Abstract
An endoplasmic reticulum (ER) retention sequence (ERS) is a characteristic short sequence that mediates protein retention in the ER of eukaryotic cells. However, little is known about the detailed molecular mechanism involved in ERS-mediated protein ER retention. Using a new surface display–based fluorescence technique that effectively quantifies ERS-promoted protein ER retention within Saccharomyces cerevisiae cells, we performed comprehensive ERS analyses. We found that the length, type of amino acid residue, and additional residues at positions −5 and −6 of the C-terminal HDEL motif all determined the retention of ERS in the yeast ER. Moreover, the biochemical results guided by structure simulation revealed that aromatic residues (Phe-54, Trp-56, and other aromatic residues facing the ER lumen) in both the ERS (at positions −6 and −4) and its receptor, Erd2, jointly determined their interaction with each other. Our studies also revealed that this aromatic residue interaction might lead to the discriminative recognition of HDEL or KDEL as ERS in yeast or human cells, respectively. Our findings expand the understanding of ERS-mediated residence of proteins in the ER and may guide future research into protein folding, modification, and translocation affected by ER retention.
Keywords: endoplasmic reticulum (ER), flow cytometry, fluorescence, protein sorting, protein trafficking (Golgi), yeast, ER retention sequence, Erd2, protein ER retention, surface display
Introduction
In eukaryotic cells, the endoplasmic reticulum (ER)3 and the Golgi are two important cell organelles mainly responsible for the folding, post-translational modification, and translocation of newly synthesized polypeptides (1). Studies have suggested that the incorrectly folded protein could subsequently cause diseases such as Parkinson's and Alzheimer's, for example (2).
To facilitate the functions of the ER and Golgi, many proteins reside in these two apparatuses, including protein disulfide isomerase, Kar2, DnaJ-related protein Scj1, etc. One interesting finding is that these ER-resident proteins share a conserved C-terminal short peptide sequence, which is reported as HDEL or KDEL in Saccharomyces cerevisiae or human, respectively (3, 4). It is speculated that protein containing a C-terminal HDEL sequence, such as Kar2, could retain in yeast ER through interaction with the yeast ER lumen protein-retaining receptors, Erd1 or Erd2 (5, 6). Similar studies reported in human cells indicate that protein possessing a C-terminal KDEL sequence interacts with human ER protein retention receptor KDELR1, KDELR2, or KDELR3 (7, 8). As the only identified receptors in cells, recent studies on Erd2 and KDELR1 indicated that deletion of ERD2 caused defective protein traffic through the Golgi (6), and KDELR1 malfunction led to T-cell homeostasis (9), cell intrinsic lymphopenia and apoptosis (10, 11).
The understanding of how ERS interacting with its receptors to promote ER retention remains in the preliminary stage, mainly due to the lack of an effective method and resolved protein structures of Erd and KDELR. Previous studies in yeast show that amino acids 51–57 in Kluyveromyces lactis Erd2 determine its interaction specificity against different ERS (12). Similar results were obtained in the human receptors, in which Asp-50 was demonstrated to be required for the efficient binding of KDEL ligands (13). This residue has further been identified as important for the function of Erd2 in S. cerevisiae (6). Moreover, studies also show that the length of ERS is 4 amino acids in both human and yeast proteins. Nevertheless, the biochemical characterization of yeast Kar2 protein suggests that FEHDEL is a stronger ERS than other HDEL-containing sequences (14). In addition, analyses of the KDEL derivatives of human ER-localized proteins also indicate that extra amino acids at positions −5 and −6 of the ERS may be involved in determining the ER localization of the protein (15).
Besides its physiological significance, the yeast ER retention effect recently came to attention by being utilized to establish pioneering high-throughput screening methods for protease engineering, including the surface display–based yeast ER sequestration system (YESS) (16) and FRET-based protease evolution via cleavage of an intracellular substrate (PrECISE) (17). In the YESS approach, FEHDEL, the original C-terminal retention sequence of Kar2, exhibited a stronger ability than HDEL to retain the heterologous protease and its substrates in the yeast ER, thus providing a broader dynamic range for protease engineering. Therefore, further characterization of the HDEL-type ERS and investigation of the mechanism of ERS interaction with ER receptors will advance the understanding of ERS-promoted protein ER retention as well as ER function in cells.
In the present studies, a new strategy for integrating the yeast surface display, flow cytometry, and protein ER retention was developed, which provided an efficient way to specifically characterize different ERS and their interactions with ER receptors in yeast ER. Combining this strategy with the I-TASSER (iterative threading assembly refinement) program guided structure simulation (18), a mechanism for aromatic residue-controlled protein ER residence in S. cerevisiae was proposed.
Results
Analyzing the ERS-promoted ER retention effect by a modified YESS approach
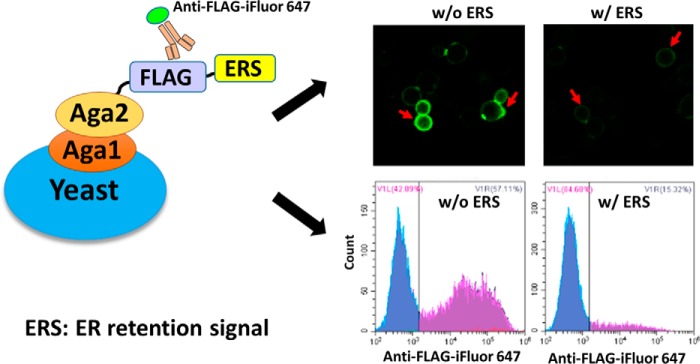
Comparing the methods of gene knockout and down-regulation (5, 6), a new strategy evolved from the YESS approach (16) was developed here so that the ER retention strength of different ERS could be evaluated quickly (Fig. 1). Using the YESS approach, we noticed that fusing ERS at the C terminus of Aga2 would not disrupt but would obviously slow down the Aga1-Aga2–mediated surface display (SD) process, thus providing a way to evaluate the ER retention strength of different ERS. Based on this strategy, different ERS were anchored at the C terminus of Aga2, with a FLAG epitope tag inserted between Aga2 and ERS to form the Aga2–FLAG–ERS cassette. The FLAG epitope tag was then recognized by the iFluor 647–conjugated anti-FLAG antibodies once the Aga2–FLAG–ERS cassette was displayed on the yeast cell surface. Through this method, the strength of ERS-mediated protein ER retention in yeast cells was converted to the fluorescent signals on the cell surface, which could be quantitated quickly by flow cytometry and fluorescence microscopy (Fig. 1). In principle, a strong ERS will cause enhanced retention of the Aga2–FLAG–ERS cassette in yeast ER, resulting in a decreased cell surface presence and thus exhibiting low fluorescent intensity after antibody labeling.
Figure 1.
Scheme of the strategy for evaluating the ERS-promoted ER retention effect in yeast. Cells bearing Aga2–FLAG–ERS cassettes were labeled with iFluor 647–conjugated anti-FLAG antibody followed by flow cytometry and fluorescence microscopy analysis. The red arrows (top right) point toward the yeast cells with surface fluorescence. w/o, without ERS; w/, with ERS.
Identifying the key residues in HDEL-type ERS
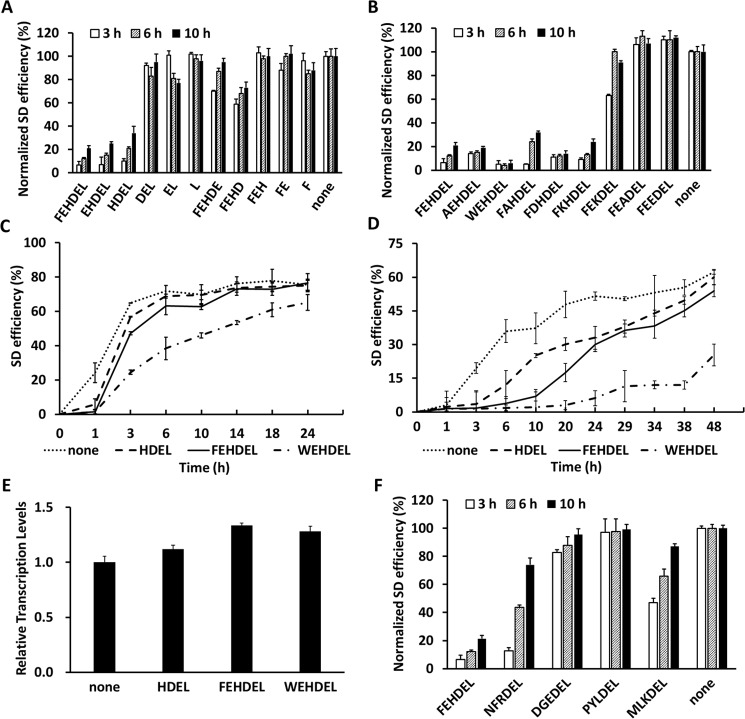
To understand HDEL-type ERS-mediated protein ER retention in yeast cells, we further characterized FEHDEL, the strongest ERS identified so far, which is also the C-terminal ERS of Kar2 that was studied previously and applied in the YESS system. First, FEHDEL derivative sequences shortened from the C or N terminus, respectively, including FEHDEL, FEHDE, FEHD, FEH, FE, F, EHDEL, HDEL, DEL, EL, and L, were investigated at 20 °C for their length effect on ER retention strength (pESD-2–1–pESD-2–11 in supplemental Table S1, Fig. 2A, and supplemental Table S2). Normalized to the control of the Aga2–FLAG cassette containing no ERS (pESD-1 in supplemental Table S1), only HDEL, EHDEL, and FEHDEL exhibited obvious ER retention ability, with FEHDEL being the strongest and showing the lowest normalized SD efficiencies of 6.5, 12.1, and 21.3% after induction for 3, 6, and 10 h, respectively. Comparably, EHDEL and HDEL exhibited higher SD efficiencies of 7.2 and 10.1% at 3 h, 15.4 and 21.4% at 6 h, and 24.8 and 33.9% at 10 h, respectively. Other than these ERS, no other FEHDEL derivatives presented clear ER retention ability, indicating that the C-terminal HDEL sequence was critical for the ER retention effect and that additional amino acids at the N terminus of HDEL might affect its ER retention ability.
Figure 2.
Characterization of the ER retention strength of different FEHDEL derivatives. The SD efficiencies of the Aga2–FLAG–ERS cassettes containing different FEHDEL derivatives were quantitated using FACS analysis after induction at 20 °C (A and B) or in a time course of 24–48 h after induction at 30 °C (C) or 20 °C (D). E, the in-cell mRNA levels of the Aag2–FLAG cassettes without ERS or with C-terminal ERS of HDEL, FEHDEL, and WEHDEL were quantitated by RT-PCR after induction for 6 h at 30 °C. F, the normalized SD efficiency of the sequences of NFRDEL, DGEDEL, PYLDEL, and MLKDEL was evaluated after induction at 20 °C. The induced cells were surface-labeled with iFluor 647–conjugated anti-FLAG antibodies. Data are presented as mean ± S.E. (n = 3 independent experiments). In A, B, and F, the data were normalized with cells bearing the Aga2–FLAG cassette. p ≤ 0.05 (Student's t test).
Following these findings, site mutagenesis against the residues at positions −4, −5, and −6 of FEHDEL was carried out to further investigate the roles of certain residues in the HDEL-type ERS (pESD-2–12 to pESD-2–19 in supplemental Table S1, Fig. 2B, and supplemental Table S3). It is worth noting that these three residues all have interesting biochemical properties, including the aromatic property and positive charge of His at position −4, the negative charge of Glu at position −5, and the strong aromatic property of Phe at position −6. In this study, His at position −4 was first mutated to Ala, Glu, and Lys, representing amino acids with simple, negatively charged, and positively charged side chains, respectively. Normalized to the control of the Aga2–FLAG cassette, the results showed that the ER retention effect was abolished in FEADEL and FEEDEL, and impaired in FEKDEL, with SD efficiencies ranging from 62.7 to 99.6% after induction at 20 °C. (The underlined letters in the ERS mutants represent amino acids with simple, aromatic, negatively charged, and positively charged side chains.) Interestingly, the decreased ER retention strength of FEKDEL suggested the involvement of aromatic property of His, as the positively charged Lys could only partially sustain its retention ability. Next, Glu at position −5 was mutated to Ala, Asp, or Lys to change this negatively charged residue to another small and neutral, negatively charged, or positively charged residue. Among these three mutants, FAHDEL presented a clearly decreased ER retention effect with surface display efficiencies of 5.3, 24.1, and 32.3% after induction for 3, 6, and 10 h, respectively, at 20 °C, indicating a preference for a charged or larger residue at position −5. Finally, Phe at position −6 was mutated to Ala or Trp, which either abolished or enhanced the aromatic property at this position. Our results showed that WEHDEL exhibited a more than 2-fold stronger ER retention strength than FEHDEL, with the SD efficiency largely decreasing to 4.6, 4.4, and 6.3% after induction for 3, 6, and 10 h, respectively, at 20 °C. These results combined suggest that besides His at position −4, an aromatic residue at position −6 could enhance the strength of HDEL-type ERS.
Changing Phe to Trp generated WEHDEL, a much stronger ERS. The detailed ER retention abilities were further evaluated in time-dependent experiments at both 30 °C (Fig. 2C and supplemental Table S4) and 20 °C (Fig. 2D, supplemental Table S5). At 30 °C, the Aga2–FLAG control cassette reached its maximum SD efficiency of 71.9% after a 6-h induction, whereas it took 10, 14, and 24 h for HDEL, FEHDEL, and WEHDEL to reach their own maximum of 69.6, 73.2 and 65.2%, respectively. Similar results were obtained at 20 °C, further demonstrating the strongest ER retention ability of WEHDEL, especially when the maximum SD efficiencies of WEHDEL were only 86 and 41% of the Aga2–FLAG control after a 24- and 48-h induction at 30 and 20 °C, respectively. At the same time, the in-cell mRNA levels of these cassettes were quantitated and found to be similar by qRT-PCR experiments (Fig. 2E).
To further confirm the decisive role of HDEL in determining the ER retention ability of ERS, the C-terminal sequences of four other proteins that contain only the DEL sequences in S. cerevisiae, including NFRDEL of Sil1, MLKDEL of Scj1, DGEDEL of Gpi17, and PYLDEL of Qcr2, were evaluated in our studies (Fig. 2F, Table 1, and pESD-2–20 to pESD-2–23 in supplemental Table S1). Similar to FEKDEL, both NFRDEL and MLKDEL had positively charged residues at position −4, thus presenting moderate ER retention abilities, which is consistent with the identified ER cellular localization of Si11 and Scj1 in yeast (19, 20). DGEDEL exhibited very weak ER retention ability even though Gpi17 was identified as an ER-localized protein (21). Comparably, PYLDEL presented no ER retention ability, consistent with the non-ER cellular localization of Qcr2 (22).
Table 1.
Proteins containing carboxyl-terminal sequence of DEL in S. cerevisiae
The sequences of 15 proteins from S. cerevisiae were extracted from the NCBI database. The italics and underlined letters represent the common sequence of HDEL motif ERS. Information regarding functional properties and localization was adapted from http://www.yeastgenome.org/. (Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.)
| C-terminal sequences | Genes | Function | Location | Strength of ERSa |
|---|---|---|---|---|
| NFRDEL | SIL1 (Ref. 19) | Nucleotide exchange factor for the ER luminal chaperone Kar2p | ER | Medium |
| DGEDEL | GPI17 (Ref. 21) | Subunit of the glycosylphosphatidylinositol transamidase complex that adds GPIs to newly synthesized proteins | ER membrane | Weak |
| PYLDEL | QCR2 (Ref. 32) | Subunit 2 of ubiquinol cytochrome-c reductase | Mitochondrion inner membrane | Weak |
| MLKDEL | SCJ1 (Ref. 20) | A homolog of bacterial chaperone DnaJ, cooperates with Kar2p to mediate maturation of proteins | ER lumen | Medium |
| AIHDEL | PDI1 (Ref. 33) | Protein disulfide isomerase, essential for disulfide bond formation in secretory and cell-surface proteins | ER lumen | Strong |
| GLHDEL | SED4 (Ref. 34) | Integral ER membrane protein that stimulates Sar1p GTPase activity | ER membrane; Golgi apparatus membrane | Medium |
| AAHDEL | CPR5 (Ref. 35) | Peptidyl-prolyl cis-trans isomerase (cyclophilin) of the ER; catalyzes the cis-trans isomerization of peptide bonds N-terminal to proline residues | ER lumen | Weak |
| TVHDEL | EUG1 (Ref. 36) | Protein disulfide isomerase of the ER lumen | ER lumen | Medium |
| VSHDEL | SEC20 (Ref. 37) | Membrane glycoprotein v-SNARE; involved in retrograde transport from the Golgi to ER | ER | Medium |
| ILHDEL | LHS1 (Ref. 38) | Molecular chaperone of the ER lumen; involved in polypeptide translocation and folding | ER lumen | Strong |
| SSHDEL | MPD2 (Ref. 39) | Member of the Pdi family; exhibits chaperone activity | ER | Weak |
| PLHDEL | KRE5 (Ref. 40) | Protein required for β-1,6-glucan biosynthesis | ER lumen | Strong |
| NKHDEL | MPD1 (Ref. 41) | Member of the Pdi family; interacts with and inhibits the chaperone activity of Cne1p | ER lumen | Weak |
| IEHDEL | YOS9 (Ref. 42) | ER quality-control lectin; integral subunit of the HRD ligase; participates in efficient ER retention of misfolded proteins in ERAD | ER membrane | Strong |
| FEHDEL | KAR2 (Ref. 43) | ATPase involved in protein import into the ER; acts as a chaperone to mediate ER protein folding and may help export soluble proteins | ER lumen | Strongb |
a The strength of the ERS was evaluated in our research.
b The high level of ER retention strength in FEHDEL was identified in both our and others' research (14).
Evaluating the interaction between different ERS with Erd1 and Erd2
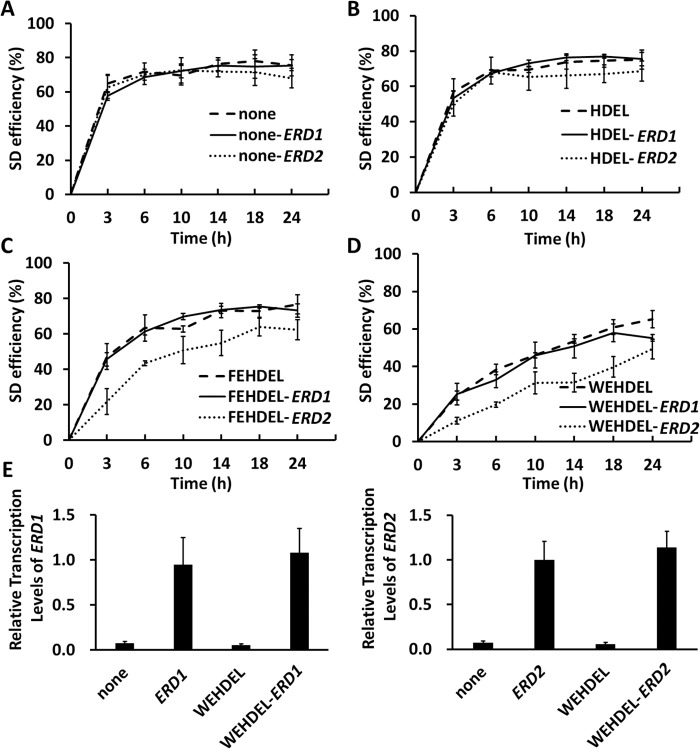
In our studies here, the interaction between different ERS with Erd1 or Erd2, including HDEL, FEHDEL, and WEHDEL, was investigated through a fast evaluating method. Based on the YESS approach, the ERD1/ERD2 and the different Aga2–FLAG–ERS cassettes were anchored downstream of GAL1 and GAL10 in the bidirectional GAL1–GAL10 promoter, respectively (pESD-3–1, pESD-3–2, and pESD-4–1 to pESD-4–6 in supplemental Table S1), providing the advantage of simultaneous expression under the induction of galactose. Overexpressed Erd1 or Erd2 might further retain the correspondent Aga2–FLAG–ERS cassette in the ER through its interaction with ERS, thus causing the decreased SD efficiencies of the Aga2–FLAG–ERS cassette. A similar bimolecular fluorescence complementation (BiFC) strategy has been used to investigate the interaction between KDEL and human KDELR in previous research (15), except that YESS is unique for yeast cells.
Using cell surface fluorescence intensity as the indicator, the flow cytometry results indicated that the SD efficiency of the Aga2–FLAG control was not affected by the overexpression of either Erd1 or Erd2 (Fig. 3A and supplemental Table S6). Similarly, the SD efficiencies of different Aga2–FLAG–ERS cassettes, including Aga2–FLAG–HDEL, Aga2–FLAG–FEHDEL, and Aga2–FLAG–WEHDEL, also were not obviously affected by Erd1 overexpression (Fig. 3, B–D, and supplemental Table S6). However, under the overexpression of Erd2, decreased SD efficiencies were recorded with the Aga2–FLAG–ERS cassettes, among which the Aga2–FLAG–HDEL and Aga2–FLAG–WEHDEL presented the highest and lowest SD efficiencies, respectively. The decreased SD efficiencies under Erd2 overexpressed condition strongly suggested that the HDEL-, FEHDEL-, and WEHDEL-meditated protein ER retention was Erd2-associated. Additionally, it was also noticed that the 24-h maximum SD efficiencies of Aga2-FLAG-WEHDEL under normal, Erd1, or Erd2 overexpression conditions were around 65.2, 55.2, and 49.3%, respectively, which were lower than those of HDEL and FEHDEL (Fig. 3, B–D), further confirming its strongest ER retention strength. Quantitated by RT-PCR, the mRNA levels of ERD1 or ERD2 in their overexpressed cells were consistently around 20-fold higher than under normal conditions and was not affected by the co-expression of either the Aga2–FLAG or the Aga2–FLAG–WEHDEL cassettes (Fig. 3E).
Figure 3.
Interaction of different ERS with Erd1 and Erd2. A–D, under conditions with or without overexpressed Erd1 or Erd2, the SD efficiencies of the Aga2–GFP–FLAG (A) or Aga2–GFP–FLAG–ERS cassettes (B–D) (ERS: HDEL, FEHDEL, or WEHDEL) were recorded at 24 h after induction at 30 °C. E, the mRNA levels of ERD1 and ERD2 in the cells bearing Aga2–GFP–FLAG or Aga2–GFP–FLAG–WEHDEL cassettes were quantitated by RT-PCR after induction for 6 h at 30 °C. The cells were surface-labeled with iFluor 647–conjugated anti-FLAG antibodies for SD efficiency quantitation. The data are presented as mean ± S.E. (n = 3 independent experiments). p ≤ 0.05 (Student's t test).
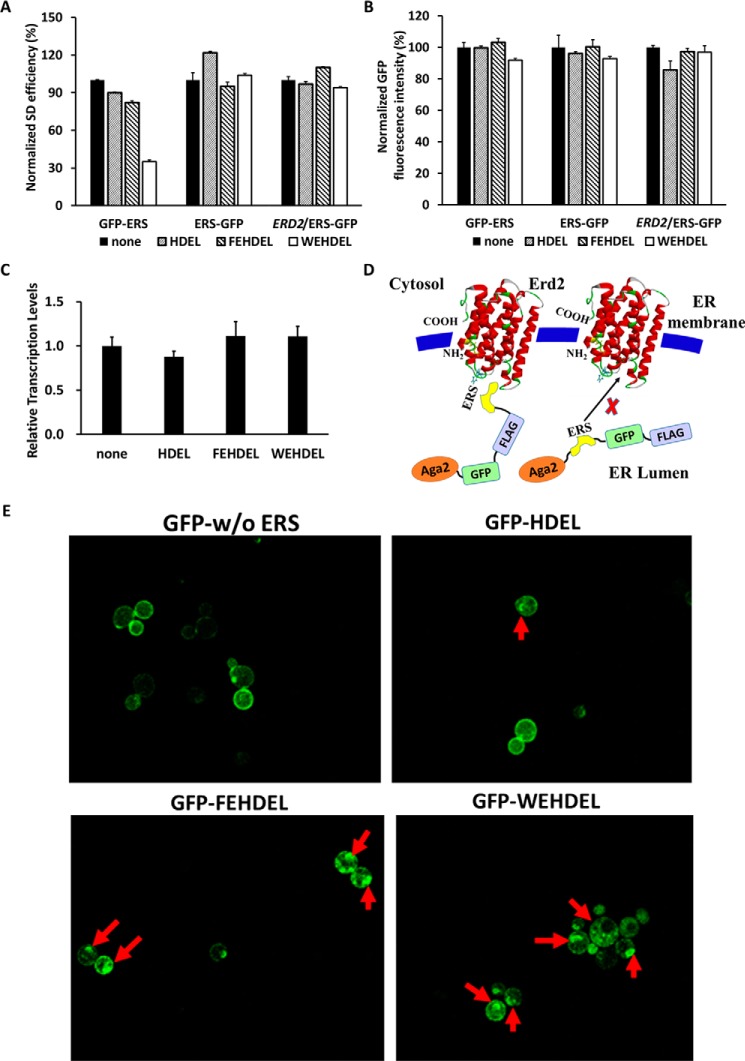
The expressed Aga2–FLAG–ERS cassettes can be transported to the cytoplasm, accumulated in the ER and Golgi, and displayed on the cell surface. Therefore, the attempt to quantify their total protein expression levels by Western blot analysis met with experimental difficulties, as protein extraction efficiencies from the ER, Golgi, and cell surface are lower than from cytoplasm. For example, a strong ERS such as WEHDEL leads to a large accumulation of ERS complexes in the ER and Golgi and decreased transportation to cell surface and cytoplasm, which subsequently causes an decreased total extracted ERS complex protein levels than the control of the Aga2–FLAG cassette without ERS. To solve this problem, ERS including HDEL, FEHDEL, and WEHDEL were inserted into either the Aga2–ERS–GFP–FLAG or the Aga2–GFP–FLAG–ERS cassettes to detect the protein expression levels of the Aga2–GFP–FLAG–ERS cassettes by evaluating the total cellular GFP fluorescence intensity (pESD-6–1 to pESD-6–3 and pESD-7–1 to pESD-7–3 in supplemental Table S1). The use of GFP here facilitated the quick quantitation of the total protein expression in cells using flow cytometry. Besides, the SD efficiencies of different Aga2–GFP–FLAG–ERS and Aga2–ERS–GFP–FLAG cassettes could still be quantitated by the iFluor 647–conjugated anti-FLAG antibodies without interference from GFP in flow cytometry, because iFluor 647 and GFP can be excited by different lasers with detection emission wavelengths that do not overlap. Due to the different ER retention abilities of ERS, the Aga2–GFP–FLAG–HDEL, Aga2–GFP–FLAG–FEHDEL, and Aga2–GFP–FLAG–WEHDEL cassettes presented SD efficiencies of 70.7, 65.1, and 27.4%, respectively, after induction for 6 h at 30 °C, which were all lower than the Aga2–GFP–FLAG control of 79.0% (Fig. 4A and supplemental Fig. S1A). However, none of the Aga2–ERS–GFP–FLAG cassettes exhibited obviously lower SD efficiencies than the Aga2–GFP–FLAG control, even under the overexpression of Erd2 (Fig. 4A and supplemental Fig. S1A). Moreover, the total GFP intensities of these cassettes were all similar (Fig. 4B and supplemental Fig. S1B), indicating that their protein expression levels may not be affected by the differences in ERS and Erd2 overexpression. At the same time, the mRNA levels of the different Aga2–GFP–FLAG–ERS cassettes were quantitated and found to be similar, which is consistent with their total cellular GFP fluorescence intensities (Fig. 4C). These combined findings demonstrate that the strong interaction between Erd2 and ERS occurred only when ERS was located at the C terminus of the ER-resident protein (Fig. 4D).
Figure 4.
Exploring the position of ERS in a protein for its interaction with ERD2. A and B, the normalized SD efficiency (A) or total cellular GFP fluorescent intensity (B) of the different Aga2–GFP–FLAG–ERS and Aga2–ERS–GFP–FLAG cassettes was analyzed after induction for 6 h at 30 °C. C, the in-cell mRNA levels of the Aag2–GFP–FLAG cassettes without ERS or with C-terminal ERS of HDEL, FEHDEL, and WEHDEL were quantitated by RT-PCR after induction for 6 h at 30 °C. D, the proposed scheme presents the interaction between Erd2 and ERS located at the C terminus of a protein complex. E, the intracellular and surface-localized GFP fluorescence of cells bearing the Aga2–GFP–FLAG or different Aga2–GFP–FLAG–ERS cassettes. The red arrows point to the accumulation of GFP fluorescence in yeast ER and Golgi. The cells were surface-labeled with iFluor 647–conjugated anti-FLAG antibodies for SD efficiency quantitation. In A and B, data are presented as mean ± S.E. (n = 3 independent experiments). p ≤ 0.05 (Student's t test).
Another advantage of GFP was that it facilitated direct visualization of the cellular localization of the Aga2–GFP–FLAG–ERS cassettes using a fluorescent microscope. Cellular GFP fluorescence evaluation showed that the Aga2–GFP–FLAG cassette was mostly secreted out and scattered evenly on the yeast cell surface without accumulating in the yeast ER or Golgi (Fig. 4E). Comparably, the yeast cells bearing Aga2–GFP–FLAG–FEHDEL and Aga2–GFP–FLAG–WEHDEL cassettes clearly presented clustered GFP fluorescence next to the edge of the cell inner membrane, which indicated an accumulation of these cassettes in the yeast ER and Golgi. This dispersion of GFP inside and outside of cells also suggested that using GFP fluorescence instead of Western blot analysis might be a more efficient way to evaluate total ERS complex expression levels in our studies.
Characterizing the residues involved in the interaction of HDEL-type ERS and Erd2
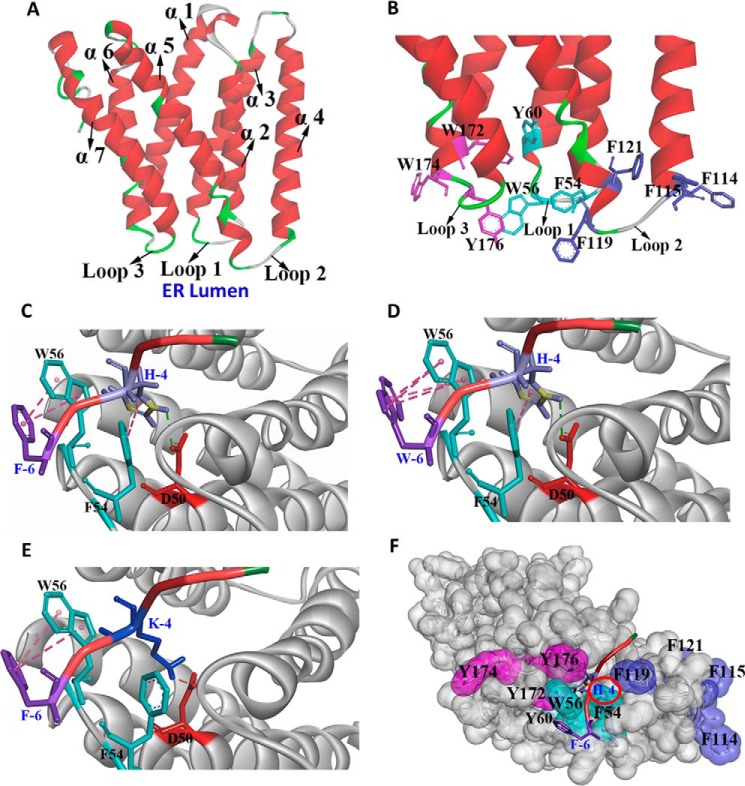
To further explore the interaction between the ERS and Erd2, the tertiary structure of Erd2 was simulated using the I-TASSER program (18), and the interaction between FEHDEL and Erd2 was deduced using the ZDOCK program to guide the subsequent biochemical characterization (Fig. 5). Erd2 structure was simulated based on a eukaryotic SWEET transporter (Protein Data Bank code: 5CTG) (23), which consisted of a seven-transmembrane α-helix (helix α1–α7) with loops 1, 2, and 3 facing the ER lumen (Fig. 5A). The Asp-50, identified previously as involved in the interaction between Erd2 and ERS (13), was located at the edge of helix α2 and loop 1. One interesting observation on the simulated Erd2 structure is that the loop regions facing the ER lumen are rich in aromatic residues, including Phe-54, Trp-56, and Tyr-60 on loop 1, Phe-114, Phe-115, Phe-119, and Phe-121 on loop 2, and Trp-172, Trp-174, and Tyr-176 on loop 3 (Fig. 5B).
Figure 5.
Structural simulation of the interaction between Erd2 and ERS. A, the overall simulated structure of Erd2 using the I-TASSER program. B, the aromatic residues located on loop 1 (blue), loop 2 (purple), and loop 3 (red) form a characteristic subdomain facing the ER lumen. C–E, the speculated interaction between Erd2 and FEHDEL (C), WEHDEL (D), and FEKDEL (E). F, the FEHDEL polypeptide was embedded into the aromatic subdomain with the His residue inserted into the cavity of Erd2.
Our biochemical studies on HDEL suggest that the His residue mainly determine the ER retention strength duo as to its positive charge and aromatic property (Fig. 2B). This finding was supported in our structure simulation showing that the His residue in FEHDEL possibly formed an H-bond with Asp-50 and a π-π stacking force with Phe-54 through its imidazole ring (Fig. 5C). Meanwhile, the aromatic ring of Phe at position −6 of FEHDEL might form a π-π stacking force with Trp-56, thereby enhancing the ER retention ability of FEHDEL beyond that of HDEL (Fig. 5C). It was also conjectured that the Trp at position −6 of WEHDEL might form an even stronger π-π stacking force with Trp-56 than that of FEHDEL (Fig. 5D). In comparison with FEHDEL, only an ionic bond was formed between the Lys residues of FEKDEL and Asp-50 in the simulated structure (Fig. 5E). More interestingly, a characteristic domain constituted mainly by aromatic residues was observed in Erd2, into which the His residue of HDEL was deeply inserted (Fig. 5F).
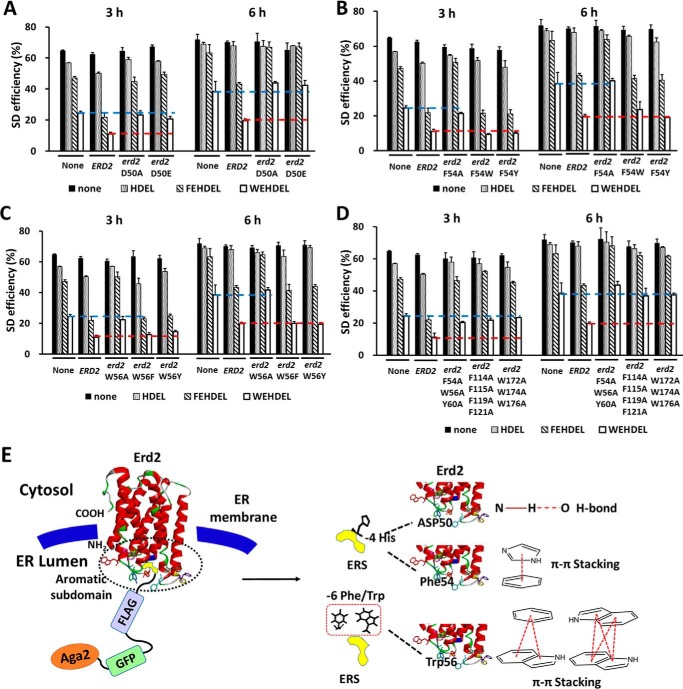
Based on this simulated interaction between ERS and Erd2, mutations on Asp-50, Phe-54, and Trp-56 were analyzed further (pESD-3–3 to pESD-3–10 and pESD-4–7 to pESD-4–30 in supplemental Table S1). Interestingly, the SD efficiencies of all of the Aga2–FLAG–ERS cassettes in yeast cells with overexpressed erd2-D50A or erd2-D50E mutants were similar to those in normal yeast cells without Erd2 overexpression, ranging from 20.8 to 70.6% after induction for 3–6 h (Fig. 6A and supplemental Table S7). These numbers were significantly higher than in those yeast cells with overexpressed Erd2, indicating the key role of Asp-50 in maintaining the function of Erd2. Similarly, mutations on Trp-56 and Phe-54 also confirmed their critical roles in maintaining the function of Erd2. The SD efficiencies of all Aga2–FLAG–ERS cassettes were similar in the yeast cells overexpressing erd2-F54A or erd2-W56A mutants or the wild-type control without ERD2 overexpression, indicating that the Erd2 structure was either disrupted or significantly changed by the F54A or W56A mutations (Fig. 6, B and C, and supplemental Table S7). Comparably, overexpressing erd2-F54W, erd2-F54Y, erd2-W56F, or erd2-W56Y mutants in yeast cells maintained the ER retention of the HDEL-type Aga2–FLAG–ERS cassettes, similar to those under the overexpression of ERD2 (Fig. 6, B and C, and supplemental Table S7). It was concluded that the erd2-F54W, erd2-F54Y, erd2-W56F, and erd2-W56Y mutants maintained the aromatic properties of the residues at positions 54 and 56 while sustaining the conformation of the aromatic subdomain.
Figure 6.
Characterization of the key residues in the interaction between HDEL-type ERS and Erd2. A–D, under conditions without overexpression of ERD2 or with overexpression of ERD2 or its mutants, the SD efficiency of the Aga2–FLAG–ERS and Aga2–FLAG–ERS cassettes (ERS: HDEL, FEHDEL, or WEHDEL in supplemental Table S1) was quantitated after induction for 3 and 6 h at 30 °C, respectively. The cells were surface-labeled with iFluor 647–conjugated anti-FLAG antibodies for FACS analysis. The red or blue dotted line represents the SD efficiency of the Aga2–FLAG–WEHDEL cassette in a normal yeast cell or with overexpressed Erd2, for comparison, respectively. E, the hypothesized mechanism for the interaction between HDEL-type ERS and Erd2 in S. cerevisiae. In A–D, the data are presented as mean ± S.E. (n = 3 independent experiments). p ≤ 0.05 (Student's t test).
Furthermore, the significance of all the aromatic properties of loop 1, loop 2, and loop 3 of Erd2 was also evaluated through combined mutations, including F54A/W56A/Y60A, F114A/F115A/F119A/F121A, and W172A/W174A/Y176A mutations on loops 1, 2, and 3, respectively (pESD-3–11 to pESD-3–13 and pESD-4–31 to pESD-4–39 in supplemental Table S1). Surprisingly, all these mutants disrupted the function of Erd2 (Fig. 6D and supplemental Table S7) providing evidence that, not only in the HDEL-type ERS, the aromatic residues in the loop 1, loop 2, and loop 3 of Erd2 also played important roles in maintaining its function as an ERS receptor.
Based on these findings, we proposed a mechanism with a characteristic subdomain constituted by the gathered aromatic residues located on loops 1, 2, and 3 into which the HDEL-type ERS is embedded, with its His residue deeply inserted to interact with Asp-50 and Phe-54. Moreover, this aromatic subdomain might also be involved in the interaction with the additional aromatic residues located at the N terminus of HDEL, in which Trp-56 plays a crucial role (Fig. 6E).
Applying WEHDEL in YESS for protease engineering
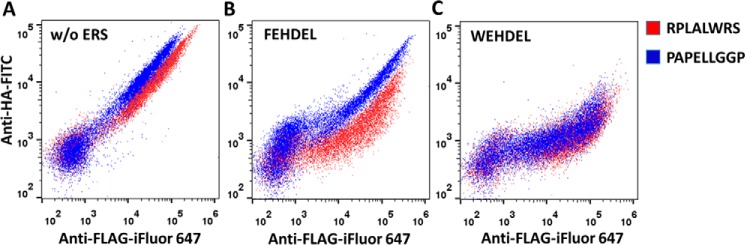
WEHDEL, the strongest ERS identified in our studies, was then applied to our recently developed YESS approach to expand its dynamic range for protease engineering. As an example, human MMP7 was used to evaluate the property of WEHDEL because of its potential therapeutic application on IgG cleavage against autoimmune diseases (24). As seen in Fig. 7A, due to the low proteolytic activity of MMP7, clear cleavage was not observed on MMP7 against either its native substrate, RPLALWRS, or the conserved IgG lower hinge sequence, PAPELLGGP, when no ERS was applied (pESD-8 and pESD-9 in supplemental Table S1). After FEHDEL was applied as an ERS, evident cleavage was recorded with MMP7 against RPLALWRS, whereas the cleavage against PAPELLGGP was still subtle (Fig. 7B). However, the utilization of WEHDEL as an ERS could lead to clear cleavage against both RPLALWRS and PAPELLGGP (Fig. 7C), indicating that WEHDEL could strongly retain the MMP7 and its substrates in yeast ER to facilitate the weak proteolytic reaction. Considering that YESS is a pioneering method for protease engineering and even for kinase engineering (16), the discovery of WEHDEL provides additional options that may assist the engineering of enzymes with slow kinetic properties.
Figure 7.
Application of WEHDEL using the YESS approach. The MMP7 against its native substrate, RPLALWRS, and the human IgG lower hinge sequence, PAPELLGGP, were tested using the YESS approach with different ERS. A, without ERS; B, FEHDEL; C, WEHDEL. Induced cells were surface-labeled with iFluor 647–conjugated anti-FLAG antibodies and FITC-conjugated anti-HA antibodies followed by FACS analysis. High APC fluorescence with little or no FITC fluorescence indicated the specific cleavage at the substrate sequence (16).
Discussion
Compared with previously published gene knock-out (5) and bimolecular fluorescence complementation methods (15), the strategy developed in our studies could convert the retention strength of different ERS to the differentiable surface display efficiencies, facilitating quantitation by the highly sensitive flow cytometry technology (Fig. 1). More importantly, the use of the bidirectional GAL1–GAL10 promoter could lead to the simultaneous expression of ERS and its receptors, thus minimizing the bias in investigating their interactions. In our studies, a total of 37 different ERS and 128 constructs (supplemental Table S1) were quantitatively evaluated using this strategy, which effectively deciphered the characteristics of HDEL-type ERS and the significant role of aromatic residues in both ERS (at position −6 and −4) and Erd2 (Phe-54, Trp-56, and other aromatic residues facing the ER lumen) in determining their interactions.
The ERS for ER-resident proteins
Our studies of FEHDEL derivatives suggested that the HDEL sequence was critical in maintaining the ER retention effect (Fig. 2, A and B). Our analysis of the C-terminal 6 amino acids of all 11 proteins that possess a C-terminal HDEL sequence in S. cerevisiae indicated that these HDEL-type ERS all present clear but different ER retention abilities (Table 1, Fig. S2A, and pESD-2–1 and pESD-2–24 to pESD-2–33 in Table S1). These results further confirmed the ER retention ability of the HDEL sequence, also indicating that the N-terminal residues at positions −5 and −6 affect the strength of different HDEL-type ERS. Similarly, the study of the human protein disulfide isomerase family member ERp18 also suggested that the amino acids at positions −5 and −6 of the N terminus of KDEL affect its ER localization in human cells (15). In addition, the C-terminal sequences of four other ER-localized proteins that do not contain the HDEL-type ERS, including Pbn1, Ero1, Erv2, and Emp65, were also evaluated in our studies (supplemental Fig. S2B and pESD-2–34 to pESD-2–37 in supplemental Table S1). Surprisingly, RSVKRE and YKLDIQ, which were extracted from Pbn1 and Ero1, respectively, both exhibited weak ER retention strength, suggesting that ERS might act in a more complex manner than just the HDEL-type. One possible explanation of how a protein with weak or no C-terminal ERS can still be ER-resident, such as Scj1, Gpi17, Sil1, Pbn1, Ero1, Erv2, and Emp65 that were tested in our studies, is that these proteins may carry out their ER-associated physiological functions through interaction with other ER-resident proteins containing strong ERS. For examples, Scj1 has been reported to cooperate with Kar2 to mediate the maturation of proteins (25).
Our results showed that ERS containing either DEL or HDEL sequences could confer different ER retention abilities (Fig. 2F, supplemental Fig. S2A, and Table 1). It needs to be pointed out that the total cellular GFP fluorescence intensities of the different Aga2–GFP–FLAG–ERS cassettes that contain these ERS are similar, indicating their similar protein expression levels in cells (supplemental Fig. S2C and pESD-6–4 to pESD-6–17 in supplemental Table S1). Combining these results, it could be concluded that the different ER retention abilities of ERS are caused by their innate property of interaction with ERD2.
Erd2 as the main receptor of HDEL-type ERS
An ERD2 knock-out strain would be ideal for the direct assessment of the effects of the various Erd2 mutations. Unfortunately, we were not able to generate the ERD2 knock-out strain even after tremendous effort. This may be because ERD2 is an essential gene, the deletion of which in the yeast genome would lead to cell death. In addition, we also tried the classic yeast two-hybrid method to analyze the interaction between ERS and Erd1/Erd2, but no positive results were obtained (supplemental Fig. S3 and pGAD-C1 and pGBDU-C1 in supplemental Table S1). The failure of the yeast two-hybrid method might have occurred because Erd are ER-localized proteins, which could not be translocated to the nucleus for evaluation. We then quantitated the intracellular interaction between different ERS and Erd1/Erd2 as an alternative strategy (Fig. 3). ERD2 or its mutants were simultaneously overexpressed in the cells to see if they would strengthen ERS-mediated protein ER retention. In Fig. 3, B–D, our results indicate that overexpressed ERD2 enhanced protein ER retention with ERS of HDEL, FEHDEL, and WEHDEL. Comparably, it had no effect on the Aga2–FLAG cassette without ERS (Fig. 3A). As a comparison, overexpression of Erd1 had no influence on protein ER retention, which could be regarded as a control method to confirm the effectiveness of our strategy (Fig. 3, A–D). Therefore, the method developed here exhibited the advantages of specifically studying the interaction between different ERS and Erd in yeast ER, which revealed the strong interaction of HDEL-type ERS against Erd2 instead of Erd1 (Fig. 3 and supplemental Table S6). In fact, this alternative strategy could lead to a fast evaluation of all Erd2 mutations by simply expressing the different ERS and Erd2 variants simultaneously, leading them to the yeast ER through the N-terminal ER signal peptide. Under such circumstances, the alternative strategy utilized here might be the best option we could choose for our studies.
One noteworthy fact is that the three identified KDEL receptors in human cells, KDELR1, KDELR2, and KDELR3, are all homologous with Erd2 (7, 8), presenting sequence similarities ranging from 47.1 to 48.9% with Erd2 (supplemental Fig. S4A). Comparably, their sequencing similarities with Erd1 range from 9.1 to 10.5% (supplemental Fig. S4B). As we confirmed that HDEL-type ERS-mediated protein ER retention is clearly Erd2-associated, it is possible that Erd1-mediated ER retention is protein-specific. In fact, the protein interaction mapping results suggested that Erd1 might carry out its function through protein interaction with Erd2 (26).
The mechanism of Erd2 interaction with HDEL-type ERS
A mechanism for interpreting the interaction between Erd2 and HDEL-type ERS was proposed based on structure simulation and subsequent biochemical analysis (Fig. 6E). Asp-50 has been demonstrated previously to be a key residue in maintaining the function of Erd2 and KDELR1 (13). However, the involvement of Phe-54 is an interesting finding, suggesting that HDEL might interact with Erd2 through not only the H-bond with Asp-50 but also the π-π stacking force with Phe-54. These interactions were caused by the special properties of the imidazole ring of the His residue in HDEL, which differentiated it from the characteristic human ERS, KDEL. In fact, replacing His with Lys in FEHDEL led to an impaired but still weak ER retention ability of FEKDEL (Fig. 2B). Moreover, the finding that D50A and F54A mutations disrupted the interaction between HDEL and Erd2, whereas F54W and F54Y mutations maintained the interaction, together provide supportive evidence for this speculation (Fig. 6, A and B).
Loops 1–3 of the simulated Erd2 were predicted to contain a total 10 aromatic residues, establishing an intense aromatic net facing the ER lumen (Fig. 5B). Considering the aromatic property of His and Phe in FEHDEL, we speculated that the π-π stacking force among the aromatic rings of ERS and Erd2 contributes to their interaction (Fig. 5C). This speculation was also supported by the evidence that mutating these aromatic amino acids to Ala disrupted the interaction between Erd2 and FEHDEL or WEHDEL (Fig. 6D). This might explain that why replacing His with Lys largely impaired the ER retention strength of FEKDEL, whereas replacing Phe with Trp significantly enhanced the strength of WEHDEL (Fig. 2B). In addition, this might also be the reason that FEHD and FEHDE both exhibited certain ER retention strength, because these two sequences keep two aromatic residues of the original FEHDEL sequence (Fig. 2A).
To provide more information on the interaction between ERS and Erd2 mutants, further structural simulation was performed (supplemental Fig. S5). A docking simulation indicated that the mutation of Asp-50, Phe-54, Trp-56, or the aromatic residues in loop 1, loop 2, or loop 3 to Ala might damage the aromatic domain in Erd2, causing the His residue in the FEHDEL polypeptide to be unable to be inserted into the cavity of the Erd2 mutants (supplemental Fig. S5, A–F). Comparably, replacing Trp-56 with another aromatic residue, such as Tyr or Phe, might not impair the aromatic net of Erd2, thus preserving its ability to interact with FEHDEL or WEHDEL (Fig. 6C).
Differential recognition of HDEL and KDEL in yeast and human cells
Erd2 exhibits distinct simulated structures in comparison with Erd1 but shares similar ones with KDELR1 (Fig. 6A and supplemental Fig. S6, A and B). One interesting question for the ER retention study was why yeast and human cells prefer different ERS, such as HDEL and KDEL, respectively. Speculation based on our biochemical analysis and structure simulation suggested that this difference might be caused by the sequence difference around Phe-54 and Trp-56 (Erd2 number) on loop 1 between Erd2 and KDELR1 (supplemental Fig. S4). KDEL could be steadily docked into the KDELR1 through ionic bonds between Lys and Asp-50 and Glu-117, and a hydrogen bond between Lys and Tyr-55 (supplemental Fig. S6C). Comparably, HDEL was docked into the KDELR1 in an unstable state in which His could form a weak π-π and π–lone pair interaction with Trp-120 and Tyr-55, respectively, without an observed interaction with Asp-50 (supplemental Fig. S6D). It also should be noted that this binding may be even weaker due to the inaccessibility of Trp-120 inside the ER membrane. Oppositely, HDEL was stably docked into Erd2 to build strong interactions (Fig. 5D), whereas KDEL docked into Erd2 revealed an unstable complex (Fig. 5E). Considering that the sequence around Phe-54 and Trp-56 played a critical role in constituting the binding cavities for the His/Lys residue, this might explain why HDEL and KDEL are preferred in the yeast and human cells, respectively. Although a weak interaction between HDEL and KDELR1 was speculated, HDEL might still be recognized as an ERS in human cells, especially because human cells contain 13 proteins with the C-terminal HDEL sequence (27).
Based on our structure simulation and biochemical analysis, the aromatic residues in both HDEL and Erd2 might together control the HDEL-mediated protein ER residence in S. cerevisiae. In addition, our studies also revealed that proteins not possessing this C-terminal characteristic sequence, such as Pbn1, Ero1, etc., could also reside in the ER, indicating a complex ER retention mechanism.
Experimental procedures
Vector construction
A pESD plasmid constructed previously was modified to evaluate the strength of different ERS (16). The AGA2 gene downstream of the GAL10 promoter was fused with a two-part cassette encoding the FLAG-tag sequence DYKDDDDK and the ERS, forming an Aga2–FLAG–ERS cassette. ERD1 and ERD2 were under the control of the GAL1 promoter for simultaneous expression in yeast cells. A total of 37 different ERS in 128 constructs was generated and analyzed, and detailed information is listed in supplemental Table S1.
FACS analysis
The constructed plasmids were transformed into EBY100 cells (URA+, leu−, trp−) followed by cultivation and induction. The cells bearing different constructs were labeled with iFluor 647–conjugated anti-FLAG antibodies (GenScript, Nanjing, China) followed by detection using similar protocols published previously (28) with a Beckman Coulter CytoFLEX flow cytometer (Beckman Coulter). The iFluor 647 fluorescent intensity was detected with the APC channel, 660/20 nm band pass, and GFP fluorescent intensity was detected with the FITC channel, 525/40 nm band pass.
The SD efficiencies of cells bearing different Aga2–FLAG–ERS cassettes were normalized by using the SD efficiency of the cells bearing an Aga2–FLAG cassette as the control. The normalized display efficiency was calculated as: [Normalized display efficiency] = ([Cell display percentage of Aga2–FLAG–ERS]/[Cell display percentage of Aga2–FLAG]) × 100%. To exhibit the real time-dependent enhancement, the cell surface display efficiencies in the time-dependent experiments were not normalized by the control.
Florescence microscopy
Yeast cells bearing pESD-Aga2–GFP–ERS cassettes were cultured overnight and then induced at 20 °C for 20 h. Yeast cells bearing pESD–Aga2–FLAG–ERS cassettes were cultured, induced, and then labeled with iFluor 647–conjugated anti-FLAG antibodies. Fluorescent images of cells were obtained with a Zeiss laser-scanning microscope (Zeiss, German) with a 488-nm argon-ion laser.
Yeast total RNA extract and qRT-PCR
Total RNA of yeast samples was extracted from induced cells after galactose induction using a yeast RNAiso kit (TaKaRa) followed by reverse transcription into cDNA in a 20-μl reaction mixture using a PrimeScriptTM II first-strand cDNA synthesis kit (TaKaRa). The cDNA levels were then analyzed using the CFX real-time PCR system (Bio-Rad). Relative expression levels against endogenous TAF10 (29) were determined with efficiency correction and associated technical errors on triplicates calculated.
Yeast two-hybrid assay
The GFP–ERS cassettes were cloned into vector pGBDU-C1 to form pGBDU-bait constructs with pGBDU-GFP as the control. ERD1 and ERD2 genes were cloned into vector pGAD-C1 to form pGAD-prey constructs. pGBDU-bait and pGAD-prey plasmids were transformed into PJ69-4α and PJ69-4a (30), respectively. The PJ69-4α and PJ69-4a strains bearing different constructs were cross-streaked on a YPD plate for mating. After a 3-h incubation at room temperature, mated cells were transferred to SD (−leucine and −uracil) plates to select for diploids. Then single colonies were streaked on SD (−leucine, −uracil, and −histidine) plates. The PJ69-4α strain (containing pGBDU-Rli1 or pGBDU-Lto1 plasmid) and the PJ69-4a strain (containing pGAD-YAE1 plasmid) were used as the positive controls (31). See detailed information of constructs in supplemental Table S1.
Structure simulation
The Erd2 structure was simulated using the I-TASSER program (18). The simulated structure with the highest C-score was chosen for later analysis using Discovery Studio software. Rigid docking between Erd2 and different ERS was carried out using the ZDock program (44), and the top 10 structures presenting the highest ZDock scores were chosen followed by further optimization using the RDock program. The top-rated structures in the RDock program were chosen for detailed analysis to guide the subsequent biochemical analysis. The structure of other proteins, including Erd1, KDELR1, KDELR2, and KDELR3, were simulated and analyzed using the same protocol as with Erd2.
Quantification and statistical analysis
The data represent the mean ± S.D. from three independent experiments. p values were calculated using Student's two-tailed t test (Microsoft Excel); p < 0.05 was considered statistically significant.
Author contributions
L. Y. and G. Z. designed the research; M. M., C. Z., Y. Z., and W. P. conducted the experiments; X. L. and Q. W. performed the structural analysis; L. M. and B. L. I. contributed to the conceptualization; L. Y., G. Z., M. M., and C. Z. analyzed the data; and M. M. and L. Y. wrote the paper.
Supplementary Material
Acknowledgments
We thank Dr. Xiangdong Gao (Wuhan University, China) for providing the yeast two-hybrid system, and Dr. Yang Zhang (University of Michigan) for providing the online I-TASSER service for protein structure simulation. We also thank Meng Dai and Xin Tao from Dr. Li Yi's research group (Hubei University, China) for assistance with experiments.
This work was supported by Grant 31540068 from the National Natural Science Foundation of China (to L. Y.) and Grant 2014AA022203C from the Ministry of Science and Technology of China (to G. Z.). The authors declared that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. S1–S6 and Tables S1–S7.
- ER
- endoplasmic reticulum
- ERS
- ER retention sequence
- YESS
- yeast ER sequestration system
- SD
- surface display.
References
- 1. Braakman I., and Bulleid N. J. (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 80, 71–99 [DOI] [PubMed] [Google Scholar]
- 2. Gregersen N., Bross P., Vang S., and Christensen J. H. (2006) Protein misfolding and human disease. Annu. Rev. Genomics Hum. Genet. 7, 103–124 [DOI] [PubMed] [Google Scholar]
- 3. Munro S., and Pelham H. R. (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48, 899–907 [DOI] [PubMed] [Google Scholar]
- 4. Pelham H. R. (1988) Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 7, 913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hardwick K. G., Lewis M. J., Semenza J., Dean N., and Pelham H. R. (1990) ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J. 9, 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Semenza J. C., Hardwick K. G., Dean N., and Pelham H. R. (1990) ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 61, 1349–1357 [DOI] [PubMed] [Google Scholar]
- 7. Lewis M. J., and Pelham H. R. (1990) A human homologue of the yeast HDEL receptor. Nature 348, 162–163 [DOI] [PubMed] [Google Scholar]
- 8. Raykhel I., Alanen H., Salo K., Jurvansuu J., Nguyen V. D., Latva-Ranta M., and Ruddock L. (2007) A molecular specificity code for the three mammalian KDEL receptors. J. Cell Biol. 179, 1193–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamimura D., Katsunuma K., Arima Y., Atsumi T., Jiang J. J., Bando H., Meng J., Sabharwal L., Stofkova A., Nishikawa N., Suzuki H., Ogura H., Ueda N., Tsuruoka M., Harada M., et al. (2015) KDEL receptor 1 regulates T-cell homeostasis via PP1 that is a key phosphatase for ISR. Nat. Commun. 6, 7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siggs O. M., Popkin D. L., Krebs P., Li X., Tang M., Zhan X., Zeng M., Lin P., Xia Y., Oldstone M. B., Cornall R. J., and Beutler B. (2015) Mutation of the ER retention receptor KDELR1 leads to cell-intrinsic lymphopenia and a failure to control chronic viral infection. Proc. Natl. Acad. Sci. U.S.A. 112, E5706–E5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamimura D., Arima Y., Tsuruoka M., Jiang J. J., Bando H., Meng J., Sabharwal L., Stofkova A., Nishikawa N., Higuchi K., Ogura H., Atsumi T., and Murakami M. (2016) Strong TCR-mediated signals suppress integrated stress responses induced by KDELR1 deficiency in naive T cells. Int. Immunol. 28, 117–126 [DOI] [PubMed] [Google Scholar]
- 12. Semenza J. C., and Pelham H. R. (1992) Changing the specificity of the sorting receptor for luminal endoplasmic reticulum proteins. J. Mol. Biol. 224, 1–5 [DOI] [PubMed] [Google Scholar]
- 13. Scheel A. A., and Pelham H. R. (1998) Identification of amino acids in the binding pocket of the human KDEL receptor. J. Biol. Chem. 273, 2467–2472 [DOI] [PubMed] [Google Scholar]
- 14. Pelham H. R., Hardwick K. G., and Lewis M. J. (1988) Sorting of soluble ER proteins in yeast. EMBO J. 7, 1757–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alanen H. I., Raykhel I. B., Luukas M. J., Salo K. E., and Ruddock L. W. (2011) Beyond KDEL: The role of positions 5 and 6 in determining ER localization. J. Mol. Biol. 409, 291–297 [DOI] [PubMed] [Google Scholar]
- 16. Yi L., Gebhard M. C., Li Q., Taft J. M., Georgiou G., and Iverson B. L. (2013) Engineering of TEV protease variants by yeast ER sequestration screening (YESS) of combinatorial libraries. Proc. Natl. Acad. Sci. U.S.A. 110, 7229–7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guerrero J. L., O'Malley M. A., and Daugherty P. S. (2016) Intracellular FRET-based screen for redesigning the specificity of secreted proteases. ACS Chem. Biol. 11, 961–970 [DOI] [PubMed] [Google Scholar]
- 18. Yang J., Yan R., Roy A., Xu D., Poisson J., and Zhang Y. (2015) The I-TASSER suite: Protein structure and function prediction. Nat. Methods 12, 7–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tyson J. R., and Stirling C. J. (2000) LHS1 and SIL1 provide a lumenal function that is essential for protein translocation into the endoplasmic reticulum. EMBO J. 19, 6440–6452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blumberg H., and Silver P. A. (1991) A homologue of the bacterial heat-shock gene DnaJ that alters protein sorting in yeast. Nature 349, 627–630 [DOI] [PubMed] [Google Scholar]
- 21. Ohishi K., Inoue N., and Kinoshita T. (2001) PIG-S and PIG-T, essential for GPI anchor attachment to proteins, form a complex with GAA1 and GPI8. EMBO J. 20, 4088–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grandier-Vazeille X., Bathany K., Chaignepain S., Camougrand N., Manon S., and Schmitter J. M. (2001) Yeast mitochondrial dehydrogenases are associated in a supramolecular complex. Biochemistry 40, 9758–9769 [DOI] [PubMed] [Google Scholar]
- 23. Tao Y., Cheung L. S., Li S., Eom J. S., Chen L. Q., Xu Y., Perry K., Frommer W. B., and Feng L. (2015) Structure of a eukaryotic SWEET transporter in a homotrimeric complex. Nature 527, 259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brezski R. J., and Jordan R. E. (2010) Cleavage of IgGs by proteases associated with invasive diseases: An evasion tactic against host immunity? MAbs 2, 212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schlenstedt G., Harris S., Risse B., Lill R., and Silver P. A. (1995) A yeast DnaJ homologue, Scj1p, can function in the endoplasmic reticulum with BiP/Kar2p via a conserved domain that specifies interactions with Hsp70s. J. Cell Biol. 129, 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller J. P., Lo R. S., Ben-Hur A., Desmarais C., Stagljar I., Noble W. S., and Fields S. (2005) Large-scale identification of yeast integral membrane protein interactions. Proc. Natl. Acad. Sci. U.S.A. 102, 12123–12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chung J. J., Yang H., and Li M. (2003) Genome-wide analyses of carboxyl-terminal sequences. Mol. Cell. Proteomics 2, 173–181 [DOI] [PubMed] [Google Scholar]
- 28. Yi L., Taft J. M., Li Q., Gebhard M. C., Georgiou G., and Iverson B. L. (2015) Yeast endoplasmic reticulum sequestration screening for the engineering of proteases from libraries expressed in yeast. Methods Mol. Biol. 1319, 81–93 [DOI] [PubMed] [Google Scholar]
- 29. Teste M. A., Duquenne M., Francois J. M., and Parrou J. L. (2009) Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol. Biol. 10, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. James P., Halladay J., and Craig E. A. (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhai C., Li Y., Mascarenhas C., Lin Q., Li K., Vyrides I., Grant C. M., and Panaretou B. (2014) The function of ORAOV1/LTO1, a gene that is overexpressed frequently in cancer: Essential roles in the function and biogenesis of the ribosome. Oncogene 33, 484–494 [DOI] [PubMed] [Google Scholar]
- 32. Einerhand A. W., Kos W., Smart W. C., Kal A. J., Tabak H. F., and Cooper T. G. (1995) The upstream region of the FOX3 gene encoding peroxisomal 3-oxoacyl-coenzyme A thiolase in Saccharomyces cerevisiae contains ABF1- and replication protein A-binding sites that participate in its regulation by glucose repression. Mol. Cell. Biol. 15, 3405–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farquhar R., Honey N., Murant S. J., Bossier P., Schultz L., Montgomery D., Ellis R. W., Freedman R. B., and Tuite M. F. (1991) Protein disulfide isomerase is essential for viability in Saccharomyces cerevisiae. Gene 108, 81–89 [DOI] [PubMed] [Google Scholar]
- 34. Hardwick K. G., Boothroyd J. C., Rudner A. D., and Pelham H. R. (1992) Genes that allow yeast cells to grow in the absence of the HDEL receptor. EMBO J. 11, 4187–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dolinski K., Muir S., Cardenas M., and Heitman J. (1997) All cyclophilins and FK506-binding proteins are, individually and collectively, dispensable for viability in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 94, 13093–13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tachibana C., and Stevens T. H. (1992) The yeast EUG1 gene encodes an endoplasmic reticulum protein that is functionally related to protein disulfide isomerase. Mol. Cell. Biol. 12, 4601–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Novick P., Field C., and Schekman R. (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 [DOI] [PubMed] [Google Scholar]
- 38. Craven R. A., Egerton M., and Stirling C. J. (1996) A novel Hsp70 of the yeast ER lumen is required for the efficient translocation of a number of protein precursors. EMBO J. 15, 2640–2650 [PMC free article] [PubMed] [Google Scholar]
- 39. Tachikawa H., Funahashi W., Takeuchi Y., Nakanishi H., Nishihara R., Katoh S., Gao X. D., Mizunaga T., and Fujimoto D. (1997) Overproduction of Mpd2p suppresses the lethality of protein disulfide isomerase depletion in a CXXC sequence-dependent manner. Biochem. Biophys. Res. Commun. 239, 710–714 [DOI] [PubMed] [Google Scholar]
- 40. Meaden P., Hill K., Wagner J., Slipetz D., Sommer S. S., and Bussey H. (1990) The yeast KRE5 gene encodes a probable endoplasmic reticulum protein required for (1–6)-β-d-glucan synthesis and normal cell growth. Mol. Cell. Biol. 10, 3013–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tachikawa H., Takeuchi Y., Funahashi W., Miura T., Gao X. D., Fujimoto D., Mizunaga T., and Onodera K. (1995) Isolation and characterization of a yeast gene, MPD1, the overexpression of which suppresses inviability caused by protein disulfide isomerase depletion. FEBS Lett. 369, 212–216 [DOI] [PubMed] [Google Scholar]
- 42. Friedmann E., Salzberg Y., Weinberger A., Shaltiel S., and Gerst J. E. (2002) YOS9, the putative yeast homolog of a gene amplified in osteosarcomas, is involved in the endoplasmic reticulum (ER)-Golgi transport of GPI-anchored proteins. J. Biol. Chem. 277, 35274–35281 [DOI] [PubMed] [Google Scholar]
- 43. Rose M. D., Misra L. M., and Vogel J. P. (1989) KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell 57, 1211–1221 [DOI] [PubMed] [Google Scholar]
- 44. Chen R., Li L., and Weng Z. (2003) ZDOCK: an initial-stage protein-docking algorithm. Proteins 52, 80–87 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.