Abstract
Purpose
To examine 12-month real-world visual acuity outcomes and treatment patterns in neovascular age-related macular degeneration (nAMD) eyes, including those with pigment epithelial detachment (PED), receiving ranibizumab or aflibercept.
Patients and methods
Electronic medical records were used to identify ranibizumab or aflibercept-treated nAMD eyes with 12 months follow-up from first prescription. The primary objective was to compare mean change in visual acuity (VA) between index and month 12, in eyes treated with ranibizumab and aflibercept to assess the non-inferiority of ranibizumab vs aflibercept using a −5 letter non-inferiority margin. The number of injections and non-injection visits during follow-up were key secondary objectives.
Results
A total of 3350 ranibizumab and 4300 aflibercept treatment-naive eyes were included. At month 12, mean change from index in VA letter score was −0.30 for ranibizumab and −0.19 for aflibercept (P=0.81). The adjusted difference of mean change was −0.14 (−0.79 to 0.51) (P=0.67) (generalized estimating equations method) confirming the non-inferiority of ranibizumab. Eyes received a similar number of injections during follow-up. The mean (±SD) number of ranibizumab and aflibercept injections were 6.70 (±2.54) and 7.00 (±2.40), respectively (P<0.0001). In PED eyes, the mean (±SD) change between baseline to month 12 was 1.25 (±11.3) for ranibizumab and −0.39 (±13.3) for aflibercept (adjusted between-group difference 1.80 (−0.71 to 4.30; P=0.16)) achieved with a mean (±SD) 7.85 (±2.68) ranibizumab and 7.47 (±2.45) aflibercept injections, (P=0.11).
Conclusions
Ranibizumab and aflibercept treatment yielded comparable VA outcomes in nAMD eyes, including those with PED, with similar treatment patterns over 12 months in real-world clinical practice.
Introduction
Age-related macular degeneration (AMD) is a chronic, degenerative eye disease causing pathological changes in the macular region of the retina and is the most common cause of permanent vision impairment and loss in older adults.1, 2, 3 Despite accounting for only about 10% of AMD cases, neovascular AMD (nAMD) is responsible for 80–90% of severe vision loss in AMD patients.3 nAMD is characterized by retinal thickening and the growth and leakage of new blood vessels resulting in scarring of the macula and irreversible loss of central vision.
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents are now the established standard of care for nAMD.4 Approved in the United States in 2006, and the European Union in 2007 following the pivotal MARINA and ANCHOR phase III trials, ranibizumab was a major breakthrough in the treatment of nAMD5 achieving VA improvement by approximately seven and eleven letters, respectively, on the Early Treatment Diabetic Retinopathy Study (ETDRS) chart at 12 months, which was generally maintained up to 24 months.6, 7 Subsequent studies highlighted that individualized treatment strategies could optimize outcomes and treatment frequency. Ninety-three percent patients receiving individualized ranibizumab dosing for 24 months in the HARBOR study achieved similar visual acuity outcomes compared to monthly dosing.8
Aflibercept was approved in the United States in November 2011 and in European Union in November 2012 following demonstration of non-inferiority of 8-weekly aflibercept to monthly ranibizumab in the VIEW 1 and 2 studies.9 Aflibercept recommended dosing in nAMD is three monthly loading doses, followed by injection once every 8 weeks.10
Occurring in up to 62% eyes with advanced AMD, retinal pigment epithelial detachment (PED) is an important marker of disease severity and predictor of vision loss in AMD patients.11 Although evidence from small, non-comparative studies suggests that both ranibizumab and aflibercept may be effective in reversing the anatomical changes associated with PED,12, 13, 14, 15, 16 there is limited evidence of effectiveness in visual acuity outcomes in PED patients.17, 18, 19
Real-world evidence (RWE) studies to date suggest that ranibizumab and aflibercept are not dosed according to recommendations and patients generally receive fewer doses than label recommendations.20, 21, 22 The consequences of individualized dosing on visual acuity outcomes in the real world is unclear. Using US electronic medical records (EMR), this study examines visual acuity linked to real-world treatment patterns in a large cohort of nAMD patients.
Methods
Information source
A retrospective, comparative, non-randomized cohort study of real-world ocular treatment with intravitreal injections of ranibizumab or aflibercept in nAMD patients was performed using data extracted from a standardized EMR system in the United States. Consistent with the US Code of Federal Regulations (45 CFR 164.514(e)), this EMR source constitutes a ‘limited data set’ in which all-patient identifiers have been completely removed and site and clinician data pseudo-anonymized. On this basis, formal ethics approval is not required. Data were extracted for the period 1 January 2011–30 June 2015.
Patients
Treatment-naive adult patients (aged ≥21 years) were included if they had a diagnosis of nAMD (recorded as ICD-9-CM code 362.52) and ≥1 ranibizumab or aflibercept injection between 01 July 2011 and 30 June 2014. This first injection date was defined as the index date. Eyes were required to have a nAMD diagnosis on or within 6 months pre-index, no record of anti-VEGF therapy 180 days pre-index and at least 1 year of post-index follow-up data. Eyes were required to have had ≥1 quantifiable VA (not: light perception, no light perception, invalid) reading on or within 30 days of the index date and ≥1 quantifiable VA reading eligible to be included as the month 12 reading. Eligible VA readings at month 12 were defined as any time between day 300 and day 420 post-index. Eyes which (in addition to nAMD) recorded ICD-9-CM 362.42 (serous PED) and 362.43 (hemorrhagic PED) were classified as having PED.
Eyes were excluded if they received any anti-VEGF treatment within 6 months prior to the index date or if discontinuation occurred, defined as a gap of more than 180 days between study injections or VA assessments during the follow-up period, or if anti-VEGF therapy was switched during the 12 months of follow-up.
Study design and analyses
The primary objective was to compare mean change in VA (letters) between index and month 12 in treatment-naive eyes receiving ranibizumab or aflibercept.
Secondary objectives included: the proportion of eyes gaining or losing ≥0, ≥5, ≥10 and ≥15 letters between index and month 12; the mean number of injections per eye during the first 12 months of treatment (follow-up period), and in the first 2 months after index injection (loading period). Non-injection visits were defined as those visits without study drug injection but during which either visual acuity or intraocular pressure was measured or when optical coherence tomography or fluorescein angiography was utilized.
Subgroup analysis included eyes stratified by baseline VA <69 and ≥69 letters (~≥20/40, a threshold for driving ability in United States) and eyes with PED.
Visual acuity recorded as Snellen fraction was adjusted for partially read lines and converted to approximate ETDRS letter scores as described previously,23 except ‘count fingers’ was treated as one letter. Age was collected in 5-year bands up until the maximum (90+ years).
Sample size
Based on observations in a previous trial with ranibizumab and aflibercept in nAMD,9 which reported a mean (standard deviation (SD)) change in BCVA at month 12 of 7.9 (15.0) with aflibercept and 8.1 (15.3) with ranibizumab, the sample size for the VA outcome analysis for non-inferiority of ranibizumab vs aflibercept was calculated assuming no difference, a non-inferiority limit of −5 letters, and a significance level of 0.05. Assuming a common SD of 15.0, 154 eyes were determined to be needed to achieve at least 90% power.
Statistical analysis
The eye was defined as the unit of analysis. Calculations were performed using SAS 9.1 statistical package. All eligible eyes with 12-month follow-up data post-index were assessed for all outcomes. Descriptive statistics included mean (SD), median, or range, where appropriate. P-values using χ2 testing for categorical variables and the Wilcoxon rank-sum test for continuous variables were reported. A P-value of <0.05 was considered statistically significant.
The primary comparison of the study was a test of non-inferiority of ranibizumab vs aflibercept, based on the primary outcome, using a margin of non-inferiority of 5 letters. A general linear model (GLM) of analysis of covariance (ANCOVA) was used to compare the two study groups. Mean difference in VA (letters) score at month 12 was the dependent variable. A generalized estimating equations (GEE) method was also used to account for demographic and clinical characteristics (including age and baseline VA) as well as repeated measurements in cases of bilateral treatment clustering at the patient level. The GEE data are presented throughout.
A two-sided Wilcoxon rank-sum test was used to compare numbers of injections and visits. Logistic regression models, adjusting for baseline VA, were used to compare binary change in VA letter score (the relative odds of gaining/losing ≥5, ≥10 and ≥15 letters at month 12).
Tests were not adjusted for multiple comparisons.
Sensitivity analyses
For month 12 measurements, a sensitivity analysis expanding the window to ±3 months was performed.
To investigate the impact of requiring eyes to have 12 months follow-up, the baseline characteristics of eyes discontinuing treatment or switching anti-VEGF therapy were also considered.
Results
Study populations
A total of 3350 ranibizumab and 4300 aflibercept-treated treatment-naive eyes were included in the study. Of these 253 ranibizumab-treated eyes and 203 aflibercept-treated eyes were classified as PED.
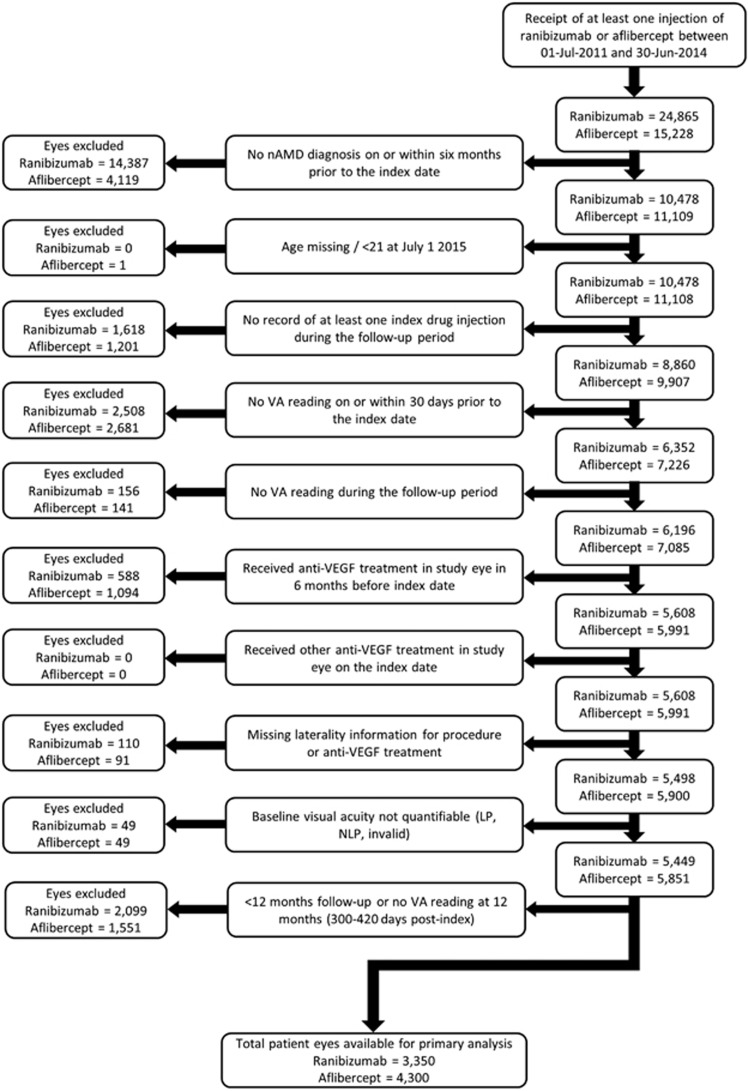
Figure 1 shows the study flow chart incorporating exclusion rules.
Figure 1.
Development and attrition of patient cohorts. LP, light perception; nAMD, neovascular age-related macular degeneration; NLP, no light perception; VA, visual acuity; VEGF, vascular endothelial growth factor.
Baseline characteristics were generally balanced between ranibizumab and aflibercept cohorts. Treatment-naive ranibizumab patients were slightly older than aflibercept patients (47.3% vs 39.9%, respectively, were aged >85 years) with slightly lower baseline mean study eye VA (57.5 (±21.2) vs 58.5 (±20.7); P=0.025). PED ranibizumab patients were also slightly older than their aflibercept counterparts (49.0% vs 38.0%, respectively, were aged >85 years) with a higher proportion of female patients (71.2% vs 58.0% P=0.007) (Table 1).
Table 1. Patient demographics and baseline characteristics.
| Characteristicsa |
Main study |
PED subgroup |
Switched Treatment |
Discontinued Treatment |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ranibizumab cohort | Aflibercept cohort | P-value | Ranibizumab cohort | Aflibercept cohort | P-value | Ranibizumab cohort | Aflibercept cohort | Ranibizumab cohort | Aflibercept cohort | |
| Number of patients | 2869 | 3630 | 208 | 176 | 847 | 442 | 882 | 697 | ||
| Number (%b) of eyes | 3350 (61.5%) | 4300 (73.5%) | 253 (7.6%) | 203 (4.7%) | 927 (17.0%) | 484 (8.3%) | 955 (17.5%) | 785 (13.4%) | ||
| Agec (years), n (%) | <0.0001 | 0.0001 | ||||||||
| ≤70 | 209 (7.3%) | 298 (8.2%) | 10 (4.8%) | 16 (9.1%) | 91 (10.7%) | 55 (12.4%) | 61 (6.9%) | 43 (6.2%) | ||
| 71–75 | 255 (8.9%) | 382 (10.5%) | 16 (7.7%) | 22 (12.5%) | 101 (11.9%) | 45 (10.2%) | 64 (7.3%) | 62 (8.9%) | ||
| 76–80 | 413 (14.4%) | 638 (17.6%) | 30 (14.4%) | 42 (23.9%) | 135 (15.9%) | 60 (13.6%) | 133 (15.1%) | 112 (16.1%) | ||
| 81–85 | 636 (22.2%) | 863 (23.8%) | 50 (24.0%) | 29 (16.5%) | 192 (22.7%) | 105 (23.8%) | 188 (21.3%) | 159 (22.8%) | ||
| 86–89 | 604 (21.1%) | 726 (20.0%) | 34 (16.3%) | 40 (22.7%) | 159 (18.8%) | 88 (19.9%) | 169 (19.2%) | 131 (18.8%) | ||
| 90+ | 752 (26.2%) | 723 (19.9%) | 68 (32.7%) | 27 (15.3%) | 169 (20.0%) | 89 (20.1%) | 267 (30.3%) | 190 (27.3%) | ||
| Min | 33.0 | 38.0 | 63.0 | 58.0 | 38.0 | 53.0 | 53.0 | 38.0 | ||
| Mean (SD) | 83.4 (8.1) | 82.4 (7.8) | 84.3 (7.1) | 81.5 (7.4) | 81.8 (8.4) | 81.9 (8.5) | 83.4 (7.9) | 83.4 (7.7) | ||
| Female (n, %) | 1887 (65.8%)d | 2312 (63.8%)e | 0.09 | 148 (71.2%) | 102 (58.0%) | 0.007 | 525 (62.5%) | 295 (67.4%) | 528 (63.8%) | 397 (61.8%) |
| Bilateral treatment | 481 (16.8%) | 670 (18.5%) | 0.11 | 45 (21.6%) | 27 (15.3%) | 0.23 | 80 (9.4%) | 42 (9.5%) | 73 (8.3%) | 88 (12.6%) |
| Study eye VA, mean letter score at index (SD) | 57.5 (21.2) | 58.5 (20.7) | 0.025 | 61.9 (18.0) | 61.7 (19.2) | 0.91 | 57.8 (19.8) | 57.8 (19.9) | 49.1 (25.2) | 51.0 (24.5) |
| VA letter score, n (%) | ||||||||||
| ≥70 | 1098 (32.8%) | 1498 (34.8%) | 0.06 | 107 (42.3%) | 89 (43.8%) | 0.74 | 290 (31.3%) | 165 (34.1%) | 237 (24.8%) | 193 (24.4%) |
| ≤35 | 639 (19.1%) | 777 (18.1%) | 0.26 | 27 (10.7%) | 26 (12.8%) | 0.48 | 174 (18.8%) | 93 (19.2%) | 320 (33.5%) | 231 (29.4%) |
| Study eye VA, mean change index to switch (SD) | −1.0 (13.9) | −1.5 (15.2) | ||||||||
Baseline VA for all treatment-naive patients (not just those with VA scores at 12 months).
Denominator for PED subgroup is eyes in main study. For all other cohorts, denominator is eyes that meet inclusion criteria but with no requirement for 12 months of follow-up or VA at month 12.
Age was collected in 5-year bands up until the maximum (90+ years). The midpoint of the band, and 92 for the highest band was used when calculating age descriptive statistics.
Gender not known for two patients.
Gender not known for five patients.
Overall, 15.4% of eyes (17.5% ranibizumab vs 13.4% aflibercept) discontinued therapy during follow-up. Eyes discontinuing treatment (compared to those with 12 months follow-up) were generally older with a higher proportion in 90+ age bracket (28.8% vs 22.7%) and had poorer baseline vision in the study eye (mean (±SD) VA 50.0 (±24.9) vs 58.1 (±20.9); P<0.0001).
An additional 12.5% of eyes (17.0% ranibizumab vs 8.3% aflibercept) switched anti-VEGF therapy and were therefore excluded. These eyes had similar baseline visual acuity compared to the non-switchers (mean (±SD) 57.8 (19.8) vs 58.1 (20.9)). Among the switchers, baseline VA was comparable by index treatment (mean (±SD) 57.8 (19.8) vs 57.8 (19.9)), with a smaller decrease in VA from baseline to switch for switchers from ranibizumab (mean (±SD) −1.0 (13.9) vs −1.5 (15.2)).
Visual acuity
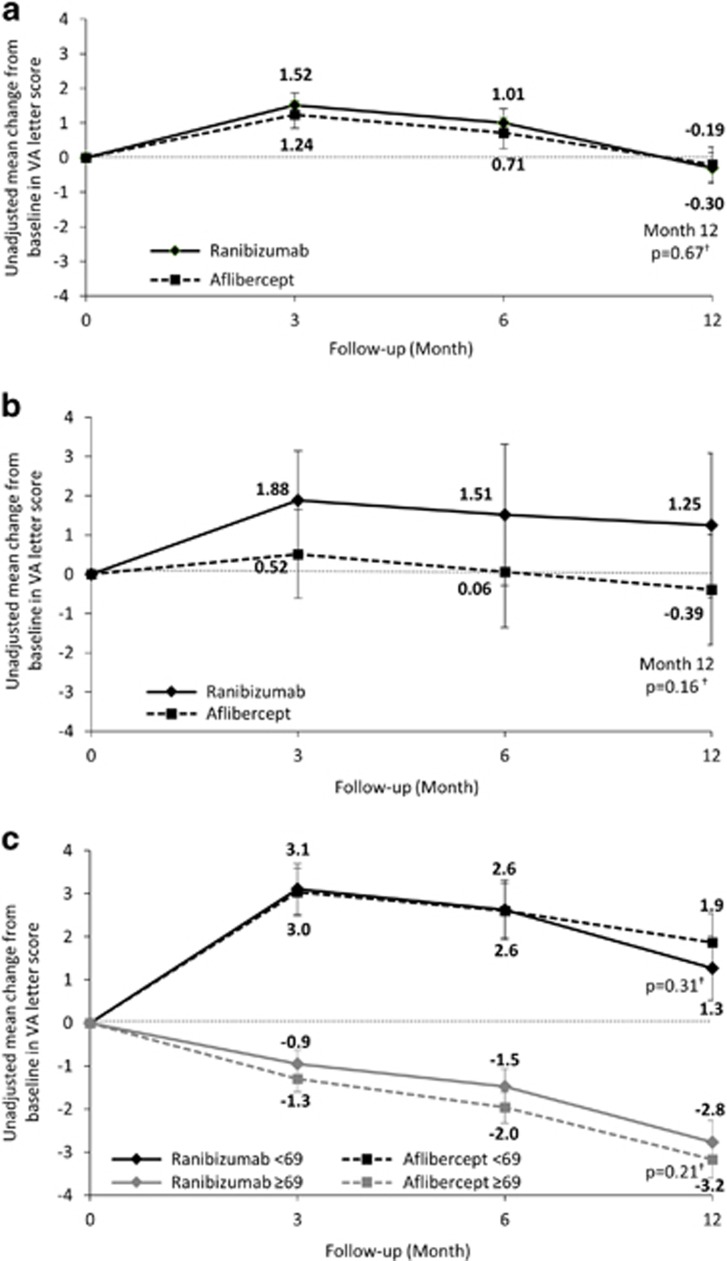
Ranibizumab and aflibercept-treated eyes achieved similar visual acuity outcomes over 12 months. At month 12, the mean (±SD) change from baseline in VA letter score (primary objective) was −0.30 (±14.8) for ranibizumab and −0.19 (±14.7) for aflibercept. Non-inferiority was confirmed by virtue of the lower boundary of the confidence interval (−0.79) being greater than the negative of the non-inferiority margin (5) (adjusted between-group difference −0.14, 95% CI: −0.79 to 0.51, P=0.67) (Figure 2a). This was confirmed in a sensitivity analysis in which the window for the month 12 measurement was expanded to ±3 months (adjusted difference −0.22, 95% CI: −0.86 to 0.43, P=0.51).
Figure 2.
Mean change from baseline in VA in eyes during 12 months follow-up; (a) in all eyes, (b) in PED eyes, and (c) in all eyes according to baseline VA letter score. Error bars represent 95% confidence intervals. †Least squares mean estimate from GEE model adjusted for baseline VA, age, and clustering at patient level.
In the PED subgroup, at month 12, the mean (±SD) change from baseline was 1.25 (±11.3) for ranibizumab and −0.39 (±13.3) for aflibercept (adjusted between-group difference 1.80, 95% CI: −0.71 to 4.30, P=0.16) (Figure 2b).
Ranibizumab and aflibercept also achieved similar visual acuity outcomes in eyes stratified by baseline VA. Eyes with a lower baseline VA (<69 letters) achieved greater improvement with either treatment than eyes with a high baseline VA (≥69 letters). In the lower-baseline VA subpopulation, the mean (±SD) change in VA letter score between index and month 12 was similar for ranibizumab and aflibercept (+1.3 (±17.2) vs +1.9 (±17.3), respectively; adjusted difference −0.51, 95% CI: −1.49 to 0.47, P=0.31). In the high baseline VA subpopulation, the mean (±SD) change in VA letter score between index and month 12 was similar for ranibizumab and aflibercept (−2.8 (±9.3) vs −3.2 (±9.1), respectively; adjusted difference 0.42, 95% CI: −0.24 to 1.08, P=0.21) (Figure 2c).
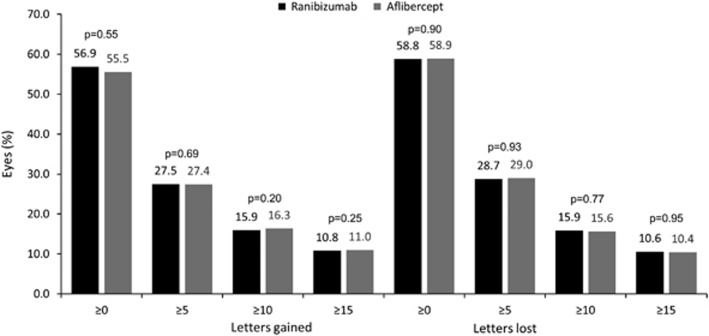
The proportion of eyes achieving predefined VA thresholds, defined as gain or loss of ≥0, ≥5, ≥10, ≥15 VA letters at month 12 compared to baseline, was similar for both treatment groups (Figure 3).
Figure 3.
Letters gained/lost at month 12 compared to baseline in treatment-naive eyes. P-values derived from a two-sided Wilcoxon rank-sum test.
Treatment patterns
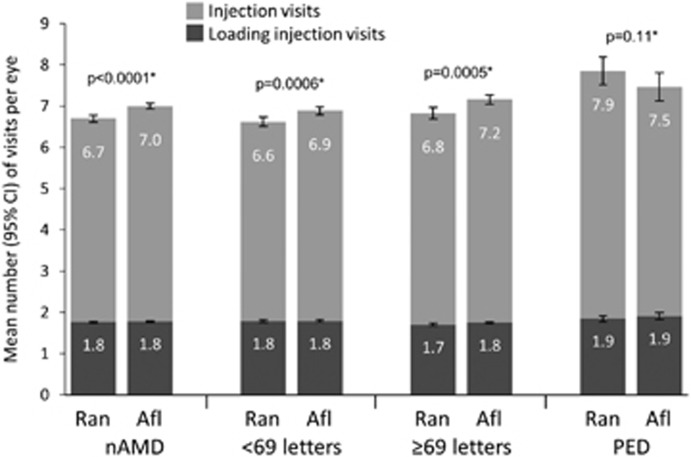
During the 12-month post-index follow-up period, ranibizumab and aflibercept-treated eyes received a similar number of injections. The mean (±SD) number of injections was 6.70 (±2.54) vs 7.00 (±2.40) for ranibizumab and aflibercept eyes, respectively (P<0.0001). The mean number of injections per eye was similar regardless of baseline VA and agent received (Figure 4). In the subgroup of patients with PED, the mean (±SD) number of injections per eye was similar for both agents in the 12-month follow-up period (ranibizumab 7.85 (±2.68) vs aflibercept 7.47 (±2.45)) (P=0.11) highlighting that PED eyes had on average one injection more than non-PED eyes during the first 12 months of treatment (Figure 4).
Figure 4.
Mean number of injection visits per eye during 12 months of follow-up. *P-values derived from a two-sided Wilcoxon rank-sum test and refer to injection visits. Error bars are 95% confidence intervals. nAMD, neovascular age-related macular degeneration; NIV, non-injection visits; PED, retinal pigment epithelial detachment.
Within the loading period of follow-up (defined as the first 2 months post-index), of a possible maximum of three injections, the mean (±SD) number of injections administered was 1.76 (±0.60) for ranibizumab and 1.78 (±0.56) for aflibercept (Figure 4). The number of injections in the loading period was also similar by agent across subgroups (Figure 4). The mean (±SD) number of non-injection visits was 2.15 for both ranibizumab (±2.86) and aflibercept (±3.08) (P=0.12) during the 12-month follow-up period (P=0.12).
Discussion
This is the first US EMR-based retrospective study to compare both treatment frequency and outcomes with ranibizumab and aflibercept in nAMD. Results show, despite differences in the label-recommended dosing, comparable numbers of ranibizumab and aflibercept injections are administered in the first year of therapy in the United States with no clinically relevant differences in visual acuity outcome at 12 months. On this basis, the non-inferiority of ranibizumab to aflibercept, the primary test of the study, was achieved. Alternative models used for comparison of the primary outcome (GLM of ANCOVA similarly concluded non-inferiority, as did the sensitivity analysis expanding window to ±3 months for the month 12 measurement.
Of note was the maintenance of visual acuity in patients with both high (≥69 letters) as well as low (<69 letters) baseline visual acuity over 12 month’s follow-up. The former group are often excluded or under-represented in clinical trials (for example in VIEW 1 and VIEW 2 clinical trials, enrollment was restricted to patients with baseline VA of ≤73 letters.9 The maintenance visual acuity in this cohort is supportive of the much advocated early detection and treatment in nAMD to ensure nAMD eyes remain in the best health state for a greatest duration of time.24, 25
With a mean 7.0 doses in the 12-month follow-up period, data from this study suggest that in clinical practice aflibercept is being administered at a similar frequency to clinical trials (7.5 doses in VIEW 1 and VIEW 2 trials).9 With a mean 6.7 doses in the first year of treatment and 2.15 non-injection visits, this study suggests that ranibizumab is generally being dosed flexibly with injection frequency comparable to previous clinical studies, which have reported a mean 12.6 ranibizumab injections over 2 years and 7.7 over 1 year26, 27 although higher than in previously reported real-world studies (mean 5.7 injections in year 1).
Recent claims database analyses have also shown similar dosing of ranibizumab and aflibercept in treatment-naive nAMD patients with 4.9 vs 5.2 and 5.8 vs 5.5 injections, respectively, over 12 months.20, 22 The higher number of injection visits in this study perhaps reflects the more complete picture of treatment provided by EMR compared to claims databases due to potential gaps in claims reporting or identification or claim adjudication at clinic level.
Of key clinical and patient relevance, this study demonstrates that with comparable dosing, ranibizumab and aflibercept were equally effective in maintaining visual acuity in real-world clinical practice. The essentially stable VA during follow-up in this study contrasts with gains reported in clinical trials such as CATT, HARBOR, and VIEW trials.9 Differences in VA outcomes between RWE studies and clinical trials are not unexpected and most likely reflect the narrow population and significant exclusion criteria in clinical trials. RWE studies would allow inclusion of eyes with a wider range of CNV lesions (for example, larger lesions) as well as those with structural damage at the fovea and may include confounders such as cataract, which would be excluded from clinical trials. Further, visual acuities recorded here are not equivalent to refracted protocol-standardised visual acuity measurements seen in clinical trials and as such may show fewer letters gained compared with a clinical trial-recorded visual acuity measurement.
There is limited prospective data with which to guide treatment for PEDs and these patients are generally excluded from clinical trials and not analyzed as a subgroup. Ranibizumab and aflibercept were equally effective in stabilizing VA over 12 months and with similar efficacy to that in non-PED patients suggesting anti-VEGF therapy is a viable treatment option in PED patients and is in agreement with a further comparative short-term study.28
Of note is the relatively low proportion of PED eyes identified in this study. At 6% of eyes in the study, this is considerably lower than would be expected. Indeed, PEDs are seen in up to 62% of eyes with advanced AMD.11 This potential under-representation is likely due to the lack of granularity in ICD-9 coding for PED. The ICD-9 codes 362.42 and 362.43 used to classify PED in this analysis refer only to serous and hemorrhagic PEDs, respectively, and hence other PED subtypes including fibrovascular and drusenoid PEDs, which might be expected to account for ~90% of PEDs29 were not necessarily captured. Serous PEDs comprise ~10% of all PEDs in nAMD29 and in this context, the 6% rate of PED identification in this study is consistent with expectations for the frequency of this subtype in nAMD. Therefore, while in this study ranibizumab and aflibercept show similar efficacy in PED, their relative efficacy in fibrovascular and drusenoid PED remain to be determined, especially as fibrovascular PED has been demonstrated to be more difficult to treat with anti-VEGF agents and with poorer visual outcomes than serous PED.30
It has been hypothesized that the wider binding capacity of aflibercept (VEGF-A, VEGF-B, and PIGF) coupled to its higher affinity for VEGF compared to ranibizumab might result in improved efficacy.31, 32, 33, 34 However, results here suggest that these properties do not translate into improvements in patient-relevant visual functioning as captured by VA in real-world clinical practice. Furthermore, this study highlights that the potential capacity advantage offered by 8-weekly aflibercept compared to monthly ranibizumab does not translate to real-world clinical practice, where treatment patterns with the two agents are similar, crucially with similar visual acuity outcomes.
To our knowledge, this is the first comparative US EMR-based retrospective study to compare outcomes and treatment frequency with ranibizumab and aflibercept in nAMD. The standardized EMR database used utilizes data entered as part of routine clinical care, which allowed data evaluation in a detailed and consistent manner from a large number of geographically diverse practices in United States. Although in the study itself, data collection was retrospective (introducing inherent biases such as misclassification, treatment bias, and confounding) data fields were predefined before any data collection started and were entered prospectively and is reflective of real-world daily clinical practice rather than of protocol-driven clinical trials.
It is important to continue to explore long-term outcomes of anti-VEGF treatment in nAMD given the chronic nature of the disease. However, it is possible that any differences in treatment may not have been manifest at 12 month’s follow-up. An extended study with longer follow-up would address this.
There was a high proportion of eyes lost to follow-up due to both treatment discontinuation and switching anti-VEGF treatment. Discontinuing eyes were generally older with poorer VA at index, which are associated with an increased mortality rate and reduced gains in visual acuity. Possibly patients with poorer visual acuity outcomes might discontinue or switch treatment thus confounding our results. The direction of any confounding would depend both on the differential discontinuation rates between treatments (ranibizumab is higher) and trend in visual acuity at switch (aflibercept has a larger decrease). The high discontinuation rate in this study has been described before in real-world studies of anti-VEGF treatment in nAMD.35 Additional reasons for discontinuation might include inconvenience and/or discomfort associated with intravitreous injection and a perceived lack of benefit35 or death. Regular intravitreal injections are associated with a significant treatment burden for patients, caregivers, and physicians, which is often unsustainable in clinical practice.36
Visual acuity post-switch for eyes that switched treatment was available but not analyzed in this study. A companion analysis for switchers with 12 months on the switch treatment found a comparable change in VA at 12 months post-switch (mean (SD) VA change −1.56 (13.4) ranibizumab to aflibercept switchers vs 0.21 (19.2) aflibercept to ranibizumab switchers; P=0.55) (manuscript in preparation).
nAMD generally occurs in those aged 50 or over. However, eyes from patients aged <50 were included in this study (0.2% of those analyzed). This finding raises the possibility of misclassification/miscoding of these younger patients, however, it is unlikely that the study results were impacted.
Further limitations also include the loss of sensitivity associated with conversion of Snellen vision recorded in this study to ETDRS letters, limited data on choroidal neovascularization classification as well as limited systemic medical history of participants and lack of match-control. The latter could be addressed with larger study cohorts in the future. An additional limitation is the lack of detail in ICD-9 classification for PEDs resulting in potential misclassification and potentially the majority of PED eyes remaining uncaptured.
Conclusions
This study represents, to our knowledge, the largest EMR database analysis comparing both treatment patterns and visual outcomes between ranibizumab and aflibercept. Despite differences in their approved treatment regimens, in real-world clinical practice, ranibizumab and aflibercept-treated nAMD eyes receive similar numbers of injections and achieve similar visual acuity outcomes in the first year of treatment. In addition, this study shows that anti-VEGF therapy is effective in maintaining vision in patients with high visual acuity not normally assessed in phase 3 clinical trials and further supports the early detection and treatment paradigm in nAMD to ensure maintenance of visual function of the eye.
Acknowledgments
Novartis Pharma AG, Basel, Switzerland funded this study. Medical writing support was provided by Jonathan Askham at Wellmera AG and was funded by Novartis.
Footnotes
PD is a consultant to Alcon, Genentech, Novartis Pharma AG, and Ophthotech. AL has received travel support from Bayer and attended advisory boards for Novartis and Bayer. AF and FM are employees of Novartis. RG is an employee of QuintilesIMS. QuintilesIMS received funding from Novartis to conduct the study.
References
- Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron 2012; 75(1): 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004; 122(4): 477–485. [DOI] [PubMed] [Google Scholar]
- Quillen DA. Common causes of vision loss in elderly patients. Am Fam Physician 1999; 60(1): 99–108. [PubMed] [Google Scholar]
- Kovach JL, Schwartz SG, Flynn Jr HW, Scott IU. Anti-VEGF treatment strategies for wet AMD. J Ophthalmol 2012; 2012: 786870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedania VS, Bakri SJ. Current perspectives on ranibizumab. Clin Ophthalmol 2015; 9: 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009; 116(1): 57–65.e5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355(14): 1419–1431. [DOI] [PubMed] [Google Scholar]
- Ho AC, Busbee BG, Regillo CD, Wieland MR, Van Everen SA, Li Z et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014; 121(11): 2181–2192. [DOI] [PubMed] [Google Scholar]
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012; 119(12): 2537–2548. [DOI] [PubMed] [Google Scholar]
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125387lbl.pdf.
- Coscas F, Coscas G, Souied E, Tick S, Soubrane G. Optical coherence tomography identification of occult choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol 2007; 144(4): 592–599. [DOI] [PubMed] [Google Scholar]
- Broadhead GK, Hong T, Zhu M, Li H, Schlub TE, Wijeyakumar W et al. Response of pigment epithelial detachments to intravitreal aflibercept among patients with treatment-resistant neovascular age-related macular degeneration. Retina 2015; 35(5): 975–981. [DOI] [PubMed] [Google Scholar]
- Iordanous Y, Powell AM, Mao A, Hooper PL, Eng KT, Schwartz C et al. Intravitreal ranibizumab for the treatment of fibrovascular pigment epithelial detachment in age-related macular degeneration. Can J Ophthalmol 2014; 49(4): 367–376. [DOI] [PubMed] [Google Scholar]
- Panos GD, Gatzioufas Z, Petropoulos IK, Dardabounis D, Thumann G, Hafezi F. Effect of ranibizumab on serous and vascular pigment epithelial detachments associated with exudative age-related macular degeneration. Drug Des Dev Ther 2013; 7: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KH, Chow CC, Rathod R, Mieler WF, Lim JI, Ulanski LJ 2nd et al. Rapid response of retinal pigment epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to bevacizumab and ranibizumab. Eye 2013; 27(5): 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Ruping J, Neubauer AS, Mayer W, Ulbig M, Haritoglou C et al. Alterations of vascular pigment epithelium detachments associated with age-related macular degeneration during upload with intravitreal ranibizumab. Retina 2013; 33(9): 1843–1849. [DOI] [PubMed] [Google Scholar]
- Freeman WR, Kozak I, Yuson RM, Nigam N, Cheng L, Mojana F. Prognosti implications of pigment epithelial detachment in bevacizumab (avastin)-treated eyes with age-related macular degeneration and choroidal neovascularization. Retina 2011; 31(9): 1812–1818. [DOI] [PubMed] [Google Scholar]
- Introini U, Torres Gimeno A, Scotti F, Setaccioli M, Giatsidis S, Bandello F. Vascularized retinal pigment epithelial detachment in age-related macular degeneration: treatment and RPE tear incidence. Graefes Arch Clin Exp Ophthalmol 2012; 250(9): 1283–1292. [DOI] [PubMed] [Google Scholar]
- Ritter M, Bolz M, Sacu S, Deak GG, Kiss C, Pruente C et al. Effect of intravitreal ranibizumab in avascular pigment epithelial detachment. Eye 2010; 24(6): 962–968. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Sagkriotis A, Olson M, Lu J, Makin C, Milnes F. Treatment frequency and dosing interval of ranibizumab and aflibercept for neovascular age-related macular degeneration in routine clinical practice in the USA. PLoS ONE 2015; 10(7): e0133968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp NM, Liu Y, Yeh WS, Chia Y, Kiss S, Almony A et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am J Ophthalmol 2014; 157(4): 825–33.e1. [DOI] [PubMed] [Google Scholar]
- Johnston SS, Wilson K, Huang A, Smith D, Varker H, Turpcu A. Retrospective analysis of first-line anti-vascular endothelial growth factor treatment patterns in wet age-related macular degeneration. Adv Ther 2013; 30(12): 1111–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina 2010; 30(7): 1046–1050. [DOI] [PubMed] [Google Scholar]
- Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV. Time to first treatment: the significance of early treatment of exudative age-related macular degeneration. Retina 2012; 32(7): 1260–1264. [DOI] [PubMed] [Google Scholar]
- Schwartz R, Loewenstein A. Early detection of age related macular degeneration: current status. Int J Retina Vitreous 2015; 1: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013; 120(5): 1046–1056. [DOI] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364(20): 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirani A, Ambresin A, Marchionno L, Decugis D, Mantel I. Factors influencing the treatment response of pigment epithelium detachment in age-related macular degeneration. Am J Ophthalmol 2015; 160(4): 732–8.e2. [DOI] [PubMed] [Google Scholar]
- Lee SY, Stetson PF, Ruiz-Garcia H, Heussen FM, Sadda SR. Automated characterization of pigment epithelial detachment by optical coherence tomography. Invest Ophthalmol Vis Sci 2012; 53(1): 164–170. [DOI] [PubMed] [Google Scholar]
- Baba T, Kitahashi M, Kubota-Taniai M, Oshitari T, Yamamoto S. Two-year course of subfoveal pigment epithelial detachment in eyes with age-related macular degeneration and visual acuity better than 20/40. Ophthalmologica 2012; 228(2): 102–109. [DOI] [PubMed] [Google Scholar]
- Browning DJ, Kaiser PK, Rosenfeld PJ, Stewart MW. Aflibercept for age-related macular degeneration: a game-changer or quiet addition? Am J Ophthalmol 2012; 154(2): 222–226. [DOI] [PubMed] [Google Scholar]
- Chong V. Biological, preclinical and clinical characteristics of inhibitors of vascular endothelial growth factors. Ophthalmologica 2012; 227(Suppl 1): 2–10. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012; 15(2): 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic JM, Lambert V, Devy L, Luttun A, Carmeliet P, Claes C et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci 2003; 44(7): 3186–3193. [DOI] [PubMed] [Google Scholar]
- Curtis LH, Hammill BG, Qualls LG, DiMartino LD, Wang F, Schulman KA et al. Treatment patterns for neovascular age-related macular degeneration: analysis of 284 380 medicare beneficiaries. Am J Ophthalmol 2012; 153(6): 1116–24.e1. [DOI] [PubMed] [Google Scholar]
- Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015; 99(2): 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]