Abstract
Macrophages are involved in angiogenesis, and might also contribute to the pathogenesis of intraocular neovascular diseases. Recent studies indicated that macrophages exert different functions in the process of intraocular neovascularization, and the polarization of M1 and M2 phenotypes plays extremely essential roles in the diverse functions of macrophages. Moreover, a large number of cytokines released by macrophages not only participate in macrophage polarization, but also associate with retinal and choroidal neovascular diseases. Therefore, macrophage might be considered as a novel therapeutic target to the treatment of pathological neovascularization in the eye. This review mainly summarizes diverse roles of macrophages and discusses the possible mechanisms in retinal and choroidal neovascularization.
Keywords: macrophage, retinal neovascularization, choroidal neovascularization, proliferative diabetic retinopathy, retinopathy of prematurity, age-related macular degeneration
INTRODUCTION
Intraocular neovascularization is a major complication that leads to vision loss[1]–[2], which comprises retinal neovascularization (RNV) and choroidal neovascularization (CNV). RNV contributes to numerous retinal diseases, such as proliferative diabetic retinopathy (PDR), retinopathy of prematurity (ROP) and retinal vein occlusions while CNV is a major complication of age-related macular degeneration (AMD)[1]–[3]. The pathological neovascularization could lead to leakage and hemorrhage, followed by fibrous proliferation, which may result in severe vision loss[4]–[6].
The main clinical treatments of intraocular neovascular diseases include laser photocoagulation, vitrectomy, drug delivery by intraocular injection, etc. In particular, drug delivery of anti-vascular endothelial growth factor (VEGF) agents have been proved to be effective in inhibiting neovascularization and are widely used for clinical applications in a group of ocular disorders[7]–[11]. However, laser photocoagulation and vitrectomy cannot fundamentally block neovascularization, while patients receiving long-term medication therapy of anti-VEGF treatment might develop resistance to those drugs with decreasing sensitivity to the therapy, and part of the patients may result in varying degrees of complications[7],[12]. Thus, in addition to anti-VEGF drugs, other novel therapeutic targets with high efficiency and safety are needed.
Macrophages, as essential angiogenic effector cells, play dual roles in tumor growth and angiogenesis[13]. Those key cells that control tumor angiogenesis are called tumor-associated macrophages (TAM), which programmed by several factors, such as macrophage colony-stimulating factor (M-CSF), VEGF-A and monocyte chemoattractant protein 1 (MCP-1)[14]–[16].
In the present review, diverse roles of macrophages in intraocular neovascular diseases are described from basic researches to clinical investigations. Possible mechanisms of macrophages in intraocular angiogenesis are also discussed.
M1 AND M2: POLARIZATIONS OF MACROPHAGES
Macrophages can be divided into at least two major phenotypes with diverse functions: pro-inflammatory M1 and anti-inflammatory M2 macrophages[17]–[19]. The division of M1 and M2 macrophages are named reflect from Th1 and Th2 activation[20]. The M1 phenotype is known as classically activated and pro-inflammatory macrophages, which has been reported to play crucial roles in destroying foreign organisms and inhibiting tumor cells. On the other hand, M2 phenotype is known as alternatively activated or immunosuppressive macrophages, which are understood to be important in debris scavenging, wound healing, chronic infections, tumorigenesis and angiogenesis[17],[21]–[26]. M1 phenotype can be polarized by lipopolysacchatide (LPS) and interferon (IFN)-γ, while other cytokines like interleukin (IL)-4, IL-10 and IL-13 can induce M2 polarization[18],[23]. It has been reported that M2, rather than M1 macrophages, enhanced angiogenesis in vivo, and M2 macrophages highly expressed basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF1), placental growth factor (PGF) and MCP-1[17]. TAM phenotype has been skewed toward M2-polarized cells, while M1 macrophages were also mixed in tumor angiogenesis[14],[27]. The dual roles of macrophages in angiogenesis have been becoming an issue of great concern in many medical aspects, thus an increasing number of studies concentrated in the polarization of macrophages. Nevertheless, the functions of different macrophage phenotypes in intraocular neovascularization still remain unclear.
MACROPHAGES IN CHOROIDAL NEOVASCULARIZATION
AMD is a leading disease that causes blindness in aged population[28], and severe visual loss of AMD patients is mostly caused by CNV (which is also called subretinal neovascularization). Macrophages were found to be attracted to Bruch's membrane in the patients of AMD[29], the presence of extracellular deposits is related to macrophage recruitment to Bruch's membrane, as well as the phenotype of resident subretinal macrophages[29]. In CNV, retinal pigment epithelium (RPE) cells express MCP-1, a cytokine that involved in macrophage recruitment[30].
Besides, although M1 and M2 chemokines are both increased in AMD maculae, it has higher transcript ratio of M1 chemokine CXCL11 to M2 chemokine CXCL22 in advanced AMD maculae compared to the control[31]. Thus, macrophage polarization might contribute to the pathogenesis of AMD[31].
Laser-induced CNV is an experimental mouse model to investigate the mechanisms of the development of CNV, which also accompany with increased macrophages[32]. The size of laser-induced CNV was significantly reduced by the depletion of macrophages using clodronate liposomes, which indicated that macrophages might be the key mediators in the formation and pathogenesis of CNV[33]–[34]. Interestingly, however, another study showed that intravitreal injection of macrophages inhibited CNV in the same model[35]. In that study, bone marrow-derived macrophages were stimulated by granulocyte macrophage colony-stimulating factor (GM-CSF), which might lead to the bias toward M1 phenotype[18], meaning that M1 macrophages may inhibit CNV rather than promote it. Another study reported that loss of Smad3 inhibited the development of laser-induced CNV by suppression of infiltrated macrophages, which also demonstrated that macrophages are vital for the pathogenesis of CNV[36]. Blockade of VEGF receptor significantly decreased retinal microglia and macrophages that infiltrated into laser-induced CNV[37], indicating that VEGF receptors might be involved in the recruitment of macrophages.
Zandi et al[23] reported that both M1 and M2 macrophages were remarkably increased in the posterior segment of the eyes with laser-treatment of CNV. The study showed that M1 macrophages inhibited CNV while M2 macrophages enhanced it, and selective Rho-associated kinase (ROCK)-2 inhibition decreased CNV by regulation of macrophage polarization. Tahiri et al[38] indicated that lymphocyte-derived microparticles modulated macrophage polarization towards M1 phenotype and also suppressed laser-induced CNV.
Hagbi-Levi et al[39] reported that delivery of M1-polarized macrophages from neovascular-AMD patients (but not unaffected controls) enhanced CNV, while M2-polarized macrophages from both neovascular-AMD patients and controls promoted CNV in a rat model. These results demonstrated that the pathogenesis of AMD might interfere the proangiogenic functions of macrophages. Yang et al[40] demonstrated different dynamic patterns of M1 and M2 macrophages in both experimental CNV mouse model and clinical patients. We recently showed the different distributions of M1 and M2 macrophages in the CNV model. During the first week after laser-treatment, M1 macrophages tended to concentrate around the laser areas and the outer layer of the retinas, and M2 macrophages were mainly recognized in the inner layer of the retinas in CNV model[41]. In addition, infiltration of M1 macrophages of the outer retina precedes damage in another AMD mouse model[42]. Thus M1 macrophages may have more direct roles in early stage of inflammatory, while M2 macrophages may be essential in advanced stage of CNV pathogenesis.
MACROPHAGES IN RETINAL NEOVASCULARIZATION
Clinical Investigations in Proliferative Diabetic Retinopathy Patients
Macrophages were predominantly found in surgically removed membranes of PDR patients[43]. Although the authors tried several markers to show macrophages from different stages, the definition of polarization has not been clearly defined at that time. In another study, VEGF was localized around macrophages in neovascular membrane of PDR patients[44]. Recently it has been reported that CD163, a marker of M2 macrophages, overexpressed in vitreous and fibrovascular membranes of patients with PDR, while the M1 marker CD80 was below the level of detection in the same samples of those patients[45]. Interestingly, in the study, there was a low correlation between M2 macrophages and VEGF, but a higher correlation between M2 macrophages and a matricelluar protein periostin[45]. M2 macrophage-related proteins M-CSF and IL-13 were remarkably higher expressed in the vitreous of patients with PDR, which supported that M2 macrophages might be involved in the pathogenesis of RNV[46].
Clinical Investigations in Retinopathy of Prematurity Patients
Cytokines including MCP-1, macrophage inflammatory protein 1 alpha (MIP-1α) and macrophage inflammatory protein 1 beta (MIP-1β) increased significantly in the serum of ROP patients compared to the health controls[47]. Preponderance of M1 over M2 macrophages was recognized in retrolental fibrous membranes of advanced ROP patients[48]. A possible reason may be that severe inflammation occurred in those ROP infants, and pro-inflammatory M1 macrophages were recruited in response of the pathogenesis in inhibiting such pathological neovascularization.
For the above disorders, the role of macrophages is still controversial. Macrophages might be recruited by pathological neovascularization after a long-term pathogenesis, but they may also promote the pathological neovascularization, which could be regarded as a positive feedback regulation. Thus, in vivo studies are required for further investigations.
Oxygen-induced Retinopathy Mouse Model
Oxygen-induced retinopathy (OIR) is a commonly used mouse model for investigating RNV[49]. Unlike laser-induced CNV model, the pathogenesis of OIR is more complicated, as it contains two types of retinal angiogenesis: pathological neovascularization and physiological revascularization. The pathological one sprouted with abnormal vessels from the inner retina into the vitreous, and the other is against avascular areas with healthy vascular regeneration[49]–[50]. In an earlier time, most of the researchers concentrated on the pathological neovascularization only, but recently, more scientists are paying attention to the physiological revascularization. It is intriguing that the above two types are usually inversely correlated: if the physiological revascularization increased, the pathological neovascularization often reduced by the same time[51].
A study showed that leukocytes mediate retinal vascular remodeling in a similar rat model of ischemia-induced retinopathy[52]. In OIR mouse model, the number of macrophages and microglia increased in the retinas at P14 and P17, and the expression of CCL2 (MCP-1) also enhanced in response to the ischemia-treatment[53]. By intravitreal injection of clodronate liposomes, the macrophages were depleted, by the same time, both avascular areas and neovascular tufts reduced significantly[54]. Another study also showed that macrophages promoted vasculogenesis of RNV in the OIR model[55]. The vitreal macrophages express VEGF in response to OIR treatment[56], and the expression of VEGF significantly decreased after depletion of macrophages[55]. However, though myeloid cells accumulate in both CNV and OIR models, they are not the major source of VEGFA, suggested that the angiogenic effects of macrophages might be based on other factors or cytokines[57]. M2 macrophages, rather than M1 phenotype, enhanced pathological neovascularization in the OIR model[50]. The possible mechanism(s) of M2 macrophages in RNV was summarized (Figure 1). The promotion of angiogenesis by M2 macrophages might be mediated by several kinds of cytokines and molecules. On the other hand, Zhu et al[58] showed that diverse polarized macrophages played active roles in contributing to different stages of RNV in the OIR model.
Figure 1. Hypothesis of the mechanisms of M2 macrophages in hypoxia-induced retinal neovascularization.
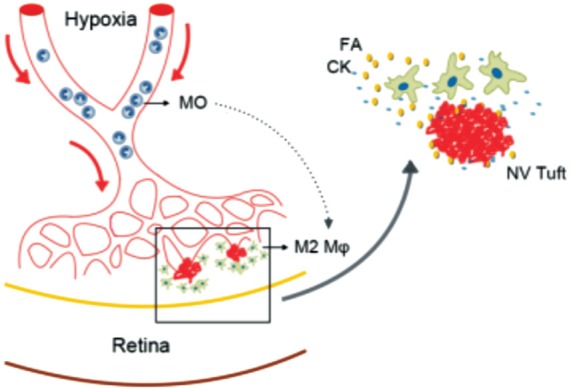
MO: Monocytes; Mφ: Macrophages; FA: Growth factors; CK: Cytokines.
It has been reported that MCP-1 could be one of the essential monocyte attractants[59]. As MCP-1 is also involved in the induction of RNV[60], it is possible that MCP-1 plays a role in attracting or modulating macrophages in the hypoxia-induced RNV. M-CSF has been used for stimulating macrophages from bone-marrow derived cells in several previous studies[23],[50],[61]. Davies et al[53] reported that M-CSF was constitutively expressed at all times in OIR mice, while GM-CSF was not present. The vitreous concentrations of M-CSF were significantly higher in PDR patients, and there was a strong positive correlation between the concentrations of M-CSF and CD163 (an M2 macrophage marker)[46]. M-CSF might be a possible factor in inducing and recruiting macrophages after hypoxia.
To sum up, in the hypoxic microenvironment, monocytes were recruited to the vitreous and retina, probably attracted by MCP-1. Several cytokines polarized and activated these monocytes to M2-phenotype macrophages, and the cells concentrated around the neovascular tufts, promoted the development and pathogenesis of RNV.
MACROPHAGE-RELATED CYTOKINES
M1 macrophages are deemed to attenuate ocular neovascularization, while M2 macrophages tend to enhance the angiogenesis in the eye. Macrophages produce a group of cytokines, and macrophage polarization could be induced by many cytokines[18]. In ocular diseases, cytokines are also considered as key factors in regulating angiogenesis. It has been proved that cytokines are involved in the pathogenesis, in recruitment of monocytes and polarization of macrophages, as well as in the effect of angiogenesis (Figure 2)[62].
Figure 2. Cytokines participate in macrophage attraction and polarization in intraocular neovascularization.
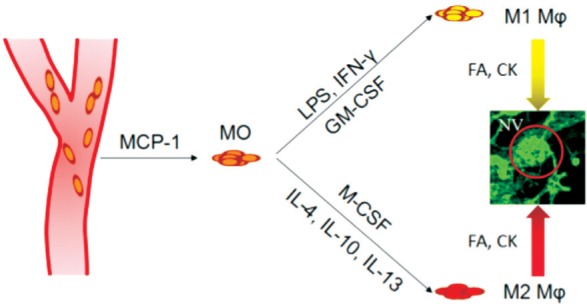
MO: Monocytes; Mφ: Macrophages; FA: Growth factors; CK: Cytokines.
High levels of IL-12, IL-18 and IL-23, and low levels of IL-10 were released by M1 macrophages[63]–[64]. However, on the other hand, M2 macrophages produced more IL-10 but less IL-12 and IL-23[65]–[66]. Th1 cytokines, such as IL-12 and IFN-γ, inhibited pathological angiogenesis in cornea, retina and choroid[23],[67]–[69]. IFN-γ probably is a mediator of the inhibitory effects of IL-12 in Th1 response, and the downstream chemokines CXCL9 and CXCL10 may play some roles in the pathogenesis[67]. IL-18 negatively regulates pathological RNV by regressing the blood vessels in the OIR mouse model[70]. However, the role played by IL-18 in laser-induced CNV remains controversial[71]–[73]. It has been reported that a typical Th2 cytokine IL-10 promoted pathological neovascularization in both CNV and OIR models[35],[63], while pretreatment of low-dose LPS suppressed CNV which possibly is regulated by the induction of IL-10[74]. Besides, Yang et al[75] reported that IL-10 suppressed experimental subretinal fibrosis formation, a following complication of CNV. All these studies provided controversial ideas of IL-10 in intraocular neovascularization. In contrast, another Th2 cytokine IL-4 attenuated laser-induced CNV, and this may resulted by different polarization and variable effects of subtypes in M2 macrophages (such as M2a, M2b and M2c)[76].
It is interesting that IL-23, as well as IL-17a neutralization inhibited ocular neovascularization[77]–[78]. The reason might be that IL-23 and IL-17 are involved in the Th17 pathway, which shows totally different effects in angiogenesis from Th1 pathway. IL-27 is involved in both induction of Th1 differentiation and inhibition of Th17 differentiation[79]. Hasegawa et al[80] demonstrated that IL-27 suppressed CNV formation via inhibition of VEGF. The immunological system of cytokines is very complicated, thus further studies are necessary to show the role played by Th17-related cytokines in macrophage polarization as well as intraocular neovascularization.
Takeuchi et al[81] reported that Th2 and Th17-related immune responses could be involved in the pathological progress of PDR. Another study by Suzuki et al[82] demonstrated that intravitreal injection of bevacizumab affects the expressions of both pro- and anti-inflammatory cytokines, which indicated that anti-VEGF treatment might also modulate immune response through the networks of such cytokines. Thus, macrophages might include more phenotypes and/or subgroups that are related to different types of cytokines, which might also be involved in the intraocular neovascularization.
CONCLUSION
Increasing evidences proved that macrophages, as well as its polarization, should play an important role in developing and/or inhibiting RNV and CNV. Recent studies demonstrated that M2 polarization of macrophages is thought to be more essential in promoting intraocular neovascularization. Moreover, a group of growth factors and cytokines are considered to participate in the pathogenesis caused by macrophages in the intraocular neovascular diseases. Therefore, specific molecular target associate with macrophages could be considered as a potential therapeutic treatment for the future clinical applications.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81371036; No.81700837); Department of Science and Technology, Hunan (No.2015TP2007); Japan Society for the Promotion of Science KAKENHI Grants (No.26293374; No.16K15734).
Conflicts of Interest: Zhou YD, None; Yoshida S, None; Peng YQ, None; Kobayashi Y, None; Zhang LS, None; Tang LS, None.
REFERENCES
- 1.Yoshida A, Yoshida S, Ishibashi T, Inomata H. Intraocular neovascularization. Histol Histopathol. 1999;14(4):1287–1294. doi: 10.14670/HH-14.1287. [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA. Ocular neovascularization. J Mol Med (Berl) 2013;91(3):311–321. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438(7070):960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 4.Hiscott P, Wong D, Grierson I. Challenges in ophthalmic pathology: the vitreoretinal membrane biopsy. Eye (Lond) 2000;14(Pt 4):549–559. doi: 10.1038/eye.2000.142. [DOI] [PubMed] [Google Scholar]
- 5.Telander DG. Inflammation and age-related macular degeneration (AMD) Semin Ophthalmol. 2011;26(3):192–197. doi: 10.3109/08820538.2011.570849. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa K, Kannan R, Hinton DR. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp Eye Res. 2016;142:19–25. doi: 10.1016/j.exer.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osaadon P, Fagan XJ, Lifshitz T, Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond) 2014;28(5):510–520. doi: 10.1038/eye.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, Miniaci S, Williams G. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR) Graefes Arch Clin Exp Ophthalmol. 2008;246(6):837–842. doi: 10.1007/s00417-008-0774-y. [DOI] [PubMed] [Google Scholar]
- 9.Mintz-Hittner HA, Kuffel RR. Intravitreal injection of bevacizumab (Avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008;28(6):831–838. doi: 10.1097/IAE.0b013e318177f934. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Li Y, Hong J. Progress of anti-vascular endothelial growth factor therapy for ocular neovascular disease: benefits and challenges. Chin Med J (Engl) 2014;127(8):1550–1557. [PubMed] [Google Scholar]
- 11.Amadio M, Govoni S, Pascale A. Targeting VEGF in eye neovascularization: what's new?: a comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol Res. 2016;103:253–269. doi: 10.1016/j.phrs.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Salam A, Mathew R, Sivaprasad S. Treatment of proliferative diabetic retinopathy with anti-VEGF agents. Acta Ophthalmol. 2011;89(5):405–411. doi: 10.1111/j.1755-3768.2010.02079.x. [DOI] [PubMed] [Google Scholar]
- 13.Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80(4):705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- 14.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunology Today. 1992;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 16.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Nakao S, Noda K, Zandi S, Sun D, Taher M, Schering A, Xie F, Mashima Y, Hafezi-Moghadam A. VAP-1-mediated M2 macrophage infiltration underlies IL-1beta- but not VEGF-A-induced lymph- and angiogenesis. Am J Pathol. 2011;178(4):1913–1921. doi: 10.1016/j.ajpath.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zandi S, Nakao S, Chun KH, Fiorina P, Sun D, Arita R, Zhao M, Kim E, Schueller O, Campbell S, Taher M, Melhorn MI, Schering A, Gatti F, Tezza S, Xie F, Vergani A, Yoshida S, Ishikawa K, Yamaguchi M, Sasaki F, Schmidt-Ullrich R, Hata Y, Enaida H, Yuzawa M, Yokomizo T, Kim YB, Sweetnam P, Ishibashi T, Hafezi-Moghadam A. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep. 2015;10(7):1173–1186. doi: 10.1016/j.celrep.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216(7):753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25(3):315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 26.Ho VW, Sly LM. Derivation and characterization of murine alternatively activated (M2) macrophages. Methods Mol Biol. 2009;531:173–185. doi: 10.1007/978-1-59745-396-7_12. [DOI] [PubMed] [Google Scholar]
- 27.Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4(4):376–389. [PMC free article] [PubMed] [Google Scholar]
- 28.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 29.Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch's membrane in age-related macular degeneration. Eye (Lond) 1990;4(Pt 4):613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 30.Grossniklaus HE, Ling JX, Wallace TM, Dithmar S, Lawson DH, Cohen C, Elner VM, Elner SG, Sternberg P., Jr Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–126. [PubMed] [Google Scholar]
- 31.Cao X, Shen D, Patel MM, Tuo J, Johnson TM, Olsen TW, Chan CC. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011;61(9):528–535. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart JM, Rakic JM, Noel A. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat Protoc. 2013;8(11):2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- 33.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(8):3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 34.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(8):3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 35.Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006;3(8):e310. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwanishi H, Fujita N, Tomoyose K, Okada Y, Yamanaka O, Flanders KC, Saika S. Inhibition of development of laser-induced choroidal neovascularization with suppression of infiltration of macrophages in Smad3-null mice. Lab Invest. 2016;96(6):641–651. doi: 10.1038/labinvest.2016.30. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Parlier R, Shen JK, Lutty GA, Vinores SA. VEGF receptor blockade markedly reduces retinal microglia/macrophage infiltration into laser-induced CNV. PLoS One. 2013;8(8):e71808. doi: 10.1371/journal.pone.0071808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tahiri H, Omri S, Yang C, Duhamel F, Samarani S, Ahmad A, Vezina M, Bussieres M, Vaucher E, Sapieha P, Hickson G, Hammamji K, Lapointe R, Rodier F, Tremblay S, Royal I, Cailhier JF, Chemtob S, Hardy P. Lymphocytic microparticles modulate angiogenic properties of macrophages in laser-induced choroidal neovascularization. Sci Rep. 2016;6:37391. doi: 10.1038/srep37391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagbi-Levi S, Grunin M, Jaouni T, Tiosano L, Rinsky B, Elbaz-Hayoun S, Peled A, Chowers I. Proangiogenic characteristics of activated macrophages from patients with age-related macular degeneration. Neurobiol Aging. 2016;51:71–82. doi: 10.1016/j.neurobiolaging.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Liu F, Tang M, Yuan M, Hu A, Zhan Z, Li Z, Li J, Ding X, Lu L. Macrophage polarization in experimental and clinical choroidal neovascularization. Sci Rep. 2016;6:30933. doi: 10.1038/srep30933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Yoshida S, Kubo Y, Yoshimura T, Kobayashi Y, Nakama T, Yamaguchi M, Ishikawa K, Oshima Y, Ishibashi T. Different distributions of M1 and M2 macrophages in a mouse model of laser-induced choroidal neovascularization. Mol Med Rep. 2017;15(6):3949–3956. doi: 10.3892/mmr.2017.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E, Feuer W, Huang D, Wen R, Hong L, Wang H, Laird JM, Sene A, Apte RS, Salomon RG, Hollyfield JG, Perez VL. Infiltration of proinflammatory M1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int J Inflam. 2013;2013:503725. doi: 10.1155/2013/503725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esser P, Heimann K, Wiedemann P. Macrophages in proliferative vitreoretinopathy and proliferative diabetic retinopathy: differentiation of subpopulations. Br J Ophthalmol. 1993;77(11):731–733. doi: 10.1136/bjo.77.11.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kakehashi A, Inoda S, Mameuda C, Kuroki M, Jono T, Nagai R, Horiuchi S, Kawakami M, Kanazawa Y. Relationship among VEGF, VEGF receptor, AGEs, and macrophages in proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2008;79(3):438–445. doi: 10.1016/j.diabres.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi Y, Yoshida S, Nakama T, Zhou Y, Ishikawa K, Arita R, Nakao S, Miyazaki M, Sassa Y, Oshima Y, Izuhara K, Kono T, Ishibashi T. Overexpression of CD163 in vitreous and fibrovascular membranes of patients with proliferative diabetic retinopathy: possible involvement of periostin. Br J Ophthalmol. 2015;99(4):451–456. doi: 10.1136/bjophthalmol-2014-305321. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida S, Kobayashi Y, Nakama T, Zhou Y, Ishikawa K, Arita R, Nakao S, Miyazaki M, Sassa Y, Oshima Y, Izuhara K, Kono T, Ishibashi T. Increased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: implications for M2 macrophage-involving fibrovascular membrane formation. Br J Ophthalmol. 2015;99(5):629–634. doi: 10.1136/bjophthalmol-2014-305860. [DOI] [PubMed] [Google Scholar]
- 47.Yu H, Yuan L, Zou Y, Peng L, Wang Y, Li T, Tang S. Serum concentrations of cytokines in infants with retinopathy of prematurity. APMIS. 2014;122(9):818–823. doi: 10.1111/apm.12223. [DOI] [PubMed] [Google Scholar]
- 48.Ma J, Mehta M, Lam G, Cyr D, Ng TF, Hirose T, Tawansy KA, Taylor AW, Lashkari K. Influence of subretinal fluid in advanced stage retinopathy of prematurity on proangiogenic response and cell proliferation. Mol Vis. 2014;20:881–893. [PMC free article] [PubMed] [Google Scholar]
- 49.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4(11):1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Yoshida S, Nakao S, Yoshimura T, Kobayashi Y, Nakama T, Kubo Y, Miyawaki K, Yamaguchi M, Ishikawa K, Oshima Y, Akashi K, Ishibashi T. M2 macrophages enhance pathological neovascularization in the mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2015;56(8):4767–4777. doi: 10.1167/iovs.14-16012. [DOI] [PubMed] [Google Scholar]
- 51.Scott A, Fruttiger M. Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye (Lond) 2010;24(3):416–421. doi: 10.1038/eye.2009.306. [DOI] [PubMed] [Google Scholar]
- 52.Ishida S, Yamashiro K, Usui T, Kaji Y, Ogura Y, Hida T, Honda Y, Oguchi Y, Adamis AP. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9(6):781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- 53.Davies MH, Eubanks JP, Powers MR. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis. 2006;12:467–477. [PubMed] [Google Scholar]
- 54.Kataoka K, Nishiguchi KM, Kaneko H, van Rooijen N, Kachi S, Terasaki H. The roles of vitreal macrophages and circulating leukocytes in retinal neovascularization. Invest Ophthalmol Vis Sci. 2011;52(3):1431–1438. doi: 10.1167/iovs.10-5798. [DOI] [PubMed] [Google Scholar]
- 55.Gao X, Wang YS, Li XQ, Hou HY, Su JB, Yao LB, Zhang J. Macrophages promote vasculogenesis of retinal neovascularization in an oxygen-induced retinopathy model in mice. Cell Tissue Res. 2016;364(3):599–610. doi: 10.1007/s00441-015-2353-y. [DOI] [PubMed] [Google Scholar]
- 56.Naug HL, Browning J, Gole GA, Gobe G. Vitreal macrophages express vascular endothelial growth factor in oxygen-induced retinopathy. Clin Exp Ophthalmol. 2000;28(1):48–52. doi: 10.1046/j.1442-9071.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 57.Liyanage SE, Fantin A, Villacampa P, Lange CA, Denti L, Cristante E, Smith AJ, Ali RR, Luhmann UF, Bainbridge JW, Ruhrberg C. Myeloid-derived vascular endothelial growth factor and hypoxia-inducible factor are dispensable for ocular neovascularization-brief report. Arterioscler Thromb Vasc Biol. 2016;36(1):19–24. doi: 10.1161/ATVBAHA.115.306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu Y, Zhang L, Lu Q, Gao Y, Cai Y, Sui A, Su T, Shen X, Xie B. Identification of different macrophage subpopulations with distinct activities in a mouse model of oxygen-induced retinopathy. Int J Mol Med. 2017;40(2):281–292. doi: 10.3892/ijmm.2017.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida S, Yoshida A, Ishibashi T, Elner SG, Elner VM. Role of MCP-1 and MIP-1alpha in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization. J Leukoc Biol. 2003;73(1):137–144. doi: 10.1189/jlb.0302117. [DOI] [PubMed] [Google Scholar]
- 61.Richards J, Gabunia K, Kelemen SE, Kako F, Choi ET, Autieri MV. Interleukin-19 increases angiogenesis in ischemic hind limbs by direct effects on both endothelial cells and macrophage polarization. J Mol Cell Cardiol. 2015;79:21–31. doi: 10.1016/j.yjmcc.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72(21):4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dace DS, Khan AA, Kelly J, Apte RS. Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PLoS One. 2008;3(10):e3381. doi: 10.1371/journal.pone.0003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beyer M, Mallmann MR, Xue J, Staratschek-Jox A, Vorholt D, Krebs W, Sommer D, Sander J, Mertens C, Nino-Castro A, Schmidt SV, Schultze JL. High-resolution transcriptome of human macrophages. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0045466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mantovani A. Macrophage diversity and polarization: in vivo veritas. Blood. 2006;108(2):408–409. [Google Scholar]
- 67.Zhou Y, Yoshida S, Kubo Y, Kobayashi Y, Nakama T, Yamaguchi M, Ishikawa K, Nakao S, Ikeda Y, Ishibashi T, Sonoda KH. Interleukin-12 inhibits pathological neovascularization in mouse model of oxygen-induced retinopathy. Sci Rep. 2016;6:28140. doi: 10.1038/srep28140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452(7187):591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87(8):581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 70.Qiao H, Sonoda KH, Ikeda Y, Yoshimura T, Hijioka K, Jo YJ, Sassa Y, Tsutsumi-Miyahara C, Hata Y, Akira S, Ishibashi T. Interleukin-18 regulates pathological intraocular neovascularization. J Leukoc Biol. 2007;81(4):1012–1021. doi: 10.1189/jlb.0506342. [DOI] [PubMed] [Google Scholar]
- 71.Ijima R, Kaneko H, Ye F, Nagasaka Y, Takayama K, Kataoka K, Kachi S, Iwase T, Terasaki H. Interleukin-18 induces retinal pigment epithelium degeneration in mice. Invest Ophthalmol Vis Sci. 2014;55(10):6673–6678. doi: 10.1167/iovs.14-15367. [DOI] [PubMed] [Google Scholar]
- 72.Hirano Y, Yasuma T, Mizutani T, Fowler BJ, Tarallo V, Yasuma R, Kim Y, Bastos-Carvalho A, Kerur N, Gelfand BD, Bogdanovich S, He S, Zhang X, Nozaki M, Ijima R, Kaneko H, Ogura Y, Terasaki H, Nagai H, Haro I, Nunez G, Ambati BK, Hinton DR, Ambati J. IL-18 is not therapeutic for neovascular age-related macular degeneration. Nat Med. 2014;20(12):1372–1375. doi: 10.1038/nm.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doyle SL, Ozaki E, Brennan K, Humphries MM, Mulfaul K, Keaney J, Kenna PF, Maminishkis A, Kiang AS, Saunders SP, Hams E, Lavelle EC, Gardiner C, Fallon PG, Adamson P, Humphries P, Campbell M. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci Transl Med. 2014;6(230):230ra244. doi: 10.1126/scitranslmed.3007616. [DOI] [PubMed] [Google Scholar]
- 74.Matsumura N, Kamei M, Tsujikawa M, Suzuki M, Xie P, Nishida K. Low-dose lipopolysaccharide pretreatment suppresses choroidal neovascularization via IL-10 induction. PLoS One. 2012;7(7):e39890. doi: 10.1371/journal.pone.0039890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y, Takeda A, Yoshimura T, Oshima Y, Sonoda KH, Ishibashi T. IL-10 is significantly involved in HSP70-regulation of experimental subretinal fibrosis. PLoS One. 2013;8(12):e80288. doi: 10.1371/journal.pone.0080288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu WK, Georgiadis A, Copland DA, Liyanage S, Luhmann UF, Robbie SJ, Liu J, Wu J, Bainbridge JW, Bates DO, Ali RR, Nicholson LB, Dick AD. IL-4 regulates specific Arg-1(+) macrophage sFlt-1-mediated inhibition of angiogenesis. Am J Pathol. 2015;185(8):2324–2335. doi: 10.1016/j.ajpath.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 77.Zhu Y, Tan W, Demetriades AM, Cai Y, Gao Y, Sui A, Lu Q, Shen X, Jiang C, Xie B, Sun X. Interleukin-17A neutralization alleviated ocular neovascularization by promoting M2 and mitigating M1 macrophage polarization. Immunology. 2016;147(4):414–428. doi: 10.1111/imm.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai Y, Tan W, Shen X, Zhu Y, Gao Y, Sui A, Lu Q, Zhong Y, Xie B. Neutralization of IL-23 depresses experimental ocular neovascularization. Exp Eye Res. 2016;146:242–251. doi: 10.1016/j.exer.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 79.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A, Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177(8):5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 80.Hasegawa E, Oshima Y, Takeda A, Saeki K, Yoshida H, Sonoda KH, Ishibashi T. IL-27 inhibits pathophysiological intraocular neovascularization due to laser burn. J Leukoc Biol. 2012;91(2):267–273. doi: 10.1189/jlb.1110603. [DOI] [PubMed] [Google Scholar]
- 81.Takeuchi M, Sato T, Tanaka A, Muraoka T, Taguchi M, Sakurai Y, Karasawa Y, Ito M. Elevated levels of cytokines associated with Th2 and Th17 cells in vitreous fluid of proliferative diabetic retinopathy patients. PLoS One. 2015;10(9):e0137358. doi: 10.1371/journal.pone.0137358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suzuki Y, Suzuki K, Yokoi Y, Miyagawa Y, Metoki T, Nakazawa M. Effects of intravitreal injection of bevacizumab on inflammatory cytokines in the vitreous with proliferative diabetic retinopathy. Retina. 2014;34(1):165–171. doi: 10.1097/IAE.0b013e3182979df6. [DOI] [PubMed] [Google Scholar]


