Abstract
AIM
To investigate whether curcumin suppressed corneal neovascularization (CNV) formation via inhibiting activation of Wnt/β-catenin pathway.
METHODS
Suture-induced CNV was established on Sprague-Dawley (SD) rats. Curcumin were daily administrated by subconjunctival injection. Phosphorylation of low-density lipoprotein receptor-related protein 6 (LRP6) and nuclear accumulation of β-catenin, two indicators of activated Wnt/β-catenin pathway, were determined by Western-blot analysis in subconfluent/proliferating human microvascular endothelial cells (HMEC) and neovascularized corneas. Wnt3a conditioned medium (WCM) were harvested from Wnt3a expressing cells. WCM-induced cell proliferation and endothelial tubular formation capacity was measured by MTT assay and Matrigel assay, respectively.
RESULTS
Phosphorylation of LRP6 and nuclear accumulation of β-catenin was significantly increased in subconfluent/proliferating endothelial cells. Activation of Wnt/β-catenin pathway by WCM markedly promotes HMEC proliferation and tubular formation. Curcumin inhibited LRP6 phosphorylation and nuclear accumulation of β-catenin. In addition, curcumin attenuated WCM-induced HMEC proliferation and disrupted tubular structure of endothelial cells on Matrigel. Meanwhile curcumin suppressed suture-induced CNV and inhibited LRP6 phosphorylation as well as β-catenin accumulation in SD rats.
CONCLUSION
Taken together, activation of Wnt/β-catenin pathway could be involved in endothelial proliferation during suture-induced CNV formation and curcumin attenuated CNV formation via inhibition of Wnt/β-catenin pathway activation.
Keywords: corneal neovascularization, Wnt/β-catenin pathway, curcumin
INTRODUCTION
Corneal neovascularization (CNV) is a leading cause of blindness worldwide, especially in developing country[1]. Transparency and avascularity is critical for cornea as refractive medium, which could be compromised by CNV formation in a variety of conditions such as infectious keratitis, immune keratitis, traumatic keratitis and systemic disorders. CNV formation can lead to visual impairment, and to more severe extent, it may result in blindness. Newly formed vessels are immature, hyperpermeable and fragile, which may lead to corneal edema, lipid deposit and the rupture of vulnerable vessels could result in stromal hemorrhage and consequently corneal scarring. Moreover, the neovessels introduce immune cells such as neutrophils and macrophages, which damage the immune privilege of cornea and loss of immune privilege is the major cause for graft rejection after therapeutic penetrating keratoplasty. Although several interventions have been utilized for CNV in clinic including steroid eyedrops, nonsteroid anti-inflammation agents, photodynamic therapy (PDT)[2] and anti-VEGF therapy[3], the effects are usually unsustained and a large amount of patients could not regain vision loss. Therefore, uncovering underlying mechanisms of CNV are urgently required, which helps to identify specific molecular targets and select promising therapeutic drugs for CNV.
Wnt/β-catenin pathway or Canonical Wnt pathway is required for embryonic development and tumorigenesis[4]–[5]. Wnts are a group of cysteine-rich secreted glycolipoproteins. In absence of Wnts, cytosolic β-catenin is phosphorylated by ‘destruction complex’ consisting of glycogen synthase kinase (GSK)-3β, Axin, adenomatous polyposis coli (APC) and casein kinase 1 (CK1). Phosphorylated β-catenin was ubiquitinated and degraded by proteasome. Wnt/β-catenin signaling pathway is initiated by Wnts binding to co-receptors formed by Low-density lipoprotein receptor-related protein 6 (LRP6) and Fzd (Frizzled receptor). Upon activation of Wnt pathway, LRP6 is phosphorylated, which results in disruption and inactivation of β-catenin “destruction complex”. This event releases β-catenin from degradation and leads to cytosolic β-catenin accumulation. Then accumulated β-catenin translocates to nuclei, binds T cell factor/Lymphoid enhancing factor (TCF/LEF) family transcription factor and promotes downstream gene expression. Wnt/β-catenin signaling pathway has also been reported to be involved in vessel growth[6]–[7]; however, its role in pathological CNV has not been elucidated.
Curcumin is a natural ingredient of Curcuma longa and is widely used in traditional medicine. It is a highly pleiotropic agent with multiple activities such as anti-inflammation, anti-oxidation and anti-cancer[8]. Plenty of studies demonstrated that curcumin also has beneficial effects on eye disorders including cataract[9]–[10], retinitis pigmentosa[11], light-induced retinal degeneration[12], diabetic retinopathy[13] and etc. Recently, curcumin has been found out to inhibit tumor angiogenesis and suppress tumor growth[14]. Similarly, curcumin could exert its anti-angiogenic effect on CNV in mice and rabbits[15]–[16]. By using a rat model of allogeneic cornea transplantation, our group also found that curcumin could inhibit CNV formation, attenuate graft rejection and promote allograft survival (unpublished data); however, the exact mechanism remains largely unknown.
It is well-accepted that inhibition of CNV, which maintains immune privilege, could attenuate corneal allograft rejection. Previous studies showed that curcumin could inhibit tumor cell proliferation via attenuation of Wnt/β-catenin pathway[17]. Therefore, in the present study, we hypothesize that activation of Wnt/β-catenin is involved suture-induced CNV formation in Sprague-Dawley (SD) rats and curcumin suppresses CNV formation via inhibition of Wnt/β-catenin pathway activation.
SUBJECTS AND METHODS
Animals
Male SD rats aged between 10 and 12wk were provided by animal facility of Medical school of Xi'an Jiaotong University. All experiments were performed in accordance with the statement of the Association for Research in Vision and Ophthalmology for the Use of Animals in Ophthalmic and Vision research and guideline of Xi'an Jiaotong University for use of animal in research. A rat model of suture-induced CNV was established. Briefly, the SD rats were deeply anesthetized by intraperitoneal injection of chloral hydrate (350 mg/kg). Eight 10-0 nylon stitches were intrastromally placed near the limbus with two stromal incursions extending over 45° of corneal circumference. Curcumin (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in dimethyl sulfoxide (DMSO, Sigma) at concentration of 40 mol/L as stock solution and diluted to 40 mmol/L with balanced salt solution (BSS) right before use. The treatment group received 100 µL curcumin (400 mmol/L) by daily subconjunctival injection and the vehicle group were given an equal volume of DMSO in BSS. Sutures were left in place for the duration of the experiments. The rat corneas without any sutures were referred to control group. The rat were daily examined with slit-lamp biomicroscopy and corneas were photographed. As desired time points, rats were kindly euthanized and corneas were harvested.
Cell Culture
Human microvascular endothelial cells (HMEC) were obtained from Lonza (Lonza, Walersville, MD, USA) and maintained in EGM (Lonza). L-cells with and without Wnt3a expression were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco's Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS, Invitrogen) and 0.4 mg/mL G418 following ATCC's instructions. Wnt3a conditioned medium (WCM) and control medium (LCM) were collected following ATCC's recommendations.
MTT Cell Proliferation Assay
Cell proliferation was measured by MTT assay following manufacturer's protocols (ATCC). Briefly, HMEC in a 24-well plate were exposed to WCM with different doses of curcumin for overnight. Totally 50 µL of MTT reagent was added into each well and incubated in CO2 incubator at 37°C. After 4h, 500 µL of detergent was applied into each well, and then the plate was covered and left in dark for overnight. The absorbance was read at 570 nm in a microplate reader.
Tube Formation Assay
24-well plate was coated with growth factor reduced Matrigel Basement Membrane Matrix (BD Biosciences, Franklin Lakes, NJ, USA) at 37°C for more than 30min. After pretreatment with different doses of curcumin, HMEC were dissociated with non-enzyme cellstripper and seeded on Matrigel. After 6h, the representative pictures were taken and branch numbers of tubular structures were quantified from different visual fields under microscope.
Nuclear Extraction
HMEC were treated with WCM for 6h in presence or absence of different doses of curcumin. Nuclear proteins were extracted from HMEC by using Nuclear Extraction Kit (Active motif, Carlsbad, CA, USA) following manufacturer's instruction. Briefly, HMEC were spun down and resuspended in hypotonic buffer for 15min. Then add detergent and vortex 10s at highest speed. After centrifuging at 14 000 g, nuclear pellets were lysed in nuclear lysis buffer for 30min on ice. Finally, centrifuge at highest speed for 10min and the nuclear fraction in the supernatant will be used in biomedical assays.
Western-blot Analysis
Corneas and cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (Santa-cruz Biotechnology, Santa-cruz, CA, USA). Protein concentration was measured with BCA assay (Thermo Scientific Pierce, Rockford, IL, USA). Fifty microgram protein were dissolved to SDS-PAGE and transferred to nitrocellulose membranes. The membranes were incubated with following antibodies: phosphorylated LRP6 and total LRP6 (Cell signaling, Danvers, MA, USA), β-catenin (Santa-cruz) for overnight. After rinsing with TBST, membranes were incubated with HRP-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA, USA). The same membranes were probed with β-actin (Sigma) as loading controls.
Statistical Analysis
Data were expressed as Mean±SD Statistical analysis was performed by using one way ANOVA with Bonferroni as post hoc test. Statistical significance was accepted with P value less than 0.05.
RESULTS
Activation of Wnt/β-catenin Pathway Implicated in Proliferation of Endothelial Cells and Formation of Corneal Neovascularization
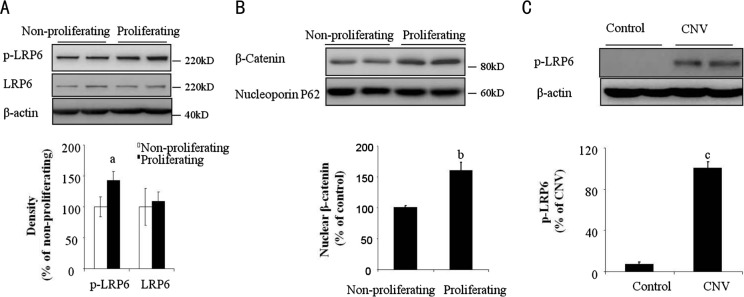
Endothelial proliferation is the initial step for angiogenesis. To address whether Wnt/β-catenin signaling pathway is involved in suture-induced CNV formation, we first investigated if Wnt/β-catenin signaling pathway is activated during proliferation of microvascular endothelial cells. We found that compared with confluent/non-proliferating HMEC, subconfluent/proliferating HMEC expressed high level of phosphorylated LRP6 (Figure 1A), which is an indicator of Wnt/β-catenin pathway activation. Upon LRP6 is phosphorylated, it will cause β-catenin accumulation and translocation into the nuclei. β-catenin, as a transcription factor, could upregulate several gene expression such as c-myc and cyclin D1, which are responsible for cell proliferation[18]–[19]. Consistent with LRP6 phosphorylation, nuclear β-catenin were also increased in subconfluent/proliferating HMEC compared to that in confluent/non-proliferating HMEC as shown in Figure 1B. Furthermore, we evaluated phosphorylation of LRP6 in suture-induced CNV of SD rats. Of note, at 7d after suture was placed, we observed that phosphorylation of LRP6 was significantly up-regulated in rat corneas (Figure 1C), indicating that Wnt/β-catenin pathway is activated during CNV formation.
Figure 1. Activated Wnt pathway in proliferating endothelial cells and neovascularized corneas.
A: Phoshorylated LRP6 was determined in non-proliferating and proliferating HMEC by Western-blot analysis and semi-quantified by densitometry. n=3, aP<0.05 vs non-proliferating; B: Nuclear β-catenin was determined in non-proliferating and proliferating HMEC by Western-blot analysis and semi-quantified by densitometry. Nucleporin p62 were used as loading control. n=3, bP<0.01 vs Non-proliferating; C: Phoshorylated LRP6 in rat corneas was determined by Western-blot analysis and semi-quantified by densitometry. n=4, cP<0.01 vs control.
Wnt3a-induced Endothelial Proliferation was Inhibited by Curcumin
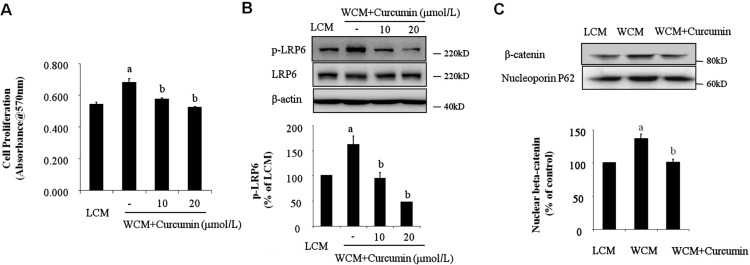
We next determined if activated Wnt/β-catenin pathway by its ligands could promote endothelial proliferation. HMEC were treated with Wnt3a conditional medium (WCM) for overnight, cell proliferation was determined by MTT assay. We found that Wnt3a significantly promoted endothelial proliferation as shown in Figure 2A. Previous study showed that curcumin could inhibit activation of Wnt/β-catenin pathway in tumor growth[20]; however, its effect on endothelial proliferation during CNV formation has not been studied. Therefore, we pretreated HMEC with different doses of curcumin and then exposed the cells to WCM. We found out that curcumin could significantly inhibit Wnt3a-situmulated endothelial proliferation in a concentration-dependent manner (Figure 2A). Furthermore, Western-blot analysis demonstrated that curcumin significantly attenuated LRP6 phosphorylation induced by WCM (Figure 2B). Consistently, WCM-induced accumulation of β-catenin in nuclei, which was significantly reversed by curcumin treatment as shown in Figure 2C. These data suggested that activation of Wnt/β-catenin pathway is responsible for endothelial proliferation and curcumin may suppress endothelial proliferation via attenuation of Wnt/β-catenin pathway activation.
Figure 2. Curcumin inhibited WCM-induced HMEC proliferation.
A: HMEC were treated with WCM for 16h with or without different doses of curcumin. Cell proliferation was determined by MTT assay. n=4, aP<0.01 vs LCM, bP<0.01 vs WCM; B: HMEC were pretreated with different doses of curcumin for 2h and then exposed to WCM for 1h. Phoshorylated LRP6 was determined by Western-blot analysis and semi-quantified by densitometry; C: HMEC were pretreated with 10 µmol/L curcumin for 2h and then exposed to WCM for 6h. Nuclear β-catenin was determined by Western-blot analysis and semi-quantified by densitometry. Nucleporin p62 were used as loading control. n=3, aP<0.01 vs LCM, bP<0.01 vs WCM.
Curcumin Suppressed Tubular Formation of HMEC
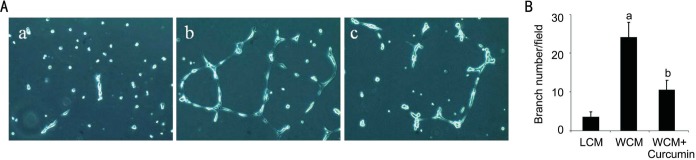
Tubular formation plays a pivotal role in angiogenesis. In order to further study curcumin's effect on CNV formation, we looked at its in vitro function on endothelial tube formation. HMEC were pretreated with WCM in presence or absence of curcumin and then seeded on growth factor-reduced Matrigel. After 6h, branch numbers of newly formed tube were counted (Figure 3B). As shown in Figure 3A, WCM treatment could promote HMEC tubular formation capacity. However, when we treated HMEC together with curcumin, WCM induced tuber structure was disturbed (Figure 3A).
Figure 3. Curcumin suppressed WCM-triggered HMEC tube formation.
HMEC were treated with WCM in presence or absence of curcumin at dose of 10 µmol/L. Then cells were seeded on growth factor reduced Matrigel. Representative pictures from LCM (A-a), WCM (A-b) and WCM+curcumin (A-c) treated groups were taken after 6 h. Branch numbers were counted from three different visual fields (B). aP<0.01 vs LCM and bP<0.05 vs WCM.
Curcumin Attenuated Suture-induced Corneal Neovascularization Formation in Mice
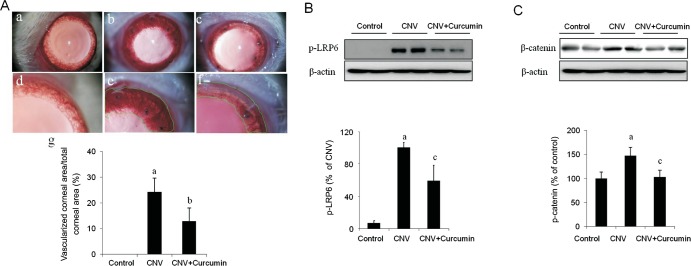
Based on our promising in vitro finding, we are moving to in vivo study. For this purpose, we looked at curcumin's effect on suture-induced CNV formation in rats. Curcumin, dissolved in DMSO and further diluted in BSS, were given by daily subconjunctival injection right after sutures were placed. An equal amount of DMSO in BSS was used as vehicle control. No toxic effects such as corneal epithelial defect were observed after subconjunctival injection of curcumin or DMSO. We found that curcumin markedly reduced corneal neovessel density at postoperating day 7 (Figure 4A). Furthermore, western-blot analysis displayed that increased phosphorylation of LRP6 were coincident with suture-induced CNV formation in the vehicle group, which were significantly attenuated in curcumin group (Figure 4B). Consistently, curcumin suppressed β-catenin accumulation in suture-induced CNV (Figure 4C). All these results suggested that curcumin may inhibit suture-induced CNV formation through suppression of Wnt/β-catenin pathway activation.
Figure 4. Subconjunctival injection of curcumin ameliorated suture-induced CNV formation in rats.
A: Curcumin markedly reduced suture-induced CNV formation in rats. Representative pictures of suture-induced CNV in mice were obtained by slit-lamp biomicroscopy with low magnification in upper panel and high magnification in lower panel. Neovascularized area were outlined and quantified by Adobe Photoshop software (a-g). n=4 for each group, aP<0.01 vs control, bP<0.05 vs CNV. Control ( A-a and A-d), CNV with vehicle (A-b and A-e) and CNV with curcumin (A-c and A-f); B: Curcumin significantly attenuated LRP6 phosphorylation in suture-induced-CNV formation. Phosphorylation of LRP6 was determined in rat corneas by Western-blot analysis at 7d after suture-induced CNV; C: Curcumin markedly inhibited β-catenin accumulation in suture-induced-CNV formation. Total β-catenin was examined in rat corneas by Western-blot analysis at 7d after suture-induced CNV. n=4 for each group, aP<0.01 vs control, cP<0.01 vs CNV.
DISCUSSION
In this study, we addressed the role of Wnt/β-catenin pathway in suture-induced CNV. Components of Wnt/β-catenin signaling pathway are expressed in human corneal epithelial cells and activation of Wnt/β-catenin pathway regulates corneal epithelial proliferation[21]–[22]. Benzalkonium chloride activated Wnt/β-catenin pathway, which may be responsible for preservative-associated corneal epithelial apoptosis[23]. Meanwhile, Wnt/β-catenin pathway activation is widely associated with development of vasculature and process of angiogenesis[7]. Multiple components of Wnt/β-catenin pathway are involved in regulating cell survival, proliferation, differentiation and motility[6]. Especially, Wnt3a promotes proliferation, migration and survival of PAEC[24]. It could also induce chick embryo angiogenesis via upregulating expression of vascular endothelial growth factor receptor 2 (VEGFR2)[25]. Chen et al[26] reported that activated Wnt/β-catenin pathway mediated pathological retinal neovascularization. However, to our knowledge, the contribution of Wnt/β-catenin pathway activation to CNV formation has not been elucidated. Therefore, we investigated that if Wnt/β-catenin was activated during suture-induced CNV in mice. We demonstrated that LRP6 phosphorylation and nuclear β-catenin accumulation were increased in subconfluent and proliferating endothelial cells as well as neovascularized corneal tissue. Furthermore, activating Wnt/β-catenin pathway by WCM could promote endothelial proliferation. All these suggested that Wnt may play a causal role in CNV formation.
LRP6, as an important member of LRP superfamily, is essential for efficient Wnt signaling transduction[27]. LRP6 is a single-pass transmembrane protein containing of four epidermal growth factor (EGF)-like repeats and three low density lipoprotein receptor (LDLR) repeats in extracellular domain and conserved PPSPXS motif in intracellular domain[28]. The extracelluar domain mediates LRP6 interaction with ligands such as Wnts, Dickkopf and Sost. Phosphorylation of LRP6 at intracellular PPSPXS motif is crucial to β-catenin stabilization and Wnt signaling transduction. Mutant LRP6 causes severe development defects in mouse embryos[29]. Previous study demonstrated that LRP6 regulates proliferation and survival in vascular smooth muscle cells and endothelial cells[30]–[31]. LPR6 were exclusively expressed by retinal vasculature during early postnatal day development[31]. In addition, up-regulation of LRP6 has observed in neovascular eye diseases such as oxygen-induced retinopathy and laser-induced choroidal neovascularization and blocking of LRP6 by specific antibody significantly inhibited retinal and choroidal neovascularization[32]–[33]. In present study, phosphorylation of LRP6 was significantly increased in rat corneas with neovascularization and inhibition of LRP6 phosphorylation correlates with reduced CNV. All these evidence suggest that targeting Wnt signaling activation at LRP6 level could be an attractive therapy for preventing ocular neovascularization.
Curcumin has been shown to affect multiple signaling transduction pathways involved in angiogenesis such as proliferation, migration and tubular structure formation. First, curcumin could directly inhibit activities of protein kinases such as protein kinase C (PKC), epidermal growth factor receptor (EGFR)-2/ErbB-2 and etc, which are closely associated with cell proliferation[34]–[35]. Second, curcumin blocked stromal-derived factor (SDF)-1α-induced human retinal endothelial cell migration[36]. Third, curcumin could suppress tube formation of vascular or lymphatic endothelial cells[37]–[38]. Therefore, curcumin's inhibitory effect on the overall process of angiogenesis favors its great potential as an anti-angiogenic drug. Of note, curcumin has entered into clinical trials and showed clinical biological activity on tumor repression[39], which may be partially inhibition of tumor vessel growth. Early study has shown that curcumin could significantly inhibit b-FGF (basic fibroblast growth factor)-induced CNV formation in vitro[15]. Recently, Kim et al[16] also demonstrated that curcumin inhibited suture-induced CNV in rabbit eye via down-regulating expression of VEGF and suppressing activation of NF-κB. Similarly, Pradhan et al[40] reported that topical delivery of curcumin nanoparticle enhanced its retention and accentuated its inhibitory effect on corneal neovascularization. These findings indicated that curcumin not only inhibited in vitro angiogenic response of endothelial cells, but attenuated angiogenesis or neovessel formation in various tissues. Our study is in agreement with previous findings and confirms curcumin's inhibitory effect on endothelial cell proliferation and its implication in CNV. Most importantly, we also examined the underlying mechanisms and found out that curcumin could abolish WCM-induced Wnt/β-catenin pathway activation and inhibit WCM-promoted endothelial proliferation. It suggested that curcumin may exert its salutary effect through modulating Wnt/β-catenin signaling transduction. In nuclei, β-catenin could bind to VEGF promoter and upregulate expression of VEGF[41]. Previous study demonstrated that curcumin could effectively inhibited VEGF expression in cultured pterygium fibroblast[42]. VEGF-mediated activation of VEGFR2 signaling plays a pivotal role in angiogenesis process, which has been also reported to be inhibited by curcumin[43]. Our study may provide a possible explanation of curcumin's inhibitory effect on VEGF production during CNV formation described by other investigator[16]. Previous studies demonstrated that curcumin suppressed expression matrix metalloproteinases (MMPs), which is clearly involved in extracellular matrix remodeling during angiogenesis[44]. β-catenin has been shown to regulates several MMPs expression such as MMP-2 and MMP-9[45]. In our study, we found that curcumin inhibited suture-induced CNV in rats and meanwhile it significantly attenuated Wnt/β-catenin pathway activation in vascularized corneas. Taken together, these results provide evidence that curcumin inhibited angiogenic response via attenuation of Wnt/β-catenin pathway activation.
Recently several studies have analyzed curcumin's effect on Wnt activation; however, results were controversial. Zhang et al[46] demonstrated that curcumin activated Wnt pathway in human neuroblastoma cell line. Similarly, activation of Wnt pathway by curcumin could suppress pre-adipocyte differentiation; while other studies showed curcumin inhibited β-catenin accumulation and attenuated its downstream signaling in several tumor cell lines such stomach adenocarcinoma cell line, human colon adenocarcinoma cell line, colorectal carcinoma cell line etc[47]–[48]. Based on the previous findings, we speculated that curcumin's modulation on Wnt pathway varies among cell types and is also related to cell status. Our results favor the concept that curcumin acts as an inhibitor of Wnt/β-catenin pathway but not an activator during endothelial proliferation and CNV formation. In the present study, we investigated short-term effect of curcumin on CNV inhibition; however, long-term effect of curcumin is still worthy of future study.
In conclusion, our study demonstrated that activation of Wnt contributes to endothelial proliferation and implicated in suture-induced CNV in mice. Thus, inhibition of Wnt activation by curcumin may provide a promising therapeutic strategy for CNV associated diseases.
Acknowledgments
Authors' contributions: Zhang YK performed the experiments; Li JM performed the experiments, analyzed data and wrote the manuscript; Qin L wrote the manuscript.
Foundations: Supported by Young Talent Research Scholar Grant (No.2016KJXX-12); Shaanxi Natural Science Grant (No.2016JM8029).
Conflicts of Interest: Zhang YK, None; Li JM, None; Qin L, None.
REFERENCES
- 1.Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88(4):495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks BJ, Ambati BK, Marcus DM, Ratanasit A. Photodynamic therapy for corneal neovascularisation and lipid degeneration. Br J Ophthalmol. 2004;88(6):840. doi: 10.1136/bjo.2003.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keating AM, Jacobs DS. Anti-VEGF Treatment of Corneal Neovascularization. Ocul Surf. 2011;9(4):227–237. doi: 10.1016/s1542-0124(11)70035-0. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 5.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5(12):997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 6.Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: a Wnt for every vessel? Curr Opin Genet Dev. 2009;19(5):476–483. doi: 10.1016/j.gde.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11(1):63–69. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28(12):1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manikandan R, Beulaja M, Thiagarajan R, Arumugam M. Effect of curcumin on the modulation of αA- and αB-crystallin and heat shock protein 70 in selenium-induced cataractogenesis in Wistar rat pups. Mol Vis. 2011;17:388–394. [PMC free article] [PubMed] [Google Scholar]
- 10.Manikandan R, Thiagarajan R, Beulaja S, Sudhandiran G, Arumugam M. Effect of curcumin on selenite-induced cataractogenesis in Wistar rat pups. Curr Eye Res. 2010;35(2):122–129. doi: 10.3109/02713680903447884. [DOI] [PubMed] [Google Scholar]
- 11.Vasireddy V, Chavali VR, Joseph VT, Kadam R, Lin JH, Jamison JA, Kompella UB, Reddy GB, Ayyagari R. Rescue of photoreceptor degeneration by curcumin in transgenic rats with P23H rhodopsin mutation. PLoS One. 2011;6(6):e21193. doi: 10.1371/journal.pone.0021193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandal MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV, Anderson RE. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46(5):672–679. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011;27(2):123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 14.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269(2):199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. 1998;4(6):376–383. [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JS, Choi JS, Chung SK. The effect of curcumin on corneal neovascularization in rabbit eyes. Curr Eye Res. 2010;35(4):274–280. doi: 10.3109/02713680903528345. [DOI] [PubMed] [Google Scholar]
- 17.Sundram V, Chauhan SC, Ebeling M, Jaggi M. Curcumin attenuates β-catenin signaling in prostate cancer cells through activation of protein kinase D1. PLoS One. 2012;7(4):e35368. doi: 10.1371/journal.pone.0035368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 19.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad CP, Rath G, Mathur S, Bhatnagar D, Ralhan R. Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/beta-catenin signaling. Chem Biol Interact. 2009;181(2):263–271. doi: 10.1016/j.cbi.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX. Wnt/β-Catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52(7):4734–4741. doi: 10.1167/iovs.10-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyu J, Joo CK. Expression of Wnt and MMP in epithelial cells during corneal wound healing. Cornea. 2006;25(10Suppl 1):S24–S28. doi: 10.1097/01.ico.0000247209.01262.4e. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Liu Q, Zhou T, Lin Z, Zong R, Liu Z, Sun F, Shao Y, Liu X, Ma JX, Liu Z. Modulation of the canonical Wnt pathway by Benzalkonium Chloride in corneal epithelium. Exp Eye Res. 2011;93(4):355–362. doi: 10.1016/j.exer.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 24.de Jesus Perez VA, Alastalo TP, Wu JC, Axelrod JD, Cooke JP, Amieva M, Rabinovitch M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J Cell Biol. 2009;184(1):83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nimmagadda S, Geetha-Loganathan P, Scaal M, Christ B, Huang R. FGFs, Wnts and BMPs mediate induction of VEGFR-2 (Quek-1) expression during avian somite development. Dev Biol. 2007;305(2):421–429. doi: 10.1016/j.ydbio.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, Willett KL, Guerin KI, Mammoto A, Campbell M, Smith LE. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation. 2011;124(17):1871–1881. doi: 10.1161/CIRCULATIONAHA.111.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 28.Brown SD, Twells RC, Hey PJ, Cox RD, Levy ER, Soderman AR, Metzker ML, Caskey CT, Todd JA, Hess JF. Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem Biophys Res Commun. 1998;248(3):879–888. doi: 10.1006/bbrc.1998.9061. [DOI] [PubMed] [Google Scholar]
- 29.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407(6803):535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Adhikari N, Li Q, Hall JL. LDL receptor-related protein LRP6 regulates proliferation and survival through the Wnt cascade in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;287(6):H2376–H2383. doi: 10.1152/ajpheart.01173.2003. [DOI] [PubMed] [Google Scholar]
- 31.Min JK, Park H, Choi HJ, Kim Y, Pyun BJ, Agrawal V, Song BW, Jeon J, Maeng YS, Rho SS, Shim S, Chai JH, Koo BK, Hong HJ, Yun CO, Choi C, Kim YM, Hwang KC, Kwon YG. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J Clin Invest. 2011;121(5):1882–1893. doi: 10.1172/JCI42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee K, Hu Y, Ding L, Chen Y, Takahashi Y, Mott R, Ma JX. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes. 2012;61(11):2948–2957. doi: 10.2337/db11-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y, Chen Y, Lin M, Lee K, Mott RA, Ma JX. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54(1):141–154. doi: 10.1167/iovs.12-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu JY, Lin SJ, Lin JK. Inhibitory effects of curcumin on protein kinase C activity induced by 12-O-tetradecanoyl-phorbol-13-acetate in NIH 3T3 cells. Carcinogenesis. 1993;14(5):857–861. doi: 10.1093/carcin/14.5.857. [DOI] [PubMed] [Google Scholar]
- 35.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122(2):267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 36.Sameermahmood Z, Balasubramanyam M, Saravanan T, Rema M. Curcumin modulates SDF-1alpha/CXCR4-induced migration of human retinal endothelial cells (HRECs) Invest Ophthalmol Vis Sci. 2008;49(8):3305–3311. doi: 10.1167/iovs.07-0456. [DOI] [PubMed] [Google Scholar]
- 37.Binion DG, Otterson MF, Rafiee P. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut. 2008;57(11):1509–1517. doi: 10.1136/gut.2008.152496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuo M, Sakurai H, Koizumi K, Saiki I. Curcumin inhibits the formation of capillary-like tubes by rat lymphatic endothelial cells. Cancer Lett. 2007;251(2):288–295. doi: 10.1016/j.canlet.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 40.Pradhan N, Guha R, Chowdhury S, Nandi S, Konar A, Hazra S. Curcumin nanoparticles inhibit corneal neovascularization. J Mol Med. 2015;93(10):1095–1106. doi: 10.1007/s00109-015-1277-z. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61(16):6050–6054. [PubMed] [Google Scholar]
- 42.Lu CW, Hao JL, Yao L, Li HJ, Zhou DD. Efficacy of curcumin in inducing apoptosis and inhibiting the expression of VEGF in human pterygium fibroblasts. Int J Mol Med. 2017;39(5):1149–1154. doi: 10.3892/ijmm.2017.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu Z, Chen X, Guan S, Yan Y, Lin H, Hua ZC. Curcumin inhibits angiogenesis and improves defective hematopoiesis induced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling pathway. Oncotarget. 2015;6(23):19469–19482. doi: 10.18632/oncotarget.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar D, Kumar M, Saravanan C, Singh SK. Curcumin: a potential candidate for matrix metalloproteinase inhibitors. Expert Opin Ther Targets. 2012;16(10):959–972. doi: 10.1517/14728222.2012.710603. [DOI] [PubMed] [Google Scholar]
- 45.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26(2):227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Yin WK, Shi XD, Li Y. Curcumin activates Wnt/β-catenin signaling pathway through inhibiting the activity of GSK-3β in APPswe transfected SY5Y cells. Eur J Pharm Sci. 2011;42(5):540–546. doi: 10.1016/j.ejps.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Park CH, Hahm ER, Park S, Kim HK, Yang CH. The inhibitory mechanism of curcumin and its derivative against beta-catenin/Tcf signaling. FEBS Lett. 2005;579(13):2965–2971. doi: 10.1016/j.febslet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, Kim DE, Park BS, Oh S. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Commun. 2008;377(4):1304–1308. doi: 10.1016/j.bbrc.2008.10.171. [DOI] [PubMed] [Google Scholar]