Abstract
The aim of the study is to evaluate the safety and efficacy of trans-epithelial accelerated corneal cross-linking (TE-ACXL) in children with progressive keratoconus. Retrospective, case-series of 23 eyes of 14 children who underwent TE-ACXL. Evaluations were performed at baseline and 1, 3, 6, 12 and 18mo postoperatively. Mean follow-up time of 23.82±3.15mo and mean age was 13.7±1.4y (range 11 to 16y). Mean preoperative uncorrected distance visual acuity changed from 0.92±0.45 logMAR (20/160) to 0.71±0.40 logMAR (20/100) (P=0.001). Mean keratometry (Km) changed from 53.87± 6.03 to 53.00±5.81 (P=0.001). Pachymetry did not have significant changes at last follow-up (P=0.30). The mean preoperative sphere was -5.58±2.48 and -4.89±4.66 D (P=0.11) at last follow-up; refractive cylinder from -5.58±2.48 to -5.02±2.23 (P=0.046). In conclusion, tomographic and refractive stability are shown in over 91% of eyes with pediatric progressive keratoconus who underwent TE-ACXL.
Keywords: corneal cross-linking, keratoconus, accelerated cross-linking, pediatric keratoconus
INTRODUCTION
Keratoconus is a chronic, bilateral, usually asymmetric, progressive ectasia of the cornea that may be present since childhood[1]–[2]. Corneal cross-linking (CXL) has shown to halt the progression of keratoconus. Progression is usually much faster in children and adolescents; hence diagnosis of keratoconus before adulthood is an important risk factor[2].
Accelerated CXL reduces irradiation time by increasing its intensity while retaining a similar biomechanical effect as conventional CXL[3]. Trans-epithelial CXL has been shown to be clinically similar in some settings by halting progression of the disease[4]. This study aims to explore the clinical effects of trans-epithelial accelerated CXL (TE-ACXL) in children with progressive keratoconus.
METHODS
Retrospective, consecutive case series of patients younger than 16y of age with progressive keratoconus who underwent TE-ACXL. Inclusion criteria were children with documented tomographic progression. Exclusion criteria included corneal thickness of <350 µm, systemic connective tissue disease, corneal scarring, surgery or trauma. At baseline and at each of the follow-up visits (1, 3, 6, 12 and 18mo) all patients underwent uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) examination, and corneal tomography with Pentacam (Occulus, Germany). Progression was defined by an increase in Kmax of at least 1.00 D and/or corneal thinning in the past year. Written informed consent was obtained from all parents prior to the procedure and the study was performed according to the tenets of the Declaration of Helsinki.
Surgical Technique
Topical anesthesia was used. The corneal epithelium was left intact in all cases. Corneal impregnation started with a 4-minute soak with 0.25% riboflavin, benzalkonium chloride (BAC), hydroxypropylmethylcellulose (HPMC, ParaCel, Avedro Inc. Waltham MS, USA) to provide the initial dose of riboflavin and a second stage with dextran-free riboflavin 0.1% with HPMC (VibeX Rapid, Avedro Inc. Waltham MS, USA) for the next 6min. Using the KXL system (Avedro Inc. Waltham MS, USA), the cornea was exposed to UV-A radiation (30 mW/cm2) during 3min. Postoperatively, topical steroids and antibiotics were used.
Statistical Analyses
Mean values, standard deviation (SD) and 95% confidence intervals (95%CI) were used for continuous variables; frequencies and percentages for categorical variables were calculated. Mean differences in K-values, logMAR visual acuity and pachymetry at baseline and during follow-up were evaluated through Student's t-test for paired data. Change in mean and maximum K-values from baseline to 18mo was classified as follows: stability (change <0.5 D), regression (decrease >1.0 D), and progression (increase >1.00 D).
RESULTS AND DISCUSSION
Twenty-three eyes of 14 patients (11 males; 3 females) were included for analysis, all attended each of their follow-up visits. Fourteen eyes (60.8%) of 8 patients had associated atopy. Mean age at the time of treatment was 13.7±1.4y (range 11-16y). Mean follow-up was 23.82±3.15mo (range 18.57 to 29.87mo).
The mean preoperative sphere of -5.58±2.48 D had a non-significant reduction 18mo after TE-ACXL treatment to -4.89±4.66 (P=0.11), whereas both the refractive cylinder and spherical equivalent had statistically significant changes from -5.58±2.48 to -5.02±2.23 (P=0.046) and from -7.90±5.39 to -7.41±4.84 (P=0.043), respectively. Preoperative UDVA was 0.92 (20/160) ±0.45 logMAR, while the final UDVA was 0.71 (20/100) ±0.40 logMAR, with a statistically significant change of -0.21±0.15 logMAR (P<0.0001) (Table 1). Preoperative CDVA was 0.27±0.30 logMAR compared to 18mo CDVA: 0.17±0.17 logMAR and had a statistically significant improvement of -0.13 ±0.26 logMAR (P=0.05) (Table 1, Figure 1A).
Table 1. Tomographic and visual acuity changes from baseline and 1, 3, 6, 12 and 18mo follow-up.
| Variables | Pre-treatment (95% CI) |
Post-treatment 1mo (P)a
(95% CI) |
Post-treatment 3mo (P)a
(95% CI) |
Post-treatment 6mo (P)a
(95% CI) |
Post-treatment 12mo (P)a
(95% CI) |
Post-treatment 18mo (P)a
(95% CI) |
| Kmin | 50.75±6.09 | 50.37±5.49 (0.08) | 50.65±6.02 (0.29) | 49.94±5.50 (0.13) | 49.78±5.23 (0.08) | 50.37±5.83 (0.06) |
| (48.12-53.39) | (47.99-52.74) | (48.04-53.25) | (47.57-52.32) | (47.52-52.04) | (47.85-52.89) | |
| Kmax | 57.34±6.65 | 56.12±6.05 (0.002) | 56.22±6.05 (0.001) | 56.39±6.01 (0.02) | 56.02±6.01 (<0.001) | 55.65±5.59 (<0.001) |
| (54.47-60.22) | (53.50-58.73) | (53.60-58.83) | (53.79-58.98) | (53.4-58.62) | (53.23-58.06) | |
| Km | 54.00±6.18 | 53.20±5.80 (0.01) | 53.49±6.14 (0.009) | 52.92±5.70 (0.03) | 52.87±5.62 (0.02) | 53.06±5.88 (0.001) |
| (51.32-56.67) | (50.69-55.7) | (50.82-56.14) | (50.46-55.39) | (50.44-55.30) | (50.51-55.60) | |
| Pachymetry | 434.64±37.84 | 436.26±39.05 (0.74) | 434.09±40.12 (0.54) | 427.96±47.07 (0.14) | 432.52±42.29 (0.41) | 430.09±54.26 (0.30) |
| (427-440) | (430-443) | (428-440) | (422-434) | (426-439) | (424-436) | |
| UDVA | 0.92±0.45 | 0.80±0.43 (0.01) | 0.85±0.45 (0.09) | 0.83±0.38 (0.04) | 0.75±0.40 (0.001) | 0.71±0.41 (<0.001) |
| (0.86-0.98) | (0.74-0.86) | (0.79-0.91) | (0.76-0.89) | (0.69-0.81) | (0.65-0.77) | |
| CDVA | 0.27±0.30 | 0.55±0.42 (0.0003)b | 0.35±0.42 (0.89) | 0.23±0.25 (0.23) | 0.16±0.16 (0.029) | 0.17±0.17 (0.05) |
| (0.18-0.35) | (0.46-0.63) | (0.27-0.44) | (0.14-0.31) | (0.07-0.24) | (0.09-0.26) |
aP value vs baseline; 95%CI: 95% Confidence interval; n=23 at each follow-up visit; bSignificant worsening; n=23 at each follow-up visit.
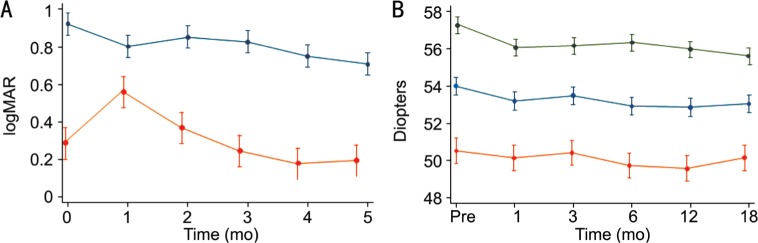
Figure 1. Visual acuity and K-values through study.
A: Line graph illustrating mean change and 95%CI in visual acuity throughout 18mo after CXL. UDVA (blue line) had a significant improvement at last follow-up (P≤0.0001), CDVA (orange line) had a significant worsening at 1mo (P=0.0003) but returned back to baseline at 3mo and significantly improved by 18mo (P=0.05), n=23 at each follow-up visit; B: Line graph depicting tomographic K-values mean change and 95%CI after TE-ACXL. Kmax (green line) had a statistically significant flattening 1mo after treatment (P=0.002) and at 18mo (P<0.001), Km (blue line) had a significant flattening at every follow-up visit (P=0.001), Kmin (orange line) had a non-significant flattening after 18mo (P=0.06), n=23 at each follow-up visit.
Mean preoperative tomographic Kmin had a non-significant improvement throughout the 18mo follow-up (P=0.06), mean preoperative tomographic Km improved significantly down (P=0.001) and the mean preoperative tomographic Kmax had a statistically significant improvement at last follow-up (Table 1, Figure 1B). Preoperative pachymetry was 434.64±37.84 µm and remained stable at 18mo with 430.09±54.26 µm (P=0.30) (Table 1). A clear and continuous demarcation line was observed in 19/23 eyes (82.6%); the average central demarcation line depth was 210.63±27.50 µm. Postoperative transient pain and mild-moderate haze were the most common side effects.
Several studies have demonstrated the arrest of progression of keratoconus with crosslinking in children[1]–[2],[4]–[9]; however, none of these studies included TE-ACXL, such as the one used in this study. The Siena CXL Pediatrics trial, with the standard epi-off protocol, is the largest prospective study of this technique, involving 77 patients age 10 to 18y, demonstrating keratoconus stabilization, with a significant visual function gained[9].
Removing the epithelium increases the penetration of riboflavin into the corneal stroma. Conversely, the removal increases risk of infection and is associated with greater pain and discomfort. Although some studies report a benefit of epithelium-off CXL[10], others have reported that both epithelium-off and trans-epithelial performed on young patients may have similar efficacy and safety, involving less pain and fewer short-term complications maintaining the epithelium[4],[7]–[8]. The addition of BAC 0.02% to the riboflavin solution can also potentially open the epithelial junctions in an intact epithelium[11].
A study with 26 eyes, with 10 from patients under 18y, used trans-epithelial CXL (3 mW/cm2 for 30min); due to significant worsening of all parameters more than half of pediatric patients were re-treated[12]. The study, however was performed without a BAC enhanced riboflavin and therefore possibly not achieving an adequate concentration.
The process behind cross-linking depends on the absorbed UV-A energy and its biological effect is proportional to the total energy dose delivered to the tissue (Bunsen-Roscoe law of reciprocity). Thus, it is possible to administer the same energy dose providing a proportional biological effect using different powers and exposure times[13]–[14]. Shetty et al[5] used epithelium-off accelerated cross-linking for the treatment of children with progressive keratoconus and found an improvement in the mean UDVA, CDVA, cylindrical refraction, and keratometry. The demarcation line in this study is similar to previous reports using accelerated cross-linking[15]; However, the significance of the demarcation line regarding progression is still debated.
In our series, TE-ACXL in children not only halted keratoconus progression but also achieved regression in some patients. We observed an improvement in UDVA at the final follow-up; CDVA worsened significantly after one month, perhaps due to post-operative corneal haze, but returned back to baseline at 3mo and continued to improve significantly. Significant Km and Kmax flattening were registered.
In total, 2 eyes (8.6%) had progression after 18mo of treatment. In addition to having a poorly defined demarcation line, both had severe stages of keratoconus with preoperative Kmax readings of >60.0 D, and allergic conjunctivitis. At the end of 18mo, thirteen eyes showed regression (>1.00 D) and had an average flattening of -2.38±1.50 D in Kmax values while the remaining 7 eyes remained stable.
Our study presents some limitations. A prospective randomized clinical trial is needed to avoid the bias caused by a retrospective design and it gives the possibility to evaluate with other standard protocols. A longer follow-up is also needed, for children that may have a disease that is more likely to progress. Finally, a larger sample size will lead to a more reliable conclusion.
Our results of TE-ACXL in children with progressive keratoconus are encouraging, with no evidence of progression in all cases with mild to moderate keratoconus during an 18mo period. Patients with severe stages of keratoconus may not be ideal candidates. TE-ACXL is a safe and effective alternative to treat keratoconus in properly selected pediatric patients.
Acknowledgments
Conflicts of Interest: Olivo-Payne A, None; Serna-Ojeda JC, None; Hernandez-Bogantes E, None; Abdala-Figuerola A, None; Pedro-Aguilar L, None; Lichtinger A, None; Ramirez-Miranda A, None; Navas A, None; Graue-Hernandez EO, None.
REFERENCES
- 1.Vinciguerra P, Albé E, Frueh BE, Trazza S, Epstein D. Two-year corneal cross-linking results in patients younger than 18y with documented progressive keratoconus. Am J Ophthalmol. 2012;154(3):520–526. doi: 10.1016/j.ajo.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Buzzonetti L, Petrocelli G. Transepithelial corneal cross-linking in pediatric patients: early results. J Refract Surg. 2012;28(11):763–767. doi: 10.3928/1081597X-20121011-03. [DOI] [PubMed] [Google Scholar]
- 3.Mrochen M. Current status of accelerated corneal cross-linking. Indian J Ophthalmol. 2013;61(8):428–429. doi: 10.4103/0301-4738.116075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magli A, Forte R, Tortori A, Capasso L, Marsico G, Piozzi E. Epithelium-off corneal collagen cross-linking versus transepithelial cross-linking for pediatric keratoconus. Cornea. 2013;32(5):597–601. doi: 10.1097/ICO.0b013e31826cf32d. [DOI] [PubMed] [Google Scholar]
- 5.Shetty R, Nagaraja H, Jayadev C, Pahuja NK, Kurian Kummelil M, Nuijts RM. Accelerated corneal collagen cross-linking in pediatric patients: two-year follow-up results. Biomed Res Int. 2014;2014:894095. doi: 10.1155/2014/894095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora R, Gupta D, Goyal JL, Jain P. Results of corneal collagen cross-linking in pediatric patients. J Refract Surg. 2012;28(11):759–762. doi: 10.3928/1081597X-20121011-02. [DOI] [PubMed] [Google Scholar]
- 7.Kumar Kodavoor S, Arsiwala AZ, Ramamurthy D. One-year clinical study on efficacy of corneal cross-linking in Indian children with progressive keratoconus. Cornea. 2014;33(9):919–922. doi: 10.1097/ICO.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 8.Salman AG. Transepithelial corneal collagen crosslinking for progressive keratoconus in a pediatric age group. J Cataract Refract Surg. 2013;39(8):1164–1170. doi: 10.1016/j.jcrs.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R, Balestrazzi A. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 2012;31(3):227–231. doi: 10.1097/ico.0b013e31822159f6. [DOI] [PubMed] [Google Scholar]
- 10.Soeters N, Wisse RPL, Godefrooij DA, Imhof SM, Tahzib NG. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial American. Journal of Ophthalmology. 2015;159(5):821–828.e3. doi: 10.1016/j.ajo.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Kissner A, Spoerl E, Jung R, Spekl K, Pillunat LE, Raiskup F. Pharmacological modification of the epithelial permeability by benzalkonium chloride in UVA/Riboflavin corneal collagen cross-linking. Curr Eye Res. 2010;35(8):715–721. doi: 10.3109/02713683.2010.481068. [DOI] [PubMed] [Google Scholar]
- 12.Salman AG. Transepithelial corneal collagen crosslinking for progressive keratoconus in a pediatric age group. J Cataract Refract Surg. 2013;39(8):1164–1170. doi: 10.1016/j.jcrs.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Wernli J, Schumacher S, Spoerl E, Mrochen M. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci. 2013;54(2):1176–1180. doi: 10.1167/iovs.12-11409. [DOI] [PubMed] [Google Scholar]
- 14.Cınar Y, Cingü AK, Turkcu FM, Yüksel H, Sahin A, Yıldırım A, Caca I, Cınar T. Accelerated corneal collagen cross-linking for progressive keratoconus. Cutan Ocul Toxicol. 2014;33(2):168–171. doi: 10.3109/15569527.2013.816724. [DOI] [PubMed] [Google Scholar]
- 15.Kymionis GD, Tsoulnaras KI, Liakopoulos DA, Grentzelos MA, Paraskevopoulos TA, Zacharioudaki ME, Zivkovic M, Kouroupaki AI, Tsilimbaris MK. Corneal stromal demarcation line determined with anterior segment optical coherence tomography following a very high intensity corneal collagen cross-linking protocol. Cornea. 2015;34(6):664–667. doi: 10.1097/ICO.0000000000000427. [DOI] [PubMed] [Google Scholar]