EVIDENCE FOR INFLAMMATION IN PSYCHIATRIC DISORDERS: IN WHOM AND HOW?
More than one-third of patients with major psychiatric illnesses such as schizophrenia and major depression are poorly responsive to standard treatments, and this contributes significantly to the global burden of disease.[1,2] Consequently, there is a need for better understanding of pathogenesis and biological underpinnings of these disorders so as to identify new treatment targets that can facilitate response and hasten clinical recovery. According to three lines of evidence which accumulated in the last decade, neuroinflammation plays a key role in the pathophysiology of major psychiatric disorders [Figure 1].[3,4,5] Notwithstanding this growing evidence, it remains unclear whether and for whom anti-inflammatory therapies may optimally work in psychiatry.
Figure 1.
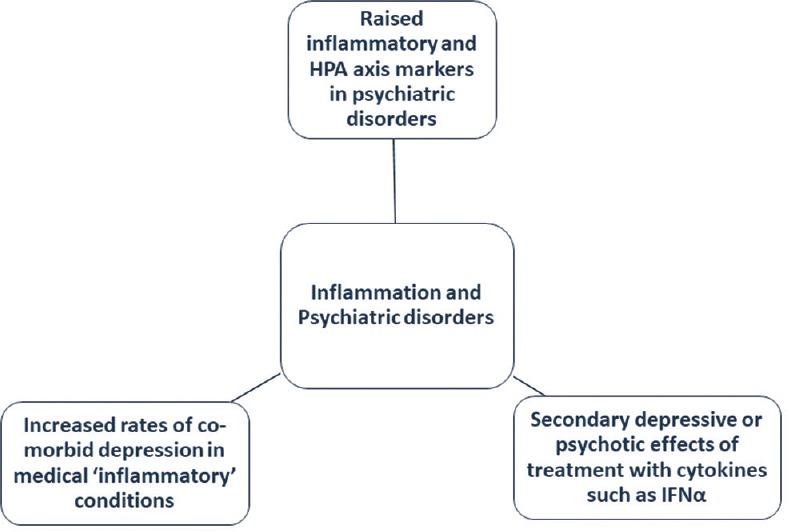
Major lines of evidence linking inflammation and psychiatric disorders
To find answers to this key treatment question, it may be helpful to understand the link between psychiatric disorders and immunoinflammatory pathways from an evolutionary perspective. A recent review on the evolutionary basis for immune-related treatment targets in depression highlights how stress-related immune responses and neural pathways work together to protect organisms from a variety of environmental threats. According to it, early life trauma may have a priming effect on the inflammatory response to subsequent stress.[6] Such at-risk individuals are prone to mount an elevated inflammatory response to stressful events, increasing their risk for depression. The elevated levels of proinflammatory cytokines consistently reported in individuals with major depression support this theory.[3,7]
As these inflammatory responses enhance host survival in a pathogen-laden environment, such individuals may have selectively survived and reproduced, leading to a genomic bias toward depression over time. But how do elevated proinflammatory cytokines exert their “depressogenic” effects? The answer may partly lie in the shared features between depression and “sickness syndrome,” a behavioral constellation secondary to cytokine outflow, characterized by low mood, lethargy, muscle aches and pain, social withdrawal, and anhedonia. Indeed, both anecdotal and empirical evidence attest to the presence of sickness behavior as an antecedent to depression and depressive spectrum conditions such as seasonal affective disorder.[8,9] These responses may serve to channelize energy and resources to fighting pathogens and infections, as elucidated in the pathogen-host defense hypothesis of depression – a theory which seeks to cohesively link the immunoinflammatory pathways and depression.[10]
A possible role for inflammatory response has also been postulated in schizophrenia. A crucial role for prenatal insults to the immune system occurring during the second trimester of pregnancy is well known.[11] Researchers have linked persistent immune response of mothers, in response to chronic infections, with increased risk of schizophrenia in the offspring.[12] Several reports describe elevated levels of proinflammatory cytokines in blood and cerebrospinal fluid of patients with schizophrenia and related conditions such as acute psychotic states when compared to nonpsychiatric controls.[13,14] Abnormalities in glutamatergic transmission and tryptophan/kynurenine pathways, established in schizophrenia, have been intricately linked to variations in cyclooxygenase-2 (COX-2) activity.[15]
Given this burgeoning evidence, one may legitimately ask the question: Are all psychiatric disorders inflammatory? The answer is no and only certain subpopulations among patients with psychiatric disorders exhibit raised levels of systemic inflammation. Thus, it is important to preselect patients based on inflammatory biomarker profile while designing treatment trials with anti-inflammatory agents. As majority of such trials have been carried out in schizophrenia and affective disorders, we examine the evidence base for anti-inflammatory therapy in these conditions separately.
SCHIZOPHRENIA
The concept of immunomodulatory therapies for schizophrenia is not new. In fact, nearly a century ago, the Nobel laureate Julius Ritter Wagner von Jauregg successfully employed immune-based vaccination therapy for psychotic patients, using vaccines that stimulated a type I immune response.[16] However, the uptake of such interventions was limited outside Germany and neighboring European nations.
In the past few decades, several researchers have examined the efficacy of COX-2 inhibitors as an adjunct to standard antipsychotic therapy [Table 1]. Of these, three trials[17,18,19] that included patients with a shorter duration of illness reported positive effects, while two trials[20,21] that studied chronically ill patients returned negative results. This seems to suggest that anti-inflammatory agents may be more useful when initiated earlier in the disease trajectory. This is further supported by evidence from animal studies that the therapeutic effects of COX-2 inhibition depend on the duration of disease process and time of initiation of COX-2 inhibitor.[22]
Table 1.
Clinical studies of anti. inflammatory agents in schizophrenia and psychotic disorders
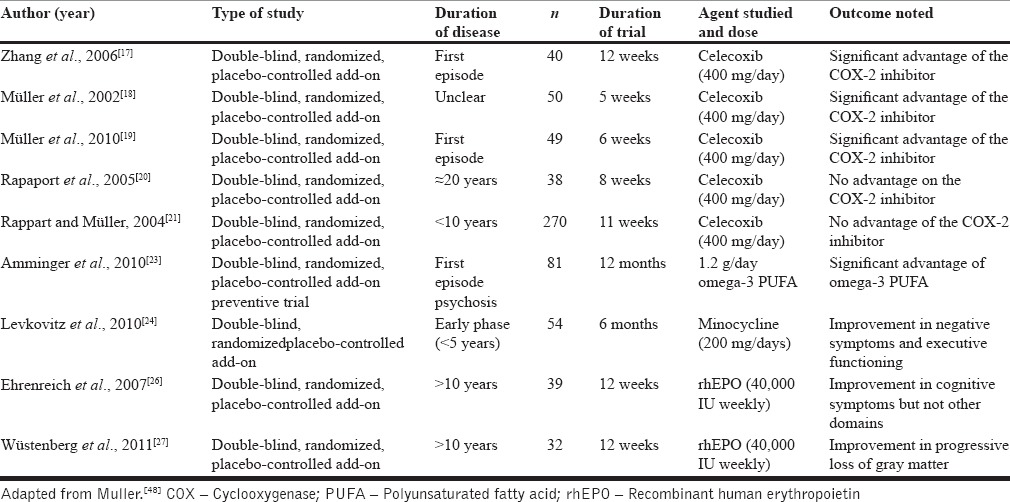
A few studies have evaluated the therapeutic effects of omega-3 fatty acids in schizophrenia, but the results have been inconsistent and effect sizes comparable to those of placebo. In this regard, the study by Amminger et al.[23] [Table 1] is worth mentioning, as it was done among a high-risk group for schizophrenia, and revealed that the group who received omega-3 fatty acids demonstrated significantly lower transition to psychosis in follow-up compared to placebo-treated controls. The results of this intriguing study suggest that inflammation may have a role to play in primary prevention strategies for schizophrenia, and this merits further investigation.
Preliminary clinical evidence also supports the beneficial effects of antibiotics such as minocycline, mainly in cognitive symptoms [Table 1].[24] Here, it is pertinent to note that minocycline has anti-inflammatory properties, which it exerts mainly by modulating the nitric oxide system and inhibiting the activation of microglia in the central nervous system.[25] These studies support the theorized links between the immune system and schizophrenia and provide a basis for further evaluation of add-on antibiotic therapy to improve symptom subsets in psychosis.
Interestingly, the therapeutic role of erythropoietin, an immunomodulatory glycoprotein secreted by the kidney, has been studied in depression in two trials [Table 1]. While one randomized controlled study provided positive evidence for benefits in cognitive symptoms, it failed to show any effect on positive and negative symptoms as well as indices of social functioning.[26] The other was a cohort study and showed that add-on rh-erythropoietin may delay loss of gray matter in schizophrenia.[27]
Put together, these findings are encouraging and seem to suggest that clinicians can consider anti-inflammatory agents as an evidence-based component of early intervention programs for schizophrenia. Further, preliminary evidence suggests that these agents may work more robustly for cognitive symptom dimension than others. This raises the question of separate neurobiological correlates for different symptom dimensions and warrants further work on developing symptom-specific treatment modalities.
AFFECTIVE DISORDERS
A few trials have examined the therapeutic efficacy of the antitumor necrosis factor (TNF)-α antibody infliximab as an adjunctive therapy in major depression as well as treatment resistant subgroups. One of these trials showed a significant effect for antidepressant efficacy in major depression comorbid with psoriasis.[28] The other placebo-controlled trial was carried out among patients poorly responsive or nonresponsive to standard antidepressant therapy and used a parenteral infliximab infusion protocol.[29] The results did not show clear superiority for the treatment when compared to the placebo arm. In the same paper, the authors made several noteworthy observations that infliximab responders also exhibited higher levels of serum C-reactive protein (CRP) and that the reductions in CRP following treatment were greater among the responders than nonresponders. Preliminary evidence also exists for central anti-inflammatory effects as well as anxiety ameliorating effects of angiotensin II type-1 receptor blocking agents in depression.[30]
A number of trials have been carried out to evaluate preventive and curative efficacy of nonsteroidal anti-inflammatory drugs targeting COX pathways on depressive symptoms [Table 2]. Majority of such trials have studied aspirin/ibuprofen (both nonselective COX inhibitors) and celecoxib (selective COX-2 inhibitor). Of the three preventive trials available with aspirin,[31,32,33] all were long-term trials (5–10 years) and showed mixed results. Two trials showed beneficial effects,[31,33] while the largest of the three trials was an epidemiological study and failed to show beneficial effects of aspirin on prevention of depression in elderly men with comorbid cardiovascular disease.[32] Notably, Almeida et al.'s study,[31] which showed beneficial effects of aspirin, included only aged men with high levels of plasma homocysteine, a marker for raised inflammatory state.
Table 2.
Preventive or curative clinical trials of anti-inflammatory agents in affective states
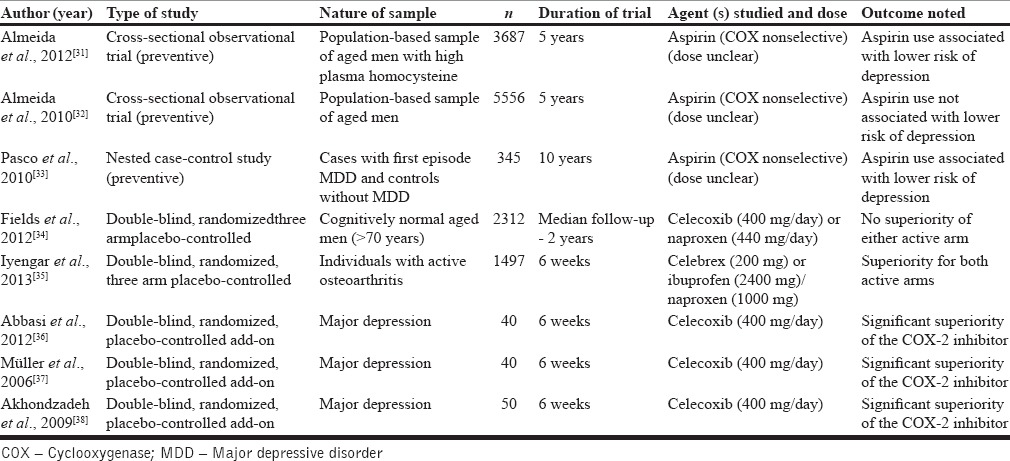
Results from two trials[34,35] that carried out a three-way comparison between nonselective COX inhibitors such as ibuprofen/naproxen, celecoxib, and placebo are particularly illuminating with regard to who may benefit from adjunctive anti-inflammatory therapies. While one of them studied effects on depression in patients with osteoarthritis,[35] a known chronic disease with an inflammatory basis, the other study had no such restrictions in inclusion criteria.[34] The former[35] showed statistical superiority for both active treatments over control in ameliorating depressive symptoms, but the latter study[34] did not detect any differences in active arms versus controls on their depression scores.
Five clinical trials are available that have evaluated the role of selective COX-2 inhibitors as a treatment/preventive strategy for depressive symptoms (either standalone or comorbid with inflammatory conditions such as osteoarthritis). Characteristics of these trials are summarized in Table 2. While four of them yielded evidence for efficacy of COX-2 inhibitors in reducing depressive symptoms,[35,36,37,38] the only preventive trial showed no benefits.[34] A recent meta-analysis of 14 trials, that included trials of nonsteroidal anti-inflammatory drugs (NSAIDs) and cytokine inhibitors, concluded that anti-inflammatory treatment reduced depressive symptoms with moderate effect size.[39]
Regarding bipolar depression, the volume of literature is relatively smaller. A recent meta-analysis, that included eight trials assessing a range of anti-inflammatory therapies such as adjunctive NSAID, omega-3 polyunsaturated fatty acids, N-acetyl cysteine, and pioglitazone, concluded that these treatments were effective in reducing symptoms of bipolar depression, with moderate effect sizes.[40] Anti-inflammatory treatment was also found to reduce posttreatment manic symptoms, with a robust effect size, in another meta-analysis of six randomized controlled trials.[41] Six trials exist on adjunctive administration of omega-3 fatty acids, and their overall results indicate a greater efficacy for reduction of depressive, but not manic, symptoms in bipolar disorder.[42]
Recently, a number of augmentation trials using the cluster of mind–body therapies (MBTs) have been carried out in depression. These include yoga, meditation, progressive muscular relaxation, and indigenous techniques such as Tai Chi. This may stem from their demonstrated anti-inflammatory effects in many disorders including depressive states.[43,44] A large meta-analysis[45] concluded that MBTs as a group are effective in reducing peripheral inflammatory markers such as CRP, though these effects are yet to be conclusively established in depression. Preliminary evidence also exists for positive effects of dietary supplements such as zinc and omega-3 fatty acids in depression, with researchers suggesting that they may also act by modulating inflammatory pathways.[46]
Overall, the available evidence indicates that simple and low-cost investigations such as CRP can be utilized to preselect patients, be it for clinical or research purpose. This stems from research findings that ant-inflammatory therapies may be more relevant for people with elevated inflammatory profile. It follows that the usage of NSAIDs cannot replace conventional antidepressant therapy and should be discouraged in the absence of systemic inflammation. Anti-inflammatory therapies in depression may be more beneficial as a treatment strategy, while evidence for their efficacy in prevention of depression is inconsistent. As a side comment, studying the effects of anti-inflammatory agents on individuals with first episode depression may be more informative than studying chronically diseased individuals as the former represent a purer phenotype.
CONCLUSION AND FUTURE DIRECTIONS
From an evolutionary perspective, inflammatory responses served the benefit of channelizing energy towards managing infections and wounds. This served to maintain behavioral selection. Extant literature supports the use of anti-inflammatory therapies as an adjunct to standard treatment in depression and schizophrenia and to a lesser extent in other affective states. Further high-quality evidence is required to make clinical recommendations advocating routine usage of anti-inflammatory agents in psychiatric disorders. Anti-inflammatory therapies appear to be relevant only in a subset of patients with inflammation and low symptom intensity. Enriching trials for patient populations with evidence of raised central or peripheral inflammatory markers and employing uniform diagnostic and scoring criteria would certainly improve the clarity of findings. The usage of diagnostic modalities such as positron emission tomographic (PET) scanning would help us understand the relationship between central and peripheral inflammation in psychiatric disorders, about which little is currently known, and it would also highlight changes in inflammation-related neurocircuitry with treatment. Treatment studies need to look closely whether changes in inflammatory marker levels parallel that of depressive symptoms, given the conflicting evidence in this regard.[47]
The next line of research should be on biomarkers that may serve to construct “inflammatory signatures” of patients who may benefit maximally from these add-on strategies. This is also in line with the increasing focus on personalized medicine approaches. There is some encouraging preliminary data in this regard, mainly about proinflammatory cytokines such as interleukin (IL)-6, IL-1 β, and TNF-α. More cost-effective biomarkers may be serum CRP, albumin, and zinc levels, considering that inflammation can also acutely alter liver function. Given the adverse effects of indiscriminate NSAID usage as well as the central role of cytokines in physiological functions such as learning and memory, the optimal duration of adjunctive anti-inflammatory therapies also needs to be conclusively established. The future may lie in more specific measures of inflammation such as translocator protein imaging ligands and following their changes concurrent with anti-inflammatory therapy in order to develop novel antidepressant therapies.[48]
REFERENCES
- 1.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muthuramalingam A, Menon V, Rajkumar RP, Negi VS. Is depression an inflammatory disease? Findings from a cross-sectional study at a tertiary care center. Indian J Psychol Med. 2016;38:114–9. doi: 10.4103/0253-7176.178772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:372. doi: 10.3389/fnins.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muneer A. Bipolar disorder: Role of inflammation and the development of disease biomarkers. Psychiatry Investig. 2016;13:18–33. doi: 10.4306/pi.2016.13.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AH, Raison CL. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–64. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuppili PP, Selvakumar N, Menon V. Sickness behavior and seasonal affective disorder: An immunological perspective of depression? Indian J Psychol Med. 2017 doi: 10.4103/IJPSYM.IJPSYM_232_17. DOI: 10.4103/IJPSYM.IJPSYM_232_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raison CL, Miller AH. Pathogen-host defense in the evolution of depression: Insights into epidemiology, genetics, bioregional differences and female preponderance. Neuropsychopharmacology. 2017;42:5–27. doi: 10.1038/npp.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer U, Schwarz MJ, Müller N. Inflammatory processes in schizophrenia: A promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132:96–110. doi: 10.1016/j.pharmthera.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Krause D, Matz J, Weidinger E, Wagner J, Wildenauer A, Obermeier M, et al. The association of infectious agents and schizophrenia. World J Biol Psychiatry. 2010;11:739–43. doi: 10.3109/15622971003653246. [DOI] [PubMed] [Google Scholar]
- 13.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahadevan J, Sundaresh A, Rajkumar RP, Muthuramalingam A, Menon V, Negi VS, et al. An exploratory study of immune markers in acute and transient psychosis. Asian J Psychiatr. 2017;25:219–23. doi: 10.1016/j.ajp.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB, et al. Autoimmune diseases and severe infections as risk factors for schizophrenia: A 30-year population-based register study. Am J Psychiatry. 2011;168:1303–10. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 16.Muller N, Schwarz J, Reidel M. COX-2 inhibition in schizophrenia: Focus on clinical effects of celecoxib therapy and the role of TNF-alpha. In: Eaton WW, editor. Medical and Psychiatric Comorbidity over the Course of Life. Washington: American Psychiatric Publishing; 2005. pp. 265–76. [Google Scholar]
- 17.Zhang Y, Chun Chen D, Long Tan Y, Zhou D. A double-blind, placebo-controlled trial of celecoxib added to risperidone in first-episode and drug-naive patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256:50. [Google Scholar]
- 18.Müller N, Riedel M, Scheppach C, Brandstätter B, Sokullu S, Krampe K, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–34. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 19.Müller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. Celecoxib treatment in an early stage of schizophrenia: Results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–24. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D, et al. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry. 2005;57:1594–6. doi: 10.1016/j.biopsych.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Rappart F, Müller N. Celecoxib add-on therapy does not have beneficial antipsychotic effects over risperidone alone in schizophrenia. Neuropsychopharmacology. 2004;29:222. [Google Scholar]
- 22.Casolini P, Catalani A, Zuena AR, Angelucci L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J Neurosci Res. 2002;68:337–43. doi: 10.1002/jnr.10192. [DOI] [PubMed] [Google Scholar]
- 23.Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: A randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–54. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 24.Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71:138–49. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- 25.van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: A quantitative (R)-[11C] PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–2. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Ehrenreich H, Hinze-Selch D, Stawicki S, Aust C, Knolle-Veentjer S, Wilms S, et al. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007;12:206–20. doi: 10.1038/sj.mp.4001907. [DOI] [PubMed] [Google Scholar]
- 27.Wüstenberg T, Begemann M, Bartels C, Gefeller O, Stawicki S, Hinze-Selch D, et al. Recombinant human erythropoietin delays loss of gray matter in chronic schizophrenia. Mol Psychiatry. 2011;16:26–36, 1. doi: 10.1038/mp.2010.51. [DOI] [PubMed] [Google Scholar]
- 28.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: Double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 29.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology. 2011;36:1–8. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almeida OP, Flicker L, Yeap BB, Alfonso H, McCaul K, Hankey GJ, et al. Aspirin decreases the risk of depression in older men with high plasma homocysteine. Transl Psychiatry. 2012;2:e151. doi: 10.1038/tp.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almeida OP, Alfonso H, Jamrozik K, Hankey GJ, Flicker L. Aspirin use, depression, and cognitive impairment in later life: The health in men study. J Am Geriatr Soc. 2010;58:990–2. doi: 10.1111/j.1532-5415.2010.02827.x. [DOI] [PubMed] [Google Scholar]
- 33.Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, et al. Clinical implications of the cytokine hypothesis of depression: The association between use of statins and aspirin and the risk of major depression. Psychother Psychosom. 2010;79:323–5. doi: 10.1159/000319530. [DOI] [PubMed] [Google Scholar]
- 34.Fields C, Drye L, Vaidya V, Lyketsos C, ADAPT Research Group. Celecoxib or naproxen treatment does not benefit depressive symptoms in persons age 70 and older: Findings from a randomized controlled trial. Am J Geriatr Psychiatry. 2012;20:505–13. doi: 10.1097/JGP.0b013e318227f4da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iyengar RL, Gandhi S, Aneja A, Thorpe K, Razzouk L, Greenberg J, et al. NSAIDs are associated with lower depression scores in patients with osteoarthritis. Am J Med. 2013;126:1017.e11–8. doi: 10.1016/j.amjmed.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 36.Abbasi SH, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: Randomized double-blind placebo-controlled study. J Affect Disord. 2012;141:308–14. doi: 10.1016/j.jad.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–4. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 38.Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: A double blind and placebo controlled trial. Depress Anxiety. 2009;26:607–11. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- 39.Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 40.Rosenblat JD, Kakar R, Berk M, Kessing LV, Vinberg M, Baune BT, et al. Anti-inflammatory agents in the treatment of bipolar depression: A systematic review and meta-analysis. Bipolar Disord. 2016;18:89–101. doi: 10.1111/bdi.12373. [DOI] [PubMed] [Google Scholar]
- 41.Husain MI, Strawbridge R, Stokes PR, Young AH. Anti-inflammatory treatments for mood disorders: Systematic review and meta-analysis. J Psychopharmacol. 2017;31:1137–48. doi: 10.1177/0269881117725711. [DOI] [PubMed] [Google Scholar]
- 42.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: Meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81–6. doi: 10.4088/JCP.10r06710. [DOI] [PubMed] [Google Scholar]
- 43.Lavretsky H, Alstein LL, Olmstead RE, Ercoli LM, Riparetti-Brown M, Cyr NS, et al. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: A randomized controlled trial. Am J Geriatr Psychiatry. 2011;19:839–50. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pullen PR, Nagamia SH, Mehta PK, Thompson WR, Benardot D, Hammoud R, et al. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail. 2008;14:407–13. doi: 10.1016/j.cardfail.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Morgan N, Irwin MR, Chung M, Wang C. The effects of mind-body therapies on the immune system: Meta-analysis. PLoS One. 2014;9:e100903. doi: 10.1371/journal.pone.0100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, et al. Adjunctive nutraceuticals for depression: A Systematic review and meta-analyses. Am J Psychiatry. 2016;173:575–87. doi: 10.1176/appi.ajp.2016.15091228. [DOI] [PubMed] [Google Scholar]
- 47.Muthuramalingam A, Menon V, Rajkumar RP, Negi VS. Effect of fluoxetine on inflammatory cytokines in drug-naive major depression: A Short-term prospective study from South India. J Clin Psychopharmacol. 2016;36:726–8. doi: 10.1097/JCP.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 48.Müller N. The role of anti-inflammatory treatment in psychiatric disorders. Psychiatr Danub. 2013;25:292–8. [PubMed] [Google Scholar]


