Abstract
Chemotherapeutic drugs such as paclitaxel cause painful peripheral neuropathy in many cancer patients and survivors. Although NMDA receptors (NMDARs) at primary afferent terminals are known to be critically involved in chemotherapy-induced chronic pain, the upstream signaling mechanism that leads to presynaptic NMDAR activation is unclear. Group I metabotropic glutamate receptors (mGluRs) play a role in synaptic plasticity and NMDAR regulation. Here we report that the Group I mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG) significantly increased the frequency of miniature excitatory postsynaptic currents (EPSCs) and the amplitude of monosynaptic EPSCs evoked from the dorsal root. DHPG also reduced the paired-pulse ratio of evoked EPSCs in spinal dorsal horn neurons. These effects were blocked by the selective mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP), but not by an mGluR1 antagonist. MPEP normalized the frequency of miniature EPSCs and the amplitude of evoked EPSCs in paclitaxel-treated rats but had no effect in vehicle-treated rats. Furthermore, mGluR5 protein levels in the dorsal root ganglion and spinal cord synaptosomes were significantly higher in paclitaxel- than in vehicle-treated rats. Inhibiting protein kinase C (PKC) or blocking NMDARs abolished DHPG-induced increases in the miniature EPSC frequency of spinal dorsal horn neurons in vehicle- and paclitaxel-treated rats. Moreover, intrathecal administration of MPEP reversed pain hypersensitivity caused by paclitaxel treatment. Our findings suggest that paclitaxel-induced painful neuropathy is associated with increased presynaptic mGluR5 activity at the spinal cord level, which serves as upstream signaling for PKC-mediated tonic activation of NMDARs. mGluR5 is therefore a promising target for reducing chemotherapy-induced neuropathic pain.
Keywords: glutamate receptor, microtubule, neurophysiology, neuroscience, neurotransmitter, pain, synaptic plasticity, tubulin
Introduction
Painful peripheral neuropathy is a major dose-limiting side effect of some commonly used chemotherapeutic agents, including paclitaxel, bortezomib, oxaliplatin, and vincristine (1, 2). Persistent and severe pain may require dose reduction or cessation of chemotherapy, which can increase cancer-related morbidity and mortality. The pathophysiology of chemotherapy-induced neuropathic pain is not fully known, and treatment options remain limited. Glutamate released from the central terminals of nociceptive primary afferents is critically involved in nociceptive transmission in the spinal dorsal horn (3, 4). Activation of glutamate-gated N-methyl-d-aspartate receptors (NMDARs)2 and the resulting Ca2+ influx through NMDAR channels are crucial for promoting synaptic plasticity in both physiological processes, such as learning and memory, and pathological conditions, such as neuropathic pain (5–7). Treatment with paclitaxel or bortezomib potentiates nociceptive input from primary afferent nerves by inducing tonic activation of presynaptic NMDARs in the spinal cord via protein kinase C (PKC) (8, 9). However, the upstream signaling mechanism that leads to activation of PKC and presynaptic NMDARs in chemotherapy-induced neuropathic pain remains unclear.
Glutamate activates 2 major types of receptors: fast-acting ionotropic glutamate receptors and G protein-coupled metabotropic glutamate receptors (mGluRs). Eight mGluR subtypes have been identified; they can be divided into 3 groups on the basis of their signal transduction mechanisms, pharmacology, and sequence homology (10). In contrast to Group II mGluRs (mGluR2 and mGluR3) and Group III mGluRs (mGluR4, mGluR6, mGluR7, and mGluR8), which are coupled to inhibitory Gαi/o proteins, Group I mGluRs (mGluR1 and mGluR5) preferentially activate phospholipase C via coupling to stimulatory Gαq/11 proteins (11). Activation of Group I mGluRs stimulates the phospholipase Cβ-associated pathway, producing inositol 1,4,5-triphosphate and diacylglycerol. Inositol 1,4,5-triphosphate releases calcium from cellular stores, activating calcium-dependent ion channels; intracellular calcium and diacylglycerol stimulate PKC and its associated downstream signaling pathways (11, 12). In the brain, Group I mGluRs are often activated when synaptic glutamate release increases (11, 13). Chemotherapy-induced tonic activation of presynaptic NMDARs potentiates synaptic glutamate release in the spinal dorsal horn (8, 9). Both Group I mGluRs and NMDARs are closely associated with maintaining long-lasting enhancement of excitatory synaptic transmission (14, 15). Nevertheless, the role of Group I mGluRs and their link to PKC-mediated presynaptic NMDAR activation in chemotherapy-induced neuropathic pain remain unknown.
Therefore, in this study, we sought to determine the role of Group I mGluRs in the regulation of glutamatergic synaptic transmission in the spinal dorsal horn in paclitaxel-induced neuropathic pain. We found that presynaptic mGluR5, a Group I mGluR, acts in concert with PKC and NMDARs to form a signaling cascade that maintains long-lasting enhancement of synaptic glutamate release to spinal dorsal horn neurons in paclitaxel-induced neuropathic pain. This new information extends our understanding of the molecular mechanisms of chemotherapy-induced neuropathic pain and points to new strategies to treat this condition.
Results
Stimulation of mGluR5 increases glutamatergic input to spinal dorsal horn neurons
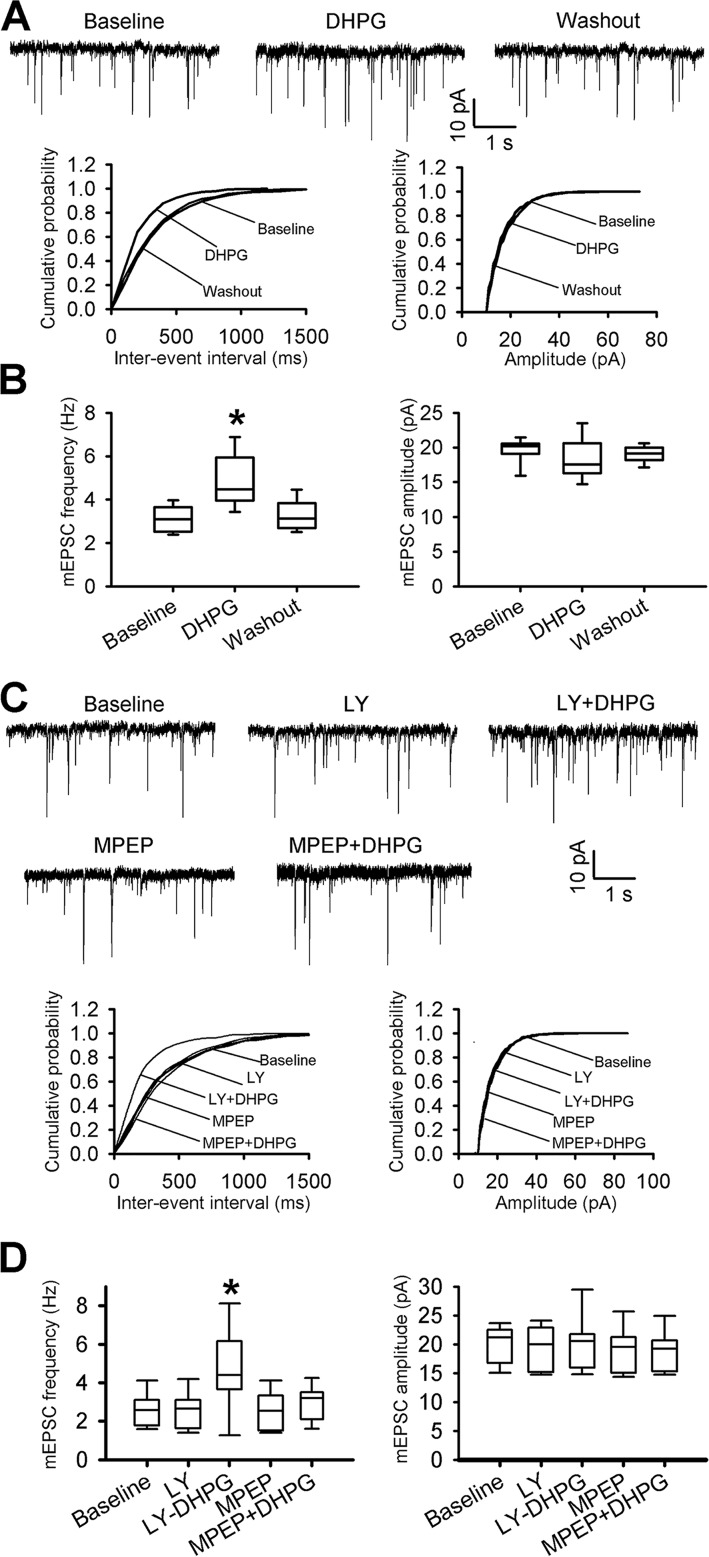
Group I mGluRs are expressed in the dorsal root ganglion (DRG) and spinal dorsal horn (12, 16, 17). We first recorded miniature excitatory postsynaptic currents (mEPSCs) in spinal cord lamina II neurons and determined whether stimulating mGluR1 or mGluR5 affects synaptic glutamate release to these neurons in naive rats. Bath application of (S)-3,5-dihydroxyphenylglycine (DHPG, 20 μm), a combined mGluR1 and mGluR5 agonist (15, 18), significantly increased the baseline frequency, but not the amplitude, of mEPSCs in all lamina II neurons examined (n = 8 neurons, Fig. 1, A and B).
Figure 1.
Stimulation of mGluR5, but not mGluR1, increases synaptic glutamate release to spinal dorsal horn neurons. A, original recording traces and cumulative plots show the effect of bath application of 20 μm DHPG on the frequency and amplitude of mEPSCs of a lamina II neuron from a naive rat. B, box-and-whisker plots show the effect of bath application of DHPG on the frequency and amplitude of mEPSCs in naive rats (n = 8 neurons, 4 rats). C, representative recording traces and cumulative plots show the effect of bath application of MPEP or LY367385 (LY) on the DHPG-induced increase in the frequency of mEPSCs of a lamina II neuron from a naive rat. D, box-and-whisker plots show the baseline and differential effect of MPEP and LY367385 on the DHPG-induced increase in the frequency of mEPSCs in naive rats (n = 10 neurons, 5 rats). *, p < 0.05 compared with the respective baseline control. Box-and-whisker plots show medians, 25th, and 75th percentiles, and ranges.
Because there are no selective agonists for mGluR1 and mGluR5, we used DHPG plus (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385) to activate mGluR5, and DHPG plus 2-methyl-6-(phenylethynyl)-pyridine (MPEP) to activate mGluR1. MPEP is the most potent and specific non-competitive antagonist for mGluR5 (16, 19), whereas LY367385 is a highly selective mGluR1 antagonist (15, 20). Bath application of DHPG (20 μm) plus LY367385 (50 μm) significantly increased the mEPSC frequency of lamina II neurons in naive rats (n = 10 neurons, Fig. 1, C and D). However, application of MPEP (20 μm) plus DHPG had no significant effect on the mEPSC frequency of lamina II neurons (n = 10 neurons, Fig. 1, C and D). These results suggest that mGluR5 activation potentiates glutamatergic excitatory input to spinal dorsal horn neurons.
Stimulation of mGluR5 increases glutamatergic input from primary afferent terminals to spinal dorsal horn neurons
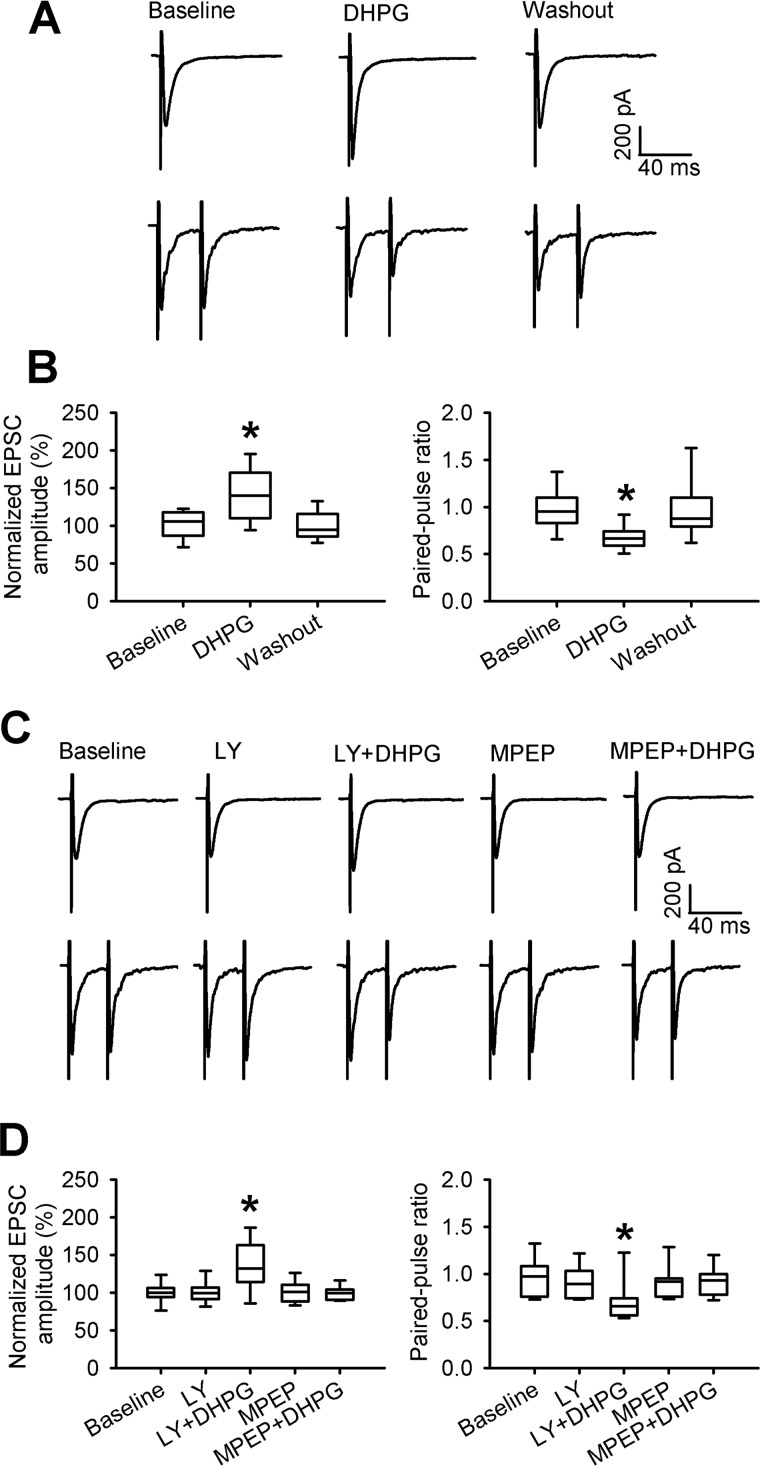
To determine specifically whether mGluR5 at primary afferent terminals is involved in regulating glutamatergic input to spinal cord dorsal horn neurons, we recorded monosynaptic EPSCs of spinal lamina II neurons evoked from the dorsal root, which correspond to induced glutamate released from primary sensory nerves (8, 21). Because the amplitude of evoked EPSCs largely depends on the intensity of the stimulation, we normalized it to the baseline amplitude for each recorded neuron. Bath application of 20 μm DHPG significantly increased the amplitude of monosynaptic EPSCs of lamina II neurons from naive rats (n = 9 neurons, Fig. 2, A and B). To confirm the presynaptic action of DHPG, we also examined the effect of DHPG on the paired-pulse ratio (PPR) of monosynaptically evoked EPSCs in spinal dorsal horn neurons. Bath application of DHPG significantly reduced the PPR of evoked EPSCs in lamina II neurons (n = 10 neurons, Fig. 2, A and B).
Figure 2.
Activation of mGluR5, but not mGluR1, potentiates glutamate release from primary afferent nerve terminals. A, representative recordings show the effect of bath application of 20 μm DHPG on monosynaptic EPSCs of a lamina II neuron evoked from the dorsal root (top) and on EPSCs evoked by a pair of pulses (bottom) in a naive rat. B, box-and-whisker plots show the effect of DHPG on the mean amplitude of evoked EPSCs (n = 9 neurons, 5 rats) and the PPR (n = 10 neurons, 5 rats) of lamina II neurons from naive rats. C, representative recordings show the effect of bath application of MPEP or LY367385 (LY) on DHPG-induced changes in the amplitude of evoked EPSCs of a lamina II neuron (top) and EPSCs evoked by a pair of pulses (bottom) in a naive rat. D, box-and-whisker plots show the effects of MPEP and LY367385 on the DHPG-induced changes in the mean amplitude of evoked EPSCs (n = 10 neurons, 4 rats) and PPR of evoked EPSCs (n = 14 neurons, 6 rats) from naive rats. *, p < 0.05 compared with the respective baseline control. Box-and-whisker plots show medians, 25th and 75th percentiles, and ranges.
In addition, we determined whether selectively stimulating mGluR1 or mGluR5 affects synaptic glutamate release from primary afferent terminals. Bath application of 20 μm DHPG plus 50 μm LY367385 (for activating mGluR5) significantly increased the amplitude of monosynaptic EPSCs of lamina II neurons from naive rats (n = 14 neurons, Fig. 2, C and D). However, bath application of DHPG plus 20 μm MPEP (for activating mGluR1) had no effect on the amplitude of evoked EPSCs of lamina II neurons (n = 10 neurons). Furthermore, we examined the effect of stimulating mGluR1 or mGluR5 on the PPR of monosynaptically evoked EPSCs in spinal dorsal horn neurons from naive rats. Bath application of DHPG plus LY367385 significantly reduced the PPR of evoked EPSCs from baseline levels (n = 14 neurons, Fig. 2, C and D). In contrast, bath application of DHPG plus MPEP had no significant effect on the PPR of evoked EPSCs (n = 14 neurons). Together, these results suggest that presynaptic mGluR5 at primary afferent terminals controls glutamatergic input to spinal dorsal horn neurons.
Presynaptic mGluR5 mediates paclitaxel-induced increases in synaptic glutamate release to spinal dorsal horn neurons
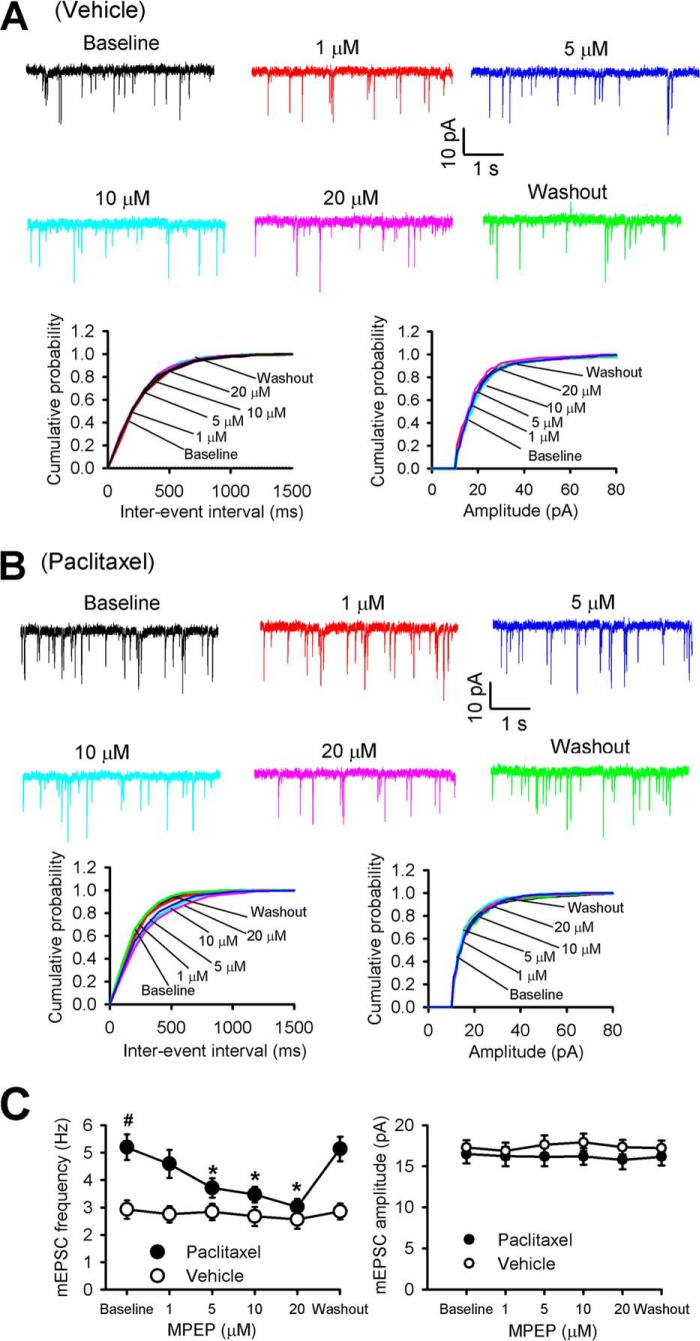
We next determined whether presynaptic mGluR5 plays a role only in the paclitaxel-induced increase in glutamatergic input to spinal dorsal horn neurons. We examined the differential effect of MPEP on the mEPSCs of lamina II neurons from vehicle- and paclitaxel-treated rats. Bath application of 1 to 20 μm MPEP decreased the frequency, but not the amplitude, of mEPSCs in lamina II neurons from paclitaxel-treated rats in a concentration-dependent manner (n = 9 neurons, Fig. 3). At 20 μm, MPEP application completely normalized paclitaxel-induced increases in the frequency of mEPSCs to the level observed in neurons of vehicle-treated rats. In contrast, MPEP had no effect on the frequency or amplitude of mEPSCs in lamina II neurons from vehicle-treated rats (n = 8 neurons, Fig. 3). These data suggest that presynaptic mGluR5 plays a critical role in the paclitaxel-induced increase in synaptic glutamate release to spinal dorsal horn neurons.
Figure 3.
mGluR5 mediates paclitaxel-induced increases in synaptic glutamate release in the spinal cord. A, representative recording traces and cumulative plots show the effect of bath application of 1, 5, 10, and 20 μm MPEP on the frequency and amplitude of mEPSCs of a lamina II neuron from a vehicle-treated rat. B, representative recording traces and cumulative plots show the effect of bath application of 1, 5, 10, and 20 μm MPEP on the frequency and amplitude of mEPSCs of a lamina II neuron from a paclitaxel-treated rat. C, plots of mean data show the concentration-dependent effect of MPEP on the frequency and amplitude of mEPSCs of lamina II neurons from vehicle-treated rats (n = 8 neurons, 4 rats) and paclitaxel-treated rats (n = 9 neurons, 5 rats). Data are expressed as mean ± S.E. *, p < 0.05 compared with the respective baseline control. #, p < 0.05 compared with the baseline in the vehicle-treated group.
mGluR5 mediates paclitaxel-induced increases in glutamatergic transmission from primary afferent nerves to spinal dorsal horn neurons
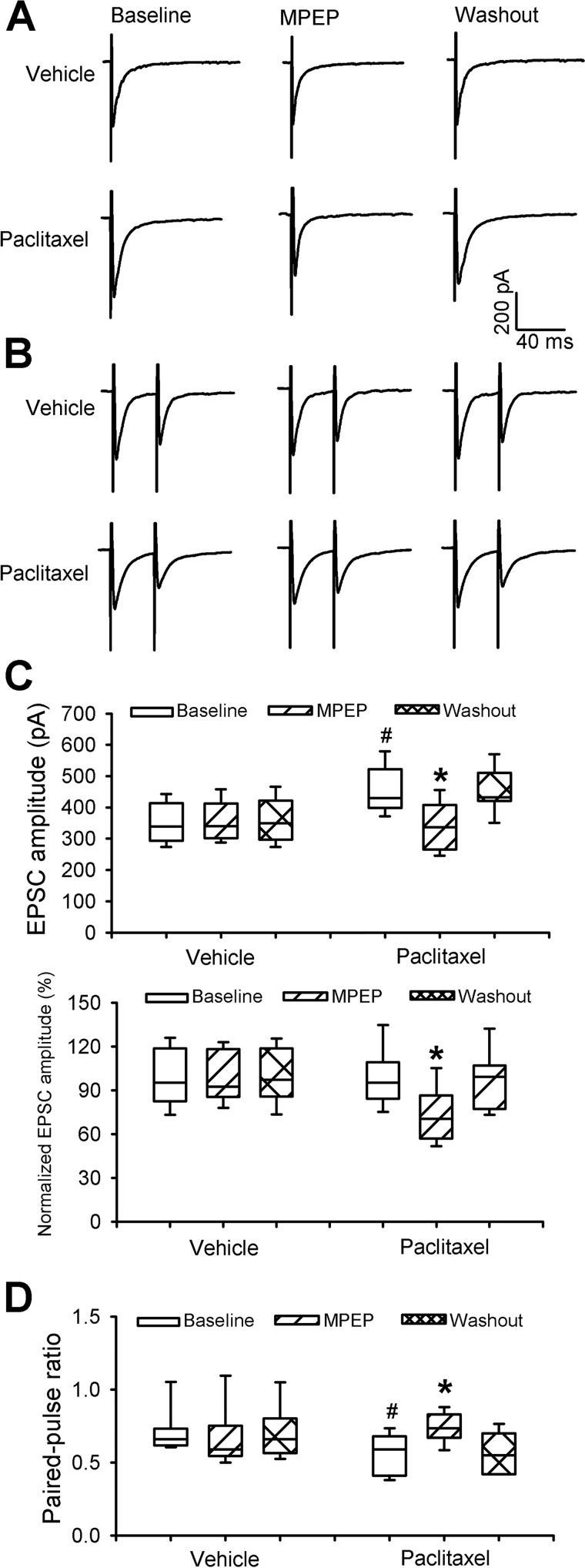
We next determined whether mGluR5 is involved in paclitaxel-induced increases in glutamatergic transmission between primary afferent nerves and spinal dorsal horn neurons. We recorded EPSCs of spinal lamina II neurons monosynaptically evoked from the dorsal root using a constant stimulation intensity. The baseline amplitude of the evoked EPSCs was significantly higher in paclitaxel-treated rats than in vehicle control rats (Fig. 4, A–C). Bath application of 20 μm MPEP normalized the increased amplitude of evoked EPSCs of lamina II neurons from paclitaxel-treated rats (n = 10 neurons, Fig. 4, A–C) but had no effect in neurons from vehicle-treated rats (n = 9 neurons). Furthermore, we examined the effect of MPEP on the PPR of monosynaptically evoked EPSCs in spinal dorsal horn neurons. Bath application of MPEP reversed the reduction in the PPR of evoked EPSCs observed in neurons from paclitaxel-treated rats (n = 10 neurons, Fig. 4, B–D) but had no effect in neurons from vehicle-treated rats (n = 9 neurons). These results suggest that increased presynaptic mGluR5 activity at primary afferent terminals mediates paclitaxel-induced potentiation of glutamatergic synaptic input to spinal dorsal horn neurons.
Figure 4.
mGluR5 mediates paclitaxel-induced increases in glutamate release from primary afferent terminals. A and B, representative recording traces show the effect of bath application of 20 μm MPEP on monosynaptic EPSCs (A) of a lamina II neuron evoked from the dorsal root and on EPSCs evoked by a pair of pulses (B) in a vehicle-treated and a paclitaxel-treated rat. C and D, box-and-whisker plots show the effect of MPEP application on the mean amplitude of evoked EPSCs (C) and the PPR of evoked EPSCs (D) in lamina II neurons from vehicle-treated rats (n = 9 neurons, 5 rats) and paclitaxel-treated rats (n = 10 neurons, 5 rats). *, p < 0.05 compared with the respective baseline control. #, p < 0.05 compared with the baseline in the vehicle-treated group. Box-and-whisker plots show medians, 25th and 75th percentiles, and ranges.
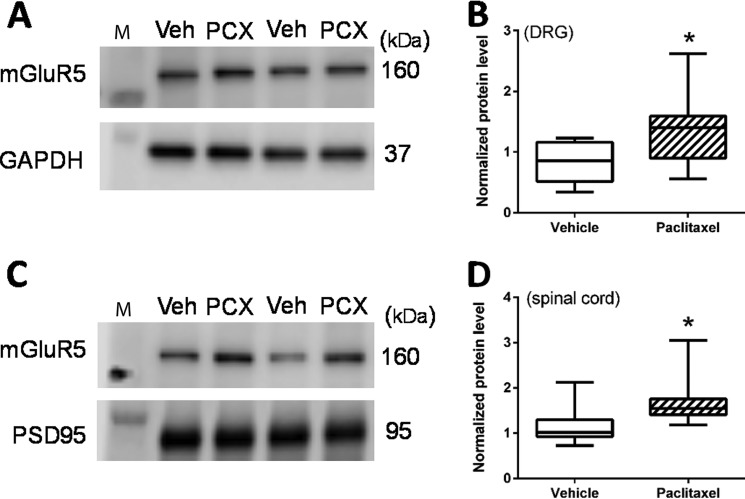
Paclitaxel treatment increases the mGluR5 protein levels in the DRG and dorsal spinal cord synaptosomes
Because mGluR5 is expressed in primary sensory neurons in the DRG (12), we quantified paclitaxel-induced changes in the level of mGluR5 protein in the DRG. Western immunoblotting detected a single band corresponding to the correct molecular mass (∼160 kDa) of mGluR5 protein in DRG tissues (Fig. 5A). The mGluR5 level in the DRG tissue was significantly higher in paclitaxel-treated rats than in vehicle-treated rats (n = 8 rats in each group, Fig. 5, A and B).
Figure 5.
Paclitaxel increases the protein levels of mGluR5 in the DRG and spinal cord synaptosomes. A and B, original gel images (A, two pairs of samples) and quantification (B) of the mGluR5 protein level in DRGs obtained from vehicle (Veh)-treated and paclitaxel (PCX)-treated rats (n = 8 rats per group). C and D, original gel images (C, two pairs of samples) and quantification (D) of the mGluR5 protein level in spinal cord synaptosomes isolated from vehicle-treated and paclitaxel-treated rats (n = 8 rats per group). In A and C, the molecular mass mark (M) is shown on the left of the gel image; the estimated molecular mass (kDa) of detected protein bands is indicated on the right side. GAPDH and PSD95 were used as loading controls for protein normalization on the same gel. *, p < 0.05 compared with the vehicle-treated group.
Because the electrophysiological recording data (Figs. 3 and 4) showed that presynaptic mGluR5 activity in the spinal dorsal horn is increased by paclitaxel treatment, we also measured mGluR5 protein levels in synaptosomes isolated from the dorsal spinal cord. Western immunoblotting showed that mGluR5 protein levels in spinal cord synaptosomes were also significantly higher in paclitaxel- than in vehicle-treated rats (n = 8 rats in each group, Fig. 5, C and D).
mGluR5 activation increases synaptic glutamate release to spinal dorsal horn neurons via PKC
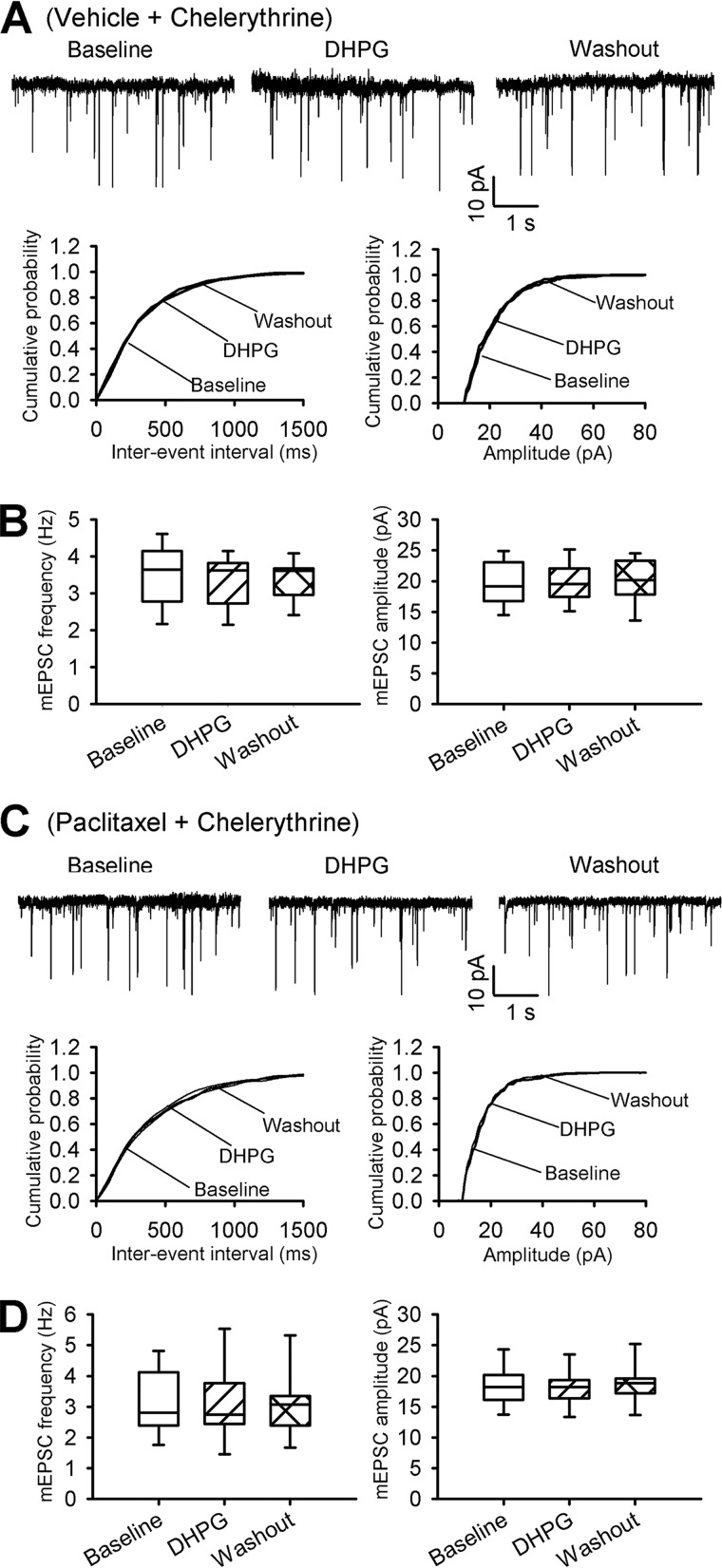
PKC plays a critical role in the paclitaxel-induced increase in synaptic glutamate release in the spinal cord (8). Because mGluR5 activation leads to increased PKC activity (11), we determined whether PKC plays a role in mGluR5-mediated increases in synaptic glutamate release. It has been shown that chelerythrine, a specific membrane-permeable PKC inhibitor, inhibits PKC activity in spinal cord slices (8, 22). In vehicle-treated rats, bath application of 20 μm DHPG had no effect on the baseline frequency or amplitude of mEPSCs in spinal cord slices that had been incubated with 10 μm chelerythrine for 30 to 60 min (n = 8 neurons, Fig. 6, A and B, versus Fig. 1, A and B). In paclitaxel-treated rats, chelerythrine treatment normalized the increased baseline frequency of mEPSCs of lamina II neurons (n = 10 neurons, Fig. 6, C and D, versus Fig. 3, B and C). In these neurons, further bath application of 20 μm DHPG had no effect on the frequency of mEPSCs. These findings suggest that PKC is critically involved in the mGluR5-mediated glutamatergic input to spinal dorsal horn neurons that is potentiated by paclitaxel treatment.
Figure 6.
DHPG increases synaptic glutamate release via PKC in the spinal dorsal horn. A and B, representative recording traces and cumulative plots (A) and box-and-whisker plots (B) show that application of 20 μm DHPG had no effect on the frequency and amplitude of mEPSCs of lamina II neurons from vehicle-treated rats' spinal cord slices incubated with 10 μm chelerythrine (n = 8 neurons, 4 rats). C and D, original recording traces and cumulative plots (C) and box-and-whisker plots (D) show that application of 20 μm DHPG had no effect on the frequency or amplitude of mEPSCs of lamina II neurons from paclitaxel-treated rats' spinal cord slices incubated with chelerythrine (n = 10 neurons, 5 rats). Box-and-whisker plots show medians, 25th, and 75th percentiles, and ranges.
mGluR5 activation increases synaptic glutamate release to spinal dorsal horn neurons via presynaptic NMDARs
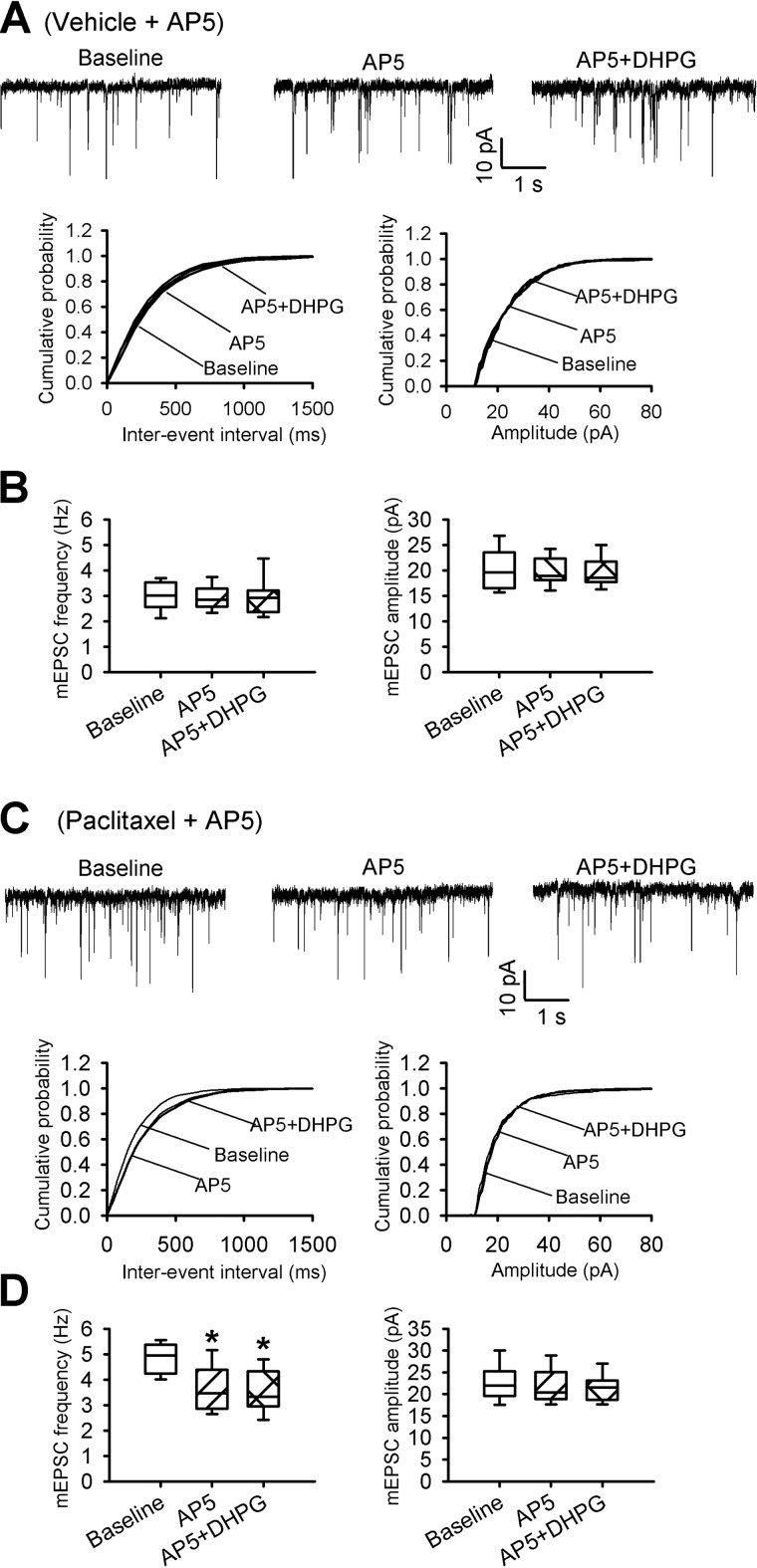
Blocking mGluR5 (Figs. 3 and 4) or NMDARs (8) normalizes paclitaxel-induced increases in synaptic glutamate release to spinal dorsal horn neurons. We next determined whether NMDARs are involved in mGluR5-medated increases in synaptic glutamate release to spinal dorsal horn neurons. In the presence of the specific NMDAR antagonist 2-amino-5-phosphonopentanoic acid (AP5, 50 μm), application of 20 μm DHPG had no significant effect on the frequency or amplitude of mEPSCs recorded from lamina II neurons of vehicle-treated rats (n = 8 neurons; Fig. 7, A and B). In another 8 lamina II neurons recorded from paclitaxel-treated rats, bath application of AP5 normalized the increased mEPSC frequency. In these neurons, subsequent application of DHPG failed to increase the frequency of mEPSCs in the presence of AP5 (n = 8 neurons; Fig. 7, C and D). These results suggest that mGluR5 activation increases the glutamatergic input to spinal dorsal horn neurons that is stimulated by paclitaxel treatment through presynaptic NMDARs.
Figure 7.
DHPG potentiates synaptic glutamate release through NMDARs in the spinal dorsal horn. A and B, representative recording traces and cumulative plots (A) and box-and-whisker plots (B) show no effect from application of 20 μm DHPG on the frequency and amplitude of mEPSCs of lamina II neurons from vehicle-treated rats' spinal cord slices incubated with 50 μm AP5 (n = 8 neurons, 4 rats). C and D, original recording traces and cumulative plots (C) and box-and-whisker plots (D) show no effect from application of 20 μm DHPG on the frequency or amplitude of mEPSCs of lamina II neurons from paclitaxel-treated rats' spinal cord slices incubated with 50 μm AP5 (n = 8 neurons, 4 rats). Box-and-whisker plots show medians, 25th, and 75th percentiles, and ranges.
Blocking mGluR5 at the spinal cord level reverses paclitaxel-induced pain hypersensitivity
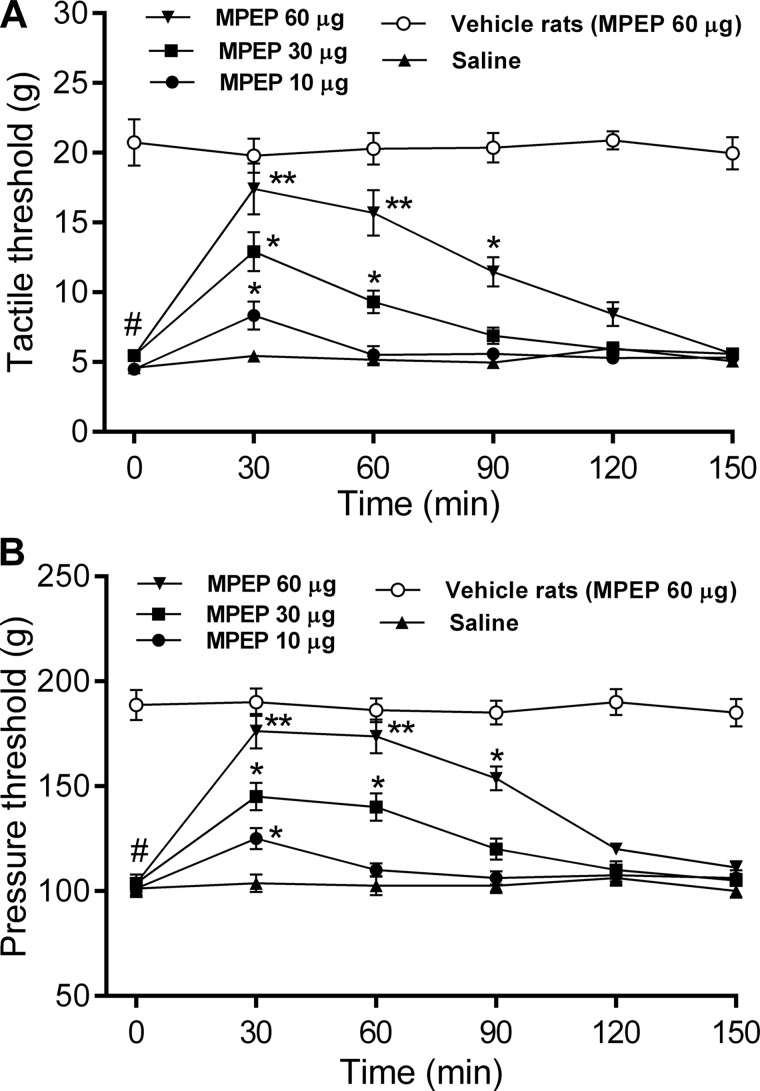
In addition, we determined the functional significance of mGluR5 in the regulation of nociceptive transmission at the spinal cord level in paclitaxel-treated rats. We tested the effect of intrathecal injection of MPEP on the tactile and nociceptive withdrawal thresholds in both vehicle- and paclitaxel-treated rats. Paclitaxel treatment caused a large reduction in the paw withdrawal threshold in response to the tactile and noxious pressure stimuli (Fig. 8). Intrathecal administration of 10–60 μg of MPEP significantly reduced tactile allodynia and mechanical hyperalgesia in paclitaxel-treated rats in a dose-dependent manner (n = 8 rats in each group, Fig. 8). The effect of MPEP reached its maximum about 30 min after intrathecal injection and lasted for about 120 min. By contrast, intrathecal injection of 60 μg of MPEP had no significant effect on the tactile or pressure withdrawal thresholds in vehicle-treated rats (n = 8 rats, Fig. 8). These data indicate that increased mGluR5 activity at the spinal cord level contributes to the pain hypersensitivity induced by paclitaxel treatment.
Figure 8.
Blocking mGluR5 at the spinal cord level reduces paclitaxel-induced pain hypersensitivity. A and B, time course of the effect of intrathecal injection of 10 to 60 μg of MPEP and saline on the paw-withdrawal thresholds measured with von Frey filaments (A) and a noxious pressure stimulus (B) in paclitaxel-treated rats and vehicle-treated rats. Data are expressed as mean ± S.E. (n = 8 rats in each group). *, p < 0.05; **, p < 0.01 compared with the baseline (time 0). #, p < 0.05 compared with the baseline in the vehicle-treated group.
Discussion
In this study, we showed that stimulation of presynaptic mGluR5, but not mGluR1, increases glutamatergic input to spinal dorsal horn neurons. In this regard, DHPG, a selective Group I mGluR (mGluR1 and mGluR5) agonist, profoundly increased the frequency of mEPSCs of spinal dorsal horn neurons. DHPG also significantly increased the amplitude of EPSCs monosynaptically evoked from the primary afferent nerves and reduced the PPR of evoked EPSCs. Furthermore, these effects of DHPG were abolished by blocking mGluR5 with MPEP, but not by blocking mGluR1 with LY367385. Our findings are consistent with those of previous molecular and biochemical studies showing that mGluR5, but not mGluR1, is expressed in DRG neurons and primary afferent nerve terminals in the superficial spinal dorsal horn (12, 17). Our electrophysiological data indicate that stimulation of mGluR5 on primary afferent nerve terminals potentiates synaptic glutamate release to spinal dorsal horn neurons.
A major finding of our study is that paclitaxel treatment induces tonic activation of presynaptic mGluR5, which is in return responsible for the increased glutamatergic input to spinal dorsal horn neurons that promotes neuropathic pain. We demonstrated that MPEP reduced the frequency of mEPSCs of neurons of paclitaxel-treated rats in a dose-dependent manner. MPEP also normalized the amplitude of EPSCs evoked from primary afferent nerves and the PPR of evoked EPSCs, both of which has been altered by paclitaxel treatment. These findings indicate that presynaptic mGluR5 is tonically activated by ambient glutamate in paclitaxel-treated rats. Furthermore, our results suggest that mGluR5 is up-regulated at the synaptic sites of the spinal dorsal horn in paclitaxel-induced neuropathic pain. Because mGluR5 is highly expressed in the DRG (12, 16), it is possible that mGluR5 is synthesized in DRG neurons and transported to the central terminals of primary afferent nerves in the spinal cord. However, we found that blocking mGluR5 activity had no effect on synaptic glutamate release from primary afferent nerves in vehicle-treated rats. This differential effect of mGluR5 blockade in paclitaxel-treated and control rats suggests that presynaptic mGluR5 is silent under normal conditions but is tonically activated at primary afferent nerve terminals after paclitaxel treatment.
It is unclear how paclitaxel treatment increases the mGluR5 protein level at presynaptic terminals in the spinal cord. mGluR5 physically interacts with tubulins (23) for microtubule-dependent active axonal transport. Paclitaxel, by causing microtubule stabilization, may impair axonal transport of mGluR5 in DRG neurons (24) to increase its accumulation in the DRG and at the primary afferent nerve terminals. Alternatively, paclitaxel-induced DRG neuronal damage may lead to augmented mGluR5 protein synthesis and transport to the presynaptic terminals in the spinal dorsal horn. The sources of glutamate for the paclitaxel-induced tonic activation of presynaptic mGluR5 probably include the same primary afferent terminals, excitatory interneurons in the dorsal horn, and/or surrounding synapses and glial cells (4, 25–27).
Data from this and other recent studies indicate that blocking mGluR5, PKC, or NMDARs normalized the increased glutamatergic input from the central terminals of primary afferent nerves caused by paclitaxel (8). However, the direct link between mGluR5, PKC, and NMDARs in the spinal dorsal horn remains elusive. PKC is involved in NMDAR phosphorylation (9) and in mGluR5-mediated potentiation of NMDAR currents in the brain (15, 28). In the present study, PKC inhibition abolished DHPG-induced increases in the mEPSC frequency of dorsal horn neurons from vehicle- and paclitaxel-treated rats. We also found that blocking NMDARs abolished the stimulatory effect of DHPG on the mEPSC frequency in spinal cord neurons from both vehicle- and paclitaxel-treated rats. Thus, presynaptic mGluR5 probably serves as an upstream signal for PKC and NMDAR activation, increasing glutamatergic input to spinal dorsal horn neurons and leading to chemotherapy-related neuropathic pain.
Our study provides new in vivo evidence about the causal relationship between augmented mGluR5 activity at the spinal cord level and pain hypersensitivity induced by paclitaxel. We showed that blocking mGluR5 at the spinal cord level profoundly attenuated tactile allodynia and mechanical hyperalgesia induced by paclitaxel. However, mGluR5 blockade had no effect on normal nociception in control rats. A similar differential effect of mGluR5 antagonists has been shown in other chronic pain models (16, 29). Our findings have important therapeutic implications, as blocking mGluR5 at the spinal cord level could conceivably be an effective strategy for treating chemotherapy-induced painful neuropathy.
In summary, our findings indicate that paclitaxel treatment causes up-regulation and tonic activation of presynaptic mGluR5, which in turn augments augmented glutamatergic input to spinal dorsal horn neurons via PKC and NMDARs. Together, the mGluR5—PKC—NMDAR–glutamate release may form a signaling cascade and a positive feedback loop leading to sustained nociceptive input and pain hypersensitivity after paclitaxel treatment. Because mGluR5 is preferentially involved in augmenting spinal nociceptive transmission in paclitaxel-induced chronic pain but not in physiological pain conditions, mGluR5 could be a desirable target for development of effective therapies specifically for chemotherapy-induced neuropathic pain.
Experimental procedures
Animal model and paclitaxel treatment
Experiments were carried out using adult male Sprague-Dawley rats (220–250 g; Harlan, Indianapolis, IN). A total of 78 rats was used for the entire study. All procedures and protocols were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. To induce peripheral neuropathy, we injected rats intraperitoneally with 2 mg/kg of paclitaxel (TEVA Pharmaceuticals, North Wales, PA) on 4 alternate days, as described previously (8, 30). Rats in the control group received intraperitoneal injection of the vehicle (Cremophor EL/ethanol, 1:1) on the same 4 alternate days. We confirmed the presence of mechanical hyperalgesia and tactile allodynia in the hindpaws of the rats 10 to 12 days after the completion of paclitaxel treatment. All terminal experiments were conducted 2 to 3 weeks after the last paclitaxel or vehicle injection.
Nociceptive behavioral tests
To determine tactile sensitivity, we placed rats individually on a mesh floor within suspended chambers and allowed them to acclimate for at least 30 min. We applied a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) perpendicularly to the plantar surface of both hindpaws with sufficient force to bend the filament for 6 s. Brisk withdrawal or flinching of the paw was considered a positive response. In the absence of a response, we applied the filament of the next greater force. After a response, we applied the filament of the next lower force. The tactile stimulus with a 50% likelihood of producing a withdrawal response was calculated using the “up-down” method (31).
To measure the mechanical nociceptive threshold, we conducted the paw pressure (Randall-Selitto) test on the left hindpaw using an analgesiometer (Ugo Basile, Varese, Italy). To activate the device, we pressed a foot pedal to activate a motor that applied a constantly increasing force on a linear scale. When the animal displayed pain by either withdrawing the paw or vocalizing, the pedal was immediately released, and the animal's nociceptive threshold was recorded (32, 33). Each trial was repeated 2 or 3 times at ∼2-min intervals, and the mean value was used as the force to produce a withdrawal response.
Spinal cord slice preparation
Rats were anesthetized with 2 to 3% isoflurane, and the lumbar spinal cord at the L3–L6 level was removed through laminectomy. The spinal cord tissues were placed in an ice-cold sucrose artificial cerebrospinal fluid presaturated with 95% O2 and 5% CO2. The artificial cerebrospinal fluid contained (in mm) 234 sucrose, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 12.0 glucose, and 25.0 NaHCO3. A vibratome was used to slice the spinal cord tissue transversely into 400-μm sections. The slices were preincubated in Krebs solution oxygenated with 95% O2 and 5% CO2 at 34 °C for at least 1 h before being transferred to the recording chamber. The Krebs solution contained (in mm) 117.0 NaCl, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 11.0 glucose, and 25.0 NaHCO3.
Electrophysiological recordings in spinal cord slices
The spinal cord slice was placed in a glass-bottomed chamber and continuously perfused with Krebs solution at 5.0 ml/min at 34 °C. Lamina II outer zone neurons were visualized on an upright fixed-stage microscope with differential interference contrast/infrared illumination and selected for recordings. Most (>85%) lamina II neurons are glutamate-releasing excitatory interneurons (34) that predominantly receive nociceptive input from primary afferent nerves (3). EPSCs were recorded using whole-cell voltage-clamp techniques (3, 35). The impedance of the glass recording electrode was 4–7 MΩ when it was filled with the internal solution containing (in mm) 135 potassium gluconate, 5 KCl, 2.0 MgCl2, 0.5 CaCl2, 5.0 HEPES, 5.0 EGTA, 5.0 ATP-Mg, 0.5 Na-GTP, 1.0 GDPβS, and 10 QX314 (adjusted to pH 7.25 with 1.0 m KOH, 280–300 mosm).
EPSCs were evoked from the dorsal root using a bipolar tungsten electrode connected to a stimulator (0.2 ms, 0.5 mA, 0.1 Hz) (27, 35). Monosynaptic EPSCs were identified on the basis of the constant latency and absence of conduction failure of evoked EPSCs in response to a 20-Hz electrical stimulation, as we described previously (27, 35). To measure the PPR, 2 EPSCs were evoked by a pair of stimuli administered at 50-ms intervals. The PPR was expressed as the ratio of the amplitude of the second synaptic response to the amplitude of the first synaptic response (8, 27). In addition, miniature EPSCs (mEPSCs) were recorded in the presence of 2 μm strychnine, 10 μm bicuculline, and 1 μm tetrodotoxin at a holding potential of −60 mV (32, 36). The input resistance was monitored, and the recording was abandoned if it changed by more than 15%. Synaptic currents were recorded using an amplifier (MultiClamp 700A, Axon Instruments, Foster City, CA), filtered at 1–2 kHz, and digitized at 10 kHz. Only 1 neuron was recorded in each spinal cord slice, and at least 3 rats were used in each recording protocol.
All drugs were freshly prepared in artificial cerebrospinal fluid before the experiments and delivered via syringe pumps to reach their final concentrations. AP5 and (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385) were purchased from HelloBio (Princeton, NJ). DHPG and MPEP were purchased from Abcam (Cambridge, MA).
Western immunoblotting
Two weeks after the end of treatment with paclitaxel or vehicle, the rats were anesthetized with 3% isoflurane, and the lumbar DRGs and dorsal spinal cord tissues were rapidly removed. The DRG tissues were homogenized in ice-cold radioimmunoprecipitation assay buffer containing mixtures of the protease and phosphatase inhibitors. The DRG homogenate was centrifuged at 12,000 × g for 10 min at 4 °C, and the supernatant was collected. The spinal cord tissues were homogenized in 10 volumes of ice-cold HEPES-buffered sucrose (0.32 m sucrose, 1 m EGTA, and 4 mm HEPES, pH 7.4) containing the protease inhibitor mixture (Sigma). The homogenate was centrifuged at 1,000 × g for 10 min at 4 °C to remove the nuclei and large debris. The supernatant was centrifuged at 10,000 × g for 15 min to obtain the crude synaptosomal fraction. The synaptosomal pellet was lysed via hypo-osmotic shock in 9 volumes of ice-cold HEPES-buffer with the protease inhibitor mixture for 30 min. The lysate was centrifuged at 25,000 × g for 20 min at 4 °C to obtain the synaptosomal membrane fraction.
Samples were subjected to 12% SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The blots were probed with rabbit polyclonal anti-mGluR5 antibody (catalog number AGC-007, Alomone Laboratories, Jerusalem, Israel). We have shown that this mGluR5 antibody detects a single protein band that matches the molecular mass (∼160 kDa) of mGluR5 (16). An ECL kit (GE Healthcare) was used to detect the protein band, which was visualized and quantified with an Odyssey Fc Imager (LI-COR Biosciences, Lincoln, NE). We used PSD95 (catalog number 75-348, NeuroMab, Davis, CA) as the protein loading control for spinal cord synaptosomes, and GAPDH (catalog number ab9485, Abcam) as the loading control for the DRG tissue. The amount of mGluR5 protein in the DRG and spinal cord synaptosomes was normalized to the amount of GAPDH and PSD95, respectively, in the same blots. The mean value of mGluR5 protein in vehicle-treated rats was considered to be 1.
Data analysis
The amplitude of the evoked EPSCs was quantified by averaging 10 consecutive EPSCs off-line with Clampfit 10.0 software (Axon Instruments). The mEPSCs were analyzed using the MiniAnalysis program (Synaptosoft, Leonia, NJ), and the cumulative probability of the amplitude and the inter-event interval of the mEPSCs were compared using the Kolmogorov-Smirnov test. The group data were presented in box-and-whisker plots, which show the minimum, 25th percentile, median, 75th percentile, and maximum values. We used a Student's t test to compare the mGluR5 protein levels in the 2 groups. We used one-way analysis of variance followed by Tukey's post hoc test to compare values in more than 2 groups. To determine the effects of treatment on hyperalgesia and allodynia, we used repeated measures analysis of variance followed by Dunnett's post hoc test. p values < 0.05 were considered to be statistically significant.
Author contributions
J. D. X. and S. R. C. conducted experiments. J. D. X., S. R. C., and H. L. P. performed data analysis. H. L. P. conceived the project and wrote the manuscript with input from the other authors.
This work was supported by National Institutes of Health Grants GM120844 and NS101880 and a grant from the N. G. and Helen T. Hawkins Endowment (to H.-L. P.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- NMDAR
- N-methyl-d-aspartate receptor
- AP5
- 2-amino-5-phosphonopentanoic acid
- DHPG
- (S)-3,5-dihydroxyphenylglycine
- MPEP
- 2-methyl-6-(phenylethynyl)-pyridine
- DRG
- dorsal root ganglion
- EPSC
- excitatory postsynaptic current
- mEPSC
- miniature excitatory postsynaptic current
- mGluR
- metabotropic glutamate receptor
- LY367385
- (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid
- PPR
- paired-pulse ratio
- GDPβS
- guanyl-5′-yl thiophosphate.
References
- 1. Sisignano M., Baron R., Scholich K., and Geisslinger G. (2014) Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat. Rev. Neurol. 10, 694–707 [DOI] [PubMed] [Google Scholar]
- 2. Grisold W., Cavaletti G., and Windebank A. J. (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro. Oncol. 14, iv45–iv54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan Y. Z., and Pan H. L. (2004) Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J. Neurophysiol. 91, 2413–2421 [DOI] [PubMed] [Google Scholar]
- 4. Yoshimura M., and Jessell T. (1990) Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J. Physiol. 430, 315–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Traynelis S. F., Wollmuth L. P., McBain C. J., Menniti F. S., Vance K. M., Ogden K. K., Hansen K. B., Yuan H., Myers S. J., and Dingledine R. (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen S. R., Zhou H. Y., Byun H. S., Chen H., and Pan H. L. (2014) Casein kinase II regulates N-methyl-d-aspartate receptor activity in spinal cords and pain hypersensitivity induced by nerve injury. J. Pharmacol. Exp. Ther. 350, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou H. Y., Chen S. R., Byun H. S., Chen H., Li L., Han H. D., Lopez-Berestein G., Sood A. K., and Pan H. L. (2012) N-methyl-d-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J. Biol. Chem. 287, 33853–33864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie J. D., Chen S. R., Chen H., Zeng W. A., and Pan H. L. (2016) Presynaptic N-methyl-d-aspartate (NMDA) receptor activity is increased through protein kinase C in paclitaxel-induced neuropathic pain. J. Biol. Chem. 291, 19364–19373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xie J. D., Chen S. R., Chen H., and Pan H. L. (2017) Bortezomib induces neuropathic pain through protein kinase C-mediated activation of presynaptic NMDA receptors in the spinal cord. Neuropharmacology 123, 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Niswender C. M., and Conn P. J. (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conn P. J., and Pin J. P. (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237 [DOI] [PubMed] [Google Scholar]
- 12. Crawford J. H., Wainwright A., Heavens R., Pollock J., Martin D. J., Scott R. H., and Seabrook G. R. (2000) Mobilisation of intracellular Ca2+ by mGluR5 metabotropic glutamate receptor activation in neonatal rat cultured dorsal root ganglia neurones. Neuropharmacology 39, 621–630 [DOI] [PubMed] [Google Scholar]
- 13. Pin J. P., and Duvoisin R. (1995) The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34, 1–26 [DOI] [PubMed] [Google Scholar]
- 14. Kotecha S. A., Jackson M. F., Al-Mahrouki A., Roder J. C., Orser B. A., and MacDonald J. F. (2003) Co-stimulation of mGluR5 and N-methyl-d-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J. Biol. Chem. 278, 27742–27749 [DOI] [PubMed] [Google Scholar]
- 15. Li D. P., Zhu L. H., Pachuau J., Lee H. A., and Pan H. L. (2014) mGluR5 Upregulation increases excitability of hypothalamic presympathetic neurons through NMDA receptor trafficking in spontaneously hypertensive rats. J. Neurosci. 34, 4309–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J. Q., Chen S. R., Chen H., Cai Y. Q., and Pan H. L. (2010) Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J. Neurochem. 112, 162–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valerio A., Rizzonelli P., Paterlini M., Moretto G., Knöpfel T., Kuhn R., Memo M., and Spano P. (1997) mGluR5 metabotropic glutamate receptor distribution in rat and human spinal cord: a developmental study. Neurosci. Res. 28, 49–57 [DOI] [PubMed] [Google Scholar]
- 18. Schoepp D. D., Goldsworthy J., Johnson B. G., Salhoff C. R., and Baker S. R. (1994) 3,5-dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. J. Neurochem. 63, 769–772 [DOI] [PubMed] [Google Scholar]
- 19. Gasparini F., Lingenhöhl K., Stoehr N., Flor P. J., Heinrich M., Vranesic I., Biollaz M., Allgeier H., Heckendorn R., Urwyler S., Varney M. A., Johnson E. C., Hess S. D., Rao S. P., Sacaan A. I., Santori E. M., Veliçelebi G., and Kuhn R. (1999) 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38, 1493–1503 [DOI] [PubMed] [Google Scholar]
- 20. Werner C. G., Scartabelli T., Pancani T., Landucci E., Moroni F., and Pellegrini-Giampietro D. E. (2007) Differential role of mGlu1 and mGlu5 receptors in rat hippocampal slice models of ischemic tolerance. Eur. J. Neurosci. 25, 3597–3604 [DOI] [PubMed] [Google Scholar]
- 21. Li L., Chen S. R., Chen H., Wen L., Hittelman W. N., Xie J. D., and Pan H. L. (2016) Chloride homeostasis critically regulates synaptic NMDA receptor activity in neuropathic pain. Cell Rep. 15, 1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao Y. L., Chen S. R., Chen H., and Pan H. L. (2012) Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-d-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J. Biol. Chem. 287, 25073–25085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serge A., Fourgeaud L., Hemar A., and Choquet D. (2003) Active surface transport of metabotropic glutamate receptors through binding to microtubules and actin flow. J. Cell Sci. 116, 5015–5022 [DOI] [PubMed] [Google Scholar]
- 24. Theiss C., and Meller K. (2000) Taxol impairs anterograde axonal transport of microinjected horseradish peroxidase in dorsal root ganglia neurons in vitro. Cell Tissue Res. 299, 213–224 [DOI] [PubMed] [Google Scholar]
- 25. Angulo M. C., Kozlov A. S., Charpak S., and Audinat E. (2004) Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 24, 6920–6927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou H. Y., Zhang H. M., Chen S. R., and Pan H. L. (2007) Increased nociceptive input rapidly modulates spinal GABAergic transmission through endogenously released glutamate. J. Neurophysiol. 97, 871–882 [DOI] [PubMed] [Google Scholar]
- 27. Zhou H. Y., Chen S. R., Chen H., and Pan H. L. (2010) Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J. Neurosci. 30, 4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang H., and van den Pol A. N. (2007) Rapid direct excitation and long-lasting enhancement of NMDA response by group I metabotropic glutamate receptor activation of hypothalamic melanin-concentrating hormone neurons. J. Neurosci. 27, 11560–11572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inquimbert P., Bartels K., Babaniyi O. B., Barrett L. B., Tegeder I., and Scholz J. (2012) Peripheral nerve injury produces a sustained shift in the balance between glutamate release and uptake in the dorsal horn of the spinal cord. Pain 153, 2422–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polomano R. C., Mannes A. J., Clark U. S., and Bennett G. J. (2001) A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 94, 293–304 [DOI] [PubMed] [Google Scholar]
- 31. Chaplan S. R., Bach F. W., Pogrel J. W., Chung J. M., and Yaksh T. L. (1994) Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 [DOI] [PubMed] [Google Scholar]
- 32. Chen S. R., Hu Y. M., Chen H., and Pan H. L. (2014) Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. J. Physiol. 592, 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen S. R., Chen H., Yuan W. X., and Pan H. L. (2011) Increased presynaptic and postsynaptic alpha2-adrenoceptor activity in the spinal dorsal horn in painful diabetic neuropathy. J. Pharmacol. Exp. Ther. 337, 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santos S. F., Rebelo S., Derkach V. A., and Safronov B. V. (2007) Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J. Physiol. 581, 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li D. P., Chen S. R., Pan Y. Z., Levey A. I., and Pan H. L. (2002) Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J. Physiol. 543, 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen S. R., Zhu L., Chen H., Wen L., Laumet G., and Pan H. L. (2014) Increased spinal cord Na+-K+-2Cl− cotransporter-1 (NKCC1) activity contributes to impairment of synaptic inhibition in paclitaxel-induced neuropathic pain. J. Biol. Chem. 289, 31111–31120 [DOI] [PMC free article] [PubMed] [Google Scholar]