Abstract
Objective:
To characterize the brain-infiltrating immune cell repertoire in Rasmussen encephalitis (RE) with special focus on the subsets, clonality, and their cytokine profile.
Methods:
The immune cell infiltrate of freshly isolated brain tissue from RE was phenotypically and functionally characterized using immunohistology, flow cytometry, and T-cell receptor (TCR) deep sequencing. Identification of clonally expanded T-cell clones (TCCs) was achieved by combining flow cytometry sorting of CD4+ and CD8+ T cells and high-throughput TCR Vβ-chain sequencing. The most abundant brain-infiltrating TCCs were isolated and functionally characterized.
Results:
We found that CD4+, CD8+, and also γδ T cells infiltrate the brain tissue in RE. Further analysis surprisingly revealed that not only brain-infiltrating CD8+ but also CD4+ T cells are clonally expanded in RE. All 3 subsets exhibited a Tc1/Th1 phenotype characterized by the production of interferon (IFN)-γ and TNF. Broad cytokine profiling at the clonal level showed strong production of IFN-γ and TNF and also secretion of interleukin (IL)-5, IL-13, and granzyme B, both in CD4+ and CD8+ T cells.
Conclusions:
CD8+ T cells were until now considered the central players in the immunopathogenesis of RE. Our study adds to previous findings and highlights that CD4+ TCCs and γδ T cells that secrete IFN-γ and TNF are also involved. These findings underline the complexity of T-cell immunity in RE and suggest a specific role for CD4+ T cells in orchestrating the CD8+ T-cell effector immune response.
Rasmussen encephalitis (RE) is a rare neurologic disorder mainly affecting children and causing drug-resistant epilepsia partialis continua, intellectual decline, and neurologic deficits in parallel with progressive hemispheric atrophy that, if untreated, will reach the final stage of the disease with fixed severe neurologic symptoms.1 Efforts to characterize the pathogenesis and identify the etiology of RE started already with the description of the disease by Rasmussen et al. in 1958.2 Both viral agents and antibody-mediated immune responses have been suspected or reported to be involved in the pathophysiology of RE with, however, inconsistent results.3–6
The most robust evidence comes from recent research, which showed that RE-affected brain tissue is characterized by clonally expanded CD8+ T-cell infiltrates in the brain tissue, suggesting a specific immunologic reaction to either exogenous or endogenous antigens.7–11 This notion is supported by several small-size treatment studies with immunosuppressive/modulatory agents, which have in part shown promising results.12–16 Given that the most effective treatment remains hemispherectomy/hemispherotomy with a significant risk of functional deterioration, there is a great need for better treatment options directed against pathophysiologic aspects and/or a potential cause of the disease.4
In this direction, we here studied the types of immune cells infiltrating the brain in RE using histopathology and ex vivo characterization of isolated cells by flow cytometry. We further investigated the presence of T-cell clonal (TCC) expansions, generated and characterized for the first time the most frequent TCCs from the brain of a patient with RE, and assessed their functional phenotype.
METHODS
Patients.
Case 1.
A 4-year-old boy developed progressive focal neurologic deficits and seizures. He was diagnosed with bilateral RE based on the clinical symptoms, radiologic findings (figure 1), and histopathologic analysis from brain biopsies derived from both hemispheres. Cerebrospinal fluid (CSF) studies revealed normal glucose and albumin quotient, normal immunoglobulin G (IgG) index, no oligoclonal bands and was negative for neurotropic viruses. The CSF was also tested 10 months later and was normal except for the presence of oligoclonal bands. The boy underwent left vertical parasagittal hemispherotomy 1 and a half year later. Part of the resected brain tissue was obtained for research analyses. Serum was tested and was negative for paraneoplastic antibodies, namely antibodies against NMDA receptor, AMPA, GABA (B), mGluR1, mGluR5, LGI1, and Caspr2. The CSF was also tested and was negative for antibodies against Hu, Ri, Yo, amphiphysin, CV2 (CRMP5), Ta/Ma2, Ma1, SOX1 and GAD, LGI1, Caspr2, and NMDA receptor (for further details, see online case description, appendix e-1, http://links.lww.com/NXI/A11).
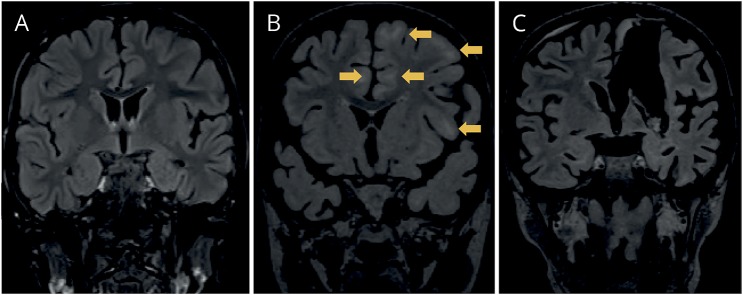
Figure 1. Coronal MRI images showing the evolution of white matter abnormality and atrophy of patient 1.
MRI (fluid-attenuated inversion recovery, FLAIR) in February 2013 (A), in September 2013 (B), and after left vertical parasagittal hemispherotomy in October 2013 (C). The arrows in B show subcortical regions with white matter FLAIR signal abnormality. Note also the progressive atrophy of the brain and the left temporal lobe in particular.
Case 2.
A 36-year-old man underwent MRI showing findings consistent with RE: hyperintense signal in the left cortical and subcortical area with cortical atrophy and enlargement of the lateral ventricle. A brain biopsy was consistent with RE and included in the study.
Case 3.
A female patient with RE underwent a partial resection of the right hemisphere at the age of 7 to treat status epilepticus. Because of drug-resistant epileptic activity, the patient underwent functional hemispherotomy at the age of 25. The brain biopsy of the latter operation was included in this study and was consistent with RE. The brain biopsy from the first operation was not available.
Standard protocol approvals, registrations, and patient consents.
The project was approved by the Cantonal Ethics Committee Zurich (no. 33-2015), informed consent was obtained accordingly from the parents of the 4-year-old boy, and approval was received for the retrospective analyses of cases 2 and 3.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded brain tissue sections of diagnostic brain biopsies (5-μm thickness) were stained on a Leica Bond III–automated immunostaining platform (Leica Biosystems) with the appropriate antibodies (table e-1, http://links.lww.com/NXI/A9) in 1% bovine serum albumin. Tissue sections were analyzed using a Nikon Eclipse 80i light microscope equipped with an Olympus UC30 camera.
Isolation of brain-infiltrating mononuclear cells.
This and all subsequent methods were performed from CNS tissue that was obtained and processed immediately after surgery from RE case 1. The brain biopsy was cut into small pieces and incubated with media containing 1 mg/mL collagenase A (Roche, Basel, Switzerland) and 0.1 mg/mL DNAse I (Roche) at 37°C for 60 minutes. Brain-infiltrating cells were isolated by Percoll density gradient centrifugation (GE Healthcare, Buckinghamshire, United Kingdom). The cells were characterized directly by flow cytometry, expanded with phytohemagglutinin (PHA)-L (Sigma-Aldrich, St. Louis, MO), or cryopreserved until further use.
Flow cytometry.
For the direct phenotypic analysis of the mononuclear brain infiltrate, Fc-binding blocking with human IgG and staining with Live/Dead Aqua (Invitrogen, Waltham, MA) was performed, followed by staining with the appropriate antibodies (table e-1, http://links.lww.com/NXI/A9). Measurements were performed on an LSR II flow cytometer (BD, Franklin Lakes, NJ), and data were analyzed with FlowJo (Ashland, OR).
High-throughput T-cell receptor sequencing.
DNA was isolated using the AllPrep DNA/RNA Mini Kit (Qiagen, Limburg, Netherlands), and high-throughput sequencing for Vβ T-cell receptor (TCR) was performed at Adaptive Biotechnologies (Seattle, WA) using the immunoSEQ platform.17–19
Expansion of brain-infiltrating mononuclear cells and generation of TCCs.
Brain-infiltrating mononuclear cells were expanded as bulk populations by seeding into 96-well plates 2,000 cells/well together with 1.5 × 105 allogeneic irradiated (3,000 radians) peripheral blood mononuclear cells, 1 μg/mL of PHA and human interleukin-2 (IL-2; supernatant kindly provided by Federica Sallusto, PhD, Bellinzona, Switzerland) in IMDM medium supplemented with 2 mM l-glutamine, 100 U/mL penicillin/streptomycin, 50 μg/mL gentamicin, and 5% human serum. IL-2 was added every 3–4 days, and at day 14, cells were pooled and cryopreserved. TCCs were generated by fluorescence activated cell sorting (FACS) (FACSAria III; BD) of specific T-cell populations stained with anti-CD4, anti-CD8, and appropriate anti-TCR Vβ antibodies (Beckman Coulter, Brea, CA) and subsequently cloned by limiting dilution (0.3 cells/well) in 96-well plates. TCCs were then expanded using the above-described protocol and rechecked for purity by sequencing of the specific TCR Vβ chain (Microsynth, Balgach, Switzerland) and flow cytometry.
Cytokine expression profiling of expanded brain-infiltrating T cells.
Brain-infiltrating mononuclear cells were analyzed for cytokine profiling after 1 expansion using intracellular cytokine staining on stimulation with 50 ng/mL phorbol myristate acetate (PMA; Sigma-Aldrich) and 1 μg/mL ionomycin (Sigma-Aldrich) in the presence of GolgiPlug (BD). After 5 hours, T cells were stained with Live/Dead Aqua, fixed and permeabilized with the Cytofix/Cytoperm Kit (BD), and stained with the appropriate antibodies (table e-1, http://links.lww.com/NXI/A9). Unstimulated cells served as controls. Measurements were performed on an LSRFortessa flow cytometer (BD), and data were analyzed with FlowJo.
Cytokine expression profiling of TCCs.
TCCs were stimulated with PMA and anti-CD3 (OKT3 antibody; Janssen-Ortho, Toronto, Canada) for 24 hours. Cytokine secretion of individual TCCs was measured in supernatants using a Th1/Th2/Th17 Cytokine Multi-Analyte ELISArray Kit (Qiagen) and a granzyme B ELISA kit (Mabtech, Nacka Strand, Sweden). Data were analyzed using GraphPad Prism (La Jolla, CA).
RESULTS
All 3 subtypes, CD4+, CD8+, and γδ, are forming the T-cell infiltrate in RE.
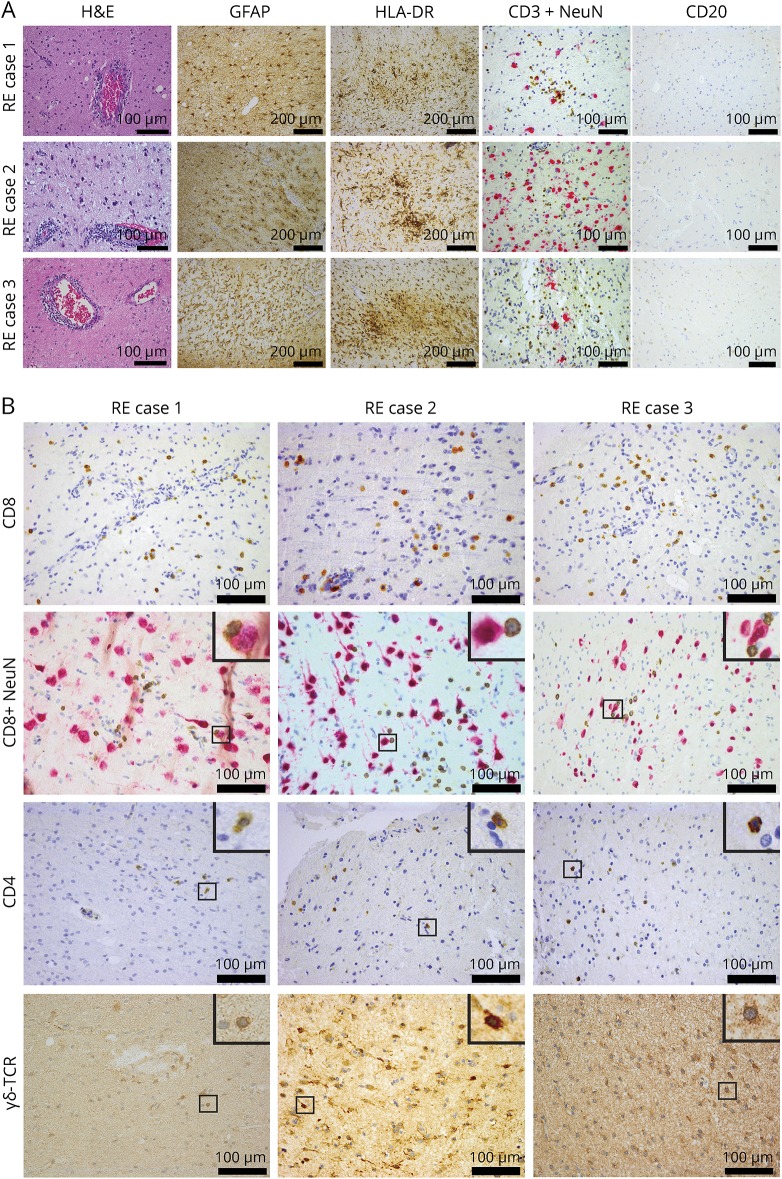
We first performed immunohistochemical analyses to investigate the similarities between the 3 RE cases. All 3 cases displayed prominent astrogliosis, strong microglial activation, T-cell infiltrates, and essential absence of B cells (figure 2A). Further characterization of the T-cell infiltrate confirmed the presence of CD8+ T cells, some of them in direct contact to neurons in accordance with what has been described before.8 The histopathologic findings were thus consistent with RE in all 3 cases. We observed also in all 3 cases the presence of CD4+ T cells both in the perivascular compartment and in the parenchyma as well as infiltrating γδ T cells (figure 2B). Apart from the T-cell staining, the γδ-TCR antibody also displayed a regional staining of glial cells (data not shown).
Figure 2. Immunopathologic analysis of all 3 Rasmussen encephalitis cases.
(A) Perivascular cuffing (hematoxylin and eosin [H&E]), prominent astrogliosis (GFAP), strong microglial activation (HLA-DR staining), T-cell infiltrates (CD3+NeuN), and absence of B cells (CD20) are shown. (B) Representative images showing CD8+ T cells infiltrating the tissue, some of them in proximity or direct contact to neurons (CD8+NeuN) and also, in all 3 cases, CD4+ T cells as well as γδ T cells (γδ-TCR) infiltrating the brain parenchyma. For CD8+NeuN, CD4, and γδ-TCR, a higher magnification of the small regions in squares is shown on the top right inset of each image. Scale bar = 100 μm except for GFAP and HLA-DR = 200 μm. GFAP = glial fibrillary acidic protein; HLA-DR = human leukocyte antigen; RE = Rasmussen encephalitis; TCR = T-cell receptor.
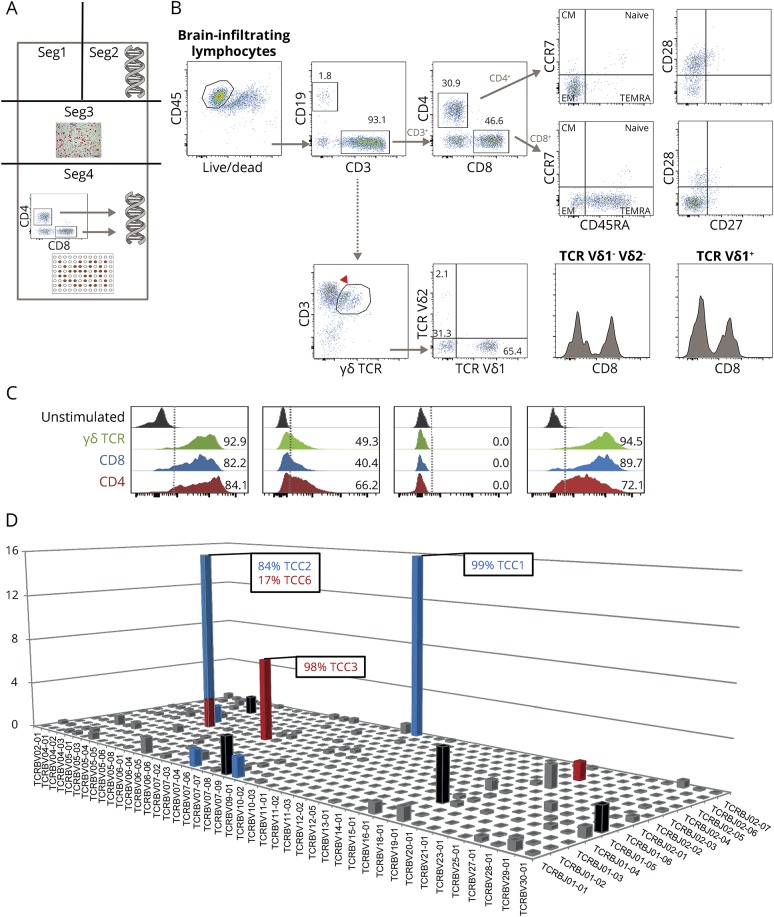
To confirm these histopathologic findings and quantitatively assess and perform a more detailed analysis of the brain-infiltrating cells, we used brain tissue resected at hemispherotomy surgery from case 1. Figure 3A shows the methodological strategy of dissecting the tissue into 4 segments (seg1–seg4) for the various analyses.
Figure 3. Methodological strategy, flow cytometric analysis, cytokine profile, and clonality of brain-infiltrating cells.
(A) Methodological strategy of dissecting the brain tissue (dimensions: 2 × 6 × 1.5 cm) for various analyses: segment (seg) 1 was kept for further analyses. Seg2 was taken for DNA extraction and subsequent high-throughput TCR sequencing. Seg3 was embedded in paraffin and used in immunohistochemical studies. Seg4 was used for isolation of brain-infiltrating mononuclear cells with subsequent (1) phenotypic analyses by flow cytometry, (2) expansion with phytohemagglutinin, FACS of CD4+ and CD8+ T cells, DNA extraction, and subsequent high-throughput TCR sequencing, and (3) T-cell cloning (TCC). (B) Flow cytometry analysis (seg4) of the brain-infiltrating mononuclear cells. (C) Cytokine profile of brain-infiltrating T cells. (D) High-throughput TCR sequencing (seg2) showing oligoclonal expansions of both CD4+ and CD8+ T-cell infiltrates. CM = central memory; EM = effector memory; TCR = T-cell receptor.
Flow cytometric analysis of leukocytes (CD45+) obtained from fresh brain tissue (seg4) revealed a predominant T-cell infiltrate (CD3+, 93.1%) consisting of CD4+ (30.9%) and CD8+ (46.6%) T cells. Both subpopulations displayed effector memory phenotypes. B cells (CD19+) comprised only 1.8% of the cell population, confirming the immunohistochemical analysis. We also found 33.1% of the infiltrating T cells to be γδ T cells with 65.4% being Vδ1+, while only a minority expressed Vδ2+. The remaining fraction of γδ T cells was Vδ1− Vδ2− (most probably Vδ3+ T cells). Both Vδ2+ and Vδ1− Vδ2− T cells were partially also CD8+ (figure 3B). Of note, γδ T cells are a small subset of T cells as compared to αβ T cells, have a distinct TCR on the surface, and constitute only 1%–5% of total blood lymphocytes being mainly Vδ2+. The presence of monocytes (CD14+, 1%) and natural killer cells (CD56+, 2.1%) was sparse (data not shown). To further characterize the brain T-cell infiltrate, CD8+, CD4+, and γδ+ T cells were in vitro PHA expanded (1 round). Analysis of the cytokine profile of these expanded brain-infiltrating T cells revealed that all 3 subpopulations (CD8+, CD4+, and γδ+ T cells) displayed a similar profile expressing the proinflammatory cytokines interferon (IFN)-γ and TNF and in addition the degranulation marker of cytotoxicity CD107a (figure 3C). We thus found that all 3 subtypes, CD4+, CD8+, and γδ, are forming the T-cell infiltrate in RE and express a proinflammatory (Th1-like) profile.
Both CD4+ and CD8+ T-cell brain infiltrates are clonally expanded.
We sequenced the TCR β-chain variable (TCRBV genes) expressed by T cells infiltrating seg2 and seg4 of the RE brain tissue. This sequencing can be used to trace clonal lineages and thus potentially identify the same clonal lineages in seg2 and seg4. Five hundred seventy-six unique productive sequences were identified in seg2. The complete list of Vβ-chain, J-chain, CDR3 sequences and frequencies is given in table e-2, http://links.lww.com/NXI/A10. The 13 most frequent TCCs are highlighted in a 3D histogram (figure 3D) and summarized in figure e-1 (http://links.lww.com/NXI/A8). To discriminate between CD4+ and CD8+ TCCs, we then sequenced the TCRBV chain expressed by sorted CD4+ and CD8+ T cells isolated from seg4 following 1 round of PHA expansion. Five hundred fifty-three and 291 unique productive sequences were identified in the CD4+ and in the CD8+ brain-infiltrating T-cell pool, respectively. The most frequent TCCs in seg2 that are present in the brain-infiltrating CD4+ T-cell pool and CD8+ T-cell pool from seg4 are shown in red and blue, respectively (figure 3D and figure e-1, http://links.lww.com/NXI/A8). Five of the 13 most frequent TCCs in seg2 were not present in the CD4+ or CD8+ compartment of the expanded pools from seg4 (shown in black). The 2 most frequent TCCs in seg2 representing 15.56% and 13.04% of the T-cell infiltrate were both CD8+. The third most expanded TCC in seg2 representing 6.81% of the T-cell infiltrate was a CD4+ TCC. The overall TCC match between seg2 and seg4 was 14.5% for CD4+ and 38.8% for CD8+ TCCs, while 46.7% of the infiltrating T cells in seg2 were not present in seg4. We thus found that both CD4+ and CD8+ T-cell brain infiltrates are clonally expanded in RE case 1.
Functional phenotype of brain-infiltrating clonally expanded TCCs.
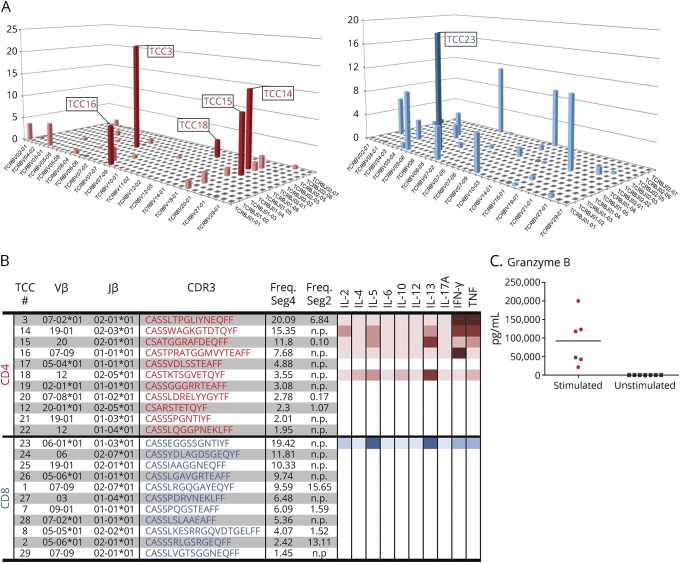
We next generated 5 CD4+ and 1 CD8+ TCC from CD4+ and CD8+ sorted and previously PHA-expanded (1 round) T cells obtained from RE brain tissue seg4. This was performed to characterize the functional phenotype of the individual TCCs found in RE case 1. The 5 CD4+ TCCs obtained were among the 6 most frequent TCCs in the pool of sorted and expanded CD4+ T cells from seg4. The CD8+ TCC was the most frequent one in the CD8+ T-cell pool (figure 4, A and B). Of interest, 2 of the CD4+ TCCs were also present in seg2. Based on our previous studies, a bias as a consequence of 1 round of PHA expansion is unlikely.20 We therefore assume that partially overlapping T-cell repertoires shape the infiltrate in physically separate areas of the brain in this RE case. A search using the BLASTP program of the nonredundant protein database (blast.ncbi.nlm.nih.gov) indicated that none of the TCR sequences found in seg2 and seg4 matched any published sequence in the database.
Figure 4. Cytokine expression profile of individual T-cell clones.
(A) The frequencies of CD4+ (red) or CD8+ (blue) TCCs that were identified in seg4 are shown in a 3D histogram. Note the similar clonality of CD4+ and CD8+ T cells. TCCs that were generated by limiting dilution and analyzed for cytokine expression are numbered and shown in bold. (B) The Vβ-chain, J-chain, and CDR3 sequence of TCRs from the most frequent TCCs as well as the cytokine profile of the derived TCCs are shown. Cytokine production is illustrated with a color gradient from pale to bolder that corresponds to lower and higher cytokine levels, respectively. (C) High levels of granzyme B secretion by both the CD4+ and CD8+ TCCs on stimulation. n.p. = not present. IFN = interferon; IL = interleukin; TCC = T-cell clone; TCR = T-cell receptor.
We examined the functional phenotype of the 6 brain-infiltrating TCCs by analyzing their cytokine profile (figure 4B). Among the CD4+ TCCs, 2 TCCs (TCC3 and TCC16) displayed a Th1 phenotype releasing mainly Th1 cytokines, 2 TCCs (TCC14 and TCC15) had a Th1/2 multifunctional phenotype, and TCC18 released mainly Th2 cytokines. The only CD8+ TCC analyzed (TCC23) released both Th1 and Th2 cytokines and hence exhibited also a multifunctional phenotype (figure 4B). Both the CD4+ TCCs and the CD8+ TCC produced granzyme B on stimulation (figure 4C). We thus observed both Th1 and Th1/2 multifunctional cytotoxic phenotypes of the individual TCCs.
DISCUSSION
In this study, we report that besides CD8+ T cells, both αβ CD4+ TCC and γδ T cells infiltrate the brain in RE. Further analyses using FACS of CD4+ and CD8+ T cells and TCR Vβ-chain high-throughput sequencing from 2 different segments of brain tissue identified that not only the brain-infiltrating CD8+ but also the CD4+ T cells are clonally expanded. Prior evidence has shown that T cell–mediated inflammation in RE is dominated by CD8+ TCCs, while an involvement of CD4+ TCCs has not been considered.7,11,21 In a seminal previous study, the investigators matched the TCR Vβ between blood and brain T cells in 5 patients and found only CD8+ T cells to be present in the RE brain.7 For this reason, our finding that a large fraction of the TCCs that were present in both brain segments, and in particular the third and sixth most frequent TCCs, were CD4+, is surprising. The reason for the discrepancy between the previous study and ours is unclear but could be explained by methodological differences. Although, the previous study included more patients, identification of brain TCCs as being CD4+ or CD8+ T cells was performed by matching them to peripheral blood CD4+ or CD8+ TCCs. On the other hand, the setup and methods used in our study with direct sorting and sequencing from 2 different segments of the brain lesion allowed phenotyping the T-cell subpopulations directly from the brain. In support of our findings, histopathologic studies have also reported the presence of CD4+ T cells in the RE brain.22–25 Our study, which documents not only the presence but also clonal expansion of CD4+ T cells, indicates that CD4+ T cells are likely involved in the disease process of RE and driven by a specific antigen. Given that this finding is derived from a bilateral RE case, further studies are needed to investigate whether this is also true for more typical RE cases in which only 1 hemisphere is affected.
In addition to the oligoclonal expansion of CD4+ and CD8+ T cells, the presence of γδ T cells suggests further complexity of the immunopathogenesis. We found that a major fraction (one-third) of the brain-infiltrating γδ T cells belong to the non-Vδ2+ subtype. Because blood γδ T cells are mainly Vδ2+, our finding indicates a specific infiltration rather than bystander recruitment into the brain. Almost half of the γδ T cells were positive for CD8, which suggests that prior histopathologic assessments of RE tissue with anti-CD8 antibodies might have overestimated the contribution of CD8+ αβ T cells because at least a significant proportion of the CD8+ T cells belong to the γδ T-cell compartment. γδ T cells constitute 1%–5% of total blood lymphocytes and approximately 50% of the lymphocytes in skin and mucosal tissues. They mediate defense mechanisms against bacteria, viruses, and protozoa and play a role in cancer, wound healing, and autoimmune diseases.26 Regarding CNS autoimmunity, previous studies have shown that γδ T cells are present in MS lesions, are oligoclonally expanded, and probably can react against heat-shock proteins.27–29 It is important that a recent study has also reported the presence of γδ (CD4−CD8−Vδ1+) T cells with a restricted T-cell repertoire in RE.30 Thus, because γδ T cells are implicated in RE and both in viral/infectious and autoimmune diseases of the CNS, they should be included in future efforts to uncover the etiology of the disease.
A previous study comparing brain tissue from patients with RE and cortical dysplasia analyzed the cytokine profile in RE using reverse transcription PCR and showed evidence for a Th1/Tc1 response.24 We here report the cytokine profile of the most frequent, CNS-infiltrating T-cell populations and individual TCCs in the brain. Individual CD4+ TCCs displayed a Th1 phenotype with IFN-γ and TNF secretion or a more complex multifunctional phenotype with additional secretion of IL-5 and IL-13. When investigating all 3 subpopulations of brain-infiltrating cells, namely CD4+, CD8+, and γδ T cells, the majority of the cells expressed IFN-γ and TNF but not IL-17.
In its most common form, RE affects only 1 hemisphere, but rare cases exist, in which the disease starts at earlier age, is more severe, and affects both hemispheres.31 In case 1 with bilateral hemispheric involvement, the clinical course of the disease, involving the typical RE prodromal period, the intractable focal seizures followed by the acute stage until surgery, the MRI findings and evolution, the absence of antibodies involved in autoimmune encephalopathies, the histopathology being in accordance with the Bien diagnostic criteria and compatible with what usually is seen in typical unilateral RE cases, as evaluated by 2 independent neuropathologists, the classic finding of T cells being in contact to neurons, and, finally, the response to surgical treatment strongly support that this is one of the very rare cases of bilateral RE.32 The presence of brain-infiltrating γδ T cells in all 3 cases also strongly suggests that bilateral and unilateral cases have a similar pathogenesis.
Taken together, our data fit well with previous knowledge and provide new insights regarding the immunopathogenesis of RE. Previous studies in RE have demonstrated that CD8+ T cells cluster around neurons that express phosphorylated STAT1 and show loss of synapses. In a model of viral encephalitis, this process of synapse elimination depended on IFN-γ production by CD8+ T cells,33 and we here provide evidence that brain-derived CD8+ T cells indeed produce IFN-γ in RE. The CD8+ TCC in addition expresses granzyme B, indicating that CD8+ T cells can induce death of astrocytes and neurons that express MHC class I through direct cell-cell contact and release of granzyme B.8,9,21 The strong expression of MHC class II and the presence of CD4+ TCCs suggest that CD4+ T cells react to specific brain antigens and secrete proinflammatory cytokines that play a role in orchestrating and/or supporting the CD8+ T-cell response. Although the helper function of CD4+ T cells may be their most important role, CD4+ TCCs produce IFN-γ and granzyme B at levels similar or even higher than CD8+ TCCs. Hence, CD4+ T cells may also act as cytotoxic effectors, i.e., by the above-mentioned IFN-γ–mediated elimination of synapses, by inducing glial apoptosis and by enhancing tissue damage via granzyme B.33–36 Finally, the identification of brain-infiltrating γδ T cells as part of the inflammatory process in RE implicates a third T-cell subtype.
Overall, we find that the T-cell infiltrate in RE is considerably more complex than previously thought. While our data do not allow to discern which of the T-cell subtypes initiates the inflammatory process, their common cytokine patterns indicate that they jointly participate in the pathogenesis of RE. It will be important now to identify the target antigens of both CD4+ and CD8+ TCCs and clarify if these are self- or foreign antigens and if molecular mimicry is involved. Future studies about the possible antigens causing or driving the disease should investigate all 3 T-cell populations, CD8+ and CD4+ TCCs, and γδ T cells. To this end, we have expanded for the first time the most prominent CD4+ and CD8+ TCCs, which can be used as important tools in unbiased approaches to identify possible antigens involved in the etiology and/or pathogenesis of RE.
ACKNOWLEDGMENT
The authors thank Nuria Vilarrasa Diaz (Neuroimmunology and Multiple Sclerosis Research Section, University Hospital of Zurich, Zurich, Switzerland) for the technical help with the brain tissue processing and Klaus Dornmair, PhD (Institute of Clinical Neuroimmunology, Ludwig-Maximilians University, Munich, Germany), for allowing them to process the brain tissue in his laboratory and Brigitte Piccapietra (Institute of Neuropathology, University Hospital Zurich, Zurich, Switzerland) for excellent technical assistance with the immunohistochemical stainings.
GLOSSARY
- IFN
interferon
- IgG
immunoglobulin G
- IL
interleukin
- PHA
phytohemagglutinin
- PMA
phorbol myristate acetate
- RE
Rasmussen encephalitis
- TCC
T-cell clone
- TCR
T-cell receptor
- TCRBV
TCR β-chain variable
AUTHOR CONTRIBUTIONS
Faiez Al Nimer and Ivan Jelcic: contributed to the design of the study, analysis and interpretation of the data, and drafting/revising the manuscript for intellectual content. Christian Kempf: contributed immunohistochemical data, neuropathologic analysis, and revising the manuscript for intellectual content. Tom Pieper: contributed clinical and radiologic data and revising the manuscript for intellectual content. Herbert Budka: contributed immunohistochemical data, neuropathologic analysis, and revising the manuscript for intellectual content. Mireia Sospedra and Roland Martin: contributed to the design of the study, analysis and interpretation of the data, and drafting/revising the manuscript for intellectual content.
STUDY FUNDING
The Neuroimmunology and Multiple Sclerosis Research Section is supported by the Clinical Research Priority Program on Multiple Sclerosis (CRPPMS) of the University of Zurich, Switzerland. The project was supported by the European Research Council Advanced Grant 340733 - HLA-DR15 in MS of R. Martin.
DISCLOSURE
F. Al Nimer received research support from the Swedish Society for Medical Research. I. Jelcic, C. Kempf, and T. Pieper report no disclosures. H. Budka received travel funding from EC, University of Edinburgh, Healthy Aging Research Centre London, ECDC, and Regional Government of Styria; served on the editorial board of PRION, The Scientific World Journal: Neurology, The Open Neurology Journal, Neuropathology, Neurology and Therapy; receives publishing royalties from CRC Press; consulted for State Attorneys or Forensic Medical Institutes in Austria; and received research support from Swiss Ministry for Health and University Hospital Zurich. M. Sospedra reports no disclosures. R. Martin served on the scientific advisory board of Biogen, Merck Serono, Teva, Genzyme, Sanofi-Aventis, CellProtect, and Neuway; received travel funding and/or speaker honoraria from Biogen, Merck Serono, Novartis, Roche, and Genzyme; holds a patent claiming the therapeutic efficacy of anti-CD25 monocolonal antibody treatment in combination with IFN-β in MS; consulted for the Myelin Repair Foundation, The Weatherall Institute of Molecular Studies, the University of Oxford, and the Hertie Foundation; is a member of the Kuratorium of the Jung Foundation for Science, Hamburg, Germany; received research support from Novartis, Biogen, the Swiss National Science Foundation, the European Union Seventh Framework Program, the European Research Council, and the University of Zurich; holds stock in CellProtect; and receives royalty payments from the NIH. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Bien CG, Widman G, Urbach H, et al. . The natural history of Rasmussen's encephalitis. Brain 2002;125:1751–1759. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen T, Olszewski J, Lloydsmith D. Focal seizures due to chronic localized encephalitis. Neurology 1958;8:435–445. [DOI] [PubMed] [Google Scholar]
- 3.Mantegazza R, Bernasconi P, Baggi F, et al. . Antibodies against GluR3 peptides are not specific for Rasmussen's encephalitis but are also present in epilepsy patients with severe, early onset disease and intractable seizures. J Neuroimmunol 2002;131:179–185. [DOI] [PubMed] [Google Scholar]
- 4.Bien CG, Schramm J. Treatment of Rasmussen encephalitis half a century after its initial description: promising prospects and a dilemma. Epilepsy Res 2009;86:101–112. [DOI] [PubMed] [Google Scholar]
- 5.Jay V, Becker LE, Otsubo H, et al. . Chronic encephalitis and epilepsy (Rasmussen's encephalitis): detection of cytomegalovirus and herpes simplex virus 1 by the polymerase chain reaction and in situ hybridization. Neurology 1995;45:108–117. [DOI] [PubMed] [Google Scholar]
- 6.Vinters HV, Wang R, Wiley CA. Herpesviruses in chronic encephalitis associated with intractable childhood epilepsy. Hum Pathol 1993;24:871–879. [DOI] [PubMed] [Google Scholar]
- 7.Schwab N, Bien CG, Waschbisch A, et al. . CD8+ T-cell clones dominate brain infiltrates in Rasmussen encephalitis and persist in the periphery. Brain 2009;132:1236–1246. [DOI] [PubMed] [Google Scholar]
- 8.Bien CG, Bauer J, Deckwerth TL, et al. . Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen's encephalitis. Ann Neurol 2002;51:311–318. [DOI] [PubMed] [Google Scholar]
- 9.Bauer J, Elger CE, Hans VH, et al. . Astrocytes are a specific immunological target in Rasmussen's encephalitis. Ann Neurol 2007;62:67–80. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Uccelli A, Laxer KD, et al. . Local-clonal expansion of infiltrating T lymphocytes in chronic encephalitis of Rasmussen. J Immunol 1997;158:1428–1437. [PubMed] [Google Scholar]
- 11.Schneider-Hohendorf T, Mohan H, Bien CG, et al. . CD8(+) T-cell pathogenicity in Rasmussen encephalitis elucidated by large-scale T-cell receptor sequencing. Nat Commun 2016;7:11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews PI, Dichter MA, Berkovic SF, Newton MR, McNamara JO. Plasmapheresis in Rasmussen's encephalitis. Neurology 1996;46:242–246. [DOI] [PubMed] [Google Scholar]
- 13.Leach JP, Chadwick DW, Miles JB, Hart IK. Improvement in adult-onset Rasmussen's encephalitis with long-term immunomodulatory therapy. Neurology 1999;52:738–742. [DOI] [PubMed] [Google Scholar]
- 14.Bien CG, Gleissner U, Sassen R, Widman G, Urbach H, Elger CE. An open study of tacrolimus therapy in Rasmussen encephalitis. Neurology 2004;62:2106–2109. [DOI] [PubMed] [Google Scholar]
- 15.Bittner S, Simon OJ, Göbel K, Bien CG, Meuth SG, Wiendl H. Rasmussen encephalitis treated with natalizumab. Neurology 2013;81:395–397. [DOI] [PubMed] [Google Scholar]
- 16.Granata T, Fusco L, Gobbi G, et al. . Experience with immunomodulatory treatments in Rasmussen's encephalitis. Neurology 2003;61:1807–1810. [DOI] [PubMed] [Google Scholar]
- 17.Robins HS, Campregher PV, Srivastava SK, et al. . Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood 2009;114:4099–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson CS, Emerson RO, Sherwood AM, et al. . Using synthetic templates to design an unbiased multiplex PCR assay. Nat Commun 2013;4:2680. [DOI] [PubMed] [Google Scholar]
- 19.Robins H, Desmarais C, Matthis J, et al. . Ultra-sensitive detection of rare T cell clones. J Immunol Methods 2012;375:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jelcic I, Jelcic I, Kempf C, et al. . Mechanisms of immune escape in central nervous system infection with neurotropic JC virus variant. Ann Neurol 2016;79:404–418. [DOI] [PubMed] [Google Scholar]
- 21.Liblau RS, Gonzalez-Dunia D, Wiendl H, Zipp F. Neurons as targets for T cells in the nervous system. Trends Neurosci 2013;36:315–324. [DOI] [PubMed] [Google Scholar]
- 22.Prayson RA, Frater JL. Rasmussen encephalitis: a clinicopathologic and immunohistochemical study of seven patients. Am J Clin Pathol 2002;117:776–782. [DOI] [PubMed] [Google Scholar]
- 23.Pardo CA, Vining EP, Guo L, Skolasky RL, Carson BS, Freeman JM. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia 2004;45:516–526. [DOI] [PubMed] [Google Scholar]
- 24.Owens GC, Huynh MN, Chang JW, et al. . Differential expression of interferon-gamma and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response. J Neuroinflammation 2013;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirones I, de Prada I, Gomez AM, et al. . A role for the CXCR3/CXCL10 axis in Rasmussen encephalitis. Pediatr Neurol 2013;49:451–457.e1. [DOI] [PubMed] [Google Scholar]
- 26.Latha TS, Reddy MC, Durbaka PV, Rachamallu A, Pallu R, Lomada D. γδ T cell-mediated immune responses in disease and therapy. Front Immunol 2014;5:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci USA 1992;89:4588–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hvas J, Oksenberg JR, Fernando R, Steinman L, Bernard CC. Gamma delta T cell receptor repertoire in brain lesions of patients with multiple sclerosis. J Neuroimmunol 1993;46:225–234. [DOI] [PubMed] [Google Scholar]
- 29.Shimonkevitz R, Colburn C, Burnham JA, Murray RS, Kotzin BL. Clonal expansions of activated gamma/delta T cells in recent-onset multiple sclerosis. Proc Natl Acad Sci USA 1993;90:923–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owens GC, Erickson KL, Malone CC, et al. . Evidence for the involvement of gamma delta T cells in the immune response in Rasmussen encephalitis. J Neuroinflammation 2015;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andermann F, Farrell K. Early onset Rasmussen's syndrome: a malignant, often bilateral form of the disorder. Epilepsy Res 2006;70(suppl 1):S259–S262. [DOI] [PubMed] [Google Scholar]
- 32.Bien CG, Granata T, Antozzi C, et al. . Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 2005;128:454–471. [DOI] [PubMed] [Google Scholar]
- 33.Kreutzfeldt M, Bergthaler A, Fernandez M, et al. . Neuroprotective intervention by interferon-gamma blockade prevents CD8+ T cell-mediated dendrite and synapse loss. J Exp Med 2013;210:2087–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhela S, Kempsell C, Manohar M, et al. . Nonapoptotic and extracellular activity of granzyme B mediates resistance to regulatory T cell (Treg) suppression by HLA-DR-CD25hiCD127lo Tregs in multiple sclerosis and in response to IL-6. J Immunol 2015;194:2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buzza MS, Zamurs L, Sun J, et al. . Extracellular matrix remodeling by human granzyme B via cleavage of vitronectin, fibronectin, and laminin. J Biol Chem 2005;280:23549–23558. [DOI] [PubMed] [Google Scholar]
- 36.Zaguia F, Saikali P, Ludwin S, et al. . Cytotoxic NKG2C+ CD4 T cells target oligodendrocytes in multiple sclerosis. J Immunol 2013;190:2510–2518. [DOI] [PubMed] [Google Scholar]