Abstract
Distinguishing safety from danger is necessary for survival, but is aberrant in individuals with post-traumatic stress disorder (PTSD). While PTSD is more prevalent in women than men, research on sex differences in safety learning is limited. Here, female rats demonstrated greater fear discrimination than males in a CS+/CS− paradigm. To determine if this sex difference transferred to fear inhibition, rats were tested for conditioned inhibition in a summation test with the CS+ and CS− presented in compound; no sex difference emerged. The results suggest sex differences in the neural mechanisms of discrimination learning but not recall of a fear inhibitor.
Discrimination between safety and danger is necessary for survival. Incorrect evaluation of a stimulus as safe when it is dangerous could result in harm, while determining a stimulus as dangerous when it is safe results in unnecessary fear and anxiety. Further, when a safety cue is well learned, it can reduce fear in the presence of a danger cue, a learning phenomenon known as conditioned inhibition of fear (Kazama et al. 2013). Overgeneralization of fear-related cues and aberrant conditioned inhibition of fear are seen in individuals with post-traumatic stress disorder (PTSD) (Jovanovic et al. 2012; Costanzo et al. 2016; Jenewein et al. 2016). Females are more likely to be diagnosed with PTSD than males (Kilpatrick et al. 2013; Kessler et al. 1995) and safety learning and discrimination are sensitive to hormonal birth control and trauma history in females compared with males (Gamwell et al. 2015; Lornsdorf et al. 2015). Translational research regarding sex differences in rodent fear discrimination is in its infancy, but has indicated greater discrimination in females compared with males, with later generalization of fear (Day et al. 2016). Biological sex is a significant factor in the expression of fear-based psychoses (Shansky 2015) and a better understanding basic behavioral differences in fear discrimination is needed to more fully realize the impact of sex for an individual's health (Shansky and Woolley 2016).
Intact male and normally cycling female adult Sprague-Dawley rats were used as follows: (n = 24/sex) for both fear discrimination acquisition and later fear discrimination recall, (n = 12/sex) for fear discrimination acquisition and conditioned inhibition, and an additional (n = 24/sex) that were given fear discrimination acquisition only, for a total N = 120. CS+/CS− discrimination conditioning adapted from Myers and Davis (2004) was used (Chen et al. 2016; Foilb and Christianson 2016; Foilb et al. 2016). Conditioning trials began with a 5 sec, 1 kHz (75 dB) tone, followed by either the CS+ or CS− for 15 sec. CS+ trials coterminated with a 500 msec, 1.2 mA scrambled foot shock. Conditioning consisted of 15 presentations of each cue in quasi-random order, so that neither cue occurred more than twice in series with a 90 sec inter-trial interval. A flashing light or white noise pip were used as conditioning stimuli. Behavioral research has noted sex differences in fear expression, where many female rats exhibit an active response—termed darting—when presented with fear stimuli, as opposed to the passive freezing response typically observed in males (Gruene et al. 2015a). A trained observer screened videos for evidence of darting, but in our experimental conditions darting occurred too infrequently to analyze. Therefore, freezing was used as a behavioral measurement of fear (Fanselow 1980) as previously (Christianson et al. 2011; Chen et al. 2016; Foilb and Christianson 2016; Foilb et al. 2016). Freezing data were analyzed by analysis of variance (ANOVA) with sex as a between subjects factor and cue type and trial as within subjects factors with Sidak post hoc contrasts. Additional methodological details are provided in the online Supplemental Material.
Experiment 1 investigated sex differences in fear discrimination acquisition (n = 60/sex; Fig. 1) and recall (n = 24/sex; Fig. 2). Fear discrimination recall was tested the day after the initial conditioning session. The test began with 2 min of baseline context exposure, followed by 30 sec presentations of CS+ and CS− cues without presentation of shock. Each cue was presented 10 times in pseudorandom order with a 30-sec inter-trial-interval. Experiment 2 investigated sex differences in the acquisition and recall of conditioned inhibition (n = 12/sex; Fig. 3). Rats received five conditioning sessions as previously (Foilb et al. 2016). Summation tests were similar to the recall test used in Experiment 1 with CS+/− trials added in which CS+ and CS− were presented in compound. The tests began with 2 min of baseline context, followed by 30 sec of each cue—CS+, CS− and CS+/− —three times each in randomized order with a 30-sec inter-trial interval.
Figure 1.

Sex differences in fear expression and discrimination during CS+/CS− conditioning. (A) Freezing averages (±SEM) to the CS+ and CS− across trial blocks of three trials in conditioning. Females significantly discriminated between the CS+ and CS− within the first trial block of each cue (P = 0.001), while males did not make this discrimination until the second trial blocks (P = 0.002). Females also displayed less freezing to the CS− than males on all trial blocks (Ps < 0.05). (*) Significant difference between CS+ and CS− within sex, (+) significant difference between males and females to the CS−, and (#) significant difference between sexes on the CS+. (B) Freezing to CS+ and CS− cues during conditioning. Freezing to each cue was averaged over 15 presentations and converted to a percentage of time (+SEM). Both males and females significantly discriminated between the CS+ and CS− (Ps < 0.0001), but females displayed significantly less average freezing to both the CS+ (P < 0.05) and CS− (P < 0.0001) compared with the males. (C) Mean (and individual replicates) discrimination indices (time freezing to CS−/time freezing to CS+ × 100) during conditioning. An index below 100 signifies reduced freezing to the CS− compared with the CS+. (*) P < 0.05, (****) P < 0.0001.
Figure 2.

Sex differences in fear recall and discrimination. (A) Timeline of recall testing where conditioning (C) occurred in the afternoon of day 1 and recall testing (T) occurred the morning of day 2. (B) Mean freezing (±SEM) to the CS+ and CS− across each of the 10 cue presentations in testing. Both sexes significantly discriminated between the CS+ and CS− on all trials (significance not marked on graph). Females displayed significantly less freezing to the CS− compared with males at the start of the test, while males reduced freezing to the CS− throughout the test. (+) Significant difference between sexes to the CS− (Ps < 0.05), and (#) significant difference between sexes to the CS+ (Ps < 0.05). (C) Average freezing (+SEM) to baseline context exposure, the CS+ and CS− during the recall test. Females displayed significantly less freezing to the baseline context and the CS− compared with males. There was no sex difference in freezing to the CS+. (D) Mean (individual replicates) discrimination indices (time freezing to CS−/time freezing to CS+ × 100) during recall testing. Females have a significantly lower discrimination index compared with males. (E) Average freezing (+SEM) to CS+ and CS− with baseline freezing subtracted. When comparing CS+ to CS− freezing, males and females showed significant discrimination (CS+ versus CS− Ps < 0.0001), while females showed greater freezing to the baseline subtracted CS+ and baseline subtracted CS− compared with males (P < 0.0001 and P < 0.05, respectively). (*) P < 0.05, (***) P < 0.001, (****) P < 0.0001.
Figure 3.
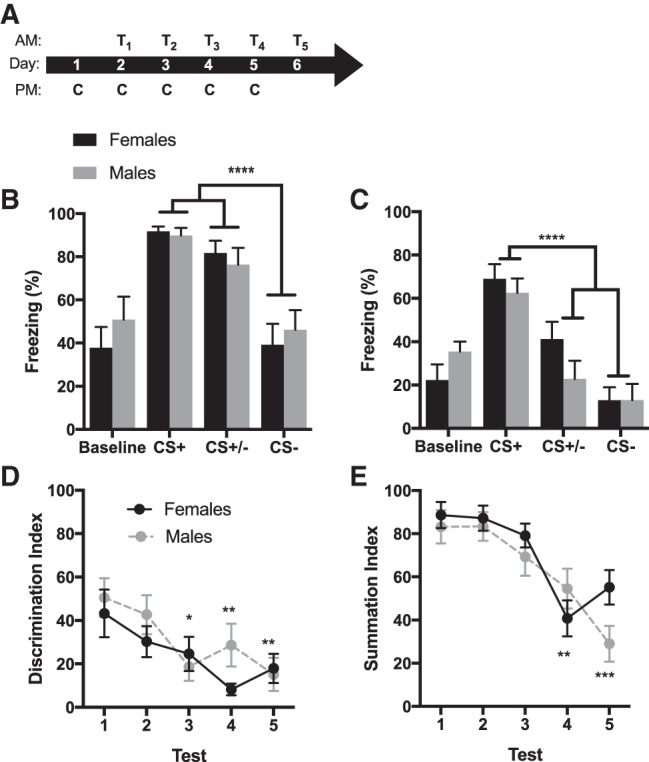
Sex differences were not evident in conditioned inhibition of fear. (A) Timeline of experiment where conditioning (C) occurred each afternoon and recall testing (T) occurred the morning after each conditioning session. (B) Average freezing (+SEM) to baseline context exposure, the CS+, CS+/− and CS− during the recall test on day 1. Animals significantly discriminated between CS+ and CS− (P < 0.0001), but did not discriminate between the CS+ and CS+/− (P = 0.33). (C) Average freezing (+SEM) to baseline context exposure, the CS+, CS+/− and CS− during the recall test on day 5. Animals significantly discriminated between all cues, with reduced freezing to the CS+/− and the CS− compared with the CS+ (Ps < 0.0001). (D) Discrimination indices (±SEM) across each of the five tests. While there was no sex difference on any test day, discrimination improved on tests 3 (P < 0.05), 4 and 5 (Ps < 0.01) compared with tests 1 and 2. (E) Summation indices (time freezing to CS+/−/time freezing to CS+ × 100; ±SEM) across each of the five tests. There were no sex differences in summation, but improved inhibitory summation on tests 4 and 5 compared with tests 1, 2, and 3 (Ps < 0.01). (*) P < 0.05, (**) P < 0.01, (***) P < 0.001, (****) P < 0.0001.
Females displayed discrimination earlier in conditioning than males (Fig. 1A). A three-way ANOVA of sex by cue by trial block (five blocks of three cue trials) revealed a main effect of trial block, F(4,472) = 40.579, P < 0.001, and interactions of trial by sex, F(4, 472) = 3.491, P = 0.008, and cue by trial, F(4,472) = 45.474, P < 0.001, but no significant interaction of trial block by cue by sex interaction, F(4,473) = 1.048, P = 0.382. Post hoc comparisons showed that females significantly discriminated between the CS+ and CS− in the first-trial block (P = 0.007), while males failed to show this discrimination until the second trial block (P = 0.769 on trial block 1; P = 0.004 on trial block 2). Females also displayed significantly reduced freezing to the CS+ compared with males on trial blocks 3, 4, and 5 (Ps = 0.039, 0.018, 0.003, respectively) as well as less freezing to the CS− compared with males on all trial blocks (P = 0.048 on the first trial block, Ps < 0.001 on trial blocks 2, 3, 4, and 5).
Freezing to each cue during conditioning is summarized in Figure 1B. ANOVA revealed main effects of sex, F(1,118) = 23.42, P < 0.0001, and cue, F(1,118) = 312.3, P < 0.0001, and a cue by sex interaction, F(1,118) = 22.15, P < 0.0001. Post hoc analyses showed that both sexes significantly discriminated between the CS+ and CS−, Ps < 0.0001, but that females displayed significantly less freezing to the CS−, P < 0.0001, compared with males. A discrimination index was calculated (freezing to CS+/freezing to CS− × 100) as a measure of animals’ ability to discriminate between the CS+ and CS− (Fig. 1C). Males and females showed significantly different discrimination indices, t(118) = 6.343, P < 0.0001, with females showing more discrimination compared with males. The sex difference was consistent across all cohorts of animals (Supplemental Fig. 1).
Sex differences were evident in fear discrimination recall tests. An ANOVA of the CS trials across test and sex (Fig. 2B), revealed a main effect of trial, F(9,414) = 14.856, P < 0.001, and cue by trial interaction, F(9,414) = 3.333, P = 0.001, but no significant interactions of sex by trial, F(9,414)= 0.421, P = 0.924, or cue by trial by sex, F(9, 414) = 1.668, P = 0.095. Females significantly discriminated between the CS+ and CS− on the first trial (P < 0.001), and continued this level of discrimination throughout the test. Males also displayed immediate discrimination on trial 1 (P = 0.004) and match the discrimination level of the females throughout the remainder of the test (P < 0.001). While females’ response to CS− was stable across trials, males reduced freezing on CS− presentations 7, 8, and 9 compared with trial 1 (Ps = 0.004, 0.003, and <0.001, respectively), as well as on trial 10 compared with trials 2 (P = 0.048) and 3 (P = 0.037), and on CS+ presentations 8, 9, and 10 compared with presentation 4 (Ps = 0.034, 0.03, and 0.007, respectively). Males and females significantly differed in their response to the CS+ on trials 1 (P = 0.019), 6 (P = 0.038), 7 (P = 0.019), 8 (P = 0.049), and 9 (P = 0.020) and showed even more differential responding to the CS−, with significantly different freezing on trials 1–6 and trial 8 (Ps < 0.032).
Figure 2C shows average freezing to each cue during recall. ANOVA revealed main of effects of cue, F(2,92) = 93.01, P < 0.0001, sex, F(1,46) = 40.33, P < 0.0001, and cue by sex interaction, F(2,92) = 12.74, P < 0.0001. Post hoc analyses showed that females continued to freeze significantly less than males to the CS− (P = 0.0003), as well as to the baseline context at the start of the test (P < 0.0001). Females froze significantly less to context than all other cues (Ps < 0.01) and froze significantly less to the CS− than the CS+ (P < 0.0001). Males also froze significantly less to the CS− and baseline context compared with the CS+ (Ps < 0.0001), but freezing to the CS− and baseline context did not significantly differ (P = 0.641). This sex by cue interaction is summarized by significant difference in discrimination index (Fig. 2D, t(46) = 3.63, P = 0.0007). To indicate that the sex differences in cue response were not simply artifacts of differential baseline fear, we subtracted baseline freezing from average freezing to each cue. Here we find main effects of cue, F(1,46) = 248.6, P < 0.0001, sex, F(1,46) = 14.05, P = 0.0005, and a cue by sex interaction, F(1,46) = 8.692, P = 0.005. These differences indicate that the sex difference in discrimination is not only due to the sex difference in baseline fear, but that there is a general sex difference in discrimination between the CS and the conditioning context (Supplemental Fig. 2).
In Experiment 2 conditioned inhibition of fear was assessed in summation tests. On test 1, analysis on average freezing to each cue (Fig. 3B) in a 4 (cue) by 2 (sex) ANOVA revealed a significant effect of cue F(3,66) = 30.06, P < 0.0001, with significantly reduced freezing to CS− compared with the CS+ and CS+/− (Ps < 0.0001) and significantly increased freezing to the CS+ and CS+/− compared with baseline context (Ps < 0.0001). Animals did not discriminate between the CS+ and CS+/− in this test (P = 0.33). There was no main effect of sex, F(1,22) = 0.1599, P = 0.6936, or cue by sex interaction, F(3,66) = 0.8828, P = 0.4547. The difference in baseline context freezing that was observed in Experiment 1 was present in this smaller sample as a trend, but did not reach significance. This may reflect an effect of different estrous status on the test day between experiments, or sampling error and the intrinsic variability in this dependent measure, which is discussed in the online Supplemental Material. The lack of sex difference in simple CS+/CS− discrimination here is likely the result of differences in cue presentation in the summation test compared with the recall test. Specifically, the first presentation of the CS− in the summation test is as a compound with the CS+. In test 5 (Fig. 3C), the same analysis revealed a significant main effect of cue, F(3,66) = 35.72, P < 0.0001, and a significant cue by sex interaction, F(3,66) = 3.15, P = 0.0307, but no main effect of sex, F(1,22) = 0.1521, P = 0.7003. Post hoc analyses showed that animals displayed greater fear to the CS+ compared with the baseline context exposure, CS− and CS+/− compound (Ps < 0.0001). Animals also froze less to the CS− compared with the CS+/− and baseline context (Ps < 0.05).
Comparing discrimination indices across all five summation tests (Fig. 3D) with a 5 (test day) × 2 (sex) ANOVA revealed a main effect of test day, F(4,88) = 5.603, P = 0.0005, but no main effect of sex, F(1,22) = 1.328, P = 0.2615, and no test day by sex interaction, F(4,88) = 0.9538, P = 0.4371. Discrimination between the CS+ and CS− was significantly improved on tests 3, 4, and 5 compared with test 1 (Ps < 0.05). A summation index was calculated as freezing to CS+/−/freezing to CS+ × 100 as a measurement of conditioned inhibition (Fig. 3D). ANOVA revealed a main effect of test day, F(4,88) = 14.9, P < 0.0001, but no main effect of sex, F(1,22) = 1.949, P = 0.1767, and no test day by sex interaction, F(4,88) = 1.751, P = 0.1460. Rats showed greater inhibition on tests 4 and 5 compared with tests 1, 2, and 3 (Ps < 0.01).
We observed a marked difference in fear discrimination between male and female rats. Differential freezing to the fear CS+ and safe CS− was greater in females compared with males during the initial conditioning and in a recall test one day later, furthermore, females exhibited greater initial discrimination between the context and the CS. These results are consistent with recent findings (Day et al. 2016), although in contrast to studies on fear discrimination in humans where females show less discrimination than males (Gamwell et al. 2015; Lornsdorf et al. 2015). Females also displayed lower contextual freezing, consistent with several prior reports in rodents (Pryce et al. 1999; Daviu et al. 2014; Pettersson et al. 2016, although see Keiser et al. 2017 for exception). When this difference in contextual freezing was subtracted from freezing to each cues, there was a larger difference in freezing to CS+, whereas the main difference in the uncorrected data was primarily in freezing to the CS−. This indicates that the sex difference may be in discrimination per se, rather than freezing to a particular cue.
The discrimination test required that rats flexibily transition between fear and safe states, which may favor the inherently more active female rats (Gruene et al. 2015a,b). However, after several days of conditioning, females may accrue more fear to the CS− (Day et al. 2016) which could manifest as a transition from more active behavior early in conditioning to more passive, i.e., male-like, with additional conditioning and stress (Foilb and Christianson 2016) and account for the lack of sex difference in conditioned inhibition summation tests. This outcome contrasts some of the results of Day et al. (2016) in which after repeated discrimination conditioning the CS− failed to pass a retardation test of conditioned inhibition. Whether these different empirical results are a consequence procedural differences or of different neural mechanism underlying summation and retardation phenomena remains unknown.
The sex difference in discrimination indicates that there may be sex differences in the neural circuitry underlying discrimination learning. Interactions between the amygdala and prefrontal cortex are critical for CS+/CS− discrimination (Likhtik et al. 2014) and sexual dimorphisms observed in humans include sex differences in amygdala anatomy (Ruigrok et al. 2014), amygdala response to negative or stressful emotions (Stevens and Hamann 2012; Kogler et al. 2015), and amygdala functional connectivity (Lopez-Larson et al. 2011; Engman et al. 2016). Sex differences have been found in the rodent medial prefrontal cortex (Baran et al. 2010; Fenton et al. 2014, 2016), a brain region critical to fear expression, extinction, and discrimination (Sotres-Bayon and Quirk 2010; Milad et al. 2014; Sangha et al. 2014). Similarly, basolateral amygdala projecting neurons of the infralimbic region of the medial prefrontal cortex appear to differently mediate fear expression in males and females (Gruene et al. 2015b). That sex differences in safety learning do not persist in conditioned inhibition of fear also suggests that conditioned inhibition of fear occurs in a neural circuit that is distinguishable from the fear discrimination circuitry.
Supplementary Material
Acknowledgments
Funding in support of this research was provided by National Institute of Mental Health MH09341, the Brain and Behavior Research Foundation grant No. 19417, and the Boston College McNair Scholars Program.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.045500.117.
References
- Baran SE, Armstrong CE, Niren DC, Conrad CD. 2010. Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learn Mem 17: 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VM, Foilb AR, Christianson JP. 2016. Inactivation of ventral hippocampus interfered with cued-fear acquisition but did not influence later recall or discrimination. Behav Brain Res 296: 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Jennings JH, Ragole T, Flyer JG, Benison AM, Barth DS, Watkins LR, Maier SF. 2011. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry 70: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M, Jovanovic T, Norrholm SD, Ndiongue R, Reinhardt B, Roy MJ. 2016. Psychophysiological investigation of combat veterans with subthreshold post-traumatic stress disorder symptoms. Mil Med 181: 793–802. [DOI] [PubMed] [Google Scholar]
- Daviu N, Andero R, Armario A, Nadal R. 2014. Sex differences in the Behavioural and hypothalamic-pituitary-adrenal response to contextual fear conditioning in rats. Horm Behav 66: 713–723. [DOI] [PubMed] [Google Scholar]
- Day HL, Reed MM, Stevenson CW. 2016. Sex differences in discriminating between cues predicting threat and safety. Neurobiol Learn Mem 133: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engman J, Linnman C, Van Dijk KR, Milad MR. 2016. Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology 63: 34–42. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. 1980. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15: 177–182. [DOI] [PubMed] [Google Scholar]
- Fenton GE, Pollard AK, Halliday DM, Mason R, Bredy TW, Stevenson CW. 2014. Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learn Mem 21: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton GE, Halliday DM, Mason R, Bredy TW, Stevenson CW. 2016. Sex differences in learned fear expression and extinction involve altered gamma oscillations in medial prefrontal cortex. Neurobiol Learn Mem 135: 66–72. [DOI] [PubMed] [Google Scholar]
- Foilb AR, Christianson JP. 2016. Serotonin 2C receptor antagonist improves fear discrimination and subsequent safety signal recall. Prog Neuropsychopharmacol Biol Psychiatry 65: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb AR, Flyer-Adams JG, Maier SF, Christianson JP. 2016. Posterior insular cortex is necessary for conditioned inhibition of fear. Neurobiol Learn Mem 134(Pt B): 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamwell K, Nylocks M, Cross D, Bradley B, Norrholm SD, Jovanovic T. 2015. Fear conditioned responses and PTSD symptoms in children: sex differences in fear-related symptoms. Dev Psychobiol 57: 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. 2015a. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4: e11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM. 2015b. Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol Psychiatry 78: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenewein J, Erni J, Moergeli H, Grillon C, Schumacher S, Mueller-Pfeiffer C, Hassanpour K, Seiler A, Wittmann L, Schnyder U, et al. 2016. Altered pain perception and fear-learning deficits in subjects with posttraumatic stress disorder. J Pain 17: 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. 2012. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama AM, Schauder KB, McKinnon M, Bachevalier J, Davis M. 2013. A novel AX+/BX- paradigm to assess fear learning and safety-signal processing with repeated-measure designs. J Neurosci Methods 214: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC. 2017. Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology 42: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. 2013. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 26: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Gur RC, Derntl B. 2015. Sex differences in cognitive regulation of psychosocial achievement stress: brain and behavior. Hum Brain Mapp 36: 1028–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. 2014. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci 17: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Anderson JS, Ferguson MA, Yurgelun-Todd D. 2011. Local brain connectivity and associations with gender and age. Dev Cogn Neurosci 1: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lornsdorf TB, Haaker J, Shümann D, Sommer T, Bayer J, Brassen S, Bunzeck N, Gamer M, Kalisch R. 2015. Sex differences in conditioned stimulus discrimination during context-dependent fear learning and its retrieval in humans: the role of biological sex, contraceptives and menstrual cycle phases. J Psychiatry Neurosci 40: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rosenbaum BL, Simon NM. 2014. Neuroscience of fear extinction: implications for assessment and treatment of fear-based and anxiety related disorders. Behav Res Ther 62: 17–23. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. 2004. AX+, BX- discrimination learning in the fear-potentiated startle paradigm: possible relevance to inhibitory fear learning in extinction. Learn Mem 11: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R, Hagsater SM, Eriksson E. 2016. Serotonin depletion eliminates sex differences with respect to context-conditioned immobility in rat. Psychopharmacology (Berl) 233: 1513–1521. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Lehmann J, Feldon J. 1999. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacol Biochem Behav 64: 753–759. [DOI] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. 2014. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 39: 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Robinson PD, Greba Q, Davies DA, Howland JG. 2014. Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology 39: 2405–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM. 2015. Sex differences in PTSD resilience and susceptibility: challenges for animal models of fear learning. Neurobiol Stress 1: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Woolley CS. 2016. Considering sex as a biological variable will be valuable for neuroscience research. J Neurosci 36: 11817–11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. 2010. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol 20: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. 2012. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia 50: 1578–1593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


