Abstract
Tumor hypoxia has been widely explored over the years as a diagnostic and therapeutic marker. Herein, we synthesized an alkyne functionalized version of evofosfamide, a hypoxia-selective prodrug. The purpose of this effort was to investigate if this novel 2-nitroimidazole phosphoramide nitrogen mustard (2-NIPAM) retained hypoxia selectivity and could be utilized in radiopharmaceutical development to significantly increase retention of conjugated agents in hypoxic cells. 2-NIPAM demonstrated good hypoxia selectivity with a 62- and 225-fold increase in cytotoxicity toward PC-3 and DU145 human prostate cancer cell lines, respectively, under hypoxic conditions. Radiolabeling of 2-NIPAM with 125I was accomplished through a Cu(I)-mediated azide–alkyne cycloaddition reaction. The 125I-conjugate demonstrated 13.6 and 17.8% lower efflux rates for DU145 and PC-3 cells, correspondingly, under hypoxic conditions, suggesting that the increased retention is likely due to the known intracellular trapping mechanism. In conclusion, these studies demonstrate the potential of 2-NIPAM in serving as a trapping agent for radiopharmaceutical development.
Keywords: 2-NIPAM, prodrug, radioiodination, prostate cancer
Hypoxia is a well-known characteristic of many solid cancers. This phenomenon is attributable to the chaotic nature of tumor vasculature, leading to the inefficient delivery of oxygen and other nutrients and resulting in a heterogeneous distribution of these materials in the tumor.1,2 Tumor hypoxia has been implicated in both drug and radiation resistance, which has caused treatment failure in numerous cancers.3,4 Given this, it is not surprising that many researchers over the years have focused on the design of prodrugs that aim to exploit tumor hypoxia for diagnostic and therapeutic applications.5−8
Up to now, various hypoxia based diagnostic (e.g., 18F-FMISO) and therapeutic (e.g., AQ4N, PR-104, and evofosfamide) prodrugs have been under development.9−12 The hypoxia selectivity of many of these agents is based on bioreductive mechanisms, leading to the transformation of these drugs into reactive species in hypoxic tissues.13 These reactive species are typically electrophilic metabolites that exhibit increased retention in hypoxic cells by forming intracellular adducts with opportunistic nucleophilic biomolecules. For hypoxia-selective diagnostic agents, the increased formation and retention of these adducts in hypoxic tissues allows the generation of target-to-nontarget ratios benefiting in vivo imaging of hypoxic tissues.14 From a therapeutic perspective, hypoxia-activated chemotherapeutic agents typically generate radicals (e.g., TPZ) or electrophilic mustard agents (e.g., PR-104 and evofosfamide) that can induce DNA damage, which ultimately prompts cancer cell death.15−17 The advantage of these prodrugs is the increased selectivity and cytotoxicity for hypoxic tumors relative to normoxic nontarget tissues.
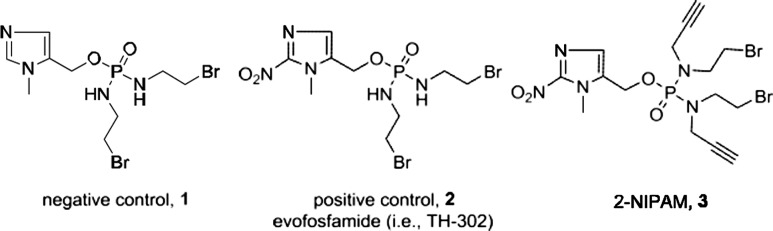
Our laboratory is interested in the development of diagnostic and radiotherapeutic agents that can utilize the hypoxic nature of cancers. Previously, we have found that when 2-nitroimdazoles, a class of hypoxia-selective prodrugs, are conjugated to receptor-targeted peptides, these bioconjugates exhibit increased long-term retention in hypoxic cells, presumably through adducts formed with intracellular biomolecules.14,18 This initial work has given us the impetus for the continued investigation and development of other prodrugs that may serve as hypoxia-selective trapping agents (HSTAs). One of the potential candidates is the 2-nitroimidazole-5-yl methyl-based compound evofosfamide (i.e., TH-302), which is depicted as 2 in Figure 1. Evofosfamide is a hypoxia-activated prodrug that has undergone several phase I/II/III clinical trials for the treatment of advanced solid cancers, including sarcomas and oesophageal adenocarcinomas.19−21 This prodrug is activated through reduction facilitated by cellular reductases (e.g., cytochrome P450) to generate a radical anion. Under hypoxic conditions, the radical anion is exposed to further reduction, leading to the fragmentation and generation of an active nitrogen mustard.17 The goal of our work is to develop a derivative of evosfosfamide that can be easily conjugated to imaging agents (e.g., fluorophores or radioisotopes) for the in vitro and in vivo detection of hypoxia or to targeting vectors to enhance the retention of these agents in hypoxic tissues. Herein, we describe the synthesis of a novel 2-nitroimidazole phosphoramide nitrogen mustard (2-NIPAM, 3) that is capable of conjugating to other moieties through the well-known azide–alkyne cycloaddition reaction. Utilizing human prostate cancer PC-3 and DU145 cell lines, we have investigated the cytotoxicity and hypoxia selectivity of this analog relative to evosfosfamide (2) and a negative control (i.e., nonactivatable, 1). In addition, we optimized the azide–alkyne cycloaddition reaction conditions for 2-NIPAM, radiolabeled the analog with 125I, and investigated the hypoxia trapping capability of this radiolabeled agent under normoxic and hypoxic conditions. The results obtained from our investigations have suggested that 2-NIPAM has the potential to serve as a hypoxia-selective imaging agent and/or trapping agent for receptor-targeted constructs.
Figure 1.
Chemical structure of phosphamide mustard negative control 1, positive control 2, and 2-NIPAM 3.
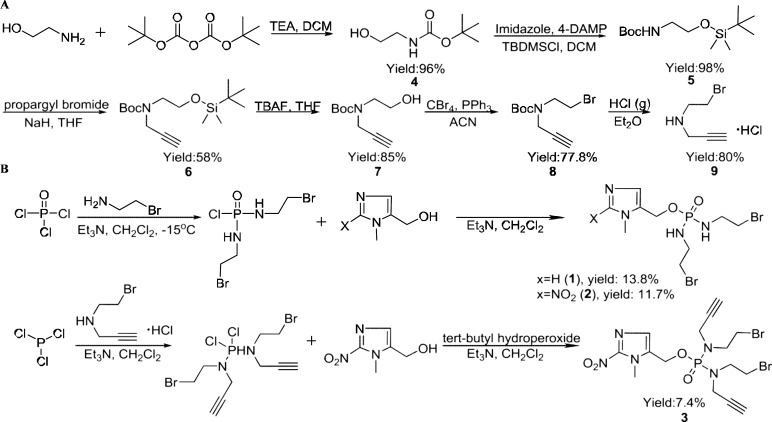
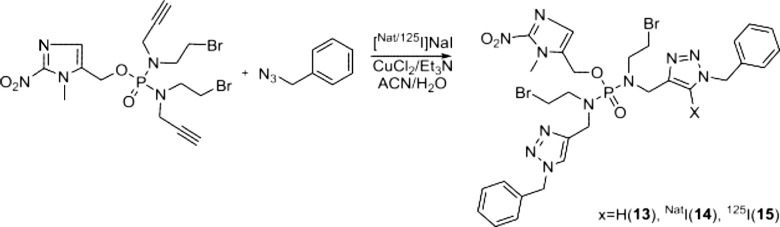
The synthesis of the phosphoramide mustards 1–3 are outlined in Scheme 1. Out first synthetic goal in the synthesis of 2-NIPAM, 3, was to produce the alkyne containing secondary amine, 9. The synthesis of compound 9 (N-(2-bromoethyl)prop-2-yn-1-amine hydrochloride), as outlined in Scheme 1A, was initiated with the dual protection of 2-aminoethan-1-ol. The amine and alcohol functional groups of the starting material were protected with Boc and tert-butyldimethylsilyl groups, respectively.12,22−27 Deprotonation of the protected amine using sodium hydride and subsequent reaction with propargyl bromide yielded the protected secondary amine 6. Deprotection of the silyl protecting groups employing a fluoride source (i.e., TBAF) provided compound 7. To this point, compound 7 was obtained in three steps with an overall yield of 85%. An Appel reaction, utilizing triphenylphosphine and CBr4, was employed to convert the alcohol into a bromide, giving compound 8. After workup, column chromatography was utilized to purify the compound and remove byproducts (e.g., POPh3) and starting reagents (e.g., CBr4). Purified 8 was dissolved in Et2O, and the Boc group was removed by the introduction of HCl gas yielding intermediate 9 in quantitative yield. The structure of 9 was confirmed by X-ray crystallography (Figure 2A), but it is noteworthy that a molecule of CBr4 also cocrystallized with the desired compound. After further investigation, it was found that additional chromatographic purifications of 8 were able to remove residual CBr4, resulting in the isolation of white crystalline 9. Overall, the synthetic route in Scheme 1A produced compound 9 in 80% yield. The structures of the compound synthesized in this scheme were confirmed by mass spectrometry and 1H and 13C NMR spectra (Figures S2, S3, and S4, correspondingly).
Scheme 1. Synthesis of Imidazole and Its Nitro-Derivative Conjugated Phosphoramidate Mustard (1–3).
Figure 2.
(A) Crystal structure of cocrystallized compound 9 and CBr4. Selected bond lengths (Å) and angles (deg): Br(1)–C(1) = 1.954(7), N(1)–C(2) = 1.497(9), N(1)–C(3) = 1.502(8), C(2)–C(1) = 1.498(10), C(3)–C(4) = 1.450(10), C(4)–C(5) = 1.181(11), C(2)–N(1)–C(3) = 114.5(5), N(1)–C(2)–C(1) = 107.6(6), C(4)–C(3)–N(1) = 109.3(6), C(5)–C(4)–C(3) = 179.1(9), C(2)–C(1)–Br(3) = 108.9(5). (B) Crystal structure of side product 11.
Initial attempts to synthesize the phosphordiamidate mustards centered on utilizing an asymmetric approach using a phosphoramic dichloride precursor (Scheme S2).26,28 Unfortunately, this approach yielded multiple products with one of the largest byproducts being a bisphosphoramidate 11. The structure of 11 was confirmed by X-ray crystallography, Figure 2B. Attempts to obtain the desired compounds by modifying the reaction conditions (i.e., substitution of different amines, varying reaction times, and temperatures) were unsuccessful.
With these setbacks, we switched our approach to focus on a symmetric phosphoramidate mustard synthesis, outlined in Scheme 1B, to obtain 3 along with our positive (2) and negative (1) controls. The negative control, 1, is an analogue of 2 that utilizes an imidazole instead of a nitroimidazole, thereby eliminating its ability to fragment and form an active phosphoramidate mustard. The controls were synthesized by reaction of two equivalents of bromoethylamine with POCl3 to yield the phosphoramidate chloride intermediate. This intermediate was subsequently reacted under basic conditions with (1-methyl-1H-imidazol-5-yl)methanol or its 2-nitroimidazole derivative to produce compound 1 in 13.8% yield and 2 in 11.7% yield, respectively. The synthesis of compound 3 was accomplished by the sequential addition of 2.2 equiv of 9 with PCl3, followed by reaction with 1-methyl-2-nitro-1H-imidazol-5-yl)methanol and oxidation via tert-butyl hydroperoxide. The structures of compound 1, 2, and 3 were confirmed by mass spectrometric, 1H, 13C, and/or 31P NMR spectra analyses (Figures S2, S3, S4, and S5, correspondingly).
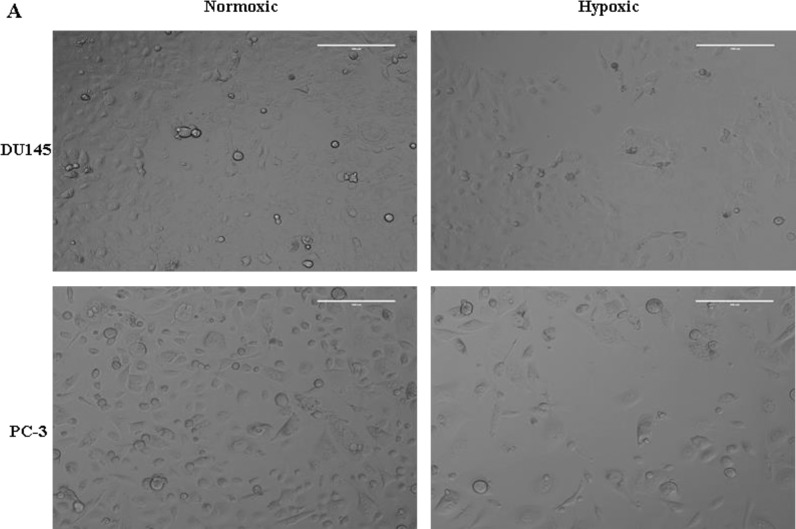
The cytotoxicity of compounds 1, 2, and 3 was evaluated in PC-3 and DU145 human prostate cancer cell lines under both normoxic and hypoxic conditions using a viability assay; results are shown in Table 1. As expected, the negative control, 1, demonstrated an IC50 that was >0.85 mM for both cell lines. Due to its inability to form an active phosphoramide mustard under hypoxic conditions, the cytotoxicity of 1 was >100-fold lower than either 2 or 3. Additionally, the negative control demonstrated no hypoxia selectivity (i.e., hypoxia cytotoxicity ratio (HCR) = 1). The positive control, 2, demonstrated increased cytotoxicity (IC50 = 4.14 ± 0.66 μM 6.49 ± 0.67 μM (PC-3)) in both cell lines under hypoxic conditions relative to normoxic conditions (IC50 = 687 ± 32.6 μM (PC-3); 842 ± 52.5 μM (DU145)). The hypoxia selectivity for 2 was found to be approximately two-fold higher in DU145 cells (HCR = 203) compared to PC-3 cells (HCR = 106). These results agree with previous reports in which evofosfamide (2) demonstrated lower hypoxia selectivity in PC-3 cells (HCR = 190) relative to DU145 cells (HCR = 240).29 Compound 3, 2-NIPAM, demonstrated slightly higher cytotoxicity (IC50 = 1.65 ± 0.09 μM (DU145); 4.70 ± 0.01 μM (PC-3)) compared to the positive control under hypoxic conditions. However, compound 3 also demonstrated three- to four-fold higher cytotoxicity under normoxic conditions relative to compound 2. This suggests that either compound 3 is more easily activated than 2 under normoxic conditions or that the “inactive” form of 3 is more cytotoxic than evofosfamide. Interestingly, relative to the positive control, the hypoxia selectivity of 3 was 2-fold lower (HCR = 62) for PC-3 cells but had similar values (HCR = 225) for DU145 cells. There is no significant difference in the HCR between compound 2 and compound 3 (P > 0.05) in the DU145 cell line, but a significant difference in PC-3 cells (P < 0.05) was observed. Representative images depicting the cytotoxicity for both cell lines incubated for 2 h with 1 × 10–5 M 2-NIPAM under hypoxic and normoxic conditions are shown in Figure 3. At any rate, these studies demonstrate that incorporation of the alkyne groups into the phosporamide structure did not substantially impact the cytotoxicity or hypoxia selectivity of 3. This strongly suggests that compound 3 is able to undergo the same activation mechanism that has already been established for 2 (i.e., evofosfamide).30
Table 1. Cytotoxicity of Prostate Cancer Cell Lines with Tested Compoundsa.
| IC50 (μmol/L) |
||||
|---|---|---|---|---|
| compd | cell lines | N2 | air | HCR |
| 1 | PC-3 | ∼1000 | ∼1000 | ND |
| DU145 | 851 ± 52.9 | 856 ± 55.2 | 1 | |
| 2 | PC-3 | 6.49 ± 0.67 | 687 ± 32.6 | 106 |
| DU145 | 4.14 ± 0.66 | 842 ± 52.5 | 203 | |
| 3 | PC-3 | 4.70 ± 0.01 | 293 ± 39.6 | 62 |
| DU145 | 1.65 ± 0.09 | 372 ± 41.6 | 225 | |
Results (mean ± SEM) are from two or more independent experiments carried out in quintuplicate. HCR: hypoxia cytotoxicity ratio; HCR = IC50(air)/IC50(N2).
Figure 3.
Microscopy images of hypoxia selective toxicity of 1 × 10–5 M 2-NIPAM with 2 h administration in PC-3 and DU145 cells (inverted microscope, ×20).
To evaluate the time-dependent cytotoxicity of compound 3, IC50 assays were performed using both cell lines under different incubation times. As shown in Figure 4A,B, the cytotoxicity of 3 was time-dependent in both cell lines with increased cytotoxicity as incubation time increased. As previously noted, higher cytotoxicity for 3 was observed in DU145 cells compared to PC-3 under hypoxic conditions. The IC50 of 3 after 6 h incubation was determined to be 0.78 μM in DU145 cells and 1.69 μM in PC-3 cells, as shown in Table 2. Enhanced cytotoxicity with elongated incubation time is likely a result of the increase in cellular adduct formation.
Figure 4.
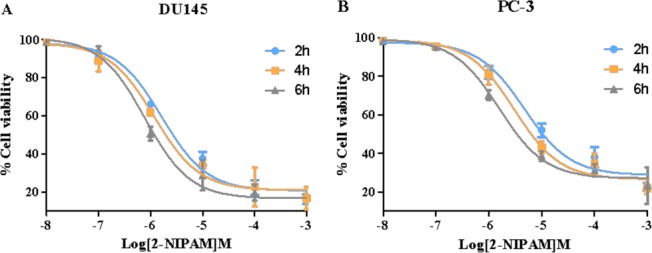
Time-dependent cytotoxicity of compound 3 (2-NIPAM) in (A) DU145 and (B) PC-3 cell lines; values are mean ± SEM (n = 5).
Table 2. Summary of Time-Dependent Cytotoxicity of Compound 3a.
| IC50 (μmol/L; N2) |
||
|---|---|---|
| compd exposure time (h) | PC-3 | DU145 |
| 2 | 4.70 ± 0.01 | 1.65 ± 0.09 |
| 4 | 3.00 ± 0.22 | 1.17 ± 0.15 |
| 6 | 1.69 ± 0.41 | 0.78 ± 0.10 |
Results (mean ± SEM) are from three or more independent experiments carried out in quintuplicate.
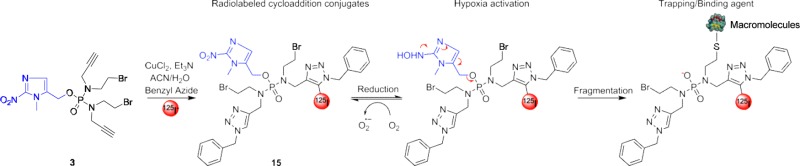
In order to investigate the conjugation and in vitro trapping efficacy of 2-NIPAM (3), we radiolabeled 3 with 125I utilizing a Cu(I)-mediated cycloaddition reaction.31 However, before proceeding, we sought to optimize the cycloaddition reaction conditions. Our previous unpublished work demonstrated that 2-nitroimidazoles can undergo rapid degradation when exposed to certain metals (e.g., Cu(II) salts). With that in mind, the stability of the 2-nitroimidazole reactants and the conjugation efficacy of the ruthenium-based and Cu(I) catalysts under various experimental conditions (i.e., temperature and reaction time) were examined. The coupling of 3 with benzyl azide utilizing two common ruthenium-based catalysts (Cp*RuCl(PPh3)2 and Cp*RuCl(COD)) did not result in the desired product as evaluated by LC–MS but instead led to multiple byproducts at both room and elevated temperatures. Our initial belief was that the ruthenium catalysts, under the conditions employed, were leading to the degradation of the 2-nitroimidazole functionality of 3. To further investigate this, we incubated the ruthenium catalyst in the presence of 2-nitroimidazole-1-acetic acid. LC–MS demonstrated that exposure of the 2-nitroimidazole analog to the ruthenium catalysts led to a gradual transformation of the analog (data not shown), presumably by reduction of the nitro group to the amine.32,33 Fortunately, the 2-nitroimidazole functionality of 3 remained stable using Cu(I)-mediated cycloaddition conditions at both room temperature and 60 °C with yields of 81.2% and 82.7%, respectively.
Following the cycloaddition optimization, we employed a reported procedure by Årstad and colleagues to incorporate 125I into the resulting triazole ring of the cycloaddition products.33 Briefly, the procedure involves a one-pot reaction that utilizes copper(II) chloride combined with Et3N to generate the cycloaddition product with the 125I incorporated on the 5-position of the triazole ring. While some mechanistic studies were performed, the precise mechanism of the 125I-incorporation remains unclear. However, some findings appear to be certain: (1) the Et3N is able to reduce a portion of the Cu(II) species in solution to Cu(I), thereby generating the needed species to carry out the cycloaddition reaction and (2) the incorporation of the 125I is carried out by a Cu(I) species. At any rate, we explored the utilization of this technique with 2-NIPAM (3), as depicted in Scheme 2. Utilizing the CuCl2/Et3N system in a water–acetonitrile mixture, we first examined the incorporation of natI (nonradioactive) into 2-NIPAM, utilizing benzyl azide. Upon completion of the 90 min reaction at 60 °C, the reaction progress was evaluated by LC–MS, as shown in Figure 5A. The cycloaddition product 13, without the incorporation of the iodide, was observed at 5.0 min, while the single iodinated product 14 was observed at 7.6 min. As can be seen from the chromatograms, the incorporation efficiency of the iodide into the triazole was modest. In order to aid separation and purification of the iodinated species (i.e., 14 and 15), the mobile phase was changed from water–acetonitrile to water–methanol. The chromatogram of 14 using the water–methanol eluent is depicted in Figure 5B. Utilizing analogous reaction conditions, the reaction of 3, benzyl azide, and 125I was carried out and evaluated by radio-HPLC using the same gradient. As expected, 15 had identical retention times compared to those of nonradioactive 14. While the overall radiochemical yield for 15 was poor (10%), the yield was more than sufficient for further in vitro characterization.
Scheme 2. Synthesis of 5-[Nat/125I]Iodo-1,2,3-trizoles (13–15).
Figure 5.
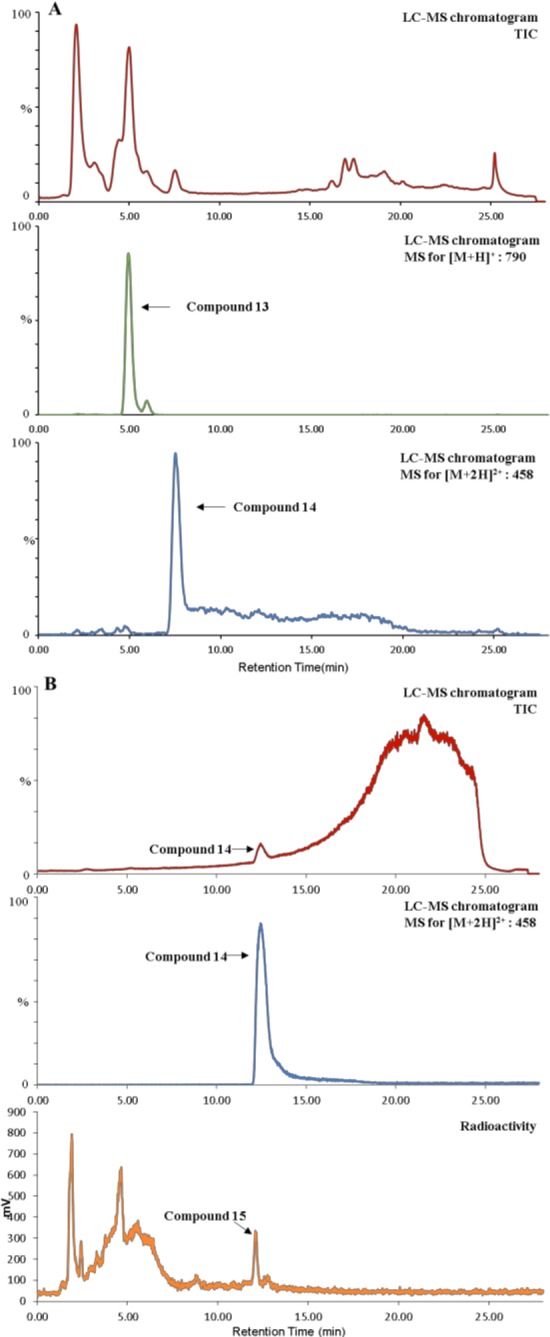
(A) LC–MS chromatogram profile of products of azide-2-NIPAM cycloaddition reaction (eluent: ACN–H2O). (B) LC–MS chromatogram profile of compound 14 aligned with the radiolabeled HPLC profile of compound 15 (eluent: methanol–H2O).
Studies were performed to determine the distribution coefficient (LogD7.4) of 15. The 125I-labeled conjugate was found to have a distribution coefficient of 0.55 ± 0.06, which demonstrated that the compound is hydrophobic enough to readily enter cells through passive diffusion. In order to evaluate the cellular trapping/retention efficacy of compound 15, we investigated the biological performance of this radiolabeled agent using in vitro efflux studies. The PC-3 and DU145 cells were initially treated with 15 for 1 h at 37 °C. Uptake of the 125I-labeled conjugate in both cell lines ranged from 2% to 4% of the total radioactivity added under both normoxic and hypoxic conditions, respectively. The efflux results for 15 over an 8 h period in both PC-3 and DU145 cells under normoxic and hypoxic conditions are given in Figure 6. For all cell lines and oxygen conditions, more than 50% of 15 was effluxed from the cells within the first 2 h. However, the 125I-labeled conjugate demonstrated lower efflux rates under hypoxic conditions in both cell lines relative to normoxic conditions. At 8 h after efflux began, 85.8% and 88.3% of the initial internalized radioactivity of 15 was externalized in normoxic condition in DU145 and PC-3 cells, respectively, compared to 72.2% and 70.5% under hypoxic conditions. The increased retention of 15 under hypoxic conditions, which is likely due to hypoxia activation and adduct formation with intracellular biomolecules, was found to be statistically significant (P < 0.05) in both cell lines when compared to normoxic data.
Figure 6.
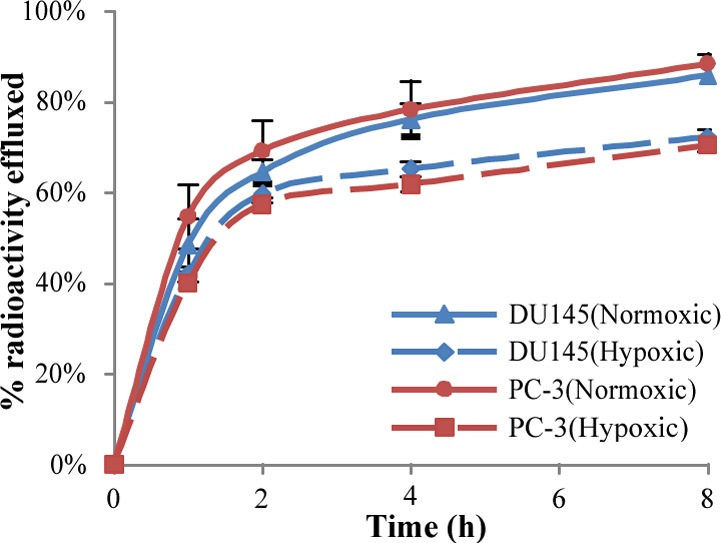
Efflux assays depicted as percentage of initial internalized activity for 125I-radioconjugate compound 15 in PC-3 and DU145 cells. Values are mean ± SD (n = 5).
To summarize, we have designed and successfully synthesized a novel 2-nitroimidazole phosphoramide mustard (2-NIPAM), modeled after evofosfamide, which allows easy conjugation of the hypoxia-selective drug to imaging moieties and other constructs through an azide–alkyne cycloaddition reaction. Our interest in 2-NIPAM, and other hypoxia-selective drugs, lies in their potential for selective activation and retention in hypoxic tissues, such as those observed with many cancers. We envision that this selectivity can be exploited for the purpose of selectively increasing the retention of diagnostic and therapeutic agents in hypoxic tumor tissues. Our studies demonstrated that 2-NIPAM had similar cytotoxicity and hypoxia selectivity compared to evofosfamide, establishing that the incorporation of the alkyne functionality did not substantially impact the activity of the agent. Using a synthesized 125I-labeled 2- NIPAM conjugate, we observed that the analog demonstrated greater cellular retention under hypoxic conditions, suggesting that retention is due to activation and adduct formation with intracellular macromolecules. Given these results, we plan on further investigating the potential of 2-NIPAM alone as a hypoxia diagnostic agent and in conjugation with receptor-targeted agents for cancer imaging and targeted radiotherapeutic applications.
Acknowledgments
We thank Ed Ezell at the Nuclear Magnetic Resonance (NMR) Facility at UNMC for assistance in collecting and interpreting the NMR data for all chemical compounds; Ying Xie and Fei Yu at the UNMC for assistance using the EVOS FL Cell Imaging System; and Dr. Shana A. Garrison for helpful advice in the preparation of this manuscript.
Glossary
ABBREVIATIONS
- TH-302
evofosfamide
- 2-NIPAM
2-nitroimidazole phosphoramide nitrogen mustard
- equiv
equivalent
- Et3N
ethanolamine
- THF
tetrahydrofuran
- ACN
acetonitrile
- TBAF
tetra-n-butylammonium fluoride
- Et2O
diethyl ester
- RCY
radiochemical yields
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00355.
Experimental procedures, compounds characterization, tables, and schemes (PDF)
Author Contributions
# These authors contributed equally.
This study was supported by the National Institutes of Health (5R01CA179059-03). No other potential conflict of interest relevant to this article was reported.
The authors declare no competing financial interest.
Supplementary Material
References
- Brown J. M.; Wilson W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- Vaupel P.; Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 2004, 9, 4–9. 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- Tredan O.; Galmarini C. M.; Patel K.; Tannock I. F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- Overgaard J. Hypoxic radiosensitization: adored and ignored. J. Clin. Oncol. 2007, 25, 4066–4074. 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- Lee H. H.; Palmer B. D.; Wilson W. R.; Denny W. A. Synthesis and hypoxia-selective cytotoxicity of a 2-nitroimidazole mustard. Bioorg. Med. Chem. Lett. 1998, 8, 1741–1744. 10.1016/S0960-894X(98)00305-9. [DOI] [PubMed] [Google Scholar]
- Sun Z. Y.; Botros E.; Su A. D.; Kim Y.; Wang E.; Baturay N. Z.; Kwon C. H. Sulfoxide-containing aromatic nitrogen mustards as hypoxia-directed bioreductive cytotoxins. J. Med. Chem. 2000, 43, 4160–4168. 10.1021/jm9904957. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Kim E. J.; Han J.; Lee H.; Shin W. S.; Kim H. M.; Bhuniya S.; Kim J. S.; Hong K. S. Hypoxia-directed and activated theranostic agent: Imaging and treatment of solid tumor. Biomaterials 2016, 104, 119–128. 10.1016/j.biomaterials.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Ware D. C.; Palmer B. D.; Wilson W. R.; Denny W. A. Hypoxia-selective antitumor agents. 7. Metal complexes of aliphatic mustards as a new class of hypoxia-selective cytotoxins. Synthesis and evaluation of cobalt(III) complexes of bidentate mustards. J. Med. Chem. 1993, 36, 1839–1846. 10.1021/jm00065a006. [DOI] [PubMed] [Google Scholar]
- Fleming I. N.; Manavaki R.; Blower P. J.; West C.; Williams K. J.; Harris A. L.; Domarkas J.; Lord S.; Baldry C.; Gilbert F. J. Imaging tumour hypoxia with positron emission tomography. Br. J. Cancer 2015, 112, 238–250. 10.1038/bjc.2014.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani A. S.; Alters S. E.; Wong A.; Albertella M. R.; Cleland J. L.; Henner W. D. Selective tumor targeting by the hypoxia-activated prodrug AQ4N blocks tumor growth and metastasis in preclinical models of pancreatic cancer. Clin. Cancer Res. 2007, 13, 2216–2225. 10.1158/1078-0432.CCR-06-2427. [DOI] [PubMed] [Google Scholar]
- Patel K.; Lewiston D.; Gu Y.; Hicks K. O.; Wilson W. R. Analysis of the hypoxia-activated dinitrobenzamide mustard phosphate pre-prodrug PR-104 and its alcohol metabolite PR-104A in plasma and tissues by liquid chromatography-mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2007, 856, 302–311. 10.1016/j.jchromb.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Duan J.-X.; Jiao H.; Kaizerman J.; Stanton T.; Evans J. W.; Lan L.; Lorente G.; Banica M.; Jung D.; Wang J. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J. Med. Chem. 2008, 51, 2412–2420. 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- Guise C. P.; Mowday A. M.; Ashoorzadeh A.; Yuan R.; Lin W. H.; Wu D. H.; Smaill J. B.; Patterson A. V.; Ding K. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Aizheng 2014, 33, 80–86. 10.5732/cjc.012.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Wagh N. K.; Ogbomo S. M.; Shi W.; Jia Y.; Brusnahan S. K.; Garrison J. C. Synthesis and in vitro and in vivo evaluation of hypoxia-enhanced 111In-bombesin conjugates for prostate cancer imaging. J. Nucl. Med. 2013, 54, 1605–1612. 10.2967/jnumed.112.117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters B. G.; Wang L. H.; Brown J. M. Tirapazamine: a new drug producing tumor specific enhancement of platinum-based chemotherapy in non-small-cell lung cancer. Ann. Oncol 1999, 10 (Suppl 5), S29–33. 10.1093/annonc/10.suppl_5.S29. [DOI] [PubMed] [Google Scholar]
- Patterson A. V.; Ferry D. M.; Edmunds S. J.; Gu Y.; Singleton R. S.; Patel K.; Pullen S. M.; Hicks K. O.; Syddall S. P.; Atwell G. J.; Yang S.; Denny W. A.; Wilson W. R. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin. Cancer Res. 2007, 13, 3922–3932. 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- Weiss G. J.; Infante J. R.; Chiorean E. G.; Borad M. J.; Bendell J. C.; Molina J. R.; Tibes R.; Ramanathan R. K.; Lewandowski K.; Jones S. F.; Lacouture M. E.; Langmuir V. K.; Lee H.; Kroll S.; Burris H. A. 3rd Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin. Cancer Res. 2011, 17, 2997–3004. 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- Wagh N. K.; Zhou Z.; Ogbomo S. M.; Shi W.; Brusnahan S. K.; Garrison J. C. Development of hypoxia enhanced 111In-labeled Bombesin conjugates: design, synthesis, and in vitro evaluation in PC- 3 human prostate cancer. Bioconjugate Chem. 2012, 23, 527–537. 10.1021/bc200600w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Wojtkowiak J. W.; Martinez G. V.; Cornnell H. H.; Hart C. P.; Baker A. F.; Gillies R. MR Imaging Biomarkers to Monitor Early Response to Hypoxia-Activated Prodrug TH-302 in Pancreatic Cancer Xenografts. PLoS One 2016, 11, e0155289. 10.1371/journal.pone.0155289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C.; Lee H. J.; Park D. J.; Lee Y. J.; Tap W. D.; Eisinger- Mathason T. S.; Hart C. P.; Choy E.; Simon M. C.; Yoon S. S. Hypoxia-activated chemotherapeutic TH-302 enhances the effects of VEGF-A inhibition and radiation on sarcomas. Br. J. Cancer 2015, 113, 46–56. 10.1038/bjc.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue R. T.; Van De Voorde L.; Berbee M.; van Elmpt W. J.; Dubois L. J.; Panth K. M.; Peeters S. G.; Claessens A.; Schreurs W. M.; Nap M.; Warmerdam F. A.; Erdkamp F. L.; Sosef M. N.; Lambin P. A phase 1 ’window-of-opportunity’ trial testing evofosfamide (TH-302), a tumour-selective hypoxia-activated cytotoxic prodrug, with preoperative chemoradiotherapy in oesophageal adenocarcinoma patients. BMC Cancer 2016, 16, 644. 10.1186/s12885-016-2709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambeiro F.; Lopez S.; Varela J. A.; Saa C. Vinyl dihydropyrans and dihydrooxazines: cyclizations of catalytic ruthenium carbenes derived from alkynals and alkynones. Angew. Chem., Int. Ed. 2014, 53, 5959–5963. 10.1002/anie.201400675. [DOI] [PubMed] [Google Scholar]
- Molander G. A.; Cormier E. P. Ketyl-allene cyclizations promoted by samarium(II) iodide. J. Org. Chem. 2005, 70, 2622–2626. 10.1021/jo047887s. [DOI] [PubMed] [Google Scholar]
- Rengasamy R.; Curtis-Long M. J.; Seo W. D.; Jeong S. H.; Jeong I. Y.; Park K. H. New building block for polyhydroxylated piperidine: total synthesis of 1,6-dideoxynojirimycin. J. Org. Chem. 2008, 73, 2898–2901. 10.1021/jo702480y. [DOI] [PubMed] [Google Scholar]
- Fedotenko I. A.; Zaffalon P. L.; Favarger F.; Zumbuehl A. The synthesis of 1, 3-diamidophospholipids. Tetrahedron Lett. 2010, 51, 5382–5384. 10.1016/j.tetlet.2010.07.140. [DOI] [Google Scholar]
- Garrido-Hernandez H.; Moon K. D.; Geahlen R. L.; Borch R. F. Design and synthesis of phosphotyrosine peptidomimetic prodrugs. J. Med. Chem. 2006, 49, 3368–3376. 10.1021/jm060142p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedejs E.; Stults J. S. Synthesis of azocine derivatives from thio aldehyde Diels-Alder adducts. J. Org. Chem. 1988, 53, 2226–2232. 10.1021/jo00245a019. [DOI] [Google Scholar]
- Ludeman S. M.; Boyd V. L.; Regan J. B.; Gallo K. A.; Zon G.; Ishii K. Synthesis and antitumor activity of cyclophosphamide analogues. 4. Preparation, kinetic studies, and anticancer screening of ″phenylketophosphamide″ and similar compounds related to the cyclophosphamide metabolite aldophosphamide. J. Med. Chem. 1986, 29, 716–727. 10.1021/jm00155a022. [DOI] [PubMed] [Google Scholar]
- Meng F.-W.; Hou G.-F.; Yu Y.-H.; Gao J.-S. 4-(2, 2-Difluoro- 1, 3-benzodioxol-4-yl)-1H-pyrrole-3-carbonitrile. Acta Crystallogr., Sect. E: Struct. Rep. Online 2012, 68, o222–o222. 10.1107/S1600536811054523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F.; Evans J. W.; Bhupathi D.; Banica M.; Lan L.; Lorente G.; Duan J. X.; Cai X.; Mowday A. M.; Guise C. P.; Maroz A.; Anderson R. F.; Patterson A. V.; Stachelek G. C.; Glazer P. M.; Matteucci M. D.; Hart C. P. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol. Cancer Ther. 2012, 11, 740–751. 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- Yan R.; Sander K.; Galante E.; Rajkumar V.; Badar A.; Robson M.; El-Emir E.; Lythgoe M. F.; Pedley R. B.; Arstad E. A one-pot three-component radiochemical reaction for rapid assembly of 125I-labeled molecular probes. J. Am. Chem. Soc. 2013, 135, 703–709. 10.1021/ja307926g. [DOI] [PubMed] [Google Scholar]
- McClelland R. A.; Panicucci R.; Rauth A. M. Products of reductions of 2-nitroimidazoles. J. Am. Chem. Soc. 1987, 109, 4308–4314. 10.1021/ja00248a028. [DOI] [Google Scholar]
- Kizaka-Kondoh S.; Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci. 2009, 100, 1366–1373. 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.