Abstract
Semaphorins form a family of membrane-bound and secreted molecules that were identified originally as axonal guidance factors during neuronal development. Given their wide distribution in many including mature tissues, semaphorin 4D (sema4D) and its main functional receptor plexin B1 (plxnB1) are expected to fulfill additional functions that remain to be uncovered. A main characteristic of the plexin receptor family is its ability to reorganize the cytoskeleton. PlxnB1 specifically may directly interact with Rho family GTPases to regulate F-actin driven pathways in cells in culture. Diurnal clearance phagocytosis by the retinal pigment epithelium (RPE) of photoreceptor outer segment fragments (POS) is critical for photoreceptor function and longevity. In this process rearrangement of RPE cytoskeletal F-actin via activation of the Rho family GTPase Rac1 is essential for POS internalization. Here, we show a novel role in POS phagocytosis by RPE cells in culture and in vivo for plexin B1 and its ligand sema4D. Exogenous sema4D abolishes POS internalization (but not binding) by differentiated RPE cells in culture by decreasing the GTP-load of Rac1. In the rat eye, Sema4D localizes to retinal photoreceptors while PlxnB1 is expressed by neighboring RPE cells. At the peak of diurnal retinal phagocytosis after light onset plxnB1 phosphorylation and sema4D levels are reduced in wild-type rat retina in situ but not in mutant RCS rat retina in which the RPE lacks phagocytic activity. Finally, increased POS phagosome content after light onset is observed in the RPE in situ of mice with either plxnB1 or sema4D gene deletion. Altogether, our results demonstrate a novel physiological function for sema4D/plxnB1- signaling in RPE phagocytosis serving as attenuating brake prior to light onset whose release enables the diurnal phagocytic burst.
Keywords: Semaphorin 4D, plexin B1, photoreceptor outer segment phagocytosis, retinal pigment epithelium
Introduction
Semaphorins form a large and diverse family of secreted or membrane associated glycoproteins defined by a cysteine-rich semaphorin protein domain [1]. The main functional transmembrane receptors for semaphorins are members of the plexin family [2,3] although a subset of secreted semaphorins requires association of neuropilin co-receptors. Semaphorins were first identified in the central nervous system and found to function as axon guidance molecules. More recently, semaphorins have been shown to also contribute to cell signaling in tissues other than the nervous system. Semaphorin 4D (sema4D) specifically is widely expressed, and binding to its high affinity binding receptor plexin B1 (plxnB1) affects many physiological functions, such as immune responses, angiogenesis, tumorigenesis and metastasis.
Plexins are large single-transmembrane spanning proteins with conserved extracellular motifs such as the sema domain [4], the cysteine-rich PSI motifs (plexin-semaphorin-integrin domain) and the IPT (Ig-like, plexins, transcription factors) domain [5]. These domains are thought to have roles in protein-protein interactions. The intracellular tail of plexins contains GTPase-activating protein (GAP) like motifs and a domain that has been shown to directly interact with Rho family GTPases such as Rnd1 and Rac1 [6–9]. Plexins may also interact with guanosine nucleotide exchange factors (GEFs) [10]. Altogether, semaphorin-plexin signaling ultimately regulates Rho GTPases in a manner dependent on cell type or cell function. PlxnB1 specifically may interact with active Rac1 in a sema4D ligand dependent manner [11,12] and may promote integrin-dependent activation of AKT through RhoA and Rho kinase (ROCK) [13].
In the vertebrate eye, daily clearance phagocytosis of spent photoreceptor outer segment fragments (POS) by the retinal pigment epithelium (RPE) is a critical part of the outer segment renewal program that maintains photoreceptor function and longevity. POS shedding every morning precisely compensates for outer segment elongation through biosynthesis to maintain constant outer segment length for life. Too much or too little removal of POS per day by RPE cells will gradually damage outer segments and ultimately harm photoreceptors and impair vision. POS clearance occurs as a burst after light onset that is synchronized by RPE signaling pathways. Exposure of phosphatidylserine “eat me” signal on outer segment tips destined to be shed recruits extracellular milk fat globule-epidermal growth factor 8 (MFG-E8) [14,15] that serves to tether POS to apical ανβ5 integrin receptors of the RPE [16]. Integrin engagement by POS binding activates tyrosine kinase signaling via focal adhesion kinase (FAK) and Mer tyrosine kinase (MerTK) [17,18]. MerTK activity is essential for internalization of bound POS. Recruitment of dynamic F-actin to transient structures called phagocytic cups beneath bound POS requires a brief burst of Rac1 activity, which is also stimulated by αvβ5 signaling albeit independently of FAK and MerTK [19]. In addition, signaling by phosphoinositide 3-kinases (PI3 kinases) and their direct downstream effectors AKT serine/threonine kinases contributes to regulating phagocytic cup size in RPE cells [20]. Here, we reveal a novel role of sema4D and its receptor plxnB1 in POS phagocytosis by RPE cells. We find that adding extracellular sema4D abolishes POS internalization by polarized, differentiated human RPE cells in culture by inhibiting Rac1. Moreover, plxnB1 phosphorylation and sema4D protein levels are reduced during the daily peak of phagocytosis in wild-type (WT) rat eyes, and this pattern is reversed in Royal College of Surgeons (RCS) MerTK-deficient rat eyes whose RPE cannot phagocytose POS. Finally, mice with sema4D or plxnB1 gene deletion show increased POS internalization at light onset. Altogether, these results support a novel inhibitory function of sema4D/plxnB1 signaling that attenuates the RPE phagocytic activity before the daily phagocytic burst.
Materials and Methods
Reagents were purchased from Sigma (St. Louis, MO) or Thermofisher (Waltham, MA) unless otherwise stated.
Antibodies
Primary antibodies against the following proteins were used: PlxnB1, RhoA and β5 Integrin (Santa Cruz Biotechnology, Santa Cruz, CA), Rac 1 (BD Biosciences, San Jose, CA), Sema4D (Millipore, Billerica, MA, and Bio-Techne, Minneapolis, MN), RPE65 (Genetex, Irvine, CA), porin, AKT and phospho-AKT (ser473) (Cell Signaling, Danvers, MA), MerTK and MFG-E8 (Bio-Techne), rhodopsin clone B6-30 [21], PSD 95 (EMD Millipore), β-actin (Sigma), and ZO-1 (Thermofisher).
Cell Culture, GTPase activity assays and adenovirus infection
All human tissue research followed the tenets of the Declaration of Helsinki and the NIH institutional review board. Fetal human eyes from donors at 16 to 22 weeks of gestation were obtained from Advanced Bioscience Resources (ABR, Alameda, CA). Primary human retinal pigment epithelial cells (hRPE) were generated and routinely maintained precisely according to published protocols [22] at 37°C and 5% CO2 in αMEM supplemented with 5% FBS, N1 supplement, glutamine-penicillin-streptomycin and nonessential amino acid solution (all 1:100). In addition, hydrocortisone (20 μg/L), taurine (250 mg/L), and triiodo-thyronin (0.013 μg/L) (THT) were dissolved in PBS and aliquots stored at −80°C until added at 1: 500 [22]. Before use for experiments, cells were grown in multi-well plates with or without glass coverslips for 25 days to yield postmitotic and polarized epithelial monolayers with high similarity to native human RPE [22].
G-LISA GTPase activity assays (Cytoskeleton) were performed following the manufacturer’s instructions on fresh lysates of hRPE cells with bound POS incubated with complete medium with 10% delipidated FBS in the presence or absence of recombinant sema4D (0.5 μM). Total sample-GTPases were detected by immunoblotting. Replication-defective, recombinant adenovirus encoding β-galactosidase or constitutively active Rac1 (RacL63, CA-Rac1) (both Cell Biolabs, San Diego, CA) were diluted in serum free αMEM and added to hRPE cells overnight. After infection, cells were incubated in complete medium for 24 hours before experiments.
POS Phagocytosis Assays
POS were purified from porcine eyes obtained fresh from a local slaughterhouse according to established procedures [23,24]. Purified POS were covalently labeled with fluorescent dye by incubation in 0.1 mg/ml Texas Red-X succinimidyl ester for 1.5 hours on a rocker in the dark at room temperature. Cells were incubated with 10 POS/cell in serum-free DMEM supplemented with 1.25 μg/ml recombinant human MFG-E8 (Bio-Techne) for 1 hour at 37°C without FBS to allow only POS binding [25]. Active human Sema4D protein fragment (Abcam, Cambridge, MA) was added to select samples.
After POS incubation, cells were washed three times with phosphate buffered saline with 0.1 mM CaCl2 (PBS-CM) and fixed with 4% paraformaldehyde in PBS-CM for 20 min or continued incubation at 37°C in DMEM with 10% delipidated FBS (Cocalico Biologicals, Reamstown, PA) to permit internalization of bound POS. After POS internalization, samples were fixed as above followed by counterstain with ZO-1 antibody and AlexaFluor-conjugated secondary antibody or with AlexaFluor-conjugated phalloidin and DAPI. Image stacks were obtained using a Leica TSP5 laser scanning confocal microscopy system and compiled in Adobe Photoshop CS4 (Adobe, San Jose, CA). Images were pseudo-colored to display POS in green, ZO-1/F-actin counterstain in red, and nuclei in blue.
Animals and Tissue Harvest
All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and reviewed and approved by according to institutional requirements. Animals were raised and housed under cyclic 12 h light/12 h dark lighting conditions and fed standard chow ad libitum.
WT Long Evans (LE) originally from Charles River Laboratories and MerTK null RCS rats [18] were bred and used for experiments at 19 days of age.
Sema4D knockout mice described previously [26] and age-matched C57Bl/6 WT mice were raised by Dr. Masayuki Mizui (Osaka University, Japan). PlxnB1 knockout mice described previously [27] and age-matched C57Bl6 WT control mice were kindly provided by Dr. Eszter Paldy (University of Heidelberg, Germany). All mice were used at 3–4 months of age.
For all experiments, animals were sacrificed by CO2 asphyxiation at defined times with respect to light onset before immediate enucleation of eyeballs, tissue fixation, or dissection.
Whole Mount Tissue Labeling and Microscopy
For detection of sema4D expression in neural retina, WT and RCS rats were sacrificed 1 hour before or after light onset followed by immediate eye enucleation and dissection in ice-cold Hanks buffered saline solution. Freshly dissected whole neural retinas were then incubated in PBS-CM with non-immune IgG or antibody to sema4D (15 μg/ml) for 1 h on ice followed by 3 washes with PBS-CM and incubation with AlexaFluor-conjugated secondary antibodies, peanut agglutinin or wheat germ agglutinin lectins. Retinas were fixed after labeling with 1% paraformaldehyde in PBS-CM for 10 minutes and mounted photoreceptor side up in Vectashield (Vector Labs, Burlingame, CA). Single photoreceptor cells were obtained by gently micropipetting immunolabeled, fixed retinas before mounting on Tissue-Tek coated glass slides and immediate imaging.
For immunostaining of plxB1 in whole mount choroid/RPE, posterior eyecups isolated from rat eyes harvested 1 hour before light onset were fixed in 4% paraformaldehyde in PBS-CM for 20 min and stained with plxnB1 and ZO-1 antibodies and AlexaFluor-conjugated secondary antibodies.
Image stacks were obtained using a Leica TSP5 laser scanning confocal microscopy system and compiled in Adobe Photoshop CS4.
Tissue sectioning, Labeling, POS quantification, and Microscopy
Eye tissue processing and ex situ POS phagosome quantification were performed exactly as published in detail recently [28]. In brief, the anterior segment of fixed eyeballs were embedded in paraffin. De-paraffinized sections were labeled with opsin antibody B6-30 and DAPI nuclei counterstain for phagosome counting or stained with hematoxylin and eosin to analyze the gross morphology of the retina. Image stacks were obtained using a Leica TSP5 laser scanning confocal microscopy system and compiled in Adobe Photoshop CS4.
Immunoprecipitation
Rat eyes were dissected 1 hour before or after light onset and lysed in 20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA and 2% Triton-X100. Cleared lysates were subject to immunoprecipitation with sepharose-bead conjugated phospho-tyrosine antibody (Cell Signaling) following the manufacturer’s instructions. Tyrosine phosphorylation of PlxnB1 was detected by immunoblotting immunoprecipitates with PlxnB1 antibody and quantified relative to total cellular PlxnB1 as detected in whole cell lysates.
Sample Lysis and Immunoblotting
Samples were lysed in 20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA and 2% Triton-X100. Cleared lysates were supplemented with reducing SDS sample buffer and boiled for 5 min before separation on SDS-polyacrylamide gels using standard protocols. Proteins were transferred to nitrocellulose membranes, incubated with primary and appropriate horseradish peroxidase-conjugated secondary antibodies followed by enhanced chemiluminescence detection (Perkin-Elmer, Waltham, MA). X-ray films were scanned and images processed using Photoshop CS4. Bands were quantified by densitometry using ImageQuant. Molecular sizes of proteins are indicated in figure legends. Bands were detected on blots at locations as predicted given proteins’ molecular sizes.
Statistical Analysis
All experiments were performed at least three times independently with duplicate samples. Samples were first analyzed using one-way ANOVA to determine significant variance among groups. If significance was established, difference of selected treated samples to control sample was determined using Bonferroni’s multiple comparison test. Student’s t-test was used to determine the difference between two samples where appropriate. P values < 0.05 were considered statistically significant differences.
Results
Sema4D abrogates POS internalization via inhibition of Rac1 in hRPE cells
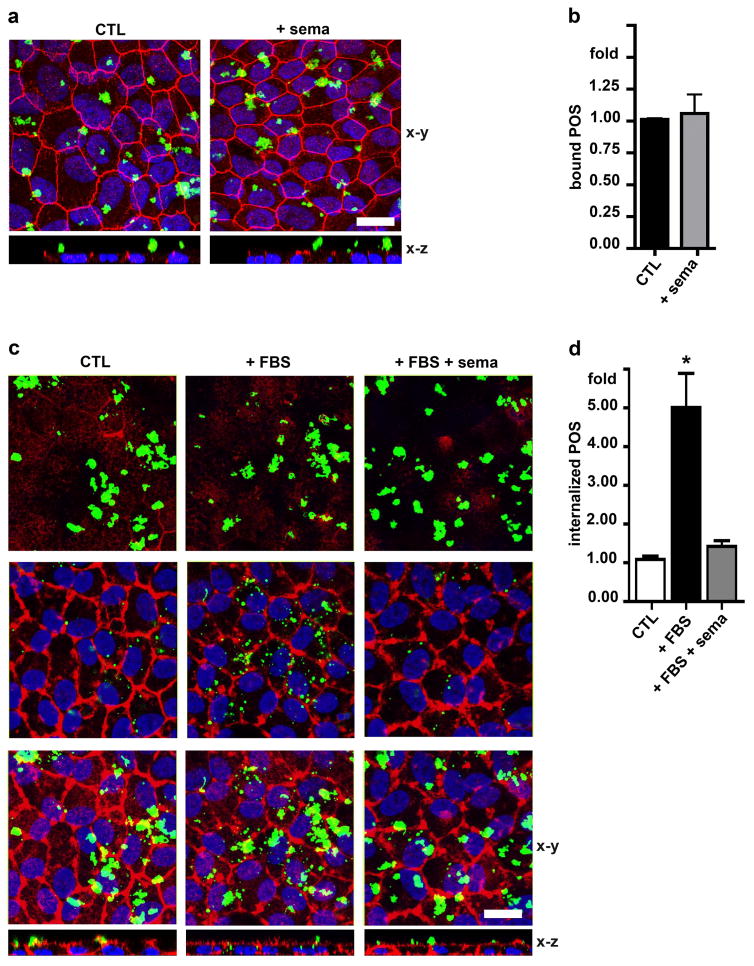
In order to directly test if sema4D/plxnB1 signaling affects the RPE phagocytic activity toward POS we performed synchronized phagocytosis assays on hRPE cells in culture with or without adding extra recombinant sema4D protein. To quantify POS binding alone, hRPE cells received fluorescent POS for 1 hour in culture medium with (+ sema) or without sema4D (CTL) with αvβ5 integrin-ligand MFG-E8 while omitting fetal bovine serum (FBS), whose MerTK ligands are needed in culture assays to stimulate internalization. Co-staining of the tight junction marker protein ZO-1 was used to delimit the apical from the basolateral plasma membrane with proteins that localize above ZO-1 considered apical in polarized cells. To quantify internalization of POS in the absence of exogenous sema4D, cells with pre-bound POS were washed to remove unbound POS and then further incubated in complete medium with 10% FBS to allow internalization for 4 hours in the presence (+FBS+sema) or absence (+FBS) of recombinant sema4D. To confirm the requirement of FBS for internalization, control cells were incubated for 4 hours with culture medium without FBS (CTL). Co-staining of the F-actin cytoskeleton was used to label the outline of cells. Figure 1a and 1b show that sema4D did not affect POS binding. X-z views of images confirmed that POS remained surface-bound (Fig. 1a, x-z). In the presence of FBS (+FBS) bound POS were internalized and were observed as fragmented POS in the cells at the level of the nuclei and in x-z views (Fig. 1c, center row and x-z). However, adding sema4D abrogated POS internalization stimulated by FBS (Fig. 1c, compare panels in middle row and x-z, and 1d).
Fig. 1.
Sema4D inhibits POS internalization without affecting POS binding. a Maximal projections of x-y confocal image stacks and a select x-z plane as indicated showing ZO-1 (shown in red), cell nuclei (shown in blue) and bound POS (shown in green) at the apical surface of hRPE cells fixed and stained following incubation with DMEM (CTL) or sema4D (+ sema) in DMEM for 1 hour at 37°C. b Quantification of bound POS after experiments as in a. c X-y images and select x-z planes showing F-actin labeling (shown in red) and POS (shown in green) internalized after incubation for 4 hours at 37°C with DMEM (CTL), DMEM with 10% FBS (+ FBS) or DMEM with 10% FBS and sema4D (+ FBS + sema). Upper row shows x-y planes at the apical surface of hRPE with bound POS, center panels shows x-y planes at the cell center with internalized POS, and lower row shows maximal projections representing the cells in their entirety. Representative fields are shown from a total of three independent experiments. d Quantification of internalized POS after experiments as in c. All bars show mean ± SD of three independent experiments with duplicate samples each. Asterisks indicate significant difference to control cells, p < 0.05 by ANOVA. Scale bars: 10 μm.
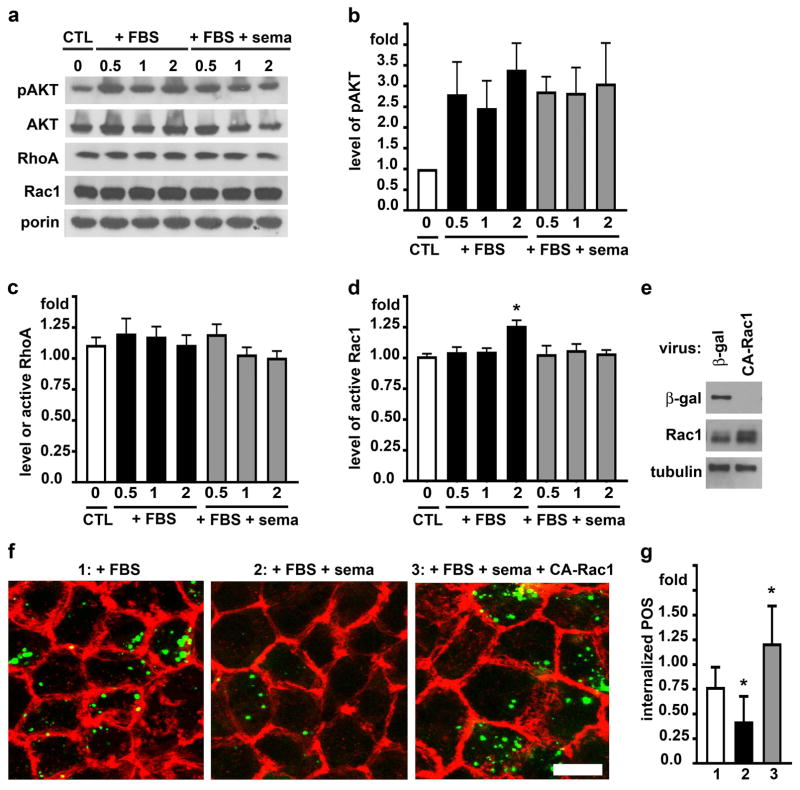
We previously reported that inhibition of AKT enhances POS internalization, which also requires activation of the F-actin regulator Rac1 GTPase [19,20]. We therefore next tested whether the presence of sema4D during POS engulfment affected RPE signaling by AKT, Rac1 or the closely related RhoA. Like in the first experiment, cells with pre-bound POS received media with FBS alone or with FBS and sema4D before quantification of signaling activities after further incubation for 0.5, 1 or 2 hours. Steady state protein levels of AKT, RhoA and Rac1 remained unchanged during POS phagocytosis (Fig. 2a). Immunoblotting for AKT phosphorylated at serine residue 473 revealed that POS challenge robustly stimulated AKT phosphorylation at this residue (as we previously reported), and this was not altered by the presence of sema4D (Fig. 2, a and b). Quantification of GTP-load of RhoA in the same RPE samples showed that sema4D did not alter RhoA activity, which did not change during the POS phagocytosis experiment (Fig. 2c). In contrast, sema4D prevented the increase in GTP-load of Rac1 during FBS-stimulated POS internalization (Fig. 2d). Furthermore, expressing a constitutively active form of Rac1 (Fig. 2e) was sufficient to restore POS internalization in the presence of sema4D (Fig. 2, f and g).
Fig. 2.
Sema4D inhibits POS internalization by preventing Rac1 activation during POS challenge. a Immunoblotting of phosphorylated AKT (60 kDa), AKT (60 kDa), RhoA (22 kDa), Rac1 (21 kDa) and porin (31 kDa) as loading control in hRPE after POS internalization for 0.5, 1 or 2 hours in the presence of FBS (+ FBS) or FBS with sema4D (+ FBS + sema). Time 0 corresponds to the end of the binding period, in which cells were fed with POS in DMEM alone (CTL). b Quantification of phosphorylated AKT (pAKT) in experiments as shown in a. c GTP-RhoA and d GTP-Rac1 levels indicating specific activity of the respective GTPase as measured by G-LISA from hRPE samples treated as in a. Bars show relative GTP-RhoA or GTP-Rac1 compared to activity at the end of the POS binding period (0) with DMEM (CTL). Data show mean ± SD of three independent experiments, each testing duplicate samples. Asterisks mark significant change in GTP-Rac1 compared to GTP-Rac1 during the binding period (p<0.05). e hRPE cells infected with recombinant adenovirus encoding β-galactosidase control protein (β-gal) or constitutively active Rac1 (CA-Rac1) were analyzed by immunoblotting. A representative immunoblot is shown of three independent experiments with duplicate samples each probed for Rac1 (~21 kDa yielding a diffuse band representing total Rac1, a mix of endogenous Rac1 and CA-Rac1), β-gal (120 kDa), and tubulin (55kDa) as loading control. Densitometry quantification showed that CA-Rac1 virus increased total Rac1 levels by an average of 47%±13%. f hRPE cells infected as in e with adenovirus encoding β-gal (left and center panels) or encoding CA-Rac1 (right panel) were subjected to 4 hour incubation at 37°C with DMEM with 10% FBS (+ FBS) or DMEM with 10% FBS and sema4D (+ FBS + sema) as indicated. Single x-y images show F-actin (shown in red) and internalized POS (shown in green) in each condition. Scale bar: 5 μm. Representative fields are shown. g Quantification of internalized POS relative to cells expressing β-galactosidase with FBS alone from experiments as in f. Bars show mean ± SD of three independent experiments with duplicate samples each. Asterisks indicate significant difference to cells expressing β-galactosidase with FBS alone, p < 0.05 by ANOVA.
Taken together these results show that sema4D inhibits POS phagocytosis by RPE cells by interfering with the activation of Rac1.
In the eye, RPE cell express PlxnB1 receptor while its sema4D ligand localizes to the photoreceptors of the adjacent neural retina
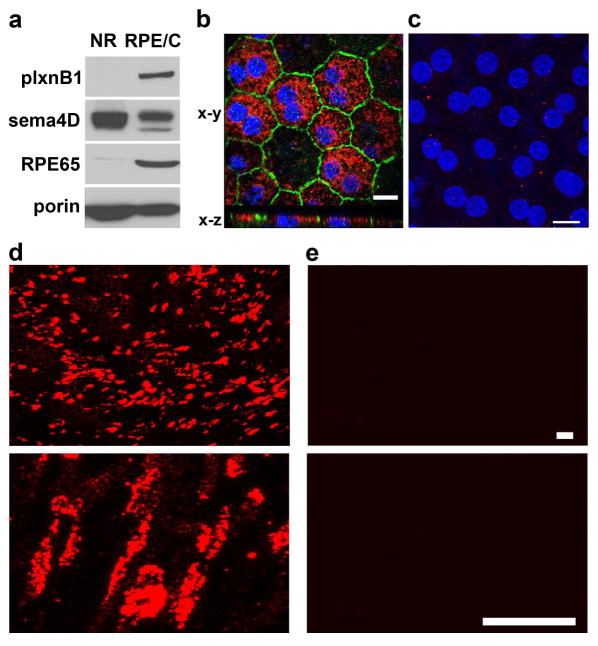
To determine if plxnB1 and its ligand sema4D localize to the site of POS phagocytosis in the mammalian eye, we performed tissue fractionation assays. As we expect proteins to be involved in POS phagocytosis to be present 1 hour before light onset, we decided to determine expression and localization of sema4D and plxnB1 at this time point. Neural retina and RPE/choroid (posterior eyecup) fractions dissected from WT rat eyes were first analyzed by immunoblotting. Figure 3a shows that plxnB1 is detected in the RPE/choroid but not in the neural retina (NR). In contrast, sema4D is enriched in the neural retina. Depletion of the RPE marker protein RPE65 from the neural retina fraction confirmed acceptable purity of isolated fractions. We next sought to complement these experiments with microscopy of fixed tissue sections. Unfortunately, despite considerable effort we were unable to obtain specific immunofluorescence signals from available commercial antibodies to these proteins in sections from rat or mouse eye tissues that were extensively processed for cryoprotection or paraffin embedding. As an alternative, we therefore immunostained live or gently fixed whole mount retinas and posterior eyecups from WT rat eyes. To study receptor localization in the RPE, we labeled plxnB1 in whole mount samples of freshly fixed posterior eyecups after removal of the neural retina. PlxnB1 was detected in RPE cells (Fig. 3b, x-y) and importantly, co-staining with marker protein ZO-1 and x-z confocal scanning showed that the receptor localizes to the apical side of the RPE (Fig. 3b, x-z).
Fig. 3.
Sema4D localizes to neural retina and the neighboring RPE in the rat eye while plexin B1 localizes to RPE only. Tissue samples were harvested 1 hour before light onset. a Immunoblotting detection of plxnB1 (200 kDa), sema4D (150 kDa), the RPE marker protein RPE65 (65 kDa) and porin (31 kDa) as loading control in neural retina (NR) and RPE/choroid (RPE/C) fractions isolated from wt rat b Immunofluorescence microscopy of flat mounted posterior eyecup RPE facing up labeled after fixation and permeabilization. Maximal projections of x-y confocal image stacks showing ZO-1 (shown in green), cell nuclei (shown in blue) and plxnB1 (shown in red) at the apical surface of RPE in WT rat eye whole mount (main panel). x-z projections show apical localization of plxnB1 as compared to ZO-1. Scale bar: 10 μm. c Maximal projections of flatmounted posterior eyecup with RPE facing up labeled live with sema4D and secondary antibodies (shown in red) followed by fixation, mounting and counterstain of nuclei with DAPI (shown in blue). Scale bar: 10 μm. d Maximal projections of x-y confocal image stacks showing live labeling with sema4D and secondary antibodies (shown in red) of freshly dissected WT rat retina at low magnification (upper panel, scale bar: 10 μm) and high magnification (lower panel, scale bar: 3.5 μm). e Control staining with non-specific primary antibodies followed by secondary antibody at the same two magnifications as d. Blot panels and microscopy fields show representative images obtained from three experiments performed independently.
Taking advantage of a sema4D antibody that recognizes the extracellular domain of the protein we next used it to stain live tissues that were chilled immediately after isolation. Sema4D antibody labeling of live posterior eyecups only yielded negligible staining of what appeared to be outer segments of photoreceptors that stuck to the RPE during neural retina removal (Fig. 3c). However, abundant Sema4D antibody labeling was seen at the exposed photoreceptor outer segment surface of the neural retina where it displayed a punctate pattern (Fig. 3d, lower panel shows higher magnification). Sema4D antibody labeling was specific as incubation of parallel samples with non-immune antibody only resulted in weak background staining (Fig. 3e). To identify in which types of photoreceptor sema4D is present, we next double labeled unfixed whole mount retinas with sema4D antibody and the fluorescent lectins peanut agglutinin (PNA) or wheat germ agglutinin (WGA), which label the extracellular sheaths of outer segments of cones and rods, respectively. Observing intact retinal whole mounts fixed immediately after labeling, we found sema4D staining at outer segments of cones but not of rods (Fig. 4, compare a and b). Moreover, observing single cones and rods after dissociating the stained retina followed by fixation and imaging, we found sema4D in all PNA-positive but not in WGA-positive photoreceptor cells (Fig. 4, compare c and d). In sema4D/WGA double labeling experiments, sema4D-positive cells were observed (but were not labeled with WGA) confirming that WGA staining did not interfere with WGA labeling (Fig. 4e). Altogether, these experiments demonstrate that sema4D localizes to cone outer segments in the rat retina.
Fig. 4.
Sema4D localizes to outer segments of cone photoreceptors. a Field shows isolated retina labeled live with sema4D antibody (shown in red) and fluorescent PNA marking cone outer segments (shown in green). Scale bar: 10 μm. b Field shows isolated retina labeled with sema4D antibody (shown in red) and WGA marking rod outer segments (shown in green). Scale bar: 5 μm. c, d, e Images of isolated photoreceptors double-stained with sema4D antibody (shown in red) and with PNA (shown in green) showing cells that are positive for both (c), while double-staining with sema4D antibody (shown in red) and WGA (shown in green) did not yield cells with both labels. WGA-positive cells were found but were negative for sema4D (d), while all sema4D-positive cells found were negative for WGA (e). Note that PNA and WGA were used merely to identify photoreceptors types; our experiment did not test if there was specific co-localization of sema4D and either label. Scale bar: 10 μm. All fields show representative maximal projections of x-y confocal image stacks obtained in three independent experiments.
Retinal sema4D ligand levels and plxnB1 receptor phosphorylation change at the peak of diurnal POS phagocytosis by rat RPE in vivo, and these changes are altered in mutant rats whose RPE cannot phagocytose POS
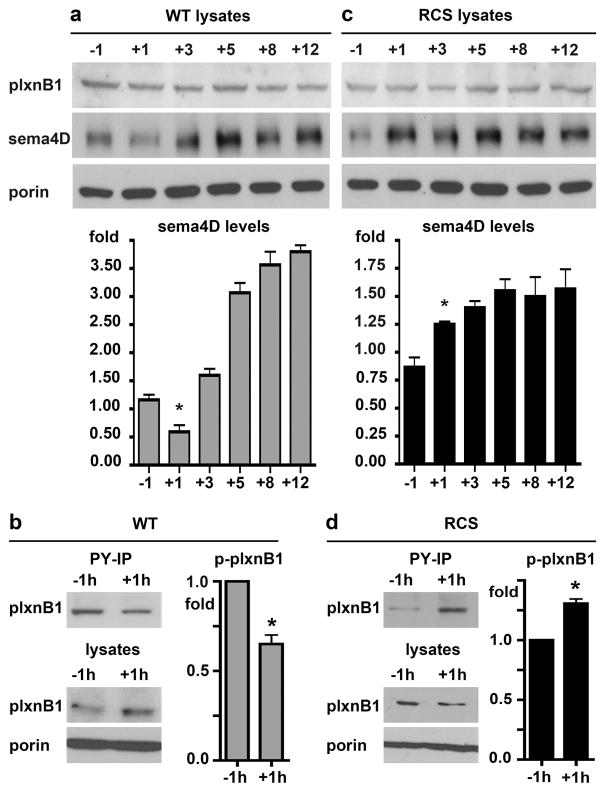
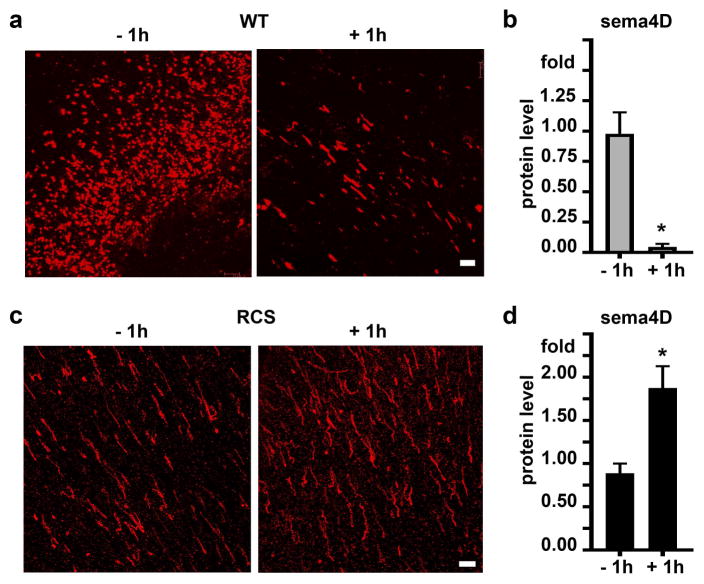
Mammalian photoreceptor outer segment shedding and RPE phagocytosis are subject to circadian regulation such that the peak of POS phagocytosis occurs daily after light onset and phagocytosis is minimal 8 hours later. To test if sema4D interaction with plxnB1 is relevant to diurnal POS phagocytosis in vivo we analyzed receptor tyrosine phosphorylation (indicative of activation by sema4D [29]) and ligand levels in retinal tissues during diurnal POS clearance. Comparison of rat retina/RPE samples harvested at different time points revealed that levels sema4D ligand in the retina significantly decreased between 1 hour before and 1 hour after light onset, which corresponds to the time of peak RPE phagocytosis activity (Fig. 5a). 3 hours after light onset, levels were similar to 1 hour before light onset (Fig. 5a). At time points past the daily burst of phagocytosis, (5, 8, and 12 hours after light onset), sema4D levels were significantly higher than at 1 hour before light onset (Fig. 5a). Like sema4D levels, plxnB1 tyrosine phosphorylation was lower at peak phagocytosis time 1 hour after light onset as compared to 1 hour before light onset (Fig. 5b). To test if these ligand and receptor changes depend on an active phagocytic pathway of the RPE, we performed the same experiments with tissues from RCS rats whose RPE fails to internalize POS. Surprisingly, both ligand level and receptor phosphorylation significantly increased by 1 hour after light onset in RCS retina/RPE and sema4D levels remained elevated at later time points tested (Fig. 5, c and d). Comparing sema4D levels specifically at outer segments by live retina whole mount tissue immunostaining (as in Fig. 4) confirmed a dramatic decrease in sema4D in WT, phagocytic rat retina from 1 hour before to 1 hour after light onset (Fig. 6, a and b). Live sema4D immunostaining in RCS whole mount retina displayed the opposite pattern (Fig. 6, c and d), with an approximate doubling of ligand staining after light onset, in agreement with the results obtained by immunoblotting.
Fig. 5.
PlxnB1 activity and sema4D protein levels decrease in WT but not RCS rat eyes at the peak time of RPE phagocytosis 1 hour after light onset. a Immunoblot of plxnB1 (200 kDa), sema4D (150 kDa) and porin (31 kDa) of WT eye samples dissected 1 hour before (−1) and 1, 3, 5, 8 and 12 hours after light onset (+1, +3, +5, +8 +12). Bar graph shows relative sema4D protein levels with time of day. b Immunoprecipitations with phosphotyrosine-antibody of WT rat eye lysates were blotted for plxnB1 yielding phospho-plxnB1 bands (upper blot, bands at 200 kDa); while immunoblotting tissue lysates (lower blots) yielded total plxnB1 (200 Da) and porin (31 kDa) as loading control. Bars show relative levels of activated phospho-plxnB1 (immunoprecipitated plxnB1 relative to total plxnB1) 1 hour after light onset compared to 1 h before light onset. c Experiments as in a analyzing sema4D and plxnB1 protein levels in RCS rat eyes. d Experiments as in b analyzing plxnB1 phosphorylation in RCS rat eyes. All experiments were performed three times independently. Representative blots are shown. Bars show densitometry quantification, mean ± SD, n = 3. Asterisks indicate significant difference of plxnB1 phosphorylation and sema4D levels 1 hour after light onset as compared to 1 hour before light, p < 0.05 by ANOVA. Differences in sema4D levels between 1 hour before and 5, 8 and 12 hour after light onset were also significant but are not marked by asterisks.
Fig. 6.
Live labeling with sema4D antibody shows change of outer retina sema4D in WT and RCS retina comparing after light onset. a Live sema4D antibody and secondary antibody labeling (shown in red) of whole mount WT retina 1 hour before and after light onset as indicated. b Quantification of signal intensity of images as in a. c Labeling of RCS rat retina as in a. d Quantification of signal intensity of images as in c. Images show representative maximal projections of x-y confocal image stacks obtained in three independent experiments. Scale bars: 10 μm. Bars show mean ± SD of three independent experiments with duplicate samples each. Asterisks indicate significant difference of sema4D levels before and after light onset, p < 0.05 by Student’s t-test.
Taken together, these results show that sema4D/plxnB1 signaling decreases specifically at the time of the phagocytic burst of the RPE and this regulation is altered in non-phagocytic RPE. The more pronounced reduction in ligand levels as observed by microscopy as compared to whole retina/RPE immunoblotting suggests that changes after light onset are likely confined to the outer segment-RPE interface.
The RPE of sema4D and plxnB1 knockout mice phagocytoses excess POS at light onset
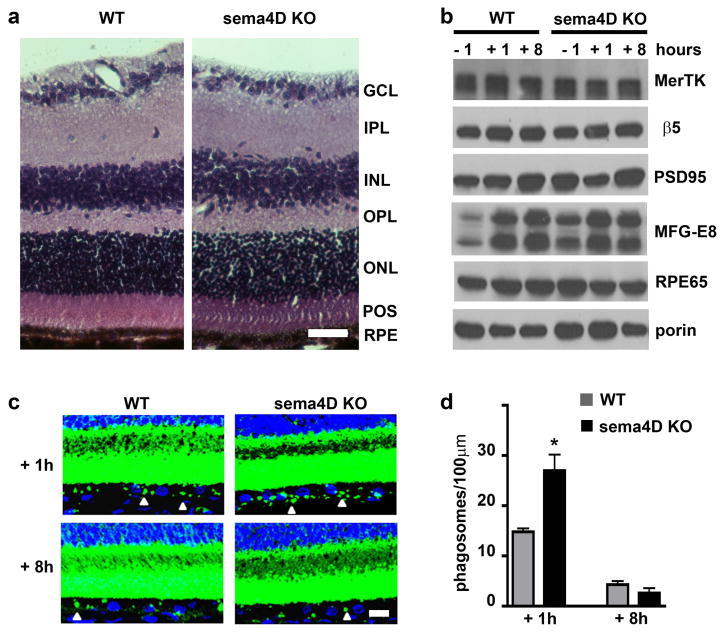
To test if sema4D/plxnB1 signaling not only responds but also contributes to the RPE phagocytosis mechanism, we next asked if lack of ligand or receptor in knockout (KO) mice affects this process. To this end, we characterized POS phagosome content of the RPE in situ 1 hour before light (just prior to POS uptake), 1 hour after light (the time of peak POS phagosome content of the RPE), and 8 hours after light (when POS phagosome content in the RPE is minimal as POS have been degraded [15]). Comparing stained sectioned retinal tissues of sema4D KO [26] and age- and strain-matched WT mice, we did not find obvious abnormalities in sema4D KO retina (Fig. 7a). Protein levels of β5 integrin and MerTK receptors, involved in POS binding and internalization, respectively, were similar in WT and sema4D KO retina/RPE extracts. The same was true for levels of PSD95 and RPE65, key photoreceptor synapse and RPE visual cycle enzyme proteins, respectively [30]. We noted 43% more MFG-E8, the physiological ligand for the POS recognition receptor αvβ5 integrin [15] before light onset in sema4D KO retina as compared to WT, but levels were comparable at the other time points (Fig. 7b).
Fig. 7.
Sema4D KO RPE harbors excess POS phagosomes at the diurnal burst of phagocytosis. a Images show comparable retinal histology in sema4D KO and WT eyes with all retinal layers present: Ganglion cell layer (GCL); inner plexiform layer (IPL); inner nuclear layer (INL); outer plexiform layer (OPL); outer nuclear layer (ONL); photoreceptor outer segments (POS); RPE. Scale bar: 30 μm. b Representative immunoblot probed for retinal and RPE markers as indicated and discussed in the text. Samples were dissected mouse eyes harvested 1 hour before (−1), 1 hour (+1) and 8 hours (+8) after light onset as indicated. Porin served as loading control. c Representative maximal projections of x-y confocal image stacks of retinal tissue sections labeled with opsin antibody (shown in green, nuclei counterstain shown in blue) obtained from mice sacrificed 1 h or 8 h after light onset as indicated. Images were intentionally overexposed to detect POS phagosomes in the RPE (arrow heads) in addition to intact outer segments. Scale bar: 10 μm. d Bars show number of phagosomes per 100 μm RPE in WT (gray bars) and sema4D KO (black bars) mouse eyes harvested 1 and 8 hours after light onset, mean ± SD, n = 5 mice per strain. Bars show number of phagosomes counted in 100 μm in each condition. Asterisks indicate significant difference between WT and KO, p < 0.05 by ANOVA.
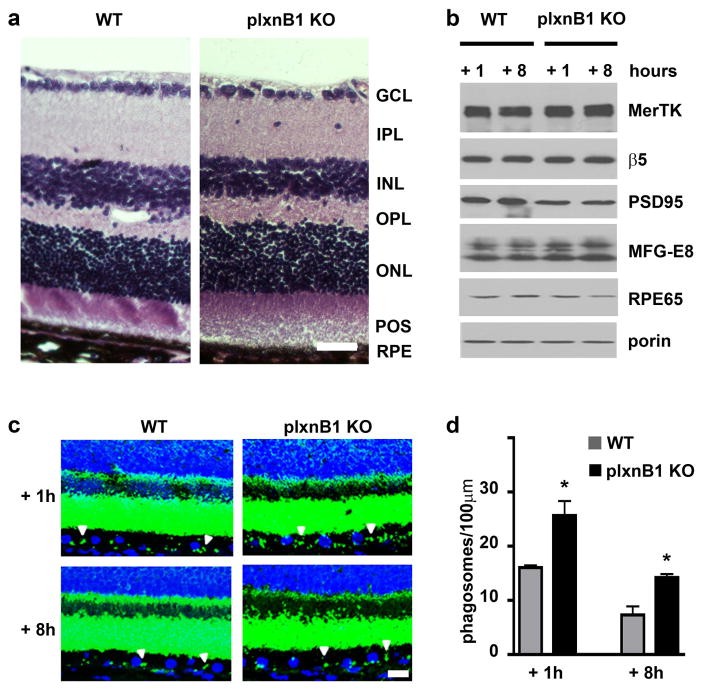
To directly compare the phagocytic activity of the RPE of sema4D KO and WT mice we next quantified the frequency of POS-opsin containing phagosomes in the RPE. In both mutant and WT retina, RPE phagosome content was significantly higher 1 hour after than 8 hours after light onset indicating that sema4D KO retina retains a diurnal rhythm of POS clearance (Fig. 7, c and d). Notably, peak phagosome frequency in sema4D KO RPE was significantly increased, by 83% as compared to WT RPE (Fig. 7d). No difference in phagosome content was observed at the late time point indicating that sema4D KO RPE was able to completely degrade engulfed POS (Fig. 7d). To determine if excess peak phagosomes in the RPE were indeed due to the specific contribution of sema4D on plxnB1 signaling we used our established experimental approaches to examine the effect of receptor KO on the process. Like for sema4D KO mice, we did not observe abnormal retinal morphology or expression of phagocytic proteins (Fig. 8, a and b). Retinal marker proteins were also normal except a ~40% reduction in PSD95. Like sema4D KO RPE, plxnB1 KO RPE contained excess phagosomes 1 hour after light onset (Fig. 8, c and d). Moreover, plxnB1 KO RPE retained significantly more phagosomes than WT RPE by 8 hours after light onset suggesting that its POS clearance may be delayed (Fig. 8d). Altogether, sema4D and plxnB1 KO retina are phenotypically similar with only minor and divergent differences in levels of specific proteins (of MFG-E8 and PSD95), the relevance of which will need to be established elsewhere. With respect to RPE phagocytosis, loss of sema4D ligand and plxnB1 receptor have the same consequence increasing peak POS phagosome levels in the RPE. Together with our findings that exogenous sema4D inhibits POS engulfment by RPE in culture (Fig. 1) and that sema4D signals through plxnB1 before but not after light onset in WT retina in vivo (Fig. 5) these results indicate that the diurnal phagocytic burst of the RPE requires inactivation of sema4D/plxnB1 signaling, which occurs in the retina after light onset.
Fig. 8.
PlxnB1 KO RPE harbors excess POS phagosomes. a Images show comparable retinal histology in PlxnB1 knockout and WT eyes with all retinal layers present: Ganglion cell layer (GCL); inner plexiform layer (IPL); inner nuclear layer (INL); outer plexiform layer (OPL); outer nuclear layer (ONL); photoreceptor outer segments (POS); RPE. Scale bar: 30 μm. b Representative immunoblot probed for retinal and RPE markers as indicated and discussed in the text. Samples were dissected mouse eyes harvested 1 hour or 8 hours after light onset as indicated. Porin served as loading control. c Representative maximal projections of x-y confocal image stacks of retinal tissue sections labeled with opsin antibody (shown in green, nuclei counterstain shown in blue) obtained from mice sacrificed 1 hour or 8 hours after light onset as indicated. Images were intentionally overexposed to detect POS phagosomes in the RPE (arrow heads) in addition to intact outer segments. Scale bar: 10 μm. d Bars show number of phagosomes per 100 μm RPE in WT (gray bars) and PlxnB1 KO (black bars) mouse eyes harvested 1 hour or 8 hours after light onset. Bars show number of phagosomes counted in 100 μm in each condition, mean ± SD, n = 5 mice per strain. Asterisks indicate significant difference between WT and KO, p < 0.05 by ANOVA.
Discussion
Expression of sema4D/plxnB1 by diverse cell types has been reported in the past years suggesting widespread functional relevance of signaling through this receptor-ligand pair. We show in this study for the first time that sema4D and plxnB1 are expressed in the retina in neighboring photoreceptor cones and RPE, respectively. In contrast to the A-subfamily plexins, which are predominantly expressed in the developing nervous system, plxnB1 are widely distributed in may tissues and cell types [31]. In the retina, we found plxnB1 receptors only in the RPE-choroid fraction. The localization of sema4D in adjacent photoreceptors matches the known mode of interaction of sema4D and plxnB1 c-termini that align in an anti-parallel fashion allowing signaling between opposing cell surfaces [32]. Sema4D dimerization in conjunction with plxnB1 complex formation [32–34] could explain the punctate pattern of sema4D that we observed in the live stained whole mount retina.
Our data reveal dynamic presence of sema4D in the retina with outer segment sema4D decreasing significantly in a diurnal fashion and after light onset. In platelets full-length transmembrane sema4D protein of ~150 kDa can be cleaved by the metalloprotease ADAM17 yielding an active extracellular soluble fragment of ~120 kDa that may interact with PlxnB1 receptors localized in distant cells [35,36]. In head and neck squamous cell carcinoma sema4D is cleaved by membrane type 1-matrix metalloproteinase (MT1-MMP) [37]. In the retina we detected by immunoblotting only a single band of ~150 kDa. The decline in sema4D levels in WT retina after light onset did not correlate with appearance of additional bands on sema4D immunoblots. Moreover, the live immunostaining of sema4D in whole mount retina using an antibody against the extracellular domain showed a punctate -rather than a diffuse- staining pattern that was restricted to cone photoreceptors. The extent of decrease after light onset observed by localized outer segment immunostaining was far greater than the decrease observed by whole retina immunoblotting suggesting that full-length sema4D may be present but not vary after light onset in areas of the retina not accessible to the live antibody labeling. Altogether, our data on localization and change after light onset lead us to conclude that sema4D in rat retina is present in the outer segment region of the neural retina as localized full-length transmembrane protein that is not cleaved by extracellular proteases to generate an extracellular fragment, which would be recognizable by our sema4D antibody. Degradation into products that cannot be recognized by our antibody likely causes the decline of detectable cone outer segment associated sema4D after light onset in WT retina. Its molecular mechanisms will need to be studied elsewhere.
The dramatic increase after light onset regulated by circadian rhythms is the primary characteristic of POS phagocytosis by the RPE in vivo. Much progress has been made identifying the basic phagocytic uptake pathway used by RPE cells that involves activating αvβ5 integrin and MerTK receptors after light onset. In contrast the underlying molecular mechanisms synchronizing the activity of this pathway to yield the daily phagocytic burst are still incompletely understood. Lack of αvβ5 integrin or its physiological ligand MFG-E8 in KO mice eliminate the daily burst resulting in a constant, non-rhythmic phagocytosis at basal levels by RPE cells instead [15,16]. Here, we show that active sema4D/plxnB1 signaling occurs mainly before light onset and inhibits POS phagocytosis. Both ligand and receptor KO mice share a phenotype of excess phagocytic burst after light onset. We propose that inhibitory sema4D/plxnB1 signaling just before light onset serves as a brake of the RPE’s phagocytic activity whose irreversible release through sema4D degradation permits the phagocytic burst.
Our experiments identify the GTPase Rac1 as one molecular target of sema4D/plxnB1 inhibitory signaling. Rac1 activity is essential and dependent on MFG-E8-αvβ5 signaling during RPE phagocytosis [19]. We found that experimentally activating Rac1 is sufficient to abolish the inhibitory effect of sema4D/plxnB1 signaling on POS phagocytosis. Thus, mechanistically, sema4D/plxnB1 signaling acts as an upstream regulatory mechanism that inhibits phagocytic integrin signaling by preventing Rac1 activation. How sema4D/plxnB1 signaling itself is synchronized remains to be studied. An important clue may be its dysregulation in retina lacking MerTK, which implies either a role for specific substrates of active MerTK or a role for molecules altered by phagocytic intake.
Taking advantage of our ability to experimentally separate the sequential steps of the phagocytic process, we determined that sema4D/plxnB1 signaling did not affect surface binding by POS, which depends on integrin αvβ5 but not MerTK. We conclude that sema4D signaling does not inhibit integrin activity of RPE cells unlike what is known from other systems [38–41]. Moreover, we did not detect any effect by sema4D/plxnB1 stimulation on Akt kinase activation by RPE cells in response to POS [20] indicating that sema4D does not act via Akt to inhibit phagocytosis although this signaling pathway activates Akt kinases in endothelial cells [13]. Finally, RhoA activity may be modulated by sema4D/plxnB1 signaling and semaphorin A39R inhibits phagocytosis by dendritic cells and neutrophils due to cell contraction and F-actin condensation that involved Rho kinase [42]. Our experiments did not detect an effect of inhibitory concentration of sema4D on levels of active RhoA in RPE cells during phagocytosis. We conclude from these signaling studies that sema4D/plxnB1 signaling inhibits POS phagocytosis by acting on Rac1 specifically.
In retina whose RPE fails to phagocytose POS such as the RCS rat retina, retinal degeneration occurs with early onset and rapid progression to photoreceptor loss and complete blindness. Although we found abnormal POS phagocytosis by RPE cells with an exacerbated phagocytic burst in both sema4D and in plxnB1 KO mice changes in retinal morphology let alone overt retinal degeneration were not apparent in either mouse strain. In sema4D and plxnB1 KO mice, excess POS phagosomes may not be immediately harmful to the RPE if their phagolysosomal systems cope with the POS load and complete digestion within 24 hours before the next phagocytic burst. These findings are similar to the overall normal tissue morphology seen in mice lacking αvβ5 integrin, whose RPE retains basal uptake activity but lacks the phagocytic burst [16]. RPE cells lacking αvβ5 gradually accumulate in their lysosomes excess autofluorescent material -often called lipofuscin- resulting in increased oxidative stress, damage to the RPE F-actin cytoskeleton, and loss of function of photoreceptors by 12 months of age [43]. For this project we were limited to studying 3–4 month-old mice, which allowed us to focus on the primary consequence of lack of sema4D/plxnB1 signaling as compared to secondary cumulative damage with age. Our data show that clearance capacity at this age is sufficient to prevent phagosome buildup. However, it is possible that daily excess phagocytic intake at light onset will eventually affect RPE turnover pathways and indirectly neural retina function in sema4D and plxnB1 KO mice.
In conclusion, we found that sema4D/plxnB1 signaling acts upstream of the known αvβ5-MerTK dependent POS phagocytosis mechanism to attenuate POS uptake by RPE cells in culture and in vivo. Our data identify sema4D and plxnB1 as physiologically relevant to the photoreceptor outer segment renewal mechanism that is essential to maintain long-term health and functionality of the retina and thus visual function.
Acknowledgments
This project was supported by NIH grant EY26215 (to S.C.F) and National Eye Institute intramural funds (to A.M.). We are grateful to Drs. Eszter Paldy and Rohini Kunar (University of Heidelberg, Germany) who generously provided the dissected tissues from plxnB1 KO mice for this study.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75(7):1389–1399. doi: 10.1016/0092-8674(93)90625-z. http://dx.doi.org/10.1016/0092-8674(93)90625-Z. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Fournier A, Nakamura F, Wang L-H, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3a receptors. Cell. 1999;99(1):59–69. doi: 10.1016/s0092-8674(00)80062-8. http://dx.doi.org/10.1016/S0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 3.Tamagnone L, Artigiani S, Chen H, He Z, Ming G-l, Song H-j, Chedotal A, Winberg ML, Goodman CS, Poo M-m, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and gpi-anchored semaphorins in vertebrates. Cell. 1999;99(1):71–80. doi: 10.1016/s0092-8674(00)80063-x. http://dx.doi.org/10.1016/S0092-8674(00)80063-X. [DOI] [PubMed] [Google Scholar]
- 4.Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Curr Op Struct Biol. 2004;14(6):669–678. doi: 10.1016/j.sbi.2004.10.010. http://dx.doi.org/10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Artigiani S, Comoglio PM, Tamagnone L. Plexins, semaphorins, and scatter factor receptors: a common root for cell guidance signals? IUBMB Life. 1999;48(5):477–482. doi: 10.1080/713803563. [DOI] [PubMed] [Google Scholar]
- 6.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The semaphorin 4D receptor plexin-B1 is a GTPase activating protein for r-ras. Science. 2004;305(5685):862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 7.Rohm B, Rahim B, Kleiber B, Hovatta I, Püschel AW. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Letters. 2000;486(1):68–72. doi: 10.1016/s0014-5793(00)02240-7. http://dx.doi.org/10.1016/S0014-5793(00)02240-7. [DOI] [PubMed] [Google Scholar]
- 8.Tong Y, Chugha P, Hota PK, Alviani RS, Li M, Tempel W, Shen L, Park H-W, Buck M. Binding of Rac1, Rnd1, and RhoD to a Novel Rho GTPase interaction motif destabilizes dimerization of the plexin-b1 effector domain. J Biol Chem. 2007;282(51):37215–37224. doi: 10.1074/jbc.M703800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong Y, Hota PK, Penachioni JY, Hamaneh MB, Kim S, Alviani RS, Shen L, He H, Tempel W, Tamagnone L, Park H-W, Buck M. Structure and function of the intracellular region of the plexin-B1 transmembrane receptor. J Biol Chem. 2009;284(51):35962–35972. doi: 10.1074/jbc.M109.056275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and Met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem. 2008;283(4):1893–1901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- 11.Vikis HG, Li W, He Z, Guan K-L. The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner. Proc Natl Acad Sci USA. 2000;97(23):12457–12462. doi: 10.1073/pnas.220421797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driessens MH, Hu H, Nobes CD, Self A, Jordens I, Goodman CS, Hall A. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol. 2001;11(5):339–344. doi: 10.1016/s0960-9822(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 13.Basile JR, Gavard J, Gutkind JS. Plexin-B1 Utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem. 2007;282(48):34888–34895. doi: 10.1074/jbc.M705467200. [DOI] [PubMed] [Google Scholar]
- 14.Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc Natl Acad Sci USA. 2012;109(21):8145–8148. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci USA. 2007;104(29):12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med. 2004;200(12):1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem. 2002;277(19):17016–17022. doi: 10.1074/jbc.M107876200. [DOI] [PubMed] [Google Scholar]
- 18.Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin- bound photoreceptors. EMBO J. 2003;22(16):4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao Y, Finnemann SC. Essential diurnal Rac1 activation during retinal phagocytosis requires αvβ5 integrin but not tyrosine kinases focal adhesion kinase or Mer tyrosine kinase. Mol Biol Cell. 2012;23(6):1104–1114. doi: 10.1091/mbc.E11-10-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulloj A, Duan W, Finnemann SC. PI 3-kinase independent role for AKT in F-actin regulation during outer segment phagocytosis by RPE cells. Exp Eye Res. 2013;113(8):9–18. doi: 10.1016/j.exer.2013.05.002. http://dx.doi.org/10.1016/j.exer.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamus G, Zam ZS, Arendt A, Palczewski K, McDowell JH, Hargrave PA. Anti-rhodopsin monoclonal antibodies of defined specificity: characterization and application. Vision Res. 1991;31(1):17–31. doi: 10.1016/0042-6989(91)90069-h. [DOI] [PubMed] [Google Scholar]
- 22.Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47(8):3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94(24):12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parinot C, Rieu Q, Chatagnon J, Finnemann SC, Nandrot EF. Large-scale purification of porcine or bovine photoreceptor outer segments for phagocytosis assays on retinal pigment epithelial cells. J Vis Exp JoVE. 2014;(94):52100. doi: 10.3791/52100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for αvβ3 and αvβ5 integrins, and protein kinase C regulates αvβ5 binding and cytoskeletal linkage. J Exp Med. 1999;190(6):861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi W, Kumanogoh A, Watanabe C, Uchida J, Wang X, Yasui T, Yukawa K, Ikawa M, Okabe M, Parnes JR, Yoshida K, Kikutani H. The Class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T Cell activation in CD100-deficient mice. Immunity. 2000;13(5):633–642. doi: 10.1016/s1074-7613(00)00063-7. http://dx.doi.org/10.1016/S1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 27.Deng S, Hirschberg A, Worzfeld T, Penachioni JY, Korostylev A, Swiercz JM, Vodrazka P, Mauti O, Stoeckli ET, Tamagnone L, Offermanns S, Kuner R. Plexin-B2, but not plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo. J Neurosci. 2007;27(23):6333–6347. doi: 10.1523/jneurosci.5381-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sethna S, Finnemann S. Analysis of photoreceptor rod outer segment phagocytosis by RPE cells in situ. Methods Mol Biol. 2013;935:245–254. doi: 10.1007/978-1-62703-080-9_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swiercz JM, Worzfeld T, Offermanns S. Semaphorin 4D signaling requires the recruitment of phospholipase C gamma into the plexin-B1 receptor complex. Mol Cell Biol. 2009;29(23):6321–6334. doi: 10.1128/MCB.00103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koulen P, Fletcher EL, Craven SE, Bredt DS, Wässle H. Immunocytochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. J Neurosci. 1998;18(23):10136–10149. doi: 10.1523/JNEUROSCI.18-23-10136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maestrini E, Tamagnone L, Longati P, Cremona O, Gulisano M, Bione S, Tamanini F, Neel BG, Toniolo D, Comoglio PM. A family of transmembrane proteins with homology to the MET-hepatocyte growth factor receptor. Proc Natl Acad Sci USA. 1996;93(2):674–678. doi: 10.1073/pnas.93.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen BJ, Robinson RA, Perez-Branguli F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature. 2010;467(7319):1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koppel AM, Feiner L, Kobayashi H, Raper JA. A 70 Amino acid region within the semaphorin domain activates specific cellular response of semaphorin family members. Neuron. 1997;19(3):531–537. doi: 10.1016/s0896-6273(00)80369-4. http://dx.doi.org/10.1016/S0896-6273(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 34.Klostermann A, Lohrum M, Adams RH, Püschel AW. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J Biol Chem. 1998;273(13):7326–7331. doi: 10.1074/jbc.273.13.7326. [DOI] [PubMed] [Google Scholar]
- 35.Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci USA. 2007;104(5):1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mou P, Zeng Z, Li Q, Liu X, Xin X, Wannemacher KM, Ruan C, Li R, Brass LF, Zhu L. Identification of a calmodulin-binding domain in sema4D that regulates its exodomain shedding in platelets. Blood. 2013;121(20):4221–4230. doi: 10.1182/blood-2012-11-470609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci USA. 2006;(24):9017–9022. doi: 10.1073/pnas.0508825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oinuma I, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1–mediated R-Ras GAP activity inhibits cell migration by regulating β(1) integrin activity. J Cell Biol. 2006;173(4):601–613. doi: 10.1083/jcb.200508204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love CA, Jones EY, Comoglio PM, Tamagnone L. Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. Faseb J. 2004;18(3):592–594. doi: 10.1096/fj.03-0957fje. [DOI] [PubMed] [Google Scholar]
- 40.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Puschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424(6947):391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 41.Walzer T, Galibert L, Comeau MR, De Smedt T. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol. 2005;174(1):51–59. doi: 10.4049/jimmunol.174.1.51. [DOI] [PubMed] [Google Scholar]
- 42.Myster F, Palmeira L, Sorel O, Bouillenne F, DePauw E, Schwartz-Cornil I, Vanderplasschen A, Dewals BG. Viral semaphorin inhibits dendritic cell phagocytosis and migration but is not essential for gammaherpesvirus-induced lymphoproliferation in malignant catarrhal fever. J Virol. 2015;89(7):3630–3647. doi: 10.1128/JVI.03634-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu C-C, Nandrot EF, Dun Y, Finnemann SC. Dietary antioxidants prevent age-related retinal pigment epithelium actin damage and blindness in mice lacking αvβ5 integrin. Free Radic Biol Med. 2012;52(3):660–670. doi: 10.1016/j.freeradbiomed.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]