Mitochondrial complex I, the largest component of the mitochondrial respiratory chain, comprises 44 subunits of which 7 are encoded by the mitochondrial genome and the remainder by the nuclear genome.1 Isolated complex I deficiencies represent a major contribution within the group of respiratory chain defects.2 We report an atypical case carrying a homozygous NDUFS4 missense mutation, with late-onset multifocal dystonia, in contrast to expected clinical phenotypes due to other NDUFS4 mutations, which have been constantly reported to be responsible for Leigh syndrome of early onset and death.3
Case report.
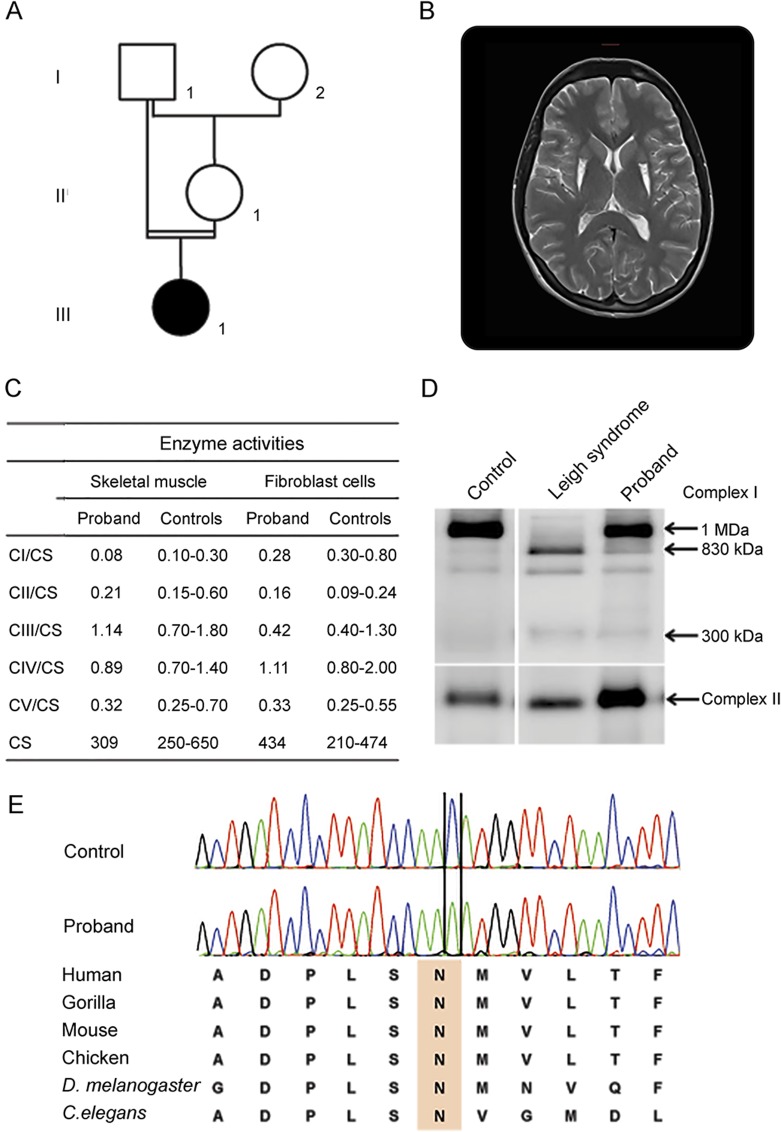
A 29-year-old woman was followed up for years in our clinics who had progressive multifocal dystonia and parkinsonism since the age of 12 years when first symptoms appeared with hand tremor and muscular contractures of both arms. She was the only child born to first-degree consanguineous asymptomatic parents with unremarkable family history (figure, A). Dystonia and bradykinesia did not progress over the following years. Examination at age 29 years revealed fixed dystonia involving shoulders and both arms and the lower face responsible for mild dysarthria, associated with severe bradykinesia of the face, amyotrophic arms and hands, and a mild restrictive respiratory failure due to chronic mild myopathy. The trunk and legs were not affected by dystonia. Gait and cognitive functions were preserved. The patient gave birth to a healthy boy at the age of 25 years with an uneventful pregnancy.
Figure. Patient's pedigree, paraclinical tests, and novel NDUFS4 gene mutation.
(A) Pedigree of the proband III1. (B) Patient brain MRI. T2-weighted MRI showed bilateral posterior putaminal atrophy and hyperintensities. (C) Mitochondrial respiratory enzyme activities of muscle and fibroblasts from the NDUFS4 patient and controls. Mitochondrial respiratory chain and citrate synthase (CS) activities were determined by spectrophotometric analysis. CS activity is expressed in nanomoles per minute per milligram. Mitochondrial respiratory chain data are expressed as the ratio of complexes/CS activities. (D) Blue native polyacrylamide gel (BN-PAGE) analysis from NDUFS4 patients: a proband and a patient with severe Leigh syndrome carrying nonsense mutations (c.262C>T; p.Gln88X and c.316C>T; p.Arg106X) and control using antibodies against NDUFB6 and NDUFS3 subunits detecting complex I and SDHA (70 kDa) revealing complex II. Primary fibroblasts were cultured from the patient and controls, and BN-PAGE analysis was performed as described elsewhere.7 (E) Identification of the c.369C>A mutation in the proband compared with wild-type and interspecies amino acid comparison, showing that the asparagine (N) at position 123 is highly conserved (in orange).
Brain MRI revealed bilateral atrophy of the posterior part of the putamen with T2 hyperintensities related to the dystonic features, without lactate peak (figure, B). Needle EMG showed diffuse myogenic polyphasic waves, compatible with a chronic muscular defect without signs of peripheral neuropathy. Lactate levels were normal both in blood and CSF. The coenzyme Q10 level was low in CSF. Electrocardiogram and ophthalmologic examination were unremarkable. Muscle biopsy did not reveal structural abnormalities. Measurement of mitochondrial enzyme activities revealed, however, a moderate reduction of complex I enzyme activity in both muscle and fibroblasts (figure, C). The patient was given coenzyme Q10 (150 mg/d) since the age of 22 years and received routine injections of botulinum toxin into dystonic muscles.
The complex I enzyme deficiency identified in muscle prompted us to look for molecular abnormalities. Blood sample was obtained from the patient after written informed consent was obtained. The entire mitochondrial genome sequence did not reveal pathogenic mutations. Thereafter, targeted resequencing of 179 nuclear-encoded mitochondrial genes, including all known structural and assembly complex I genes, was performed (table e-1, http://links.lww.com/NXG/A10), revealing a novel homozygous c.369C>A mutation in exon 4 of NDUFS4, confirmed by Sanger sequencing, changing an evolutionary conserved asparagine in lysine at position 123 (p.Asn123Lys) (figure, E). No other candidate mutation was identified in complex I genes. The c.369C>A mutation has robust bioinformatics damaging predictions absent from publicly available databases and classified as a likely pathogenic variant according to the American College of Medical Genetics and Genomics criteria (table e-2, http://links.lww.com/NXG/A11).
To date, NDUFS4 has been implicated only in individuals having Leigh syndrome who were compound heterozygous for null mutations.4 The late onset and the mild phenotype observed in the patient could be due to the homozygozity for the c.369C>A missense mutation.
To further support the pathogenicity of this novel variant, the effects of the mutation on complex I assembly were investigated in the skin fibroblasts of the patient. Blue native polyacrylamide gel revealed a moderate assembly defect with the presence of assembly intermediates of 830 kDa and 300 kDa, respectively, of the N module of complex I in the patient's cells compared with control cells. By contrast, a patient with typical severe Leigh syndrome carrying NDUFS4 compound heterozygous nonsense mutations showed complete absence of the complex I holoenzyme and stronger accumulation of complex I assembly intermediates (figure, D).
Discussion.
It has been recently stated that NDUFS4 patients are invariably affected with neonatal and early-onset Leigh syndrome characterized by a severe complex I deficiency leading to early death occurring before the age of 3 years.3 Moreover, in previous studies, all patient cells carrying NDUFS4 mutations had a much more severe complex I enzyme deficiency with a constant and similar abnormal complex I assembly profile.4
In addition, brain MRI was also atypical in our patient, without brain stem abnormalities considered as one of the most specific signs pointing to a nuclear-encoded complex I deficiency.4–6
Dystonia appears to be a common feature in complex I–deficient patients due to mutations in nuclear-encoded genes, present in approximately 32% of 130 patients from a recently reported series.2 Our results indicate that dystonia and movement disorders can also be caused by NDUFS4 mutations, emphasizing the need for extensive genetic investigation, thanks to the new sequencing technologies. These findings underscore the role of NDUFS4 in the dysfunction of respiratory complex I in patients having mitochondrial disorders and expand the clinical phenotype due to NDUFS4 mutations.
Footnotes
Author contributions: Celine Bris: acquisition and interpretation of data. Tiphaine Rouaud and Magalie Barth: clinical investigation and analysis and interpretation of data. David Goudenege: in silico analysis and analysis and interpretation of data. Valerie Desquiret-Dumas and Naig Gueguen: study concept and design and acquisition of data. Patrizia Amati-Bonneau: analysis and interpretation of data. Guy Lenaers, Dominique Bonneau, and Pascal Reynier: critical revision of the manuscript for intellectual content. Anne-Sophie Lebre: study concept and design and critical revision of the manuscript for intellectual content. Vincent Procaccio: study concept and design, critical revision of the manuscript for intellectual content, and study supervision.
Study funding: Study funded by Angers University Hospital, GIS Institut des Maladies Rares A12119KS, and Association contre les Maladies Mitochondriales.
Disclosure: C. Bris and T. Rouaud report no disclosures. V. Desquiret-Dumas and N. Gueguen have received research support from Fondation Maladies Rares. D. Goudenege, M. Barth, D. Bonneau, P. Amati-Bonneau, G. Lenaers, P. Reynier, and A.-S. Lebre report no disclosures. V. Procaccio has received research support from Angers University Hospital, GIS Institut des Maladies Rares (A12119KS), and Association contre les Maladies Mitochondriales. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was funded by the authors.
References
- 1.Fassone E, Rahman S. Complex I deficiency: clinical features, biochemistry and molecular genetics. J Med Genet 2012;49:578–590. [DOI] [PubMed] [Google Scholar]
- 2.Koene S, Rodenburg RJ, van der Knaap MS, et al. . Natural disease course and genotype-phenotype correlations in Complex I deficiency caused by nuclear gene defects: what we learned from 130 cases. J Inherit Metab Dis 2012;35:737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortigoza-Escobar JD, Oyarzabal A, Montero R, et al. . Ndufs4 related Leigh syndrome: a case report and review of the literature. Mitochondrion 2016;28:73–78. [DOI] [PubMed] [Google Scholar]
- 4.Assouline Z, Jambou M, Rio M, et al. . A constant and similar assembly defect of mitochondrial respiratory chain complex I allows rapid identification of NDUFS4 mutations in patients with Leigh syndrome. Biochim Biophys Acta 2012;1822:1062–1069. [DOI] [PubMed] [Google Scholar]
- 5.Leshinsky-Silver E, Lebre AS, Minai L, et al. . NDUFS4 mutations cause Leigh syndrome with predominant brainstem involvement. Mol Genet Metab 2009;97:185–189. [DOI] [PubMed] [Google Scholar]
- 6.Lebre AS, Rio M, Faivre d'Arcier L, et al. . A common pattern of brain MRI imaging in mitochondrial diseases with complex I deficiency. J Med Genet 2011;48:16–23. [DOI] [PubMed] [Google Scholar]
- 7.Leman G, Gueguen N, Desquiret-Dumas V, et al. . Assembly defects induce oxidative stress in inherited mitochondrial complex I deficiency. Int J Biochem Cell Biol 2015;65:91–103. [DOI] [PubMed] [Google Scholar]