Abstract
Objective
The purpose of this study was to evaluate pressurized wideband acoustic immittance (WAI) tests in children with Down syndrome (DS) and in typically developing children (TD) for prediction of conductive hearing loss (CHL) and patency of pressure equalizing tubes (PETs).
Design
Audiologic diagnosis was determined by audiometry in combination with distortion-product otoacoustic emissions, 226-Hz tympanometry and otoscopy. WAI results were compared for ears within diagnostic categories (Normal, CHL and PET) and between groups (TD and DS).
Study Sample
Children with DS (n=40; mean age 6.4 yrs.), and TD children (n=48; mean age 5.1 yrs.) were included.
Results
Wideband absorbance was significantly lower at 1–4 kHz in ears with CHL compared to NH for both TD and DS groups. In ears with patent PETs, wideband absorbance and group delay (GD) were larger than in ears without PETs between 0.25–1.5 kHz. Wideband absorbance tests performed similarly for prediction of CHL and patent PETs in TD and DS groups.
Conclusions
Wideband absorbance and group delay revealed specific patterns in both TD children and those with DS that can assist in detection of the presence of significant CHL, assess the patency of PETs, and provide frequency-specific information in the audiometric range.
Introduction
Evaluation of hearing in children with Down Syndrome (DS) is an important, yet challenging undertaking, since it is critical to identify presence and type of hearing loss in these children (Ypsilanti et al., 2005). Hearing loss may exacerbate underlying speech production and language deficits in children with DS (Dodd & Thompson, 2001). Cognitive delay and behavioral challenges have been reported to result in poorer reliability of behavioral results, and more difficulty completing tympanometry and otoacoustic emission tests (Maurizi et al., 1985; Rupa, 1995). Otoscopy and tympanometry tests are complicated by frequent ear canal stenosis and cerumen impaction that obstruct visualization of the tympanic membrane (Shott, 2006). In the first year of life, the incidence of conductive hearing loss is 34%–38%, while the incidence of sensorineural hearing loss (SNHL) is 6% and mixed hearing loss (MHL) is 3% (Park et al., 2012; Raut et al., 2011). A population-based study in Norway (Austeng et al., 2013) reported that 35% of eight-year-old children with DS had hearing levels greater than 25 dB HL in the better ear, with a higher incidence of SNHL (18%), compared to CHL (16%) and MHL (6%). Paulson et al. (2014) reported that 28% of children with DS had mild to profound hearing loss in the worst ear after the insertion of pressure equalizing tubes (PETs). Conversely, Shott et al. (2001) reported that 98% of children with DS treated with PETs early and aggressively had normal hearing levels after PET surgery. Comparison with operative findings shows that OME was responsible for 60% of participants’ CHL and imaging revealed a permanent CHL in the remaining 40% due to middle-ear anomalies (Balkany, 1979; Shott, 2006). More recently, Intrapiromkul et al. (2012) reported a 75% incidence of inner ear anomalies in DS for patients with SNHL based on high resolution computerized tomography scanning, thus the possibility of underlying permanent conductive or SNHL is relevant to distinguishing type of hearing loss in these children.
Middle-ear dysfunction is typically assessed using standard, pure-tone tympanometry in combination with medical history, otoscopy, and audiologic findings (Margolis & Hunter, 2000). However, the sensitivity and specificity of standard pure-tone tympanometry has been questioned in studies showing better sensitivity of wideband acoustic immittance (WAI) in newborns and children (Sanford et al., 2009; Hunter et al., 2010; Keefe et al., 2012).
WAI is an advanced middle ear assessment technique that includes a battery of absorbance, group delay (GD) and acoustic stapedial reflex (AR) tests using a broader frequency range (0.2 to 8 kHz) than standard tests. The pressure spectrum P(f) as a function of frequency f at the probe tip is the sum of the forward pressure spectrum wave PF(f) moving towards the tympanic membrane and the reverse pressure spectrum wave PR(f) moving back towards the probe. The pressure reflectance R(f) of the ear is defined as by ratio of the reverse pressure to the forward pressure, i.e., R(f) = PR(f)/PF(f). The energy reflectance ER is defined as ER = |R(f)|2, and absorbance is 1 − ER. The group delay (GD), also termed D(f), is defined using the phase ϕ(f) of R(f) by . GD measures how rapidly the phase varies with frequency, with units of time delay in μs (1 μs is 10−6 seconds). Absorbance and GD are measured either at ambient air pressure in the ear canal or at varying tympanometric air pressures (Keefe et al., 2015).
The wideband acoustic reflex threshold (WB-ART) test uses the same click stimulus as for the absorbance test, and an additional pulsed broadband noise signal to activate the AR. WB-ARTs have been measured in infants by monitoring absorbance changes across several frequency bandwidths (Feeney & Sanford, 2005) in a click signal in the presence of a reflex activator signal compared to a click signal in quiet. A more sensitive and objective WB-ART test was developed and evaluated in normal ears by Keefe et al. (2010) based on pressure shifts in click responses in quiet and after presentation of a pulsed activator signal to elicit an AR. This test was further refined to use shifts in absorbed sound power in a baseline click relative to later clicks that followed presentation of activator pulses (Keefe et al., 2017). This test showed lower ARTs in normal adult ears and promising results in neonatal ears (Hunter et al. 2017), and was used in the present study.
Wideband tympanograms and ambient absorbance in NH children with typical development (TD) show highly significant differences compared to children with CHL that are related to the presence of CHL (Keefe et al., 2012; Ellison et al., 2012). Ambient absorbance is able to detect CHL in all infants, children and adults, and was superior to single frequency tympanometry (Prieve et al., 2013). Keefe and Simmons (2003) reported ambient and tympanometric WAI measures and 226-Hz tympanometry to predict CHL in TD children. Comparing measures at a fixed specificity of 90%, sensitivity was lowest for static admittance at 226 Hz (28%), intermediate for ambient-pressure absorbance (72%), and highest for pressurized absorbance (94%). Pressurized absorbance predicted CHL with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.95. Beers et al. (2010) studied wideband energy reflectance (ER) in 64 TD children (average age = 6.3 years) with diagnosed middle-ear conditions and CHL compared with 78 TD children without middle-ear conditions (average age = 6.2 years). ER at 1.25 kHz had the best test performance (sensitivity of 96%, specificity of 95%). AUC exceeded 0.90 for ER across frequency bands between 800 and 5000 Hz. Compared with traditional admittance at 226 Hz, ER results had significantly better test performance in distinguishing between healthy ears and ears with CHL due to OME.
Only two studies of WAI in children with DS have been published, and none have studied tympanometric absorbance or WB-ART. One study in children with DS showed that 63% had abnormal ambient ER in the presence of normal 226 Hz tympanograms (Kaf, 2011). A study of 22 children with DS (2 to 16 years) analyzed four clinical groups: normal tympanograms, flat tympanograms, mild negative pressure tympanograms, and severe negative pressure tympanograms (Soares et al., 2016). Twelve control children aged 3 to 13 years were compared to the children with DS on 226-Hz tympanometry and wideband ambient ER measurements. ER responses at 1,000 and 1,600 Hz correctly classified the participants’ data based on ER in 60% of cases with DS.
The overall goal of this study was to assess the effectiveness of the WAI test battery to predict CHL and PET patency in children with DS and developmental delay compared to analogous predictions in TD children. Based on previous studies, significant WAI differences were hypothesized between participants in three diagnostic categories (NH, CHL, patent PET) and two groups (DS and TD).
Methods - Participants
Children with DS and TD children were enrolled through the Audiology and Otolaryngology (ENT) clinics at Cincinnati Children’s Hospital Medical Care Center (CCHMC) using posted flyers and recruitment in person. Patient charts were reviewed to obtain audiologic and otolaryngology records to determine eligibility. In order to be included in the study, children were between 9 months and 18 years old, with a diagnosis of DS, or they were TD with no known developmental delays or major neurologic diagnoses. Exclusion criteria were inability to cooperate with audiologic assessments, known SNHL or otologic abnormalities other than OME (such as cholesteatoma, external ear or ossicular anomalies). The CCHMC Institutional Review Board (IRB) approved the study. Parents of eligible children were invited to participate and were given verbal and written information about the study. Interested parents provided written consent prior to testing. Children who were age 12 years or older and able to understand their role in the study provided assent. Participants were compensated for time participating in the study.
Test Protocol
Study assessments included pneumatic otomicroscopy or pneumatic otoscopy, 226 Hz tympanometry, distortion product otoacoustic emissions (DPOAE), visual reinforcement audiometry (VRA), conditioned play audiometry (CPA) or standard audiometry, depending on developmental age. Otomicroscopy or otoscopy was performed by a pediatric otolaryngologist, who diagnosed OME and judged PET patency. Children with blocked PE tubes were not enrolled in the study. For normal controls (TD with no history of recurrent OM) otoscopy was completed by an audiologist prior to hearing testing. All hearing testing was completed by a licensed audiologist with pediatric experience, using a test assistant to provide visual and behavioral control when necessary due to developmental age. All audiometry was done with ear-specific transducers, unless the child refused to respond under earphones. In a small number of these cases, sound field audiometry was used instead. Speech reception thresholds (SRT) or speech awareness thresholds (SAT) were obtained first using monitored live voice. Ear-specific air -conduction pure tone thresholds were then obtained between 500–4000 Hz for children using VRA and CPA procedures, and between 250–8000 Hz for standard audiometry. Bone-conduction thresholds were obtained between 500–4000 Hz for all three procedures. Insert earphones (Etymotic ER-3A) were used for air conduction testing, and a RadioEar B71 transducer for bone conduction testing, with contralateral masking as needed. Standard tympanograms (226-Hz probe tone) were obtained using either an Interacoustics Titan or a Zodiac 901 tympanometer. Equivalent ear-canal volume (Veq), tympanometric peak pressure (TPP), peak-compensated static acoustic admittance magnitude (Ytm) and tympanometric width (TW) were measured. Criteria for normal middle ear function (226 Hz probe) were peak-compensated acoustic admittance magnitude ≥0.3 mmho relative to the positive tail and TW ≤ 250 daPa (Hunter & Blankenship, 2017).
DPOAE tests were performed using the Vivosonic Integrity system or Interacoustics Titan system. Signal and noise values were recorded at primary tone f2 frequencies of 2, 3, 4, 5.5, and 8 kHz with the primary tone f1 frequency selected so that f2/f1 was equal to 1.22. Frequencies below 2 kHz have poor test performance due to internal body noise in children (Gorga et al., 2005), so were not tested. The corresponding primary tone levels (L1 and L2) were set at 65 and 55 dB SPL, respectively. In-situ calibration was performed prior to testing each ear, followed by a DP-gram acquisition. Two trials were run when possible depending on the child’s cooperation, and the overall better of the two tests was chosen to optimize signal to noise ratio (SNR). Pass criteria were SNR of 6 dB or greater at 3 of 5 (f2) frequencies (2, 3, 4, 5.5, 8 kHz) and DP levels above the 10th percentile of the normative range (Gorga, 2005).
After completing hearing and clinical tests, the WAI test battery was completed. The test computer had a two-channel sound card (CardDeluxe, sample rate 22.05 kHz) and RS-232 serial port to communicate between the computer and tympanometric pump. A Titan ear probe (Interacoustics) was used for all WAI measurements. Custom software was used to calibrate the system and measure WAI responses. The system was calibrated daily using data collected with the probe inserted into two lengths and two diameters of tubing to approximate infant (<3 years) and adult ear canal diameters (>3 years). The calibration was performed over the analysis frequencies of the click stimulus between 0.2 and 8 kHz (Liu et al., 2008).
Absorbance and GD tests in the battery were performed using methods described in Keefe et al. (2015), and summarized below. These were measured first as a tympanogram with downswept pressure, then at ambient pressure, and finally as a tympanogram with upswept pressure. Tympanometry sweeps ranged between −315 and +220 daPa at a rate of approximately 100 daPa/s, with data analyzed between −300 and +200 daPa. Artifact rejection was performed across all click responses for extreme outliers. Ambient and tympanometric responses were smoothed across frequency, and tympanometric responses were also smoothed over air pressure. Absorbance and GD were measured for each click (intervals of 46 ms) as a function of ear canal air pressure and frequency. Tympanometric features were extracted from the low-frequency averaged absorbance tympanogram: Minimum (Amin) and maximum (Amax) absorbance across the air pressure range, TPP, and tympanometric width (TW). The TW was the range of air pressure over which the low-frequency averaged absorbance decreased to one-half of the difference in its maximal value at TPP to the average absorbance at positive and negative tail pressures.
The absorbance tympanogram was averaged into pass bands of lower and higher frequency bandwidths (denoted as LP and HP, respectively), and analyzed as a function of air pressure. For children of 0.5 years and older up to 3 years, the LP band was 0.35–1.4 kHz and the HP band was 1.4–8 kHz. For children of age 3 years and older through adults, the LP band was 0.38–2 kHz and the HP band was 2–8 kHz, as used in previous studies (Liu et al., 2008; Keefe et al., 2015; Hunter et al., 2015). This variation in the LP and HP bandwidths introduced a complication in the study design, as the age ranged from 8 months to 18 years, and thus covered the transition age of 3 years in LP and HP bandwidths. This complication was accepted inasmuch as normal baselines do vary with age.
Ipsilateral WB-ARTs were measured with activator stimuli presented to the same ear as the probe click stimulus, using the same Titan probe system described by Keefe et al. (2015) and summarized below. To control for any hysteresis present in the tympanogram that might vary with the polarity of the pressure sweep, the average TPP from downswept and upswept tympanograms was used to set ear-canal pressure for the WB-ART test. Data were recorded using two trials of the stimulus set at increasing activator levels in 5 dB steps up to a maximum of 80 dB SPL in some of the ears in the study, and up to a maximum of 90 dB SPL in other ears. The starting level varied from quiet in some ears or 50 dB SPL in other ears. The changes were due to refinements in test protocol over time. The SPL of the BBN activator was calibrated to the SPL measured in a 2 cm3 coupler. The upper limit was conservatively set at 80 or 90 dB SPL, respectively in consideration of the smaller ear canals in infants and children compared to adults. To detect a WB-ART, a significant negative shift in absorbed sound power was required that was synchronous with the onset of the initial click, and that increased above a critical level of absorbed sound power. An AR shift was judged as present if the minimum difference in absorbed sound power level for the final of 4 clicks relative to the baseline click was at least 0.7 dB.
All tests were optionally paused when excessive noise or body movement occurred. Such pauses were repeated as needed after the subject quieted down and the probe fit was checked. Real-time artifact rejection was used to exclude intermittently noisy tests (Keefe et al. 2015). For all tests, the best quality (i.e. lowest overall noise) recording was selected for data analyses.
Results
A total of 88 children (42 female) were included in the study; 18 were African-American, 67 were white/Caucasian, and 3 were other/unknown race. A total of 48 TD children (9 months to 15 years, average age 4 years, 6 mos., standard deviation (SD) = 40 months) and 40 children with DS (9 months to 17 years, average age 7 yrs., 9 mos., SD = 41 months) were included. Thus, the TD group was younger than the DS group, due to difficulties in reliably testing younger children with DS. There were 72 DS ears with complete diagnostic results (21 NH, 25 CHL, 26 PETs), and 86 TD ears with complete diagnostic results (36 NH, 16 CHL, 34 PETs). 17 ears were excluded due to incomplete audiogram and DPOAE data, and 1 ear was excluded due to possible SNHL. The breakdown by ears and age in each group for each diagnostic category is detailed in Table 1, along with 226-Hz tympanometry results and WB-ARTs.
Table 1.
Descriptive statistics for the diagnostic classifications for typically developing (TD) and Down syndrome (DS) groups. The diagnostic classifications are Normal Hearing (NH), Conductive Hearing Loss (CHL), and Pressure Equalizing Tubes (PET). Single frequency tympanometry (0.226-kHz probe) variables are given for each group and category: Equivalent Volume of the ear canal (Veq); Tympanometric Peak Pressure (TPP); Peak compensated static admittance at +200 daPa (SA +200); Tympanometric width (TW); Wideband acoustic reflex threshold (WB ART).
| Subgroup | Age (mos.) | Veq (ml) | TPP (daPa) | SA +200 (mmho) | TW (daPa) | WB ART (dB SPL) | |
|---|---|---|---|---|---|---|---|
| DS NH (n=21) | AVE | 94.6 | 0.60 | −31.67 | 0.54 | 99.67 | 82.50 |
| SD | 41.2 | 0.25 | 75.43 | 0.53 | 46.75 | 14.82 | |
| DS CHL (n=25) | AVE | 53.9 | 0.46 | −138.54 | 0.31 | 134.29 | 82.17 |
| SD | 39.6 | 0.14 | 103.24 | 0.44 | 95.52 | 17.98 | |
| DS PET (n=26) | AVE | 90.5 | 1.64 | * | * | * | 80.60 |
| SD | 67.9 | 0.78 | * | * | * | 19.55 | |
| TD NH (n=36) | AVE | 79.4 | 0.78 | −20.00 | 0.72 | 86.20 | 73.86 |
| SD | 44.5 | 0.18 | 40.12 | 0.48 | 35.11 | 14.61 | |
| TD CHL (n=16) | AVE | 11.8 | 0.43 | −27.19 | 0.13 | 75.00 | 80.63 |
| SD | 3.4 | 0.06 | 39.34 | 0.23 | 93.34 | 9.64 | |
| TD PET (n=34) | AVE | 67.9 | 2.15 | * | * | * | 81.21 |
| SD | 57.2 | 1.38 | * | * | * | 11.18 |
Not measurable due to PET.
Individual Cases
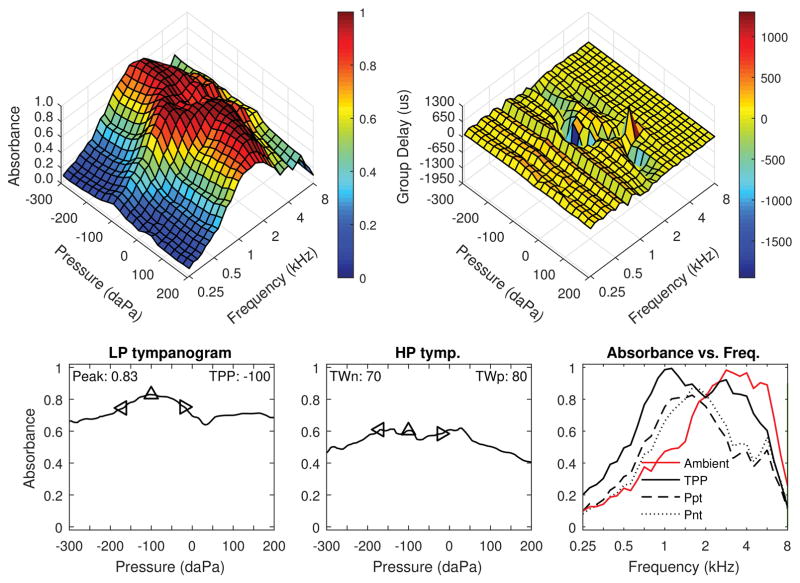
Cases demonstrating typical results in each diagnostic category from the DS group are shown in Figures 1–3. Although not shown, these examples are also typical of the TD group. In Figure 1, ambient and tympanometric absorbance are shown for the right ear of a 64 month-old male with DS, normal hearing (10–15 dB HL across frequencies) and normal DPOAEs. The three dimensional tympanogram (top left panel) shows absorbance by frequency on the y-axis. Absorbance was lowest below 1 kHz, increasing to a maximal peak in the 2–4 kHz range, then decreasing above 4 kHz. This is a typical pattern expected for negative pressure but otherwise normal middle-ear function. Absorbance as a function of ear-canal air pressure along the x-axis showed a well-defined peak with slightly negative pressure. The average low-frequency absorbance tympanogram is shown in the bottom left panel, with peak absorbance of 0.83 and peak pressure of −100 daPa. The single absorbance peak at low frequencies is similar to the peak in compensated admittance magnitude in standard tympanometry. For the average high frequency absorbance tympanogram, there was a broader peak with a tympanometric width of 150 daPa (positive and negative half-widths combined). In the top right panel, GD is shown as a function of frequency and air pressure. In this normal ear, a ridge in GD corresponding to the frequency region of maximal absorbance around 2 kHz was seen. The GD was more negative at frequencies with higher absorbance (compare to top left panel). This peak absorbance and GD relationship indicated the resonant coupling of these two measurements, which are related to pressure reflectance magnitude and phase, respectively. Absorbance was substantially greater below 2 kHz for the TPP condition compared to ambient pressure, which is consistent with the negative pressure in this ear. The measured WB-ART was 90 dB SPL in this ear, which is at the top of the normal range.
Figure 1.
Right ear of a 64 month-old male with Down syndrome and normal hearing (10–15 dB HL across frequencies) and normal DPOAE. Top left: Tympanometric absorbance (downswept pressure). Top right: Tympanometric GD. Bottom left: Average low-frequency tympanometry. Bottom middle: Average high-frequency tympanometry. Bottom right: Absorbance by frequency plots (at ambient, TPP, and the positive and negative tympanogram tails.)
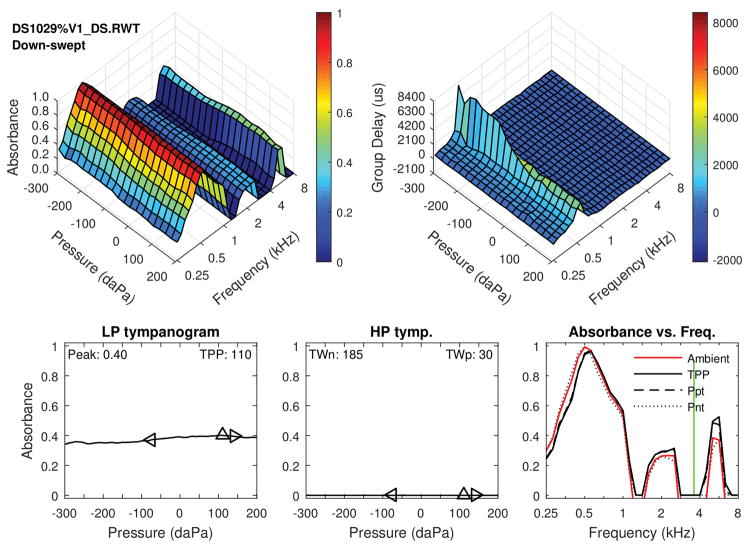
Figure 3.
The following figure shows individual case data for a patient with DS and patent PE tube. Note the high multiple peaks in the three dimensional tympanograms and ambient absorbance. Top left: Tympanometric absorbance (downswept pressure). Top right: Tympanometric GD. Bottom left: Average low-frequency tympanometry. Bottom middle: Average high-frequency tympanometry. Bottom right: Absorbance by frequency plots (at ambient, TPP, and the positive and negative tympanogram tails.)
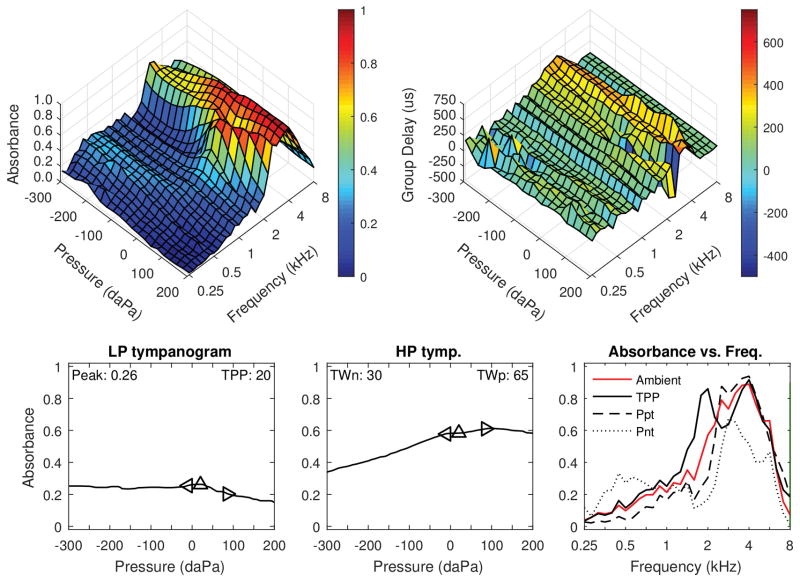
Figure 2 shows data from the left ear of a 35 month-old female with DS, who was diagnosed with OME and elevated air conduction hearing levels (50 dB at 1 kHz, 20 dB at 4 kHz). Bone conduction testing was not completed due to attention problems. This child previously had a normal ABR test, thus the hearing loss was most likely conductive given the low frequency slope and otologic findings. Although not shown, DPOAEs were absent from 1–3 kHz and present from 4–8 kHz, consistent with audiometry. Standard 226-Hz tympanometry showed low-normal static admittance (0.2 mmho) and normal tympanometric width (81 daPa). Compared to the normal absorbance responses in Figure 1, this ear had much lower absorbance between 0.25 to 2 kHz in the ambient and tympanometric responses. GD was generally reduced in this ear compared to the normal ear shown in Figure 1, except for the ridge in GD around 2–4 kHz in the region of maximal absorbance. Wideband ARs were absent, consistent with the presence of middle-ear dysfunction. Thus, the WBT and WB-ARTs were abnormal and consistent with the behavioral audiogram and DPOAE results. The presence of abnormal WBT and absent reflexes in the presence of normal 226-Hz tympanometry is consistent with findings of Kaf (2011).
Figure 2.
Left ear of a 35 month-old female with DS, who was diagnosed with OME and had elevated air conduction hearing levels. DPOAEs were absent from 1–3 kHz, and present from 4–8 kHz. Standard 0.226-kHz tympanometry (not depicted) showed low-normal static admittance (0.2 mmho) and normal tympanometric width (81 daPa). Top left: Tympanometric absorbance (downswept pressure). Top right: Tympanometric GD. Bottom left: Average low-frequency tympanometry. Bottom middle: Average high-frequency tympanometry. Bottom right: Absorbance by frequency plots (at ambient, TPP, and the positive and negative tympanogram tails.)
Figure 3 shows results for the right ear of a 36 month-old with DS and a patent PET. This example is typical of the WBT pattern for patent PETs; large peaks in absorbance are present across frequencies (i.e., near 0.5, 2 and 5 kHz in this ear) for the tympanometric and ambient tests. The WBT is essentially invariant with changes in pressure for LP and HP tympanograms. WB ARTs were absent, as in about 25% of ears with patent PETs.
Diagnostic categorization
A clinical test battery classified hearing and middle ear status as normal or abnormal based on four sources of clinical data: pneumatic otoscopy or otomicroscopy, standard tympanometry, audiometry and DPOAEs. The criteria for the classification of NH were that the pure tone average (0.5–4 kHz) was no greater than 20 dB HL (or presence of normal DPOAEs if audiometry was incomplete; this was the case in 8 ears), and that otoscopy and/or standard tympanometry were normal. An ear was classified as CHL if the PTA exceeded 20 dB HL or had an average air-bone gap of 10 dB or greater, combined with abnormal otoscopy and/or tympanometry. Masked bone conduction was successfully obtained in 44% of the DS children with hearing loss; and in 56% of TD children with hearing loss. Because masked bone conduction testing was not reliable in all cases, otoscopy and tympanometry were also used to determine presence of CHL. It is recognized that mixed hearing loss was possible in some of these CHL cases. Classification of a patent PET was based on the otolaryngology examination.
Statistical analysis
Due to the large number of potential WBT variables across frequency and pressure, an a priori statistical analysis approach was planned using exploratory analyses of the variables of theoretical interest. Extreme outliers were first excluded from analysis, descriptive statistics were completed, and the data distribution and confidence intervals (CIs) between diagnosis categories were examined graphically. Variables that showed apparent differences in the 95% CI between the diagnostic categories (NH, CHL, and PET) were selected for further analysis. Wideband variables were analyzed for significant differences to test for prediction of diagnostic categories, with DS or TD, ear side, and age included in the models as covariates. Diagnostic categories were analyzed using descriptive statistics and mixed effects models. Standard tympanometry, DPOAE and audiometry were analyzed using a non-parametric Wilcoxon rank sum test since the distributions were non-normal.
Audiometry
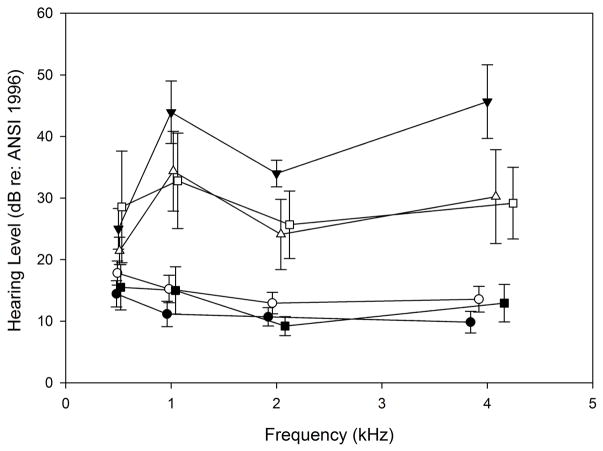
Comparisons for air conduction pure tone average (PTA, 0.5–4 kHz) were performed for each of the diagnostic categories combined with the TD and DS groups, as depicted in Figure 4. An overall Kruskal-Wallis One Way Analysis of Variance on Ranks was significant for the 6 subgroups (p < 0.001), so post-hoc comparisons were done with correction by Dunn’s method. The PTA for ears in the DS-NH category was approximately 3–4 dB poorer than the TD-NH category across 0.5–4 kHz as shown in Figure 4, but their average thresholds were not significantly different. Average air conduction results were elevated by 10–30 dB across 0.5–4 kHz for ears with CHL compared to those with NH in both the TD (p < 0.001) and DS groups (p = 0.003). Although the TD group with CHL appeared to have poorer hearing thresholds than the DS group with CHL, the difference was not significant. In the DS group, air conduction thresholds were significantly elevated for ears with patent PETs compared to CHL ears (p = 0.001) and NH ears (p = 0.024). In the TD group with patent PETs, thresholds were similar to the NH category (p > 0.5). The TD-PET category had significantly better thresholds than the DS-PET category (p < 0.002). The TD group with PET had better hearing thresholds than the TD group with CHL (p < 0.001). In summary, the DS group had poorer thresholds for the PET category compared to the TD group, but no significant threshold differences from the TD group within categories of NH and CHL.
Figure 4.
Average air conduction thresholds in each diagnostic category. The graph illustrates average thresholds as a function of frequency. TD: typically developing; DS: Down syndrome; NH: normal hearing; CHL: conductive hearing loss; TUBE: pressure equalization tube. Symbols are jittered around the frequency to allow error bars to be visualized.
DPOAE
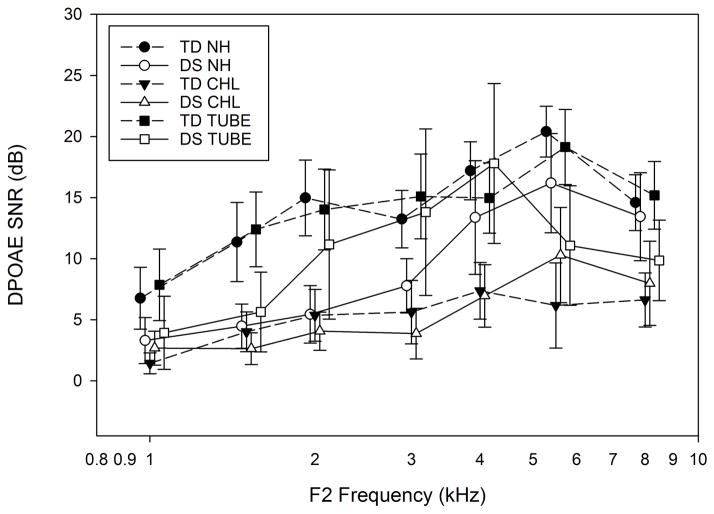
Average DPOAE signal-to-noise ratio (SNR) is shown in Figure 5 for each diagnostic category. For normal hearing categories, DPOAE SNR was not significantly different for the DS compared to the TD group, except at 2 kHz (p = 0.009). In both DS and TD groups, the average DPOAE SNR was significantly lower from 1.5 to 8 kHz for ears with CHL, compared to NH ears (p <0.001 for the overall Kruskal-Wallis ANOVA and pairwise comparisons). DPOAE SNRs for ears with PET were similar to NH ears from 1 to 8 kHz. The SNRs were significantly higher in ears with PET than in ears with CHL from 1.5 to 8 kHz (p< 0.01).
Figure 5.
Average DPOAE Signal to noise ratio (SNR) in each diagnostic category. The graph illustrates average SNR as a function of frequency. Error bars are ±1 SD. TD: typically developing; DS: Down syndrome; NH: normal hearing; CHL: conductive hearing loss; TUBE: pressure equalization tube. Symbols are jittered around the frequency to allow error bars to be visualized.
Standard tympanometry
Standard 226-Hz tympanometry was evaluated in both groups and all three diagnostic categories. Due to an inability to obtain a seal, results could not be analyzed from 13 of 34 ears (38%) of TD children with patent PETs and 10 of 26 ears (38%) in DS children with patent PETs. Children with DS appeared to have smaller ear canal volumes compared with TD children in all diagnostic categories, but these differences were not significant. As expected, ears with CHL had lower static admittance as compared to NH ears (0.46 vs. 0.71 mmho respectively, p <0.001). However, 8 out of 40 ears (20%) had normal static admittance and tympanometric width despite having a CHL.
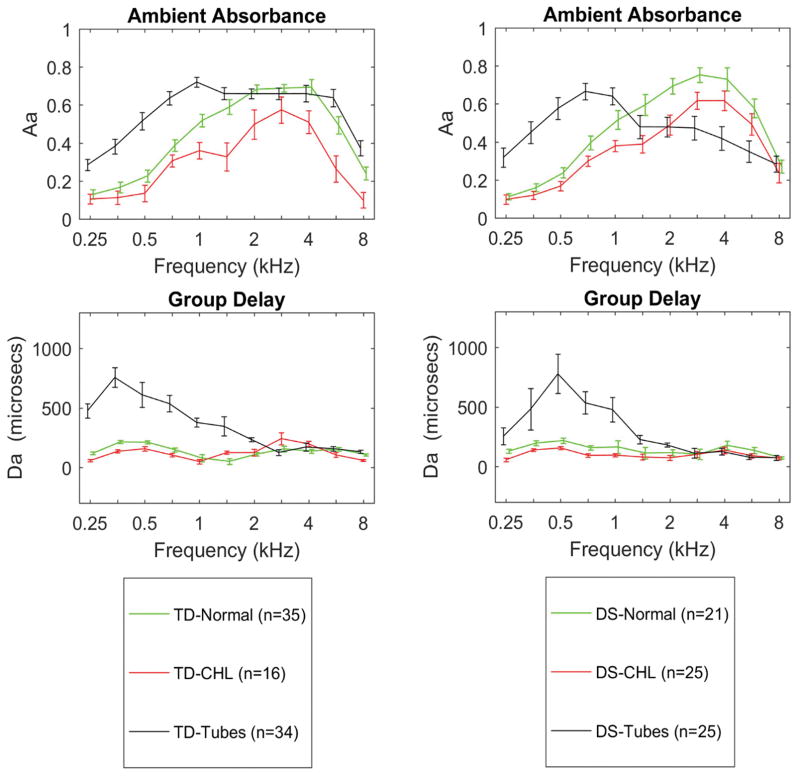
Ambient-pressure absorbance and GD
As shown in Figure 6 (top panels), the three diagnostic categories (NH, CHL and PET) had different mean values of ambient-pressure absorbance that were frequency-specific and similar for TD (left panel) and DS (right panel) groups (see statistical comparisons in Table 2). Ears with CHL had significantly lower ambient absorbance between 1 and 2 kHz and at 4 kHz, with no significant effects for DS, ear or age. GD was not significantly different between CHL and NH ears. Ears with patent PETs had significantly higher ambient absorbance from 0.25 to 1 kHz, with no significant effect due to DS, ear or age. GD at ambient pressure was similar for TD and DS groups for each diagnostic category (Figure 6, bottom panels). In ears with PET, GD was significantly longer from 0.25 kHz to 1 kHz. There was no significant effect of DS or ear for GD, but there was a significant effect of age.
Figure 6.
Comparison of ambient absorbance in TD (left top panel) and DS (right top panel) for diagnostic categories of NH, CHL and patent PETs. Bottom panels: comparison of GD for TD (left) and DS (right). Error bars are 95% CI.
Table 2.
Results for mixed models with comparisons between diagnostic categories for tests for NH versus patent PET in the top panel, and tests for NH versus CHL categories in the bottom panel. Models included Group (DS and TD), with ear and age as covariates. Tympanometry refers to data from wideband downswept absorbance.
| Variables | Ambient Absorbance | Ambient Group Delay | Absorbance at Ppt Downswept |
|---|---|---|---|
| Frequencies (kHz) | Normal v Tubesa | Normal v Tubesb | Normal v Tubesc |
| 0.25 | 0.001 | 0.003 | 0.0004 |
| 0.35 | <0.0001 | <0.0001 | <0.0001 |
| 0.5 | <0.0001 | <0.0001 | <0.0001 |
| 0.7 | <0.0001 | <0.0001 | <0.0001 |
| 1.0 | <0.0001 | <0.0001 | 0.0006 |
| Variables | Ambient Absorbance | Group Delay at TPP Downswept | Absorbance at TPP Downswept |
|---|---|---|---|
| Frequencies (kHz) | Normal v CHLa | Normal v CHLb | Normal v CHLc |
| 0.7 | NA | 0.29 | NA |
| 1.0 | 0.02 | 0.15 | 0.0004 |
| 1.4 | 0.0003 | 0.19 | <0.0001 |
| 2 | 0.006 | 0.24 | 0.0003 |
| 2.8 | 0.13 | NA | 0.009 |
| 4 | 0.03 | NA | 0.24 |
| Overall Tympanometry: | Normal v CHLd | ||
| DALnt | <0.0001 | ||
| DALpt | 0.001 |
DS included, not significant (p=0.57); Age in months included, not statistically significant (p=0.15); Ear included as random effect, not significant as fixed effect (p=0.15)
DS included, not significant (p=0.59); Age in months included, statistically significant (p=0.003); Ear included as random effect, not significant as fixed effect (p=0.17)
DS included, statistically significant (p=0.036); Age in months included, statistically significant (p<0.0001); Ear included as random effect, not significant as fixed effect (p=0.85)
DS included, not significant (p=0.08); Age in months included, not statistically significant (p=0.72); Ear included as random effect, not significant as fixed effect (p=0.29)
DS included, not statistically significant (p=0.16); Age in months included, not statistically significant (p=0.13); Ear included as random effect, not significant as fixed effect (p=0.48)
DS included, not significant (p=0.31); Age in months included, not statistically significant (p=0.68); Ear included as random effect, not significant as fixed effect (p=0.71);
Wilcoxon Rank Sum test
NA - not available, did not test for significance
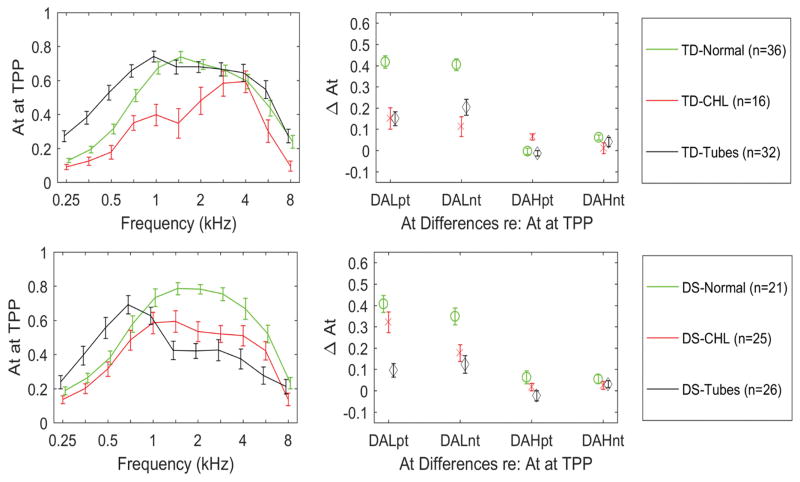
Tympanometric absorbance
Tympanometric absorbance was compared at TPP. Mean differences in up-swept tympanometric data were similar to those in down-swept data, and so are not described. The downswept tympanometric results are depicted in Figure 7 (left panels) for diagnostic categories within the DS and TD groups, and statistical comparisons are shown in Table 2. Ears with CHL had significantly lower absorbance at TPP than NH ears from 1 to 2.8 kHz. There were no significant effects of DS, ear or age on absorbance at TPP. For ears with CHL, the TD group had lower absorbance at TPP between 1–2 kHz than the children with DS. For ears with PET, the children with DS had poorer hearing levels than TD children, and lower absorbance from 1–4 kHz.
Figure 7.
Comparison of tympanometric absorbance at the TPP, in TD (top left panel) and DS (bottom left panel) for diagnostic categories of NH, CHL and patent PETs. The downswept averaged low frequency tympanograms, referenced to the positive (DALpt) and negative tail values (DALnt) are shown in the top right panels for the DS group, and bottom right panel for the TD group. The downswept averaged high frequency referenced to the positive (DAHpt) and negative tail values (DAHnt) are also shown for each category. Error bars are 95% CI.
In the right panels of Figure 7 of tympanometric absorbance data, the averaged difference is shown for absorbance at TPP relative to absorbance at either the positive- or negative-tail pressure. The plotted measures include the LP absorbance difference relative to the negative tail (DALnt) and positive tail (DALpt), and the HP absorbance difference relative to the negative tail (DAHnt) and positive tail (DAHpt). The leading “D” in each name denotes a “difference”, “A” for absorbance, “L” and “H” for LP and HP, respectively, and “nt” and “pt” for negative tail and positive tail, respectively. Statistical comparisons for DALnt and DALpt are provided in Table 2. The DALnt and the DALpt were each significantly larger for NH compared to CHL in the DS and TD groups combined. Ears with CHL had significantly lower peak to tail differences (both negative and positive tails) for both DS and TD groups. Mean differences in DAHpt and DAHnt were small between categories as shown in Figure 7, and so were not tested for statistical significance.
ROC curve analyses, including the AUC were performed for ambient and tympanometric absorbance to predict CHL compared to NH. The best prediction variable was DALnt, which had an AUC of 0.85 (p<0.0001). The optimal cut point for DALnt to classify ears as CHL or NH was 0.35. The highest ambient-pressure absorbance AUCs were 0.74 at 1.4 kHz and 0.71 at 2 kHz. For absorbance at TPP, AUCs were 0.71 at 1.4 and 2 kHz. The addition of other significant reflectance variables was explored, but combining variables did not improve performance compared to DALnt.
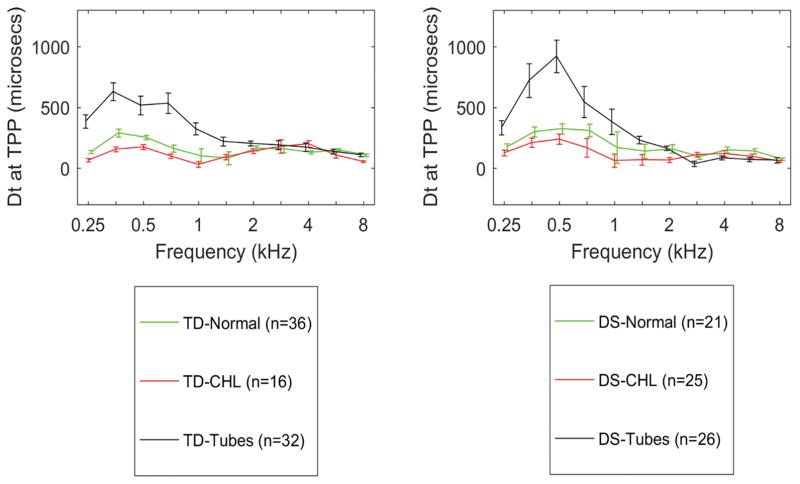
Tympanometric GD
The mean GD measured at the TPP for the diagnostic categories is shown in Figure 8. There were no significant differences in mean GD for ears with CHL compared to NH, and no effect of DS, ear or age. The mean GD in the PET category was significantly larger from 0.25 to 1 kHz than in the NH and CHL categories, with significant effects of DS and age, but not ear. GD differences were not examined for predictive accuracy because their magnitudes in CHL and NH groups were more similar than the absorbance differences.
Figure 8.
Comparison of tympanometric GD at the TPP, in TD (left panel) and DS (right panel) for diagnostic categories of NH, CHL and patent PETs. Error bars are 95% CI.
WB-ARTs
No significant difference was found in median WB-ARTs in NH, CHL or PET categories for either the TD or DS group (Kruskal-Wallis H=4.302, p=0.116).
Discussion
The goal of this study was to evaluate clinical results of WAI tests in children with DS to children who are TD, categorized into three diagnostic categories: NH, CHL, and children with patent PETs. The population included racial diversity, which makes it generalizable to similar populations. However, the study had limitations. Recruitment of children from ENT clinics meant that children who fell into different diagnostic categories tended to fall into different age ranges as well. It was not possible to match ages in the different diagnostic categories. The failure to match ages between TD and DS groups was primarily due to difficulty in both recruiting and reliably testing hearing in younger children with DS. The number of PET surgeries, and duration of PET presence was variable, and may have contributed to the variability in hearing levels, especially for children with DS. Despite this issue, the lack of significant confounding effects of age or DS diagnosis means that the results are likely to be clinically generalizable to a wide age range of children who are TD or who have DS.
In the present sample, NH children with DS had similar absorbance across a wide frequency range compared to the TD group with NH. Absorbance patterns were also similar in the diagnostic categories with CHL in the DS and TD groups, and age was not a significant covariate. GD tended to be slightly less in CHL ears compared to NH ears for low frequencies, but was not significantly different. Interestingly, the TD group with CHL had poorer hearing thresholds than the DS group with CHL (see Figure 4).
Results for the ears with patent PETs showed increased absorbance and increased GD relative to NH and CHL ears. These changes are likely related to the decreased acoustic coupling in ears with patent PETs between the ear canal and the middle-ear cavity. For ears with patent PETs, the hearing thresholds were poorer in the DS group than the TD group (Figure 4), and equivalent volumes tended to be smaller in the DS group with PET compared to the TD group (Table 1). The ambient absorbance in the DS group with PET was significantly lower at all frequencies than the absorbance in the TD group for the ambient-pressure test (Figure 6) and for the tympanometric test at TPP (Figure 7). Thus, lower tympanometric absorbance in the DS group was related to poorer hearing thresholds and smaller equivalent volumes, providing some explanation for the CHL observed in some DS ears in the presence of patent PETs. This may have been because the subjects in the DS group with patent PETs were older, and may have had more longstanding, chronic OME histories. Thus, having a PET placed at an older age may be due to an unresolved, long-standing middle ear disease in children with DS. This finding of hearing loss in some children with DS, despite having patent PETs, has been reported in other studies (Austeng et al., 2013; Paulson et al., 2014; Manickam et al., 2016). Some DS cases had apparent sensorineural components by bone conduction at 2–4 kHz, but these could also represent “Carhart notching” (altered bone conduction transmission due to a middle ear effect) that can occur in the presence of CHL (Stenfelt, 2015). It is possible that some of the children with DS and patent PETs had a mixed hearing loss, since 56% of our sample were unable to provide reliable masked bone conduction thresholds. Iino et al. (1999) reported that hearing thresholds in children with DS were poorer after PET treatment compared with control children, but the children in that study received their first PET at older ages, and about half were tested after the PET had extruded. Manickam et al (2016) reported an overall decrease in hearing function as children with DS age, with normal hearing was present in 44% (right ear) and 38% (left ear). They also reported that SNHL or MHL was present in 11% (right ear) and 9% (left ear). Thus, older children with DS are more likely to have hearing loss despite treatment for middle ear disease, and they often develop a sensory (cochlear) component at older ages.
DPOAEs for children with DS and patent PETs in the present study were lower than the TD group with patent PETs except in the 3–4 kHz range, which was consistent with their overall poorer audiometric thresholds. Because children with DS tended to have PETs for longer durations, the lower DPOAE level in the high frequencies (6–8 kHz) observed in those ears could have been affected by the mass of the long PET lumen. Alternatively, SNHL in the high frequencies is a long-term sequela of chronic otitis media (Hunter et al., 1996; Margolis et al., 2000; Gravel et al., 2006), and DPOAE levels are lower in DS than TD children after the middle-ear disease has been resolved (Hassmann et al., 1998).
Despite the fact that the children with DS and PET were older (8.5 yrs.) than the TD group with PET (3.6 yrs.), and thus would be expected to have larger middle-ear spaces, their tympanometric equivalent volumes were similar. Such a result might occur when there is a smaller middle-ear space, residual tissue or edema due to a longer time period of otitis media (OM) in these children with DS. Smaller equivalent ear-canal volumes in the presence of PET have been associated with more severe OM (Le et al., 1994), poorer pure tone thresholds on post-operative audiometry (Sidell et al., 2014), and higher risk for recurrence of OM (Takasaka et al., 1996).
Both the ambient and tympanometric absorbance at a frequency resolution of one-sixth octave showed multiple narrow peaks in absorbance at frequencies of 0.5, 2 and 5 kHz for a DS case in the patent PET category (Figure 3). This pattern is similar to that found in the ears of TD children with patent PETs, in which ambient absorbance peaks were typically observed in the range of 0.6 and 4 kHz (Sanford & Brockett, 2014). These unusually high peaks were not seen in NH or CHL ears, only in ears with patent PETs. In the present study in both DS and TD groups, the mean ambient absorbance showed an increase in PET ears at frequencies in the range of 0.25 to 1 kHz, with a corresponding increase in GD at all frequencies up to 1.4 kHz (see Figure 6). In the tympanometric data at the TPP, the mean absorbance and mean GD were also elevated in the PET category compared with NH and CHL categories, but to a lesser degree (see Figure 7). While the one-half octave analyses were helpful in testing for statistically significant differences, a diagnostic test for a patent PET might be analyzed over a more narrow frequency range (e.g., one-sixth octave or finer resolution) so as to resolve the more narrow peaks indicative of the patent PET. The present findings extend the results from TD to DS groups.
The characteristic pattern of narrow notches in tympanometric absorbance for patent PETs potentially adds to the clinical utility of WAI to identify patent PETs in children with DS. The children in this study were examined using a gold standard of otomicroscopy, and children who did not have adequate otologic examinations were not enrolled. It is often difficult to identify PET patency through otoscopy due to the small ear canal size and frequent cerumen impaction in children with DS. While tympanometry may be difficult to perform on patients with PET due to inability to obtain a seal (nearly 40% of the cases in our study had inadequate seals in the presence of patent PETs), wideband tympanometry results were obtained on all participants in our study using the research system. WAI provided additional information on frequency ranges in the presence of patent PETs in the DS group.
Ears with CHL in both TD children and children with DS had decreased absorbance across a broad range of frequencies (1–8 kHz) at ambient and peak tympanometric pressures, indicating that the CHL was not due simply to negative pressure. Decreased absorbance in the CHL category may be due either to impedance increases caused by the presence of middle-ear fluid, or some other middle-ear anomaly that may be obstructing the pathway of sound. Further, decreased wideband absorbance correlated with increased air conduction thresholds and decreased DPOAE SNR across a wide range of frequencies for groups with CHL. Importantly, the differences between NH and CHL ears were similar for the DS and TD groups. The low-frequency averaged WBTs had significantly greater absorbance for NH compared to CHL for both DS and TD groups. This finding has clinical relevance, and the commercially available Titan WAI system provides such a low-frequency averaged tympanogram for clinical use. Conversely, high-frequency averaged tympanograms did not show group differences. Low-frequency averaged tympanograms showed that the largest group difference between NH and CHL occurred in the 1–1.4 kHz frequency range. Across a number of wideband variables for absorbance and GD, significant differences were found that theoretically relate to decreased mid-frequency middle-ear transmission with shorter GD in ears with CHL, and increased low-frequency absorbance with longer GD in patent PETs. These findings help to explain the underlying physical reasons for differences in the behavioral audiometry results between groups.
No significant differences were found in median WB-ARTs in NH, CHL or PET categories for either the TD or DS group. The ability to measure the WB-ARs in ears with PET improves over single-frequency immittance methods, which typically show absent reflexes. However, we expected to see increased WB-ARTs based on a recent study using the same instrumentation in infants with CHL (Hunter et al., 2017).
The similarity of wideband absorbance profiles in NH and CHL ears in this cohort, for both TD children and children with DS, validates ambient and tympanometric absorbance as a clinical tool in assessing middle-ear and hearing status. WAI enhances the capacity to predict CHL in children who have intellectual disability and stenotic ear canals, using average absorbance patterns that are distinct from patterns in TD children. WAI may also help detect patent PET through distinct patterns seen in absorbance and GD responses. The fact that similar patterns were apparent in both TD and DS cases as shown in Figs. 1–3 means that despite the multiple challenges facing clinicians assessing children with DS, WAI is a valid tool in this special population. Improvements in identification of CHL in this population may help to initiate earlier treatment, with the goal of reducing hearing loss and associated speech and language deficits.
An advantage of using WAI tests clinically is the use of signal averaging to objectively determine responses instead of relying on subjective visual characteristics of tracings. The research WAI system (Keefe et al., 2015; Keefe et al., submitted) employs signal averaging and artifact rejection, making interpretation of test results more robust.
Conclusions
In summary, the three diagnostic categories showed distinctive patterns for individual recordings that were consistent with group averages. Wideband ambient and tympanometric absorbance showed significantly lower levels in the frequency region from 1–4 kHz for both DS and TD groups with CHL compared with NH, with no overall effects of ear or age. Ears with patent PETs had significantly higher absorbance in the low frequency region for both TD and DS groups (< 1 kHz). Thus, wideband tympanometry was able to distinguish ears with CHL and intact eardrums from ears with patent PETs on the basis of wideband patterns in the low frequencies. The mean GD was larger for ears with patent PETs for both ambient and tympanometric measures, which is a distinct addition to tympanometric absorbance, since GD measures phase effects while absorbance is a magnitude measure. WAI revealed specific patterns for normal hearing, CHL and patent PETs, which may assist clinicians in accurate diagnosis for children with DS. While WAI did not determine the degree of CHL, it is a tool that can detect the presence of significant CHL, assess the patency of PE tubes, and provide frequency-specific information that standard pure tone tympanometry does not provide.
Acknowledgments
Acknowledgments and Funding Source: Research reported in this publication was supported by the National Institute of Deafness and other Communication Disorders of the National Institutes of Health under Award Number R01 DC010202 and an ARRA supplement (DC010202-01S1). Co-author Keefe is involved in commercializing devices to assess middle-ear function. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The content of this article does not represent the views of the Department of Veterans Affairs or of the United States Government.
List of Abbreviations
- AR
acoustic reflex
- ART
acoustic reflex threshold
- AUC
area under the ROC curve
- BBN
broad band noise
- CHL
conductive hearing loss
- CI
confidence interval
- CPA
conditioned play audiometry
- DPOAE
distortion product otoacoustic emission
- DS
Down syndrome
- ER
Energy Reflectance
- Veq
Equivalent volume of the ear canal
- GD
group delay
- HP
high pass
- LP
low pass
- MHL
mixed hearing loss
- OME
otitis media with effusion
- PET
pressure equalization tube
- PTA
Pure tone Average
- ROC
Receiver Operator Characteristic
- SD
standard deviation
- SNHL
sensorineural hearing loss
- SNR
signal to noise ratio
- SPL
sound pressure level
- SA
Static Admittance
- TD
typically developing
- TPP
tympanometric peak pressure
- TW
tympanometric width
- VRA
visual reinforcement audiometry
- WAI
wideband acoustic immittance
- WB-ART
wideband acoustic reflex threshold
References
- Austeng ME, Akre H, Falkenberg ES, Overland B, Abdelnoor M, et al. Hearing level in children with Down syndrome at the age of eight. Res Dev Disabil. 2013;34:2251–2256. doi: 10.1016/j.ridd.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Balkany TJ, Mischke RE, Downs MP, Jafek BW. Ossicular Abnormalities in Down’s Syndrome. Otolaryngol Head Neck Surg. 1979;87:372–384. doi: 10.1177/019459987908700317. [DOI] [PubMed] [Google Scholar]
- Dodd B, Thompson L. Speech disorder in children with Down’s syndrome. J Intell Disabil Res. 2001;45:308–316. doi: 10.1046/j.1365-2788.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- Ellison JC, Gorga M, Cohn E, Fitzpatrick D, Sanford CA, et al. Wideband acoustic transfer functions predict middle-ear effusion. The Laryngoscope. 2012;122:887–894. doi: 10.1002/lary.23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney MP, Sanford CA. Detection of the acoustic stapedius reflex in infants using wideband energy reflectance and admittance. J Am Acad Audiol. 2005;16:278–290. doi: 10.3766/jaaa.16.5.3. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Dierking DM, Johnson TA, Beauchaine KL, Garner CA, Neely ST. A validation and potential clinical application of multivariate analysis of distortion product otoacoustic emission data. Ear Hear. 2005;26:593–607. doi: 10.1097/01.aud.0000188108.08713.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel JS, Roberts JE, Roush J, Grose J, Besing J, et al. Early otitis media with effusion, hearing loss, and auditory processes at school age. Ear Hear. 2006;27:353–368. doi: 10.1097/01.aud.0000224727.45342.e9. [DOI] [PubMed] [Google Scholar]
- Hassmann E, Skotnicka B, Midro AT, Musiatowicz M. Distortion products otoacoustic emissions in diagnosis of hearing loss in Down syndrome. Int J Pediatr Otorhinolaryngol. 1998;45:199–206. doi: 10.1016/s0165-5876(98)00106-2. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Blankenship CM. Middle ear measures. San Diego, CA: Plural; 2017. [Google Scholar]
- Hunter LL, Margolis RH, Rykken JR, Le CT, Daly KA, et al. High frequency hearing loss associated with otitis media. Ear Hear. 1996;17:1–11. doi: 10.1097/00003446-199602000-00001. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Keefe DH, Feeney MP, Fitzpatrick DF, Lin L. Longitudinal development of wideband reflectance tympanometry in normal and at-risk infants. Hear Res. 2016;340:3–14. doi: 10.1016/j.heares.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LL, Keefe DH, Feeney MP, Brown DK, Lin L, Fitzpatrick DF. Wideband pressurized acoustic stapedial reflex thresholds in newborns and infants: normal development and relationship to newborn hearing screening. Accepted for publication in J Association for Research in Otolaryngology. [Google Scholar]
- Iino Y, Imamura Y, Harigai S, Tanaka Y. Efficacy of tympanostomy tube insertion for otitis media with effusion in children with Down syndrome. Int J Pediatr Otorhinolaryngol. 1999;49:143–149. doi: 10.1016/s0165-5876(99)00117-2. [DOI] [PubMed] [Google Scholar]
- Intrapiromkul J, Aygun N, Tunkel DE, Carone M, Yousem DM. Inner ear anomalies seen on CT images in people with Down syndrome. Pediatr Radiol. 2012;42:1449–1455. doi: 10.1007/s00247-012-2490-3. [DOI] [PubMed] [Google Scholar]
- Kaf WA. Wideband energy reflectance findings in presence of normal tympanogram in children with Down’s syndrome. Int J Pediatr Otorhinolaryngol. 2011;75:219–226. doi: 10.1016/j.ijporl.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Bulen JC, Arehart KH, Burns EM. Ear-canal impedance and reflection coefficient in human infants and adults. J Acoust Soc Am. 1993;94:2617–2638. doi: 10.1121/1.407347. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Fitzpatrick D, Liu YW, Sanford CA, Gorga MP. Wideband acoustic-reflex test in a test battery to predict middle-ear dysfunction. Hear Res. 2010;263:52–65. doi: 10.1016/j.heares.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Hunter LL, Patrick, Feeney M, Fitzpatrick DF. Procedures for ambient-pressure and tympanometric tests of aural acoustic reflectance and admittance in human infants and adults. J Acoust Soc Am. 2015;138:3625–3653. doi: 10.1121/1.4936946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Feeney MP, Hunter LL, Fitzpatrick DH. Aural acoustic stapedius-muscle reflex threshold procedures to test human infants and adults. doi: 10.1007/s10162-016-0599-z. Accepted for publication in J Association for Research in Otolaryngology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Sanford CA, Ellison JC, Fitzpatrick DF, Gorga MP. Wideband aural acoustic absorbance predicts conductive hearing loss in children. Int J Audiol. 2012;51:880–891. doi: 10.3109/14992027.2012.721936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le CT, Hunter LL, Margolis RH, Daly KA, Lindgren BR, et al. A clinical profile of otitis media without an intact tympanic membrane. Arch Otolaryngol Head Neck Surg. 1994;120:513–516. doi: 10.1001/archotol.1994.01880290025005. [DOI] [PubMed] [Google Scholar]
- Liu YW, Sanford CA, Ellison JC, Fitzpatrick DF, Gorga MP, et al. Wideband absorbance tympanometry using pressure sweeps: system development and results on adults with normal hearing. J Acoust Soc Am. 2008;124:3708–3719. doi: 10.1121/1.3001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickam V, Shott GS, Heithaus D, Shott SR. Hearing loss in Down Syndrome revisited - 15 years later. Int J Pediatr Otorhinolaryngol. 2016;88:203–7. doi: 10.1016/j.ijporl.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Margolis RH, Hunter LL. Acoustic immitance measurements. New York: Thieme; 2000. [Google Scholar]
- Margolis RH, Saly GL, Hunter LL. High-frequency hearing loss and wideband middle ear impedance in children with otitis media histories. Ear Hear. 2000;21:206–211. doi: 10.1097/00003446-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Maurizi M, Ottaviani F, Paludetti G, Lungarotti S. Audiological findings in Down’s children. Int J Pediatr Otorhinolaryngol. 1985;9:227–232. doi: 10.1016/s0165-5876(85)80038-0. [DOI] [PubMed] [Google Scholar]
- Park AH, Wilson MA, Stevens PT, Harward R, Hohler N. Identification of hearing loss in pediatric patients with Down syndrome. Otolaryngol Head Neck Surg. 2012;146:135–140. doi: 10.1177/0194599811425156. [DOI] [PubMed] [Google Scholar]
- Paulson LM, Weaver TS, Macarthur CJ. Outcomes of tympanostomy tube placement in children with Down syndrome--a retrospective review. Int J Pediatr Otorhinolaryngol. 2014;78:223–226. doi: 10.1016/j.ijporl.2013.10.062. [DOI] [PubMed] [Google Scholar]
- Prieve BA, Feeney MP, Stenfelt S, Shahnaz N. Prediction of conductive hearing loss using wideband acoustic immittance. Ear Hear. 2013;34(Suppl 1):54S–59S. doi: 10.1097/AUD.0b013e31829c9670. [DOI] [PubMed] [Google Scholar]
- Raut P, Sriram B, Yeoh A, Hee KY, Lim SB, et al. High prevalence of hearing loss in Down syndrome at first year of life. Ann Acad Med Singapore. 2011;40:493–498. [PubMed] [Google Scholar]
- Rupa V. Dilemmas in auditory assessment of developmentally retarded children using behavioural observation audiometry and brain stem evoked response audiometry. J Laryngol Otol. 1995;109:605–609. doi: 10.1017/s002221510013083x. [DOI] [PubMed] [Google Scholar]
- Sanford CA, Brockett JE. Characteristics of wideband acoustic immittance in patients with middle-ear dysfunction. J Am Acad Audiol. 2014;25:425–440. doi: 10.3766/jaaa.25.5.2. [DOI] [PubMed] [Google Scholar]
- Shott SR. Down Syndrome: Common otolaryngologic manifestations. American Journal of Medical Genetics. 2006;142C:131–140. doi: 10.1002/ajmg.c.30095. [DOI] [PubMed] [Google Scholar]
- Shott SR, Joseph A, Heithaus D. Hearing loss in children with Down syndrome. Int J Pediatr Otorhinolaryngol. 2001;61:199–205. doi: 10.1016/s0165-5876(01)00572-9. [DOI] [PubMed] [Google Scholar]
- Stenfelt S. Inner ear contribution to bone conduction hearing in the human. Hear Res. 2015;329:41–51. doi: 10.1016/j.heares.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Sidell D, Hunter LL, Lin L, Arjmand E. Risk Factors for Preoperative and Postoperative Hearing Loss in Children Undergoing Pressure Equalization Tube Placement. Otolaryngol Head Neck Surg. 2014;150:1048–1055. doi: 10.1177/0194599814529080. [DOI] [PubMed] [Google Scholar]
- Soares JC, Urosas JG, Calarga KS, Pichelli TS, Limongi SC, Shahnaz N, Carvallo RM. Wideband reflectance in Down syndrome. Int J Pediatr Otorhinolaryngol. 2016;87:164–71. doi: 10.1016/j.ijporl.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Takasaka T, Hozawa K, Shoji F. Tympanostomy tube treatment in recurrent otitis media with effusion. In: Lim BCDJ, Casselbrandt M, Klein JO, Ogra PL, editors. Recent Advances in Otitis Media. Hamilton, Ontario, Canada: BC Decker; 1996. pp. 197–199. [Google Scholar]
- Ypsilanti A, Grouios G, Alevriadou A, Tsapkini K. Expressive and receptive vocabulary in children with Williams and Down syndromes. J Intellect Disabil Res. 2005;49:353–364. doi: 10.1111/j.1365-2788.2005.00654.x. [DOI] [PubMed] [Google Scholar]