulty, age group, and stimulus type with the difficult face discrimination giving rise to more errors in the 60- and 80-year-olds than for any other condition or age group.
All age groups perform substantially more poorly on the difficult trials than on any of the other conditions, and this is so to a greater extent for the faces than for the cars. Of relevance though is that the 60- and 80-year-olds make significantly more errors on faces than they do on cars, and performance is almost at chance in the difficult condition (faces: 60-year-olds: 46.5% errors; 80-year-olds: 48% errors). Note that performance for the older individuals is not hampered by time constraints as the pair of stimuli was shown for an unlimited exposure duration and because accuracy served as the dependent measure, participants were not under time pressure to respond.
Relationship between DTI and Behavior
We have established that there is an age-dependent reduction in white matter connectivity in the tracts that run through the fusiform gyrus in the ventral visual cortex, especially evident in the right IFOF but somewhat evident in the left hemisphere tracts, too, as revealed by the decrease in FA values. We also observe an age-related decrement in perceptual processing of visual stimuli in these individuals, to a greater extent for faces than for cars. The remaining issue is whether there is a direct relationship between these two findings, the behavioral, on the one hand, and the DTI measures, on the other. To explore the association between the tract measurements and the behavioral data, we examined correlations between behavioral performance and the normalized percentage of fibers, normalized percentage of voxels and average FA values in the ILF and IFOF in each hemisphere. The dependent measure for behavior was accuracy, and these correlations were done separately for faces and for cars, at all levels of difficulty. To further evaluate the specificity of any significant correlations, we also examined the relationship between behavior, whole-brain fiber and volume measurements. Because so many correlational analyses are done (72 excluding whole brain), we have adopted a p < .001 as a significant threshold (where correlation exceeds ±0.5) and we report only those that survive the corrected threshold.2
Based on the behavior and DTI findings, thus far, we make the following predictions. For the right hemisphere, age accounts for a significant amount of the variance on all three dependent measures (% fibers, % voxels, and FA) for the IFOF but not for the ILF; hence, we expect a clear association between behavior and DTI measures for the right IFOF but not the ILF. Moreover, on the hypothesis that an age-related change in white matter connectivity is implicated in the decline in face perception with age, we expect the correlation between DTI measures of the right IFOF to be higher for face than for car performance. For the left hemisphere, only FA showed age-related changes, although these changes were evident in both the IFOF and the ILF. If these age-related changes contribute to the decline in face perception with age, mean FA in the left hemisphere will be correlated with face performance but not with car performance. However, we do not expect the left hemisphere correlations to be significant, based on previous evidence implicating the right hemisphere to a greater degree in face perception than the left hemisphere.
Interestingly, for faces, there are two separate findings, both of which reveal negative associations but one coming from performance on easy faces and one from performance on difficult faces. On the easy face trials, the negative association is such that the greater the percentage of fibers in the right IFOF, the lower the error rate in these easy discriminations. The same pattern holds for percentage of voxels, but not for the FA, in the right IFOF. On the difficult face trials, the negative association is such that the lower the right IFOF FA and the percent voxels (but not the percent fibers), the greater the error rate on the difficult discriminations. There are no significant correlations with performance on the medium face trials. These findings indicate that to the extent that there is residual integrity of the right IFOF, so behavior will be better preserved.
For cars, there is a negative association in both the right and left IFOF only on the FA values on the easy car trials and for the right IFOF FA on the medium and difficult car trials, too. These findings seem somewhat nonspecific and perhaps difficult to interpret with only the FA reductions in both hemispheres associated with lower performance. No other associations with performance on car trials is apparent.
Taken together, the clearest finding from the behavioral–DTI correlations is that there is a clear association between the ability to discriminate between faces and the macro- and microstructural integrity of the IFOF in the RH. There may be some other associations in the other tracts as well, but the findings are inconsistent and unclear (perhaps because of the relatively small sample) and further investigation is needed to explore these associations more fully. We also note that these IFOF fibers are not necessarily exclusive for processing faces. The IFOF fibers traverse visual association areas other than the fusiform gyri (e.g.,, inferior and middle temporal gyri) and are likely involved in the processing of other visual stimuli in addition to faces (but perhaps with greater involvement for faces). This too remains to be fully explicated.
DISCUSSION
The goal of the current study is to explore whether changes in white matter and its structural connectivity might account for the apparent decline in face perception that accompanies aging. The use of white matter tractography to evaluate connectivity is currently enjoying much excitement in the neuroscience and neuroimaging community as it provides, for the first time, noninvasive measures for reconstructing structural trajectories in the human brain. This approach is particularly appealing in the study of age-related neural alterations given the changes in structural connectivity already known to be present in the aging brain (Pfefferbaum & Sullivan, 2003).
In this study, we acquired DTI data from a group of 28 individuals aged 18 to 86 and measured the number of fibers and the number of voxels through which the fibers pass as well as their average FA. Measurements were taken from the whole brain but also from each of the two major association tracts that pass through the pre-eminent face processing regions of the ventral visual cortex, the ILF, and the IFOF, in the left and right hemispheres separately (normalized to take into account any generalized changes across the whole brain). In addition, a subset of the participants completed perceptual tasks designed to assess their accuracy in making fine-grained discriminations between pairs of faces and between pairs of cars. Finally, to assess the relationship between the structural and behavioral changes observed, we performed a series of correlation analyses between the DTI measures and the error rate in car and in face discrimination.
The first major result of the current investigation is the replication of previously reported findings of reduction in the overall number and volume of white matter fibers as a function of age (Marner et al., 2003). The second major result, of greater interest for the current study, is the documentation of more specific age-related changes in white matter integrity, especially in the right hemisphere and, to a greater extent, in the tract that connects the posterior ventral cortex with regions of the frontal cortex, the IFOF, than in the ILF, which terminates in the anterior temporal lobe. Although tracts other than the right IFOF show some alteration on the DTI measures, age accounts for a disproportionate amount of the variance in explaining the reduction in white matter in the right IFOF on all of the DTI-dependent measures. It was also the case that the slope describing the association between DTI measures in the right IFOF and age was different from slopes derived for the right ILF, left ILF, and left IFOF, further attesting to the specific age-related vulnerability of this tract.
The behavioral findings reinforce the relative specificity of this fasciculus in accounting for the perceptual profile: Although there was a main effect of age in the perceptual discrimination of both faces and cars, exaggerated under more difficult perceptual conditions, the age-related decline was more apparent for faces than for cars. But, perhaps most importantly, this behavioral profile correlated highly with DTI structural measures derived from the right IFOF, although again there are also associations between behavioral profile and other fiber tracts, albeit not to the same extent and not with the same selectivity. The specificity of the behavioral–right IFOF relation is further revealed in that the correlation between the perception of faces and the structural measures did not hold to the same extent between another perceptual class of stimuli, the cars, and the right IFOF. In summary, there is an age-related change in white matter connectivity and this might be specifically implicated in the decline in face perception observed in older individuals via a reduction in integrity of the right IFOF.
At a general level, several recent studies have documented a decline in white matter integrity in aging using noninvasive imaging techniques such as DTI. One obvious candidate that might mediate this age-related change is myelin, known to undergo deterioration with age. Among the proposed myelin changes that occur with age are localized splitting of the myelin lamellae or cavitation of the cytoplasm and subsequent ballooning of the myelin sheath (for recent reviews, see Sullivan & Pfefferbaum, 2006; Wozniak & Lim, 2006; Peters, 2002b, 2002c). It is the case, however, that there are certain regions of the cortex which are more vulnerable to these myelin changes (Bartzokis et al., 2001), especially late-myelinating association regions and long-range tracts (Yakovlev & Lecours, 1967). The IFOF is a long-range tract and may indeed be more susceptible to structural alteration subsequent to myelin perturbations. Thus, although there may be whole-brain changes in myelin and in conduction velocity in the neural system, there may also be regions that are more vulnerable and the long-range tracts through the fusiform may be somewhat more susceptible to these changes.
The Face Network and Aging
At a more specific level, two immediate questions surface: (1) Why is there an age-related face perception decline when fusiform activation appears to be intact in functional imaging? and (2) Why does the right hemisphere IFOF play a central a role in the face perception decline? We address each in turn. In the few studies that have been done, fusiform activation in response to faces seems not to differ between younger and older observers (Grady et al., 2000). In light of this, the presence of a behavioral decline may seem surprising given the preeminent role in face processing played by the fusiform gyrus. There appears to be growing recognition, however, that activation of the fusiform gyrus per se may be insufficient for normal face processing. Surprisingly, the imaging studies show equivalent activation in younger and older individuals (Grady et al., 1994, 2000), but there is a host of behavioral data showing how older individuals perform more poorly than their younger counterparts on face tasks (Habak et al., in press; Boutet & Faubert, 2006). By the same token, imaging studies from individuals who are prosopagnosic, either congenitally (Avidan, Hasson, Malach, & Behrmann, 2005; Hasson, Avidan, Deouell, Bentin, & Malach, 2003) or as a result of an acquired lesion (Steeves et al., 2006), have reported normal fusiform activation for faces, as reflected in the site, extent, and amplitude of activation. In the study by Avidan et al., the normal blood oxygenation level-dependent signal is acquired at the very same time that the individual’s face processing performance is significantly impaired, relative to matched controls. The conclusion from these studies, increasingly supported by theoretical accounts that favor more distributed networks mediating face perception (Gobbini & Haxby, 2006; Ishai et al., 2005; Rossion et al., 2003; Haxby, Petit, Ungerleider, & Courtney, 2000), is that normal face processing requires the integrated function of multiple regions, including “core” areas such as the fusiform gyrus and superior temporal sulcus, but also more far-flung or extended regions such as the frontal cortex. The IFOF, then, especially in the right hemisphere, may play a crucial role in propagating signals from posterior regions of the ventral cortex to other parts of the distributed network and into the frontal cortex.
The reason that the right IFOF might be so central is that it traverses the fusiform gyrus and runs the posterior–anterior extent of the cortex all the way to the frontal cortex, and there is growing recognition from a host of different perspectives that the frontal cortex is implicated in various aspects of face processing (Kranz & Ishai, 2006). For example, several studies have shown selective frontal (and prefrontal) cortical activation when individuals perform working memory tasks with faces (Druzgal & D’Esposito, 2003; Haxby et al., 2000). Other studies have demonstrated involvement of the frontal cortex in the perceptual analysis of faces: One relevant current fMRI study shows that when subjects make decisions based on second-order relations of faces (when pairs of faces have identical features but the spacing between the features differs compared with faces whose features themselves differ) in order to decode the identity of faces, an extensive set of regions in the right frontal cortex is activated (Maurer et al., 2007). Yet other studies have implicated the right frontal cortex in discriminating one’s self from familiar faces (Platek et al., 2006). Finally, other recent research has shown that ventral/orbital frontal areas modulate activation in the fusiform gyrus as perceptual difficulty increases, further indicating its critical coupling with regions of the ventral visual cortex, not just in a feedforward fashion but in a feedback fashion, too, possibly for predictive purposes (Rolls, 2007; Bar et al., 2006; Summerfield et al., 2006). Summerfield et al. (2006) documented face-selective signals in the medial frontal cortex and suggested that perceptual decisions, including those about faces, involve a top–down signal from the frontal cortex to face-sensitive visual areas to assist in resolving perceptual ambiguity early and to match predicted and observed evidence for the presence of faces. Although the exact contribution of the frontal cortex is somewhat unclear, taken together, these studies underscore not just the connectivity of the face network more generally, but the importance of connections from more posterior regions to more frontal regions and vice versa for the purpose of face processing.
Given the involvement of the frontal cortex in face processing demonstrated by the studies reviewed above, a reduction in connectivity as a function of aging would have adverse consequences for all of the above processes. Surprisingly, however, we note that some imaging studies have reported increased, rather than decreased, prefrontal activation in older observers. A possible interpretation of this is that as the task becomes more difficult, increased frontal recruitment to boost cognitive resources is observed (as Grady et al., 2000, 2002, argued in their studies). This increase in frontal activation has also been documented in an fMRI study in individuals with congenital prosopagnosia (Avidan et al., 2005). It is also possible, of course, that under conditions of difficulty, there might be an attempt to predict what the input is, thus exploiting a greater top-down signal to resolve the ambiguous input—the greater frontal activity might then reflect this enhanced top-down signal. Clearly, future research is required to elucidate the contribution of the frontal cortex (and the subregions of the frontal cortex) to face processing and the distributed neural circuit. Interestingly, the IFOF is a fiber system that is unique to humans, as revealed in direct comparisons between human and macaque white matter tracts (Schmahmann & Pandya, 2006), and this leads to the obvious prediction that age effects such as those reported here would only be evident in humans and not in other primates. This claim too remains to be verified.
Suffice it to say, then, at this stage, that the disruption in connectivity in a long tract such as the IFOF can give rise to a “disconnection” of widely distributed neural networks, such as the one postulated to subserve face processing, as well as other cognitive functions (for similar finding in domain of memory function, see Persson et al., 2006). This approach in which the relationships between specific tracts and behavioral differences are assessed is becoming increasingly informative in investigations of cognitive alterations; as a further illustrative example, the relationship between anisotropy and reading (dis)ability in children has been well evaluated and mapped out (Beaulieu et al., 2005; Klingberg et al., 2000).
Before concluding, a number of caveats are warranted. The first of these concerns the ongoing debate and controversy regarding the anatomy of the fiber tracts passing through the occipito-temporal cortex. The debate concerns the ILF to a greater degree than the IFOF and the extent to which the ILF represents direct projections between the occipital and anterior temporal cortex or whether the connections between the two regions are entirely indirect, conveyed by the occipito-temporal projection system—a chain of U-shaped association fibers (Catani et al., 2003; Tusa & Ungerleider, 1985). This issue is perhaps less critical for the present investigation given the disproportionate involvement of the IFOF rather than the ILF, and it may also be the case that whether the projections are direct or indirect, this may not alter the fundamental claims regarding the disruption in connectivity in aging.
A further issue concerns the claim that it is the IFOF that is more affected than the ILF. As a fiber tract increases in length, noise errors may accumulate, resulting in poorer extraction of fibers. The ILF is shorter than the IFOF and so the IFOF may be more susceptible to disruption purely because of technical or artifactual reasons in extracting the fibers. Although it would be important in future studies to examine the integrity of other longrange fibers, such as the superior fronto-occipital fasciculus (SFOF), to discount any artifactual reasons for the particular vulnerabilities of long-range fibers, in the current study, it is predominantly the right IFOF that is affected. Were all long-range fibers particularly vulnerable, we might not have observed an asymmetry in the left versus right IFOF. Nevertheless, although the data appear to be robust, further verification and replication of the findings are clearly necessary.
Another issue that deserves consideration is also one of the technical limitations associated with tractography. The disclaimers regarding the pitfalls of tractography are well known but are worth stating again. One of the pitfalls concerns the overinterpretation of the actual values yielded by the tractography—absolute values may not be that meaningful and may be dependent on the particular measure and method used. Thus, although we do not make any direct claims about the meaning of the FA values or the numbers of fibers and voxels, we place our emphasis on the group differences rather than on the absolute values. It is also the case that the disruption uncovered by the tractography method could arise for a host of reasons including narrowing of the tract, partial narrowing, perturbation by crossing fibers, and lower anisotropy (Mori, personal communication). That we see less affected fibers in the left than in right hemisphere, especially in the IFOF, somewhat offsets general concerns about limitations in method but, again, caution must be exercised in interpretation until further replications of these (and other related findings) are in hand.
The final issue concerns the notions that there is a reduction in myelination as a function of age, an assumption we have made in this article. Some DTI studies have shown that myelin itself is not the essential component for anisotropic diffusion in fiber tracts. Although myelin is clearly critical, the axons themselves may undergo change as a function of age rather than simple changes in the myelin itself (Beaulieu, 2002). Whether it is myelin per se or the axonal geometry that is modified with age is not central to our claim; rather, it is the notion that the structural integrity of the fibers that link disparate cortical regions may be altered with age and that this may mediate neurocognitive decline in processes such as face perception. The major claim is that cognitive decline may well be associated with structural as well as functional disruption, with the former perhaps being primary and the latter (such as increased activation in different cortical regions in older and younger individuals) as a response to the disrupted network connectivity.
Conclusion
There has been growing interest in exploring the associations between the integrity of the white matter and the pattern of age-related cognitive decline. The findings from these investigations have been modest, sometimes fragile, and not always consistent (Raz, Williamson, Gunning-Dixon, Head, & Acker, 2000). Most of these studies, however, have focused on white matter hyperintensities or volume. With the adoption of noninvasive methods, such as diffusion tensor imaging, we have had the opportunity to explore the structural–behavioral relations with new precision. These DTI studies have revealed more specific abnormalities in white matter deterioration and the ways in which these changes may be associated with fairly specific changes in cognitive competence. In this article, we report a clear association between a decrement in white matter integrity in the right inferior fronto-occipital fasciculus and demonstrate that this decrement is significantly correlated with a decrease in accuracy in face discrimination. Although there are some other less obvious changes in other tracts and there are other alterations in perceptual performance, too, as shown on a car discrimination task, the relationship between the right IFOF and the profile of face perception decline stands strong and robust across multiple measures. We note that the right IFOF degradation is not necessarily sufficient or even causal for the age-related decrement in face processing. Correlation is clearly not causation and further investigations are needed to uncover the nature of the association between the behavioral decrement and the age-related changes in DTI measures.
Acknowledgments
We thank Drs. S. Mori and H. Jiang of Johns Hopkins University who provided advice and guidance at various stages of this project regarding the development of the DTI protocol and method of analysis. This study was funded by grants from the National Institutes of Mental Health (MH54246) to M. B. and by awards from the National Alliance of Autism Research to C. T. and K. J. H. and from the Cure Autism Now foundation to K. J. H. We thank Scott Kurdilla and Debbie Viszlay of the Brain Imaging Research Center for their help in the acquisition of the imaging data and Stephanie Manchin and Grace Lee Leonard for help preparing the stimuli. We also thank Dr. Carol Barnes who contributed to some of the initial discussions that prompted this research. Faces were used from the AR database (A. M. Martinez and R. Benavente, “The AR face database,” CVC Tech. Report #24, 1998) and we thank the Computer Vision Laboratory, Faculty of Computer and Information Science, University of Ljubljana, Slovenia and the Secondary School Center, Velenje, Slovenia, for allowing us to use the CVL face Database (www.lrv.fri.uni-lj.sl/facedb.html).
Footnotes
The absolute number of axonal fibers in a voxel cannot be determined at the current resolution of DTI. The voxel size in our acquisitions is 3 mm and in a cube of such dimensions, there will be several axons. However, the fiber value generated by the tractography algorithm is a mathematical abstraction of the density and macrostructural properties of a tract of interest in an individual. In DTIstudio, the fiber-tracking algorithm performs a minimum fiber length testing, which is set at 8 pixels in order to remove extremely short tracts from further consideration (Jiang, personal communication). The implication of this is that if a line can be propagated through eight contiguous voxels that have an FA value >0.20 and the angle of principle eigenvector of the neighboring voxel is <40 degrees, the set of pixels is considered as one fiber.
A full Bonferroni with multiple correction yields an alpha for each test of .000712153. With so few subjects and data points, it is very difficult to meet this criterion so we have adopted a conservative threshold at p < .001, but it is not fully adjusted for the family-wise correction.
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lobaugh NJ. Insights into brain connectivity using quantitative MRI measures of white matter. In: Jirasa V, McIntosh AR, editors. Handbook of brain connectivity. Berlin: Springer-Verlag; 2007. pp. 221–271. [Google Scholar]
- Avidan G, Hasson U, Malach R, Behrmann M. Detailed exploration of face-related processing in congenital prosopagnosia: 2. Functional neuroimaging findings. Journal of Cognitive Neuroscience. 2005;17:1150–1167. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, et al. Top-down facilitation of visual recognition. Proceedings of the National Academy of Sciences, USA. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Archives of General Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance Bulletin. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR in Biomedicine. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, et al. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Gao F, Black S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cerebral Cortex. 2007;17:2354–2363. doi: 10.1093/cercor/bhl144. [DOI] [PubMed] [Google Scholar]
- Boutet I, Faubert J. Recognition of faces and complex objects in younger and older adults. Memory & Cognition. 2006:854–864. doi: 10.3758/bf03193432. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Cercignania M, Bozzalia M, Iannuccia G, Comib G, Filippia M. Magnetisation transfer ratio and mean diffusivity of normal appearing white and grey matter from patients with multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry. 2001;70:311–317. doi: 10.1136/jnnp.70.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaby L, Jemel B, George N, Renault B, Fiori N. An ERP study of famous face incongruity detection in middle age. Brain and Cognition. 2001;45:357–377. doi: 10.1006/brcg.2000.1272. [DOI] [PubMed] [Google Scholar]
- Chang WM, Amesur NB, Klatzky RL, Zajko AB, Stetten GD. Vascular access: Comparison of US guidance with the sonic flashlight and conventional us in phantoms. Radiology. 2006;241:771–779. doi: 10.1148/radiol.2413051595. [DOI] [PubMed] [Google Scholar]
- Crook TH, Larrabee GJ. Changes in face recognition memory across the adult life span. Journal of Gerontology. 1992;47:138–141. doi: 10.1093/geronj/47.3.p138. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ, D’Esposito M. Dissecting contributions of prefrontal cortex and fusiform face area to face working memory. Journal of Cognitive Neuroscience. 2003;15:771–784. doi: 10.1162/089892903322370708. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Filley CM. The behavioral neurology of cerebral white matter. Neurology. 1998;50:1535–1540. doi: 10.1212/wnl.50.6.1535. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2006;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Rapoport SI. Age-related changes in the neural correlates of degraded and non-degraded face processing. Cognitive Neuropsychology. 2000;17:165–186. doi: 10.1080/026432900380553. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K. The functional organization of the ventral visual pathway and its relationship to object recognition. In: Kanwisher N, Duncan J, editors. Attention and performance: Functional brain imaging of visual cognition. London: Oxford University Press; 2003. [Google Scholar]
- Habak C, Wilkinson F, Wilson HR. Aging disrupts the neural transformation that link facial identity across views. Vision Research. doi: 10.1016/j.visres.2007.10.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Avidan G, Deouell LY, Bentin S, Malach R. Face-selective activation in a congenital prosopagnosic subject. Journal of Cognitive Neuroscience. 2003;15:419–431. doi: 10.1162/089892903321593135. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:380–391. doi: 10.1006/nimg.2000.0592. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang JY, van Zijl PCM, Mori S. Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. Magnetic Resonance in Medicine. 2004;52:559–565. doi: 10.1002/mrm.20147. [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Research Bulletin. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Jiang HY, van Zijl PCM, Kim J, Pearlson GD, Mori S. DTIstudio: Resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods and Programs in Biomedicine. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O’Sullivan M, et al. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Human Brain Mapping. 2006;27:230–238. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JDE, Moseley ME, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Current Biology. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Lamont AC, Stewart-Williams S, Podd J. Face recognition and aging: Effects of target age and memory load. Memory & Cognition. 2005;33:1017–1024. doi: 10.3758/bf03193209. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. Journal of Neuroscience. 2000;20:878–886. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lori NF, Akbudak E, Shimony JS, Cull TS, Snyder AZ, Guillory RK, et al. Diffusion tensor fiber tracking of human brain connectivity: Acquisition methods, reliability analysis and biological results. NMR in Biomedicine. 2002;15:494–515. doi: 10.1002/nbm.779. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. Journal of Comparative Neurology. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Maurer D, O’Craven K, Le Grand R, Mondloch CJ, Springer MV, Lewis TL, et al. Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia. 2007;45:1438–1451. doi: 10.1016/j.neuropsychologia.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Valentine T. Linear and nonlinear effects of aging on categorizing and naming faces. Psychology and Aging. 1992;7:317–323. doi: 10.1037//0882-7974.7.2.317. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Ungerleider LG, Haxby JV, Rapoport SI, Horwitz B. Network analysis of cortical visual pathways mapped with PET. Journal of Neuroscience. 1994;14:655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows JC. The anatomical basis of prosopagnosia. Journal of Neurology, Neurosurgery, and Psychiatry. 1974;37:489–501. doi: 10.1136/jnnp.37.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PCM. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PCM. Fiber tracking: Principles and strategies—A technical review. NMR in Biomedicine. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Boldt C. Aging and low-contrast vision: Face perception. Investigative Ophthalmology & Visual Science. 1981;21:362–365. [PubMed] [Google Scholar]
- Parkin A. Memory: Phenomena, experiment and theory. Oxford: Blackwell; 1993. [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, et al. Structure–function correlates of cognitive decline in aging. Cerebral Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: A review. Journal of Neurocytology. 2002a;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes in the normally aging cerebral cortex of primates. Progress in Brain Research. 2002b;136:455–465. doi: 10.1016/s0079-6123(02)36038-2. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes that occur during normal aging of primate cerebral hemispheres. Neuroscience and Biobehavioral Reviews. 2002c;26:733–741. doi: 10.1016/s0149-7634(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Platek SM, Loughead JW, Gur RC, Busch S, Ruparel K, Phend N, et al. Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping. 2006;27:91–98. doi: 10.1002/hbm.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual–motor skill. Microscopy Research and Technique. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The representation of information about faces in the temporal and frontal lobes. Neuropsychologia. 2007;45:124–143. doi: 10.1016/j.neuropsychologia.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. What and when of cognitive aging. Current Directions in Psychological Science. 2004;13:140–144. doi: 10.1177/0963721414535212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. Brain. 1992;115:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Shapiro PN, Penrod S. Meta-analysis of facial identification studies. Psychological Bulletin. 1986;100:139–156. [Google Scholar]
- Steeves JK, Culham JC, Duchaine BC, Pratesi CC, Valyear KF, Schindler I, et al. The fusiform face area is not sufficient for face recognition: Evidence from a patient with dense prosopagnosia and no occipital face area. Neuropsychologia. 2006;44:594–609. doi: 10.1016/j.neuropsychologia.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Adalsteinsson E, Swan GE, Carmelli D. Differential rates of regional brain change in callosal and ventricular size: A 4-year longitudinal MRI study of elderly men. Cerebral Cortex. 2002;12:438–445. doi: 10.1093/cercor/12.4.438. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J. Predictive codes for forthcoming perception in the frontal cortex. Science. 2006;314:1311–1314. doi: 10.1126/science.1132028. [DOI] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Jung KJ, Behrmann M. Reduced structural connectivity, revealed by diffusion tensor imaging and tractography, in congenital prosopagnosia (submitted) [Google Scholar]
- Tusa RJ, Ungerleider LG. The inferior longitudinal fasciculus: A reexamination in humans and monkeys. Annals of Neurology. 1985;18:583–591. doi: 10.1002/ana.410180512. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang HY, Nagae-Poetscher LM, van Zijl P, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Medical Image Analysis. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Lim KO. Advances in white matter imaging: A review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience and Biobehavioral Reviews. 2006;30:762–774. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R, van Zijl PCM, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magnetic Resonance in Medicine. 1999;42:1123–1127. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific Publications; 1967. pp. 3–70. [Google Scholar]


