Abstract
Purpose
Dry eye syndrome (DES) causes significant morbidity. Trials of blood-derived products in treatment of the condition show promising results. However, their production is expensive and time-consuming. We investigate fingerprick autologous blood (FAB) as an alternative low-cost, readily accessible treatment for DES.
Patients and methods
Prospective, non-comparative, interventional case series. In total, 29 eyes of 16 DES patients (2 males and 14 females) from two NHS sites in the United Kingdom. Patients instructed to clean a finger, prick with a blood lancet, and apply a drop of blood to the lower fornix of the affected eye(s), 4 times daily for 8 weeks then stop and review 4 weeks later. Follow-up visits occurred ~3 days, 2, 4, 8 weeks into therapy, and 4 weeks post-cessation. At each visit, visual acuity, corneal staining, Schirmer’s test, tear break-up time (TBUT), and ocular comfort index (OCI) were measured, and photographs taken. Results were analysed using Student’s paired t-test.
Results
At 8 weeks, there was improvement in mean Oxford corneal staining grade (3.31 to 2.07 (P<0.0001)), TBUT (5.00 to 7.80 s (P<0.05)), visual acuity (0.08 to 0.01 LogMAR equivalent (P<0.05)), and OCI score (56.03 to 39.72 (P<0.0001)). There was no statistically significant change in Schirmer’s test results. Four weeks post-cessation versus immediately after completion of FAB therapy, mean staining grade worsened from 2.07 to 2.86 (P<0.0001). OCI score worsened from 39.72 to 44.67 (P<0.05).
Conclusions
In our limited case series FAB appears to be a safe and effective treatment for DES.
Introduction
The tear film is crucial to sight through its multiple functions including maintenance of the corneal epithelium, provision of a smooth optical surface, mechanical removal of debris, and antibacterial activity. A reduction in the quality and/or quantity of the tear film results in a loss of these functions, leading to dry eye syndrome (DES/keratoconjunctivitis sicca), which affects almost 5 million Americans over the age of 501 and causes significant morbidity to sufferers. Disease can arise from deficiency of the mucoaqueous film layers, as in Sjogren’s syndrome or goblet cell damage, or more commonly deficiency of the lipid layer through Meibomian gland or adnexal dysfunction.
Standard non-surgical treatment for DES focuses on three main approaches – artificial tears for lubrication, hydration and corneal protection, punctal occlusion to reduce tear drainage, and anti-inflammatories to reduce the pro-inflammatory nature of the tear film in DES secondary to hypertonicity and other factors.2 Patients with severe DES, often of an immune-mediated nature, may fail to respond to these treatments, however. Artificial tears fail to take into account the extraordinarily complex composition of the natural tear film, and thus research over the past decade has focused on attempts to create a tear substitute more closely mimicking the tear film to treat DES. Tears contain an extensive range of growth factors, immunoglobulins, enzymes, cytokines, vitamins, amino acids, carbohydrates, and electrolytes which have been shown to be essential to the maintenance of corneal epithelial cells and physiological corneal homoeostasis (Supplementary Table 4).3
An obvious source for a tear mimic is blood. The numerous factors contained in tears are also found in blood plasma, albeit in differing concentrations (Supplementary Table 5). Several blood-derived products, such as autologous serum (AS), allogeneic serum, umbilical cord serum and platelet-rich plasma, have been studied as tear substitute candidates. The most extensively studied of these, AS, was first trialled as a preservative-free tear substitute by Fox et al4 with encouraging subjective results. Since then several studies have shown beneficial effects of AS in the treatment of DES,5 persistent epithelial defect,6, 7, 8 neurotrophic keratopathy,9 recurrent corneal erosion syndrome,10 Graft versus Host disease,11, 12 and superior limbic keratoconjunctivitis.13 However, there are several barriers to the widespread use of AS.3, 14 In the United States, AS has not yet been tested for official Food and Drug Administration (FDA) approval, leading to reluctance amongst pharmacies to manage it as a non-FDA approved product.14 In the United Kingdom, treatment can only be commenced after approval from the NHS Blood and Transplant Tissue Service and once funding is sourced, a process that usually takes several months. AS therapy is not possible for patients in whom blood donation is contraindicated. There is no consensus presently regarding a standardised AS production method, with remaining uncertainty over whether AS should be diluted before administration to reduce the concentration of tumour growth factor β,15, 16 which has been shown to inhibit epithelial cell proliferation (supplementary Table 4). Estimated total cost to produce a five months’ supply of AS drops from one blood donation is £1600 (£320/month), including the price of collection, processing, testing, and distribution.14 Once produced, AS must be kept in a freezer, and stability of the growth factors within AS after 6 months’ storage is unknown.17 Complications such as conjunctivitis,18, 19 marginal infiltrates, Candida infectious crystalline keratopathy, coagulase-negative Staphylococcus keratitis,20 limbitis,21 and immunoglobulin deposition22 have been reported.
There have been no studies thus far investigating the use of whole blood itself as a tear substitute. Fresh autologous blood, obtained by pricking one’s finger, presents several theoretical advantages over other blood-derived products: treatment cost is limited to the purchase of wipes and lancets to perform regular fingerpricks (£12 per month), no production steps are required and thus there is no delay before starting treatment, and no storage is necessary. The constituents of whole blood differ from serum and other blood-derived products however (Supplementary Table 5), and the effects of erythrocytes, leucocytes, platelets, clotting factors, and numerous other factors whose concentration differs between blood and blood-derived products, on the ocular surface has yet to be fully elucidated. With these potential benefits and uncertainties in mind, we conducted a pilot study to establish the feasibility of fingerprick autologous blood (FAB) as a novel treatment for DES.
Materials and methods
Patient recruitment
Ethics committee approval was obtained for a pilot study to assess the feasibility and efficacy of FAB therapy in cases of severe DES. Potential patients were recruited from cornea and external eye disease clinics at Moorfields eye unit at Bedford and Birmingham NHS trusts between April 2014 and June 2016. These patients were clinically assessed to determine suitability to enter the study.
Inclusion criteria for DES patients are detailed in Table 1. If only one eye met these inclusion criteria, then only that eye was included in the study. Exclusion criteria are detailed in Table 1.
Table 1. Dry Eye Syndrome (DES) Inclusion criteria (1, 2, 3, and 4 required).
| 1 | TBUT <5 s AND Schirmer’s <5mm |
|---|---|
| OR | |
| Punctate fluorescein staining of ocular surface | |
| OR | |
| OCI score >80% | |
| 2 | Artificial tear application at least QDS |
| 3 | Ciclosporin drops in use or refused |
| 4 | Punctal plugs in place or refused |
| Exclusion criteria | |
| Needle phobia or unwillingness to perform regular fingerprick | Progressive corneal melt of immunological aetiology |
| Finger infection | Active microbial infection of ocular surface |
| Systemic infection | Vitamin A deficiency |
| Systemic antibiotic use | Recurrent corneal erosion syndrome |
| Bleeding disorder, excluding anticoagulant use | Pregnant or breastfeeding women |
| Children <16 years old | |
Informed consent was gained from eligible participants and the study carried out in accordance with the Declaration of Helsinki.
Study design
Initial assessment was performed on entry to the study, and follow-up assessments ~3 days, 2 weeks, 4 weeks, and 8 weeks into therapy, and 4 weeks post-cessation of therapy. At initial assessment, a full history of the patient’s presenting complaint, past medical, drug and allergy, family, and social history were noted. At each assessment, an ocular comfort index (OCI) questionnaire was completed by the patient based on their current symptoms. The OCI was chosen based on superior validity, reliability, and Rasch-based psychometric properties in comparison to other dry eye questionnaire tools.23 Best corrected visual acuity (Snellen chart) and Schirmer’s test (without anaesthetic) were then noted. Slit-lamp biomicroscopy was performed, fluorescein 1 mg wetted strip was instilled and the anterior segment photographed with and without fluorescein filters. The eye was examined on the slit lamp by a single-experienced ophthalmologist and corneal staining graded using the Oxford Scheme for grading ocular surface staining in dry eye.24 Tear break-up time (TBUT) and then intraocular pressure (IOP) were subsequently assessed. These tests, along with an interview to determine compliance and elicit any problems with treatment regimen, and full examination to assess for any potential complications of FAB therapy, were repeated at each follow-up.
Therapy regimen in the trial comprised application of FAB four times a day for a period of 8 weeks, commencing after initial assessment. Patients were trained in the method of FAB application and given an information leaflet containing instructions (Supplementary Appendix 1) on entering the trial: patients were instructed to wash their hands with soap and water, dry with a clean towel, and then wipe a finger of the non-dominant hand with an alcohol street, although some patients used their dominant hand. Patients then used a new lancet to draw a drop of blood from their fingertip and applied the drop to the inferior conjunctival fornix. Patients then wiped their finger again with an alcohol steret and were provided with a sharps bin to dispose of the lancet. A separate finger was used for each eye. Patients were told to continue their routine conventional dry eye therapy, as recorded during the initial assessment, during the trial. Patients were told to cease FAB therapy and contact their ophthalmologist immediately if they developed any new symptoms in their eyes or fingers whilst on therapy.
Outcome measures
Primary outcome: improvement in symptoms (OCI score) or signs (Oxford Scheme corneal staining grade, Schirmer’s test, TBUT and visual acuity) after 8 weeks of FAB therapy.
Data analysis
Snellen chart best corrected visual acuities were converted into logMAR equivalents. OCI values were scored according to maximum-likelihood iterative procedures as per Johnson et al.23 Mean values for logMAR acuity, Schirmer’s test, TBUT, OCI score, Oxford Scheme grading, and IOP were compared between trial commencement and completion of 8 weeks of FAB therapy, and between completion of therapy and 4 weeks post-cessation, using two-tailed Student’s paired t-test. The effect of age (> or ≤50 years at time of entry into the study) and DES aetiology (Sjogren versus non-Sjogren) on outcomes was assessed using two-tailed Student’s unpaired t-test. Results with P value <0.05 were deemed statistically significant.
Results
In total 17 DES patients were recruited to the study. One patient declined to continue with the study after 4 week follow-up as she found regular assessments inconvenient and uncomfortable, although she tolerated the treatment regimen well. At week 4, corneal staining grade and TBUT had improved in the left eye and remained stable in the right eye, whilst OCI had improved from 63.90 to 59.08. This patient was excluded from further analysis. Details and conventional dry eye therapies used at baseline of the remaining 16 patients are summarised in Table 2. All patients had been diagnosed with severe DES prior to entry and had suffered with symptoms for at least 2 years. Sjogren’s syndrome diagnosis was clinically based on symptoms of severe DES with usually dry mouth, and known systemic autoimmune disease or positive auto-antibody screen for antinuclear antibody and/or rheumatoid factor. Topical ciclosporin therapy was used by 8/16 (50%) patients – high intolerance and refusal rates were secondary to use of off-label 0.2% ciclosporin ointment in the majority of patients, as 0.1% Ikervis achieved National Institute for Health and Clinical Excellence (NICE) approval only 6 months before end of the recruitment period. One patient was a soft contact lens wearer. Disease entry criteria were satisfied unilaterally in three patients, hence 29 eyes were studied. The study population comprised 14 female and 2 male patients. Mean±SD age was 54.4±15.0 years. Median age was 51.5 years (range 22–83).
Table 2. Patient demographics and pre-treatment therapy continued whilst on FAB.
| Patient No. | Sex | Age | Eye | Diagnosis | Artificial tears | Ciclosporin | Punctal Plugs | Other pre-treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 52 | R, L | Primary Sjogren's disease | Lacri-lube, Hyaluronic Acid | Intolerant to 0.2% ointment (pain) | Refused | Acetylcysteine |
| 2 | F | 51 | R, L | Primary Sjogren's disease | Hyaluronic acid | 0.2% ointment | OD, OS | Tranquil eyes goggle |
| 3 | M | 47 | L | Sjogren's disease secondary to rheumatoid arthritis | Optive, Hyaluronic acid | Refused 0.2% ointment (pain concern) | OD, OS | Nil |
| 4 | F | 45 | R, L | Sjogren's disease secondary to rheumatoid arthritis | Hyaluronic acid, Vitapos, Lacri-lube | Refused 0.2% ointment (pain concern) | OD, OS | Acetylcysteine |
| 5 | F | 52 | R, L | Primary Sjogren's disease | Carmellose, Hydromoor, Lacri-lube | Intolerant to 0.2% ointment (pain) | OS | Maxidex, Omega-3 oils, lid massage |
| 6 | M | 49 | R | Dry eye syndrome secondary to radiotherapy in proximity to right lacrimal gland | Vitapos, Lacrilube, Hyaluronic acid | Refused 0.2% ointment (pain concern) | OD | Botox lower and upper lid, complete tarsorrhaphy |
| 7 | F | 53 | R, L | Sjogren's disease secondary to rheumatoid arthritis | Hyaluronic acid | Intolerant to 0.2% ointment (pain) | Refused | Nil |
| 8 | F | 61 | R, L | Sjogren's disease secondary to rheumatoid arthritis | Liquifilm | Refused 0.2% ointment (pain concern) | Refused | Nil |
| 9 | F | 40 | R, L | Sjogren's disease secondary to SLE | Hylo-tears | 0.2% ointment | Refused | Nil |
| 10 | F | 83 | R, L | Sjogren's disease secondary to rheumatoid arthritis | Clinitas | Intolerant to 0.2% ointment (pain) | Punctae Cauterised | Acetylcysteine |
| 11 | F | 78 | R | Dry eye syndrome | Celluvisc, Lacri-lube, Viscotears | 0.2% ointment | OD, OS | Nil |
| 12 | F | 67 | R, L | Dry eye syndrome | Hyaluronic Acid | 0.2% ointment | OD, OS | Nil |
| 13 | F | 76 | R, L | Dry eye syndrome | Hylo-tears, Viscotears | 0.2% ointment | OD, OS | Nil |
| 14 | F | 46 | R, L | Primary Sjogren's disease | Hylo-forte, Clinitas Soothe Multi, Xailin | Ikervis 0.1% | Refused | Catacrom Soft contact lens use |
| 15 | F | 22 | R, L | Sjogren's disease secondary to rheumatoid arthritis | Hyaluronic Acid | 0.2% ointment | Refused | Nil |
| 16 | F | 49 | R, L | Primary Sjogren's disease | Thealoz Duo | Ikervis 0.1% | Removed due to intolerance | Nil |
Mean±SD period from baseline assessment and commencement of FAB therapy was 3.5±2.10 days for first follow-up, 15±4.34 days for 2 week follow-up, 32.42±7.82 days for 4 week follow-up, 58.29±8.64 days for 8 week follow-up, and 88.07±8.22 days for 12 week follow-up.
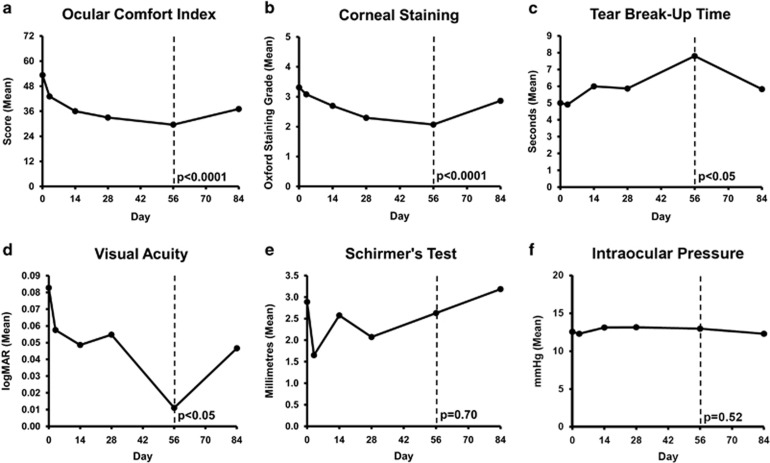
Study outcomes are shown in Figure 1. After 8 weeks FAB therapy, subjective symptoms, scored by OCI questionnaire, improved from a mean value±1 SD of 56.03±8.69 to 39.72±9.76 (P<0.0001) (Figure 1a). Objectively, corneal staining, measured using Oxford Scheme grading, improved from a mean score of 3.31±1.44 to 2.07±1.28 (P<0.0001) (Figure 1b). TBUT improved from a mean time of 5.00±4.4 to 7.80±5.53 s (P<0.05) (Figure 1c). Mean visual acuity improved from LogMAR equivalent 0.08±0.25 to 0.01±0.19, (P<0.05) (Figure 1d). Change in Schirmer’s test (2.89±4.38–2.63±2.67 mm, P=0.70) (Figure 1e) lacked statistical significance.
Figure 1.
(a) Mean change in Ocular Comfort Index score after 8 weeks on fingerprick autologous blood therapy (dashed line) and then 4 weeks after cessation. (b) Mean change in Oxford corneal staining grade after 8 weeks on fingerprick autologous blood therapy (dashed line) and then 4 weeks after cessation. (c) Mean change in tear break-up time after 8 weeks on fingerprick autologous blood therapy (dashed line) and then 4 weeks after cessation. (d) Mean change in visual acuity (LogMAR) after 8 weeks on fingerprick autologous blood therapy (dashed line) and then 4 weeks after cessation. (e) Mean change in Schirmer’s score after 8 weeks on fingerprick autologous blood therapy (dashed line) and then 4 weeks after cessation. (f) Mean change in intraocular pressure after 8 weeks on fingerprick autologous blood therapy (dashed line) and then 4 weeks after cessation.
Four weeks post-cessation of FAB therapy, mean OCI score worsened from 39.72±9.76 to 44.67±9.18 (P<0.05). Mean corneal staining grade worsened from 2.07±1.28 to 2.86±1.55 (P<0.0001). Change in mean TBUT (7.80±5.53 to 5.83±6.5 s (P=0.10)), Schirmer’s test (2.63±267 to 3.19±7.03mm, P=0.63) and visual acuity (logMAR equivalent 0.01±0.19 to 0.05±0.24, P=0.09) lacked statistical significance.
Age and DES aetiology did not significantly affect outcomes after 8 weeks FAB therapy (Table 3).
Table 3. Effect of age and DES aetiology on FAB outcomes. Mean+SD change at FAB completion (Day 56) versus baseline.
| n (Patients) | n (Eyes) | OCI | Oxford Staining Grade | TBUT (seconds) | Visual Acuity (logMAR equivalent) | Schirmer Score (mm) | ||
|---|---|---|---|---|---|---|---|---|
| Age | >50 | 9 | 17 | −17.11±11.94 | −1.00±0.87 | 2±4.10 | −0.06±0.17 | 0.53±3.46 |
| ≤50 | 7 | 12 | −15.27±13.31 | −1.58±1.31 | 3.63±7.06 | −0.09±0.15 | −1.25±3.41 | |
| P | 0.77 | 0.16 | 0.49 | 0.58 | 0.19 | |||
| Aetiology | Sjogren | 12 | 23 | −16.34±13.52 | −1.35±1.03 | 3.16±6.05 | −0.05±0.15 | −0.10±3.10 |
| Non-Sjogren | 4 | 6 | −16.19±8.28 | −0.83±1.33 | 1.20±3.27 | −0.14±0.18 | −0.83±4.96 | |
| P | 0.98 | 0.31 | 0.5 | 0.27 | 0.66 |
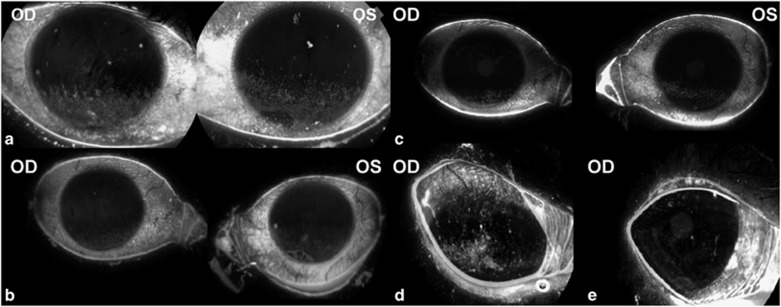
No ocular or fingertip complications were noted in any patients during the trial. Mean intraocular pressure showed no significant change throughout the trial (P=0.52) (Figure 1f). Patients found the technique to be straightforward and easy to perform. All patients complied well with treatment regimen according to questioning at each follow-up. Staining images are shown for two patients in Figure 2. Although patients were advised to continue to use their artificial tear drops as prescribed, the majority reported a decrease in use.
Figure 2.
Corneal staining of right and left eyes of patient 1 (a) at baseline (b) reduction in stain seen after 8 weeks on FAB therapy (c) staining increase 4 weeks after cessation; right eye only of patient 6 (d) at baseline (e) after 8 weeks on FAB therapy.
Discussion
Lack of adequate tear supply to the surface of the eye causes a constellation of surface pathology and symptoms that comprise DES. The broad range of growth factors and other nutrients found in natural tears have been shown to play a crucial role in maintenance of the physiological ocular surface (Supplementary Table 4). The existence of a significant population of severe DES sufferers in whom standard DES treatment is inadequate has driven research into tear substitutes which more closely mimic natural tears. Numerous blood-derived products have been trialled to variable degrees of success, but all share similar problems of high production cost, production time, legislative barriers, and stringent storage requirements.
The results of our pilot study indicate that FAB is a viable therapy for recalcitrant DES unresponsive to conventional therapy, and that continued application of blood is required to maintain improvements in both symptoms and signs seen during therapy. In total 16 patients had DES severe enough to meet the inclusion criteria of the study, and in these patients, all experienced subjective improvement in their symptoms, as measured by OCI score. Two patients reported reduced photophobia, not accounted for in the OCI questionnaire. Objectively, epithelial surface damage, as measured by degree of corneal staining on the Oxford grading scheme, improved in all but three eyes (in two of which staining grade remained constant), correlating well with subjective scores. Tear film stability, as measured via TBUT, improved in 19 of 29 eyes. Visual acuity improved in 18 eyes and was maintained in 7 eyes. Schirmer’s test showed no statistically significant change. It is possible that our study lacked the power to detect changes in this parameter owing to the small sample size. Four weeks post-cessation of FAB therapy versus immediately after completion of FAB therapy, corneal staining increased in 16 of 29 eyes. OCI score worsened in 11 of 16 patients. There was no statistically significant change in TBUT, visual acuity or Schirmer’s test after therapy cessation. The worsening of signs and symptoms after therapy cessation suggest that long-term therapy is required to maintain observed benefits. Age ≥50 years, and Sjogren’s or non-Sjogren’s aetiology, did not affect FAB efficacy. No complications were noted in any patient throughout the trial duration, and no effect on IOP was noted. The therapy regimen was tolerated well by all patients according to recorded questioning at each follow-up visit, with exception of the single patient who discontinued treatment due to inconvenience of required regular follow-up visits. A key concern from clinicians is the acceptability to patients of having to prick their fingers several times a day to provide relief for their eyes. All 16 patients have continued FAB therapy of their own accord without reported complications after study cessation, the longest for 28 months.
There are several limitations to this pilot study. The small sample size reduces external validity. Internal validity is limited by lack of a control group and absence of blinding of both researcher and patient. Both observer bias and placebo effect cannot therefore be excluded as potential contributors to observed outcomes. Analysis of both eyes in cases of severe bilateral DES as independent subjects risks overestimating statistical significance of observed differences, although any between-eye correlation is unlikely to account for the observed changes in OCI score and corneal staining grade on FAB therapy in light of the level of significance achieved. Furthermore, the decrease in the use of their artificial tears was not formally recorded. Cytological evidence of corneal epithelial anatomical changes secondary to FAB is presently lacking.
A randomised controlled trial is required to provide stronger evidence to assess the efficacy of FAB. Participant blinding will not be possible owing to the nature of FAB therapy, a problem which similarly affects AS trials. A randomised controlled crossover trial would therefore be most suited to providing this evidence.
What is the mechanism of action of FAB?
Blood is a complex medium comprising cells and plasma. Plasma constituents include water, proteins, nutrients, cytokines, electrolytes, steroid hormones, and waste products. The effect of each individual component of blood on the ocular surface has not been fully elucidated – to calculate the effect of whole blood on the surface of the eye by the sum of its parts would be a daunting task. However, we know that after injury to the corneal surface, controlled neovascularisation of the damaged surface and nerve regrowth occurs, contributing to regeneration of lost epithelium and repair of stroma.25, 26 The supply of whole blood to the damaged corneal surface is a physiological part of the repair process. It is therefore unsurprising that supplementation of blood via fingerprick should encourage that same process in dysfunctional corneal epithelium secondary to DES, with the additional benefit of avoiding the neovascularisation and scarring of that physiological process.
Blood-derived products have been extensively studied in the treatment of DES and other diseases leading to corneal epithelial damage. Their aim is to supply the components of physiological tears which promote corneal epithelial growth and maintenance. Efforts have focused on ensuring adequate concentrations of growth factors are present in the blood-derived products trialled. In whole blood, the concentration of growth factors within the plasma is low.27 Growth factors are found in high concentration within platelet α-granules,28 awaiting release upon activation of the clotting cascade at sites of tissue damage, in order to promote repair via locally increased growth factor concentration. Activation of platelets has therefore been an integral step in the manufacture of blood-derived products intended for the ocular surface – in AS by allowing collected whole blood to coagulate, and in platelet-rich plasma by centrifugation to increase platelet concentration and addition of calcium and thrombin prior to application to activate platelets.
In the present study, FAB is produced via dermal puncture of capillaries of the fingertip with a fingerprick. The nature of the blood produced can be inferred from previous studies concerning capillary blood and plasma composition.
Regarding the cellular component of FAB, analysis of paired capillary (via fingerprick) and venous blood samples by Yang et al29 showed no significant difference in the concentration of erythrocytes between the samples, and an increased concentration of leucocytes, particularly granulocytes, in capillary blood. Platelet concentration in capillary versus venous blood is currently disputed, with studies demonstrating decreased30, 31, 32 or unchanged29, 33 concentrations in capillary blood. Bond et al34 have shown that platelet count decreases for each successive drop of blood milked from a single puncture wound, and hypothesise this may reflect local platelet consumption at the site of trauma after fingerprick.
Regarding the plasma component of FAB, there have been no studies thus far concerning the concentration of growth factors in fingerprick blood plasma. In terms of venous blood plasma, Hartwig et al27 showed that concentration of growth factors in venous blood plasma is low, especially in comparison to serum (EGF 0.01 ng/ml plasma versus 0.7 ng/ml serum, PDGF 1.4 ng/ml plasma versus 14.5 ng/ml serum). Fibronectin and vitamin A concentrations were also shown to be lower in plasma than serum. The group also demonstrated reduced immortalised corneal epithelial cell line growth when incubated with plasma as opposed to serum. Is the composition of fingerprick blood plasma comparable to venous plasma? The clinical improvement seen on FAB in this study suggests that the concentration of growth factors and other factors crucial to corneal epithelial maintenance might be greater than that found in venous plasma. It is conceivable that platelet activation and hence growth factor release occurs during FAB application. Traumatic injury to capillaries and tissue via lancet,34 blood stasis during expression on the fingertip, and prolonged contact of blood with the ocular surface all present opportunities for platelet activation to occur. Indeed, it has been noted by patients on the trial that small clots can collect in the inferior conjunctival fornix after FAB application. Further study of the composition of FAB is necessary to test this hypothesis.
It is a theoretical concern that concentration of growth factors and nutrients in FAB, and hence potentially the efficacy of FAB, might vary between patients with DES of autoimmune versus non-autoimmune aetiology. However, it has been shown that the concentration of major epitheliotrophic factors in serum prepared from patients with chronic autoimmune disease is no different from that of control patients.35 It has also been demonstrated that growth factor concentrations in serum from patients suffering from DES of any aetiology is the same as that of controls.36 It is therefore unlikely that DES aetiology impacts upon the therapeutic potential of FAB.
It is not presently clear how the erythrocytes and leucocytes of FAB affect the ocular surface. There is evidence that erythrocytes, usually considered to be passive cells, are able to release growth factors and regulate neighbouring T cells, fibroblasts, and dendritic cells,37 and this is likely to have an impact on the corneal surface in FAB therapy. The effect of autologous leucocytes on the ocular surface is largely unknown – one study showed accelerated corneal ulcer healing after topical application of autologous mononuclear cells,38 but research is necessary to provide further detail in this area.
Although our study did not highlight any complications of FAB therapy, theoretical complications exist. The risk of ocular infection through transfer of skin pathogens via repeated close contact between finger and eye exists, though this is minimised by diligent cleaning of the finger with an alcohol steret. The risk of transmission of blood-borne pathogens to the anterior eye is biologically plausible, hence the exclusion criterion of existence of systemic infection. Repeated fingerpricks might lead to tissue damage at the fingertips, and traumatic neuroma formation at the site of fingerprick has been previously reported.39 On account of study design, only short-term complications could be assessed for, and longer follow-up is required to elucidate possible long-term complications.
In summary, our pilot study provides evidence in favour of the use of FAB in the treatment of severe DES refractory to conventional treatment. FAB presents a cheap, practical, and effective therapy which avoids the need for blood donation and specialist processing. A randomised controlled trial to compare FAB therapy with conventional topical therapy and AS is necessary to validate our initial findings. There are several questions regarding the biological mechanism of FAB therapy which remain unanswered and require further investigation, both at the cellular and clinical level. Potential complications of FAB therapy need to be explored further in future studies.
Summary
Acknowledgments
Infrastructural support from the NIHR Moorfields Biomedical Research Centre.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
The authors declare no conflict of interest.
Supplementary Material
References
- The Epidemiology of Dry Eye Disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocular Surf 2007; 5: 93–107. [DOI] [PubMed] [Google Scholar]
- Rauz S, Saw VP. Serum eye drops, amniotic membrane and limbal epithelial stem cells—tools in the treatment of ocular surface disease. Cell Tissue Bank 2010; 11: 13–27. [DOI] [PubMed] [Google Scholar]
- Yamada C, King KE, Ness PM. Autologous serum eyedrops: literature review and implications for transfusion medicine specialists. Transfusion 2008; 48: 1245–1255. [DOI] [PubMed] [Google Scholar]
- Fox RI, Chan R, Michelson JB, Belmont JB, Michelson PE. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum 1984; 27: 459–461. [DOI] [PubMed] [Google Scholar]
- Noble BA. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol 2004; 88: 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader S, Wedel T, Moll R, Geerling G. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefes Arch Clin Exp Ophthalmol 2006; 244: 1345–1349. [DOI] [PubMed] [Google Scholar]
- Young AL, Cheng ACO, Ng HK, Cheng LL, Leung GYS, Lam DSC. The use of autologous serum tears in persistent corneal epithelial defects. Eye 2004; 18: 609–614. [DOI] [PubMed] [Google Scholar]
- Chiang CC, Chen WL, Lin JM, Tsai YY. Allogeneic serum eye drops for the treatment of persistent corneal epithelial defect. Eye (Lond) 2009; 23: 290–293. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y. Autologous serum application in the treatment of neurotrophic keratopathy*1. Ophthalmology 2004; 111: 1115–1120. [DOI] [PubMed] [Google Scholar]
- del Castillo JMB, de la Casa JM, Sardiña RC, Fernandez RM, Feijoo JG, Gomez AC et al. Treatment of recurrent corneal erosions using autologous serum. Cornea 2002; 21: 781–783. [DOI] [PubMed] [Google Scholar]
- Fernando AI, Burton BJL, Smith GT, Corbett MC. Autologous serum drop-dependent re-epithelialisation following penetrating keratoplasty in chronic graft vs host disease. Eye (Lond) 2005; 19: 823–825. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Okamoto S, Mori T, Yamada M, Mashima Y, Watanabe R et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant 2003; 31: 579–583. [DOI] [PubMed] [Google Scholar]
- Goto E, Shimmura S, Shimazaki J, Tsubota K. Treatment of superior limbic keratoconjunctivitis by application of autologous serum. Cornea 2001; 20: 807–810. [DOI] [PubMed] [Google Scholar]
- Partal A, Scott E. Low-cost protocol for the production of autologous serum eye drops by blood collection and processing centres for the treatment of ocular surface diseases. Transfus Med 2011; 21: 271–277. [DOI] [PubMed] [Google Scholar]
- Cho YK, Huang W, Kim GY, Lim BS. Comparison of autologous serum eye drops with different diluents. Curr Eye Res 2013; 38: 9–17. [DOI] [PubMed] [Google Scholar]
- López-García JS, García-Lozano I, Rivas L, Ramírez N, Raposo R, Méndez MT. Autologous serum eye drops diluted with sodium hyaluronate: clinical and experimental comparative study. Acta Ophthalmol 2014; 92: e22–e29. [DOI] [PubMed] [Google Scholar]
- Fischer KR, Opitz A, Böeck M, Geerling G. Stability of serum eye drops after storage of 6 months. Cornea 2012; 31: 1313–1318. [DOI] [PubMed] [Google Scholar]
- Rocha EM, Pelegrino FSA, de Paiva CS, Vigorito AC, de Souza CA. GVHD dry eyes treated with autologous serum tears. Bone Marrow Transplant 2000; 25: 1101–1103. [DOI] [PubMed] [Google Scholar]
- Tananuvat N, Daniell M, Sullivan LJ, Yi Q, McKelvie P, McCarty DJ et al. Controlled study of the use of autologous serum in dry eye patients. Cornea 2001; 20: 802–806. [DOI] [PubMed] [Google Scholar]
- Poon AC. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol 2001; 85: 1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welder JD, Bakhtiari P, Djalilian AR. Limbitis secondary to autologous serum eye drops in a patient with atopic keratoconjunctivitis. Case Rep Ophthalmol Med 2011; 2011: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell PJ, Schanzlin DJ, Rao NA. Immunoglobulin deposition in the cornea after application of autologous serum. Arch Ophthalmol 1988; 106: 1423–1425. [DOI] [PubMed] [Google Scholar]
- Johnson ME, Murphy PJ. Measurement of ocular surface irritation on a linear interval scale with the ocular comfort index. Invest Ophthalmol Vis Sci 2007; 48: 4451–4458. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003; 22: 640–650. [DOI] [PubMed] [Google Scholar]
- Chang J-H, Garg NK, Lunde E, Han K-Y, Jain S, Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol 2012; 57: 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce NC. Cell cycle status in human corneal endothelium. Exp Eye Res 2005; 81: 629–638. [DOI] [PubMed] [Google Scholar]
- Hartwig D, Herminghaus P, Wedel T, Liu L, Schlenke P, Dibbelt L et al. Topical treatment of ocular surface defects: comparison of the epitheliotrophic capacity of fresh frozen plasma and serum on corneal epithelial cells in an in vitro cell culture model. Transfus Med 2005; 15: 107–113. [DOI] [PubMed] [Google Scholar]
- Blair P, Flaumenhaft R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev 2009; 23: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZW, Yang SH, Chen L, Qu J, Zhu J, Tang Z. Comparison of blood counts in venous, fingertip and arterial blood and their measurement variation. Clin Lab Haematol 2001; 23: 155–159. [DOI] [PubMed] [Google Scholar]
- Podgórski T, Bartkowiak U, Pawlak M. Comparison of hematological parameters of venous and capillary blood in athletes. Trends Sport Sci 2014; 21. [Google Scholar]
- Kim MJ, Jin JH, Kwon YS, Jun YH, Kim SK. Comparison of blood counts in capillary and venous blood in children. Korean J Hematol 2009; 44: 237. [Google Scholar]
- Leppänen E. Experimental basis of standardized specimen collection: The effect of the site of venipuncture on the blood picture, the white blood cell differential count, and the serum albumin concentration. Eur J Haematol 2009; 41: 445–448. [DOI] [PubMed] [Google Scholar]
- Daae LNW, Hallerud M, Halvorsen S. A comparison between haematological parameters in ‘capillary’ and venous blood samples from hospitalized children aged 3 months to 14 years. Scand J Clin Lab Invest 1991; 51: 651–654. [DOI] [PubMed] [Google Scholar]
- Bond MM, Richards-Kortum RR. Drop-to-drop variation in the cellular components of fingerprick blood. Am J Clin Pathol 2015; 144: 885–894. [DOI] [PubMed] [Google Scholar]
- Phasukkijwatana N, Lertrit P, Liammongkolkul S, Prabhasawat P. Stability of epitheliotrophic factors in autologous serum eye drops from chronic Stevens-Johnson syndrome dry eye compared to non-autoimmune dry eye. Curr Eye Res 2011; 36: 775–781. [DOI] [PubMed] [Google Scholar]
- Bradley JC, Bradley RH, McCartney DL, Mannis MJ. Serum growth factor analysis in dry eye syndrome. Clin Exp Ophthalmol 2008; 36: 717–720. [DOI] [PubMed] [Google Scholar]
- Antunes RF, Brandão C, Maia M, Arosa FA. Red blood cells release factors with growth and survival bioactivities for normal and leukemic T cells. Immunol Cell Biol 2011; 89: 111–121. [DOI] [PubMed] [Google Scholar]
- Zapuskalov I, Krivosheina O, Elegesheva O. [Use of autologous blood mononuclear cells in the complex treatment of corneal ulcers]. Vestn Oftalmol 2008; 124: 32–35. [PubMed] [Google Scholar]
- Kahraman S, Rezai SM, Dogu H, Sayan MA, Akar Z. Painful traumatic neuroma after a finger stick. Anesth Analg 2005; 100: 1414–1415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.