Abstract
Non-human primates harbor diverse species of malaria parasites, including the progenitors of P. falciparum and P. vivax. Cross-species transmission of some malaria parasites—most notably the macaque parasite, P. knowlesi—continues to this day, compelling the scientific community to ask whether these zoonoses could impede malaria control efforts by acting as a source of recurrent human infection. Host-restriction varies considerably among parasite species and is governed by both ecological and molecular variables. In particular, the efficiency of red blood cell invasion constitutes a prominent barrier to zoonotic emergence. Although proteins expressed upon the erythrocyte surface exhibit considerable diversity both within and among hosts, malaria parasites have adapted to this heterogeneity via the expansion of protein families associated with invasion, offering redundant mechanisms of host cell entry. This molecular toolkit may enable some parasites to circumvent host barriers, potentially yielding host shifts upon subsequent adaptation. Recent studies have begun to elucidate the molecular determinants of host-specificity, as well as the mechanisms that malaria parasites use to overcome these restrictions. We review recent studies concerning host tropism in the context of erythrocyte invasion by focusing on three malaria parasites that span the zoonotic spectrum: P. falciparum, P. knowlesi, and P. vivax.
Introduction
Malaria is an infectious disease caused by a diverse clade of vector-borne Apicomplexan parasites that replicate asexually in the red blood cells of vertebrate hosts. Although over 500 malaria parasites have been isolated from avian, reptilian, and mammalian hosts [1], only five species—Plasmodium falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi—commonly produce human infections (Figure 1) [2]. While cross-species transmission of most mammalian Plasmodium species is rarely observed in nature, the expansion of human populations into biodiversity hotspots has presented opportunities for exposure to novel zoonotic parasites. This paradigm is underscored by the growing health burden posed by P. knowlesi, a parasite of macaque monkeys, which now accounts for over 70% of human malaria infections in parts of Southeast Asia [3,4].
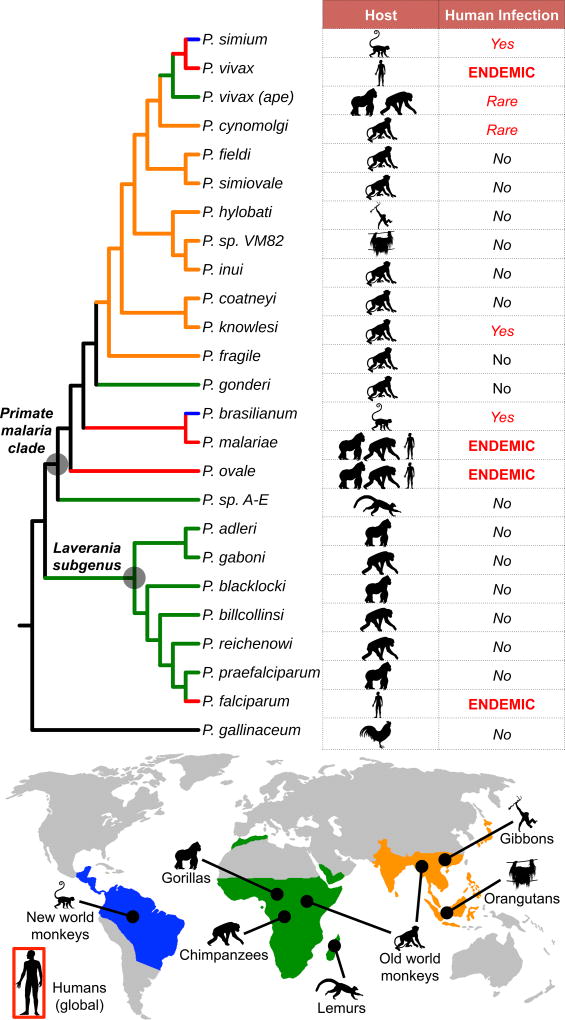
Figure 1. Evolutionary relationships of primate malaria parasites.
Neighbor-joining tree was derived from a 3486-bp gapped mtDNA alignment encompassing the cytochrome oxidase subunit 3, cytochrome oxidase subunit 1-like, and cytochrome b genes of 24 previously published non-human primate malaria parasite sequences and outgroup P. gallinaceum (GenBank accession numbers: AB354571-AB354575, AB434918, AB434919, AB444136, AB489194, AJ251941, AY791692, AY800109, AY800110, EU880499, FJ895307, GQ355477, GQ355484, HM235302, HM235369, HM235379, HQ712054, JF923761, JQ308531, and NC_008288). Lineages are colored corresponding to geographic origin of host species (blue=South America; green=Africa; orange=Asia). Human-adapted lineages, which are globally distributed, are colored red. Lineages are labeled according to host species and degree of specificity for the human host in natural settings.
However, recent advances in molecular diagnostics and the expanded sampling of wild primates have revealed that P. knowlesi is not the only malaria parasite to transcend species boundaries. The pre-civilization origins of both P. falciparum [5,6] and P. vivax [7,8]—pandemic malaria parasites, which underlie the majority of human malaria infections globally [2,9]—have recently been traced to African great apes, and cross-species transmission of other malaria parasites continues to this day (Figure 1). Indeed, the observation that most, if not all, contemporary human malaria parasites originated in non-human primate hosts has compelled the scientific community to ask whether these zoonotic reservoirs could impede malaria control efforts by acting as a source of recurrent human infection [10–12].
While it is true that the primate origins of the human malaria parasites suggest that we are predisposed to these host shifts on evolutionary timescales, the low frequency of contemporary cross-species transmission indicates that most malaria parasites must nevertheless overcome substantial ecological and molecular barriers to cross species boundaries (Box 1). Ecological factors may present barriers to cross-species transmission by influencing the probability of human exposure to the zoonotic parasite. For example, the incidence of infection in the primate reservoir, the host biting preferences of the mosquito vector, and the degree of spatial overlap between human and reservoir hosts are all ecological factors that mediate cross-species transmission potential. Given a sufficient magnitude of exposure, molecular factors may present additional barriers to emergence by influencing the efficiency of parasite replication and transmission among human hosts. Although all stages of the complex Plasmodium lifecycle (reviewed elsewhere [2]) offer opportunities for molecular incompatibility, most remain chronically understudied in the context of host-specificity. In particular, species barriers to liver stage infection and molecular incompatibilities between parasite and mosquito vector constitute important avenues for future research.
Box 1| Cross-species transmission of primate malaria parasites.
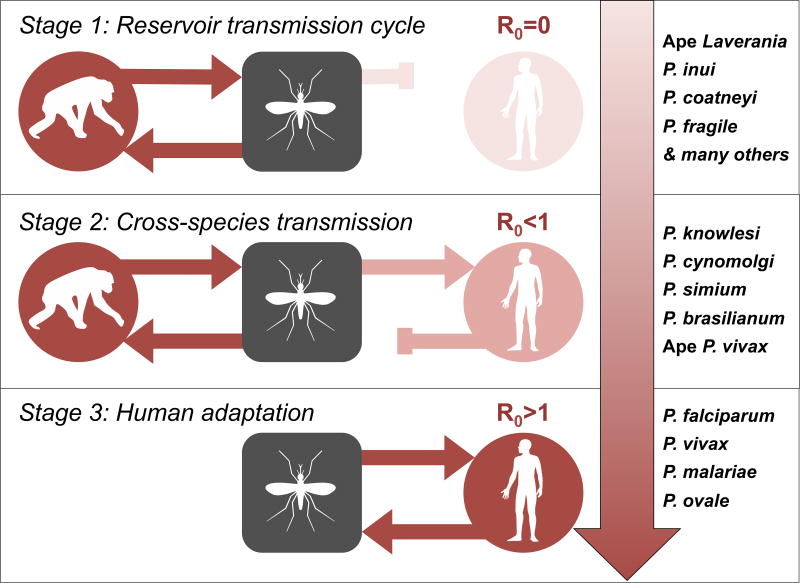
The process of zoonotic emergence is typically conceptualized as an adaptive continuum, whereby parasites are classified into one of three stages: (1) parasites transmissible within the animal reservoir, (2) parasites transmissible from animal to human (or vice versa), and (3) parasites transmissible among humans [76]. The zoonotic potential of a parasite (i.e., its capacity to transition from Stage 1 to Stage 2), is a function of both the magnitude of exposure and the probability of infection per exposure. Accordingly, barriers to cross-species transmission may relate to the epidemiology of the parasite in the reservoir host (e.g., incidence in the reservoir, vector biting preferences, geographic overlap of hosts) or to the degree of molecular compatibility at the host-parasite interface.
The basic reproductive number, R0 (i.e., the average number of secondary cases that arise from an initial primary case in a wholly susceptible population), of a parasite in the novel host defines its zoonotic potential. While many zoonotic spillover events are either incapable of forward transmission (R0=0) or produce stuttering transmission chains that eventually burn out (R0<1), subsequent adaptation may permit efficient replication and transmission within the novel host population, yielding an evolutionary host shift (i.e., Stage 3; R0>1). The natural diversity of primate malaria parasites spans this zoonotic spectrum. Four malaria parasites (i.e., P. falciparum, P. vivax, P. malariae, P. ovale) have adapted to the human population and are transmissible among human hosts [2]. Cross-species transmission of three primate species (i.e., P. knowlesi [3,4], P. simium [72], P. brasilianum [77]) have been documented with increasing frequency during recent years. While it appears that these species are either incapable of human-to-human transmission or produce stuttering transmission chains in the human population, subsequent adaption may facilitate emergence. Although several other species (i.e., P. cynomolgi [49], ape P. vivax lineages [8]) have been isolated from human hosts, these appear to be particularly rare events. Finally, most primate species (e.g., the ape Laverania [5]) appear to be either incapable of crossing species boundaries or do so inefficiently. Although P. malariae and P. ovale parasites have also been isolated from African great apes [5,78,79], the directionality of transmission (i.e., ape-to-human or human-to-ape) currently remains unclear.
Most research concerning the host-specificity of primate malaria parasites has focused upon the process of red blood cell invasion. Indeed, all malaria pathogenesis is attributable to complications of blood stage infection, and the resulting evolutionary pressures have selected for red blood cell heterogeneities that likely contribute to host restriction. In this review, we highlight recent research that has begun to elucidate the molecular mechanisms that govern variation in the host-specificity of primate malaria parasites with a particular emphasis on red blood cell invasion—the font of all malaria pathogenesis.
The cellular biology of erythrocyte invasion by the malaria parasite
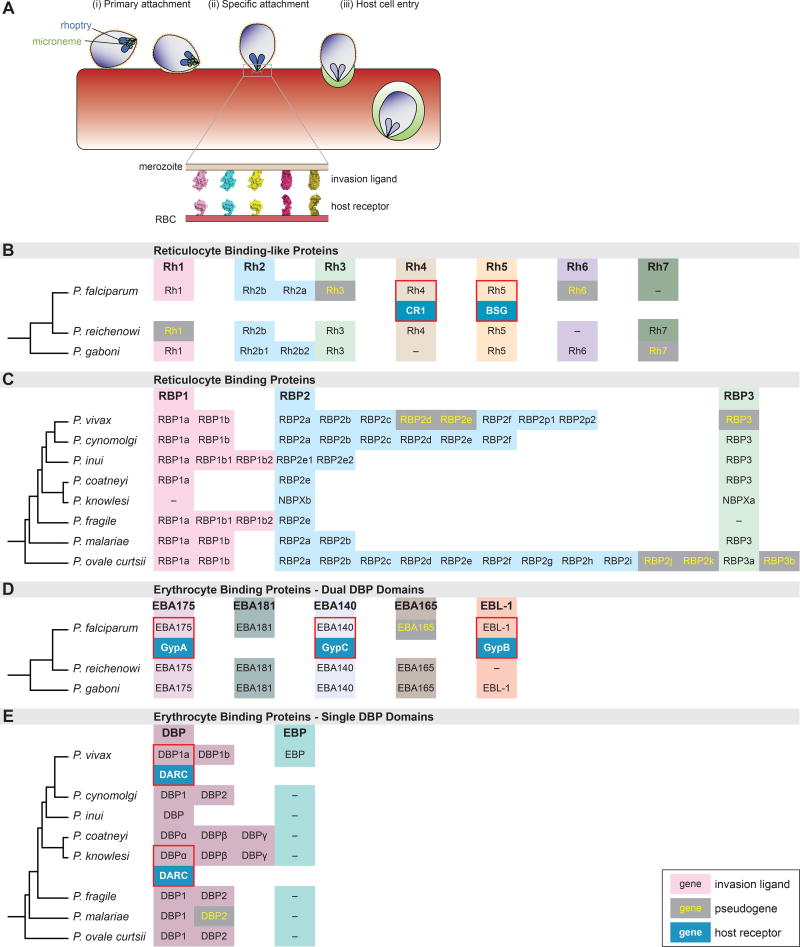
The process of erythrocyte invasion is comprised of an ordered sequence of molecular interactions between parasite ligands expressed upon the surface of the invasive merozoite and host receptors expressed upon the surface of the erythrocyte membrane (Figure 2a). Two parasite protein families play a prominent role in host tropism: (1) the erythrocyte binding-like (EBL) proteins and (2) the reticulocyte binding like (RBL) proteins [13,14]. Each EBL and RBL protein binds specifically to a receptor expressed upon the surface of the erythrocyte, irreversibly committing the parasite to invasion [13]. Despite their functional relevance, the receptors of most EBL and RBL ligands have yet to be elucidated (Figure 2b–e).
Figure 2. Malaria parasite invasion into red blood cells.
(A) Free merozoites undergo a series of events during the process of invasion beginning with (i) primary attachment, followed by (ii) specific attachment to the RBC surface and finally (iii) host cell entry [14,80,81]. Specific attachment is mediated by interactions between parasite-expressed invasion ligands (secreted from micronemes and rhoptries) and cognate host receptors on the surface of the erythrocyte.
(B–D) Diversity of parasite invasion ligands. The two major invasion ligand families involved in specific attachment, the reticulocyte binding-like proteins (RBLs) (B, C) and erythrocyte binding-like proteins (EBLs) (D, E) are shown. The invasion ligands families are further sub-divided based on the relatedness of the parasite species into either Laveranian parasites (B, D) or monkey-malaria parasites (C, E). Major subfamilies of invasion ligands (e.g. RBP1, RBP2, RBP3) are highlighted with different colors and expansions within subfamilies in each parasite species are shown in the same row as the parasite name. Invasion ligand identities were obtained from PlasmoDB. Pseudogenes, where present, are shown in grey boxes with yellow text. Where host receptors have been identified, these are indicated by blue boxes beneath the respective invasion ligand and are highlighted by a red box.
Given the magnitude of the selective pressure imposed by malaria pathogenesis during recent human evolution, it is perhaps unsurprising that proteins associated with the red blood cell constitute primary targets of parasite-induced selection [15,16]. Quintessential examples of molecular evolution in humans (e.g., sickle-cell anemia, Duffy-negativity, G6PD deficiency) are attributable to selection for polymorphisms that reduce susceptibility to severe malaria [15,17]. As a result of these selective pressures, many receptors of the EBL and RBL ligands exhibit considerable genetic diversity both within and among host species [18,19].
A growing body of evidence suggests that malaria parasites have adapted to this host cell heterogeneity via an evolutionary expansion of the EBL and RBL gene families. All Plasmodium species for which genomes have been sequenced encode multiple EBL and RBL proteins (Figure 2b–e). While genetic and expression-level variation in the EBL and RBL protein families has been implicated in immune evasion [20,21], these gene expansions also offer a degree of plasticity in host cell invasion by enabling the parasite to use multiple alternative ligand-receptor interactions—known as invasion pathways—for host cell entry. In the following sections, we explore the role that this adaptive mechanism plays in the host-specificity of three parasite species: (1) P. falciparum, (2) P. knowlesi, and (3) P. vivax.
Plasmodium falciparum and the Laverania: a highly host restricted clade of malaria parasites
Plasmodium falciparum is the most prevalent and malignant human malaria parasite, responsible for 200 million cases and 400,000 deaths annually, mostly in sub-Saharan Africa [9,22]. Recently, non-invasive sampling of non-human primate fecal samples from across equatorial Africa has revealed that the origin of P. falciparum is traceable to a sub-genus of African great ape malaria parasites, known as the Laverania [5,6] (Figure 1). Global P. falciparum isolates form a monophyletic clade embedded within the diversity of P. praefalciparum, strongly suggesting that this pandemic resulted from a single gorilla to-human cross-species transmission event [5], which occurred ~50,000 years ago [23], coinciding with the expansion of humans out of Africa.
Despite the zoonotic origins of P. falciparum, a defining feature of the Laverania is the high degree of contemporary host-specificity that these parasites exhibit. The Laverania cluster into host species-specific clades with little or no evidence of cross-species transmission, despite geographical overlap in host distributions [5,24,25]. Mosquito vectors of the ape Laverania readily bite humans, indicating that human exposure to these parasites occurs with regularity in parts of equatorial Africa [26]. This observation suggests that barriers to cross-species transmission are primarily molecular, rather than ecological. However, some human-to-ape and primate-to-primate transmission has been documented in captive environments [27,28], demonstrating that these barriers may be porous under particular epidemiological scenarios.
Plasmodium falciparum expresses an extensive repertoire of EBL and RBL invasion ligands, offering a high degree of functional redundancy during human erythrocyte invasion (Figure 2b–e). Although it was traditionally believed that no single ligand was necessary for erythrocyte invasion by all P. falciparum strains, functional experiments have since identified Rh5 as an essential determinant of erythrocyte invasion [29,30]. Hayton et al. [30] demonstrated that polymorphism in the Rh5 ligand was associated with binding to Aotus monkey erythrocytes, suggesting that this ligand may also play an important role in host tropism. The Rh5 receptor was subsequently identified to be the Ok blood group protein, Basigin (BSG) [31].
Given these findings, Wanaguru et al. [32] used a series of protein binding assays to test the hypothesis that variation in PfRh5 affinity for non-human primate BSG orthologs underlies the strong tropism of P. falciparum for human red blood cells. Indeed, PfRh5 bound to human BSG with ten-fold greater affinity relative to the chimpanzee ortholog and did not bind to gorilla BSG. Site-directed mutagenesis revealed that several amino acid substitutions in BSG accounted for this pattern of species-specific binding. A subsequent population genetic/phylogenetic analysis conducted by Forni et al. [33] found that several of these residues have undergone positive selection in great apes, while a PfRh5 residue that stabilizes the interaction with BSG has also been positively selected.
Although these findings suggest that a co-evolutionary arms race between Rh5 and BSG structures the host tropism of P. falciparum, future studies will be necessary to determine whether this mechanism reciprocally governs the host tropism of the ape Laverania. Interestingly, it was recently found that the contemporary PfRh5 allele was horizontally transferred to the P. falciparum/P. praefalciparum ancestor from a more distantly related Laveranian parasite (i.e., P. adleri), which may have enhanced its zoonotic potential [34]. While striking, this event cannot explain why P. praefalciparum is apparently incapable of infecting humans.
Moving forward, functional erythrocyte invasion assays will be necessary to confirm this host-specific phenotype in vitro, particularly in the context of multiple ligand-receptor interactions. For example, it is conceivable that while Rh5-BSG forms the foundation for Laveranian host tropism, invasion is enhanced by other receptor-ligand interactions. The recently sequenced genomes of the ape Laverania [23,35] are likely to offer additional insights. In particular, the hypothesis that pseudogenization of the PfRh3 and PfEBA165 ligands and/or duplication of PfRh2 were necessary prerequisites for expression of alternative, human-permissive invasion pathways warrants further investigation.
Monkey in the middle: the emerging public health threat posed by P. knowlesi zoonosis
In contrast to the human-adapted P. falciparum parasite, P. knowlesi is a zoonotic malaria parasite of macaque monkeys, which is responsible for a growing number of human cases in Southeast Asia [3,4]. Long mistaken for P. malariae due to its morphological similarity, molecular methods have since implicated zoonotic P. knowlesi in up to 70% of infections in rural Malaysia [3,4]. Although two reservoir hosts—long-tailed macaques (Macaca fascicularis) and pig-tailed macaques (M. nemestrina)—each harbor a high prevalence of P. knowlesi infection [36], parasites of each reservoir species appear to be reproductively isolated [37]. Although this parasite population structure appears to stem from geographic isolation, it remains unclear whether molecular restriction also plays a role.
During recent years, efforts to eliminate P. falciparum and P. vivax from Southeast Asia have coincided with an increase in the incidence of P. knowlesi, a finding that warrants additional investigation [38]. Human P. knowlesi cases form three distinct clusters—originating in long-tailed macaques in Malaysian Borneo, pig-tailed macaques in Malaysian Borneo, and long-tailed macaques in peninsular Malaysia, respectively—with no evidence of contemporary gene flow among them [39]. Although human-to-human P. knowlesi transmission has not been conclusively demonstrated in natural settings, this parasite produces transmission stages in human blood, suggesting that larger outbreaks with human-to-human transmission are not inconceivable [40]. In this section, we highlight two mechanisms that may facilitate the emergence of P. knowlesi in human hosts.
While post-translational modification of host receptors do not appear to influence erythrocyte binding of most RBL ligands, many EBL ligands rely upon the presence of sialic acids—acidic sugars that terminate glycan chains on vertebrate proteins [41]—for host cell binding. Two primary sialic acid variants are expressed on mammalian cells: N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) [41]. Neu5Ac is converted to Neu5Gc by the cytidine monophosphate Neu5Ac hydroxylase (CMAH) gene, which has been pseudogenized in the human lineage [41]. Thus, Neu5Ac is the major sialic acid variant found on human proteins, while Neu5Gc is the principal sialic acid of apes and Old World monkeys.
To evaluate the role that sialic acid plays in P. knowlesi zoonosis, Dankwa et al. [42] expressed the functional chimpanzee CMAH gene in human hematopoietic stem cells. Comparison of human cells expressing Neu5Ac to those expressing Neu5Gc revealed that the presence of the latter strongly enhances P. knowlesi invasion. In particular, the authors found that two EBL ligands—PkDBPβ and PkDBPγ— bind to erythrocytes expressing Neu5Gc, but not those expressing Neu5Ac. In contrast, PkDBPα bound to erythrocytes in a sialic acid-independent manner.
These results suggest that the usage of alternative invasion assays underlies the affinity of zoonotic P. knowlesi for human erythrocytes. While sialic acid-dependent pathways (i.e., PkDBPβ and PkDBPγ) appear to enhance P. knowlesi invasion efficiency in its natural simian host, the indifference of the PkDBPα pathway to this post-translational modification serves to bridge the fitness valley associated with the foreign cellular surface of the human host red blood cell. Nevertheless, Dankwa et al. [42] also suggest that P. knowlesi invasion and replication is less efficient in human cells relative to macaque cells, indicating that further adaptation may be necessary for widespread emergence.
Lim et al. [43] posited that expansion of P. knowlesi’s cellular niche in the human host may constitute one such adaptation. Although most human malaria parasites tend to exploit either younger reticulocytes (P. vivax [44], P. ovale [45]) or older normocytes (P. malariae [46]), P. falciparum achieves elevated parasitemia via relaxation of this restriction [47]. Lim et al. [43] demonstrated that while P. knowlesi is indifferent to the age of macaque erythrocytes, invasion is limited to reticulocytes in humans. However, long-term in vitro adaptation of P. knowlesi to human blood was associated with enhanced invasion of older erythrocytes, producing a marked increase in the rate of parasite proliferation.
Interestingly, genetic changes in P. knowlesi invasion ligands during long-term adaptation experiments appear to facilitate invasion into human erythrocytes. Dankwa et al. [42] demonstrated that amplification of the PkDBPα gene accompanied adaptation of P. knowlesi to human erythrocytes. Increased reliance upon this invasion ligand was functionally demonstrated by inhibition with the specific cytokine MGSA [42]. Moon et al. [48] have since demonstrated that although deletion of the RBL ligand PkNBPXa facilitated long-term culture of P. knowlesi in cynomologous macaque blood, doing so restricted replication in human cells. This observation suggests that PkNBPXa may be an essential determinant of human erythrocyte invasion by P. knowlesi.
These studies demonstrate that while multiple axes of host cell variation constitute potential barriers to emergence, the expression of redundant invasion pathways has endowed P. knowlesi with the functional toolkit necessary to circumvent this host restriction. As the incidence of P. knowlesi infection increases, additional research will be necessary to elucidate the compensatory ligand adaptations that underlie relaxation of reticulocyte restriction. Experimental comparison with P. cynomolgi will likely prove to be a productive avenue for future research. Plasmodium cynomolgi is a closely related zoonotic macaque parasite that has recently been isolated from human hosts [49]. Like P. knowlesi, P. cynomolgi invades macaque erythrocytes indiscriminately, but is reticulocyte-restricted in human blood, potentially limiting its zoonotic potential [50]. Kosaisavee et al. [50] revealed that this phenotype is attributable to the reliance of this parasite upon transferrin receptor 1 and the Duffy antigen/receptor for chemokines (DARC) during human erythrocyte invasion. Long-term in vitro culture of P. cynomolgi in human blood and comparison with P. knowlesi may reveal convergent mechanisms of adaptation to the human host and offer novel insight into process of zoonotic emergence.
Plasmodium vivax: a long neglected pandemic
Plasmodium vivax accounts for ~50% of all human malaria cases outside of Africa [9]. Once believed to have emerged from macaque monkeys [51,52], improved sampling of non-human primates has revealed that this pandemic originated in African great apes [7]. Indeed, human P. vivax isolates form a monophyletic clade embedded within the diversity of closely related ape P. vivax parasites. Though this pattern is superficially reminiscent of P. falciparum’s relationship to the ape Laverania, P. vivax appears to transcend species boundaries more promiscuously. First, P. vivax is readily transmissible between chimpanzees and gorillas [7], while the ape Laverania form host-specific clades [5]. Second, though rare, ape-to-human P. vivax transmission has been reported in Central Africa [8]. Finally, P. vivax has been secondarily transmitted from humans to New World monkeys multiple times, yielding a post-Columbian host shift (deemed P. simium) less than 500 years ago [12,53,54]. These observations suggest that further research into the host promiscuity of P. vivax is warranted.
The interaction between PvDBP (an EBL ligand) and DARC has long been thought to be an essential determinant of P. vivax invasion (Figure 2e) [55,56]. Indeed, P. vivax pathogenicity has favored the proliferation of a mutation in the DARC promoter, which abrogates expression of this protein on human erythrocytes [56,57]. This allele, which arose less than 33,000 years ago [58], can now be found at >95% frequency in parts of sub-Saharan Africa and underlies the near complete absence of P. vivax in human populations on the African continent [59].
However, evidence of DARC-negative P. vivax invasion has begun to accumulate in recent years [60–63], the mechanistic basis of which is a source of current debate. Gunalan et al. [64] recently analyzed P. vivax isolates from two DARC-negative Ethiopians and described a novel copy number expansion of the PvDBP gene. The authors proposed that this expansion may facilitate binding to a low-affinity receptor, though experimental support through the identification of this receptor is currently lacking. To the contrary, Hostetler et al. [65] recently demonstrated that P. vivax isolates with this PvDBP duplication are globally widespread and are unlikely to be associated with the African DARC-negative phenotype. Rather, these authors proposed that upregulation of an alternative invasion pathway—perhaps mediated by the recently described PvEBP ligand [66] or the greatly expanded PvRBP family [67,68]— may facilitate invasion of DARC-negative erythrocytes. Given the important public health implications of these findings for the spread of P. vivax in Africa, evaluation of these hypotheses will be a productive avenue for future inquiry.
Further, the functional determinants of P. vivax host-specificity remain largely unexplored. While P. vivax infection of Old World monkeys has not been documented, New World monkeys appear to be highly susceptible [69,70]. Interestingly, the CMAH gene was independently pseudogenized in the ancestor of New World monkeys [71], potentially offering a permissive, human-like cellular environment for human-adapted parasites that require the expression of Neu5Ac for invasion. If confirmed, New World monkeys could represent an ongoing source of human P. vivax/P. simium infection [70,72], potentially forming a major hurdle to malaria control efforts in South America. However, functional assays will be necessary to confirm this hypothesis, and progress may be slow until a continuous in vitro P. vivax culture system is developed [73].
Conclusions and future directions
Efforts to eliminate malaria from the human population have achieved considerable success in recent years, including a 90% reduction in the incidence of P. falciparum in Africa during the last decade [9,22]. During this period, P. knowlesi zoonosis has increased in Southeast Asia [38], and a growing body of evidence suggests that monkey-to-human transmission of P. simium has produced malaria outbreaks in areas of Brazil where elimination had been achieved [72]. While the public health community has traditionally ignored animal reservoirs in the context of malaria eradication, it is our position that the impetus to systematically evaluate the risk of malaria zoonosis has never been greater.
Moving forward, it is critical that we adopt a holistic view of malaria zoonosis that simultaneously considers the ecological and molecular barriers to emergence. Field studies will be necessary to quantify the ecological drivers of zoonosis. In particular, longitudinal sampling of high-risk human populations, primate reservoirs, and mosquito vectors will offer measures of zoonotic incidence and exposure. These epidemiological approaches will inform and contextualize laboratory studies of host-specificity at the cellular scale. Functional approaches will benefit from the recent generation of immortalized erythroid progenitor cell lines [74,75], which offer genetically tractable in vitro culture systems for the study of host-specificity. Finally, mathematical models of malaria zoonosis will be necessary to link transmission dynamics across these disparate scales of analysis. Without this integrated approach, efforts to elucidate the zoonotic potential of this diverse parasite clade are unlikely to be successful.
Highlights.
The origins of human malaria parasites have been traced to primate reservoirs.
Ecological and molecular variables govern zoonotic potential.
Erythrocyte invasion efficiency is a primary determinant of host-specificity.
Expansion of invasion-related protein families offers redundant invasion pathways.
Invasion pathway flexibility enables the parasite to overcome host barriers.
Acknowledgments
Erik J. Scully was supported by fellowships from Harvard University, the National Science Foundation, and the Cora DuBois Charitable Trust. Usheer Kanjee was supported by a Canadian Institutes of Health Research Postdoctoral Fellowship. Manoj T. Duraisingh was supported by National Institutes of Health 1R01HL139337.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
References
- 1.Borner J, Pick C, Thiede J, Kolawole OM, Kingsley MT, Schulze J, Cottontail VM, Wellinghausen N, Schmidt-Chanasit J, Bruchhaus I, et al. Phylogeny of haemosporidian blood parasites revealed by a multi-gene approach. Molecular Phylogenetics and Evolution. 2016;94:221–231. doi: 10.1016/j.ympev.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 2.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu Oa, Dondorp AM. Malaria. Lancet. 2014;383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 3.Cox-Singh J, Davis TME, Lee K-S, Shamsul SSG, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. Plasmodium knowlesi Malaria in Humans Is Widely Distributed and Potentially Life Threatening. Clinical Infectious Diseases. 2008;46:165–171. doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clinical Microbiology Reviews. 2013;26:165–184. doi: 10.1128/CMR.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W, Li Y, Learn GH, Rudicell RS, Robertson JD, Keele BF, Ndjango J-BN, Sanz CM, Morgan DB, Locatelli S, et al. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature. 2010;467:420–425. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prugnolle F, Durand P, Neel C, Ollomo B, Ayala FJ, Arnathau C, Etienne L, Mpoudi-Ngole E, Nkoghe D, Leroy E, et al. African great apes are natural hosts of multiple related malaria species, including Plasmodium falciparum. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1458–1463. doi: 10.1073/pnas.0914440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, Li Y, Shaw KS, Learn GH, Plenderleith LJ, Malenke Ja, Sundararaman Sa, Ramirez Ma, Crystal Pa, Smith AG, et al. African origin of the malaria parasite Plasmodium vivax. Nature communications. 2014;5:3346. doi: 10.1038/ncomms4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prugnolle F, Rougeron V, Becquart P, Berry A, Makanga B, Rahola N, Arnathau C, Ngoubangoye B, Menard S, Willaume E, et al. Diversity, host switching and evolution of Plasmodium vivax infecting African great apes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8123–8128. doi: 10.1073/pnas.1306004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Malaria Report. 2015 [Google Scholar]
- 10.Keeling PJ, Rayner JC. The origins of malaria: there are more things in heaven and earth…. Parasitology. 2014 doi: 10.1017/S0031182014000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rayner JC, Liu W, Peeters M, Sharp PM, Hahn BH. A plethora of Plasmodium species in wild apes: a source of human infection? Trends in parasitology. 2011;27:222–229. doi: 10.1016/j.pt.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramasamy R. Zoonotic malaria - global overview and research and policy needs. Frontiers in public health. 2014;2:123. doi: 10.3389/fpubh.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowman AF, Berry D, Baum J. The cell biology of disease: The cellular and molecular basis for malaria parasite invasion of the human red blood cell. The Journal of Cell Biology. 2012;198:961–971. doi: 10.1083/jcb.201206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tham W, Healer J, Cowman AF. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends in Parasitology. 2012;28:23–30. doi: 10.1016/j.pt.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Carter R, Mendis KN. Evolutionary and Historical Aspects of the Burden of Malaria. Clinical Microbiology Reviews. 2002;15:564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. American Journal of Human Genetics. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockett Ka, Clarke GM, Fitzpatrick K, Hubbart C, Jeffreys AE, Rowlands K, Craik R, Jallow M, Conway DJ, Bojang Ka, et al. Reappraisal of known malaria resistance loci in a large multicenter study. Nature Genetics. 2014;46:1197–1204. doi: 10.1038/ng.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demogines A, Truong KA, Sawyer SL. Species-specific features of DARC, the primate receptor for Plasmodium vivax and Plasmodium knowlesi. Molecular Biology and Evolution. 2012;29:445–449. doi: 10.1093/molbev/msr204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HY, Tang H, Shen CKJ, Wu CI. Rapidly Evolving Genes in Human. I. The Glycophorins and Their Possible Role in Evading Malaria Parasites. Molecular Biology and Evolution. 2003;20:1795–1804. doi: 10.1093/molbev/msg185. [DOI] [PubMed] [Google Scholar]
- 20.Persson KEM, Fowkes FJI, McCallum FJ, Gicheru N, Reiling L, Richards JS, Wilson DW, Lopaticki S, Cowman AF, Marsh K, et al. Erythrocyte-Binding Antigens of Plasmodium falciparum Are Targets of Human Inhibitory Antibodies and Function To Evade Naturally Acquired Immunity. The Journal of Immunology. 2013;191:785–794. doi: 10.4049/jimmunol.1300444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyer JK, Amaladoss A, Ganesan S, Preiser PR. Variable expression of the 235 kDa rhoptry protein of Plasmodium yoelii mediate host cell adaptation and immune evasion. Molecular Microbiology. 2007;65:333–346. doi: 10.1111/j.1365-2958.2007.05786.x. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto TD, Gilabert A, Crellen T, Böhme U, Arnathau C, Sanders M, Oyola S, Okouga AP, Boundenga L, Wuillaume E, et al. Genomes of an entire Plasmodium subgenus reveal paths to virulent human malaria. 2016 doi: 10.1038/s41564-018-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Délicat-Loembet L, Rougeron V, Ollomo B, Arnathau C, Roche B, Elguero E, Moukodoum ND, Okougha A-P, Mve Ondo B, Boundenga L, et al. No Evidence for Ape Plasmodium Infections in Humans in Gabon. Plos One. 2015;10:e0126933. doi: 10.1371/journal.pone.0126933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundararaman SA, Liu W, Keele BF, Learn GH, Bittinger K, Mouacha F, Peeters M, Sharp PM, Bushman FD, Hahn BH. Plasmodium falciparum-like parasites infecting wild apes in southern Cameroon do not represent a recurrent source of human malaria. Proceedings of the National Academy of Sciences. 2013;110:7020–7025. doi: 10.1073/pnas.1305201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Makanga B, Yangari P, Rahola N, Rougeron V, Elguero E, Boundenga L, Moukodoum ND, Okouga AP, Arnathau C, Durand P, et al. Ape malaria transmission and potential for ape-to-human transfers in Africa. Proceedings of the National Academy of Sciences. 2016 doi: 10.1073/pnas.1603008113. This article used large-scale entomological sampling and molecular diagnostics to identify the Anopheles mosquito vectors of African great ape malaria parasites in Gabon. Human landing catches revealed that vectors of ape parasites readily bite humans, suggesting that the barriers to cross-species transmission of ape malaria parasites are primarily molecular, rather than ecological. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacheco MA, Cranfield M, Cameron K, Escalante Aa. Malarial parasite diversity in chimpanzees: the value of comparative approaches to ascertain the evolution of Plasmodium falciparum antigens. Malaria journal. 2013;12:328. doi: 10.1186/1475-2875-12-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngoubangoye B, Boundenga L, Arnathau C, Mombo IM, Durand P, Tsoumbou TA, Otoro BV, Sana R, Okouga AP, Moukodoum N, et al. The host specificity of ape malaria parasites can be broken in confined environments. International Journal for Parasitology. 2016;46:737–744. doi: 10.1016/j.ijpara.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Baum J, Chen L, Healer J, Lopaticki S, Boyle M, Triglia T, Ehlgen F, Ralph SA, Beeson JG, Cowman AF. Reticulocyte-binding protein homologue 5 – An essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. International Journal for Parasitology. 2009;39:371–380. doi: 10.1016/j.ijpara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Hayton K, Gaur D, Liu A, Takahashi J, Henschen B, Singh S, Lambert L, Furuya T, Bouttenot R, Doll M, et al. Erythrocyte Binding Protein PfRH5 Polymorphisms Determine Species-Specific Pathways of Plasmodium falciparum Invasion. Cell Host and Microbe. 2008;4:40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–7. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanaguru M, Liu W, Hahn BH, Rayner JC, Wright GJ. RH5-Basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20735–40. doi: 10.1073/pnas.1320771110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *33.Forni D, Pontremoli C, Cagliani R, Pozzoli U, Clerici M, Sironi M. Positive selection underlies the species-specific binding of P. falciparum RH5 to human basigin. Molecular Ecology. 2015 doi: 10.1111/mec.13354. This study used a population genetic/phylogenetic approach to identify signatures of selection in the P. falciparum Rh5 invasion ligand and its corresponding receptor, BSG. Residues implicated in stabilizing the Rh5-BSG interaction were demonstrated to be under positive selection, suggesting that host-parasite co-evolution structures the host-specific binding of P. falciparum. [DOI] [PubMed] [Google Scholar]
- **34.Sundararaman SA, Plenderleith LJ, Liu W, Loy DE, Learn GH, Li Y, Shaw KS, Ayouba A, Peeters M, Speede S, et al. Genomes of cryptic chimpanzee Plasmodium species reveal key evolutionary events leading to human malaria. Nature Communications. 2016;7:11078. doi: 10.1038/ncomms11078. This study sequenced the Rh5 locus of great ape parasites in the Laverania sub-genus and used phylogenetic methods to reveal that the Rh5 gene was horizontally transferred from P. adleri to the ancestor of P. falciparum/P. praefalciparum. These results suggest that this rare evolutionary event may have facilitated the emergence of P. falciparum in the human population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otto TD, Rayner JC, Böhme U, Pain A, Spottiswoode N, Sanders M, Quail M, Ollomo B, Renaud F, Thomas AW, et al. Genome sequencing of chimpanzee malaria parasites reveals possible pathways of adaptation to human hosts. Nature communications. 2014;5:4754. doi: 10.1038/ncomms5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K-S, Divis PCS, Zakaria SK, Matusop A, Julin RA, Conway DJ, Cox-Singh J, Singh B. Plasmodium knowlesi: reservoir hosts and tracking the emergence in humans and macaques. PLoS pathogens. 2011;7:e1002015. doi: 10.1371/journal.ppat.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Divis PCS, Singh B, Anderios F, Hisam S, Matusop A, Kocken CH, Assefa SA, Duffy CW, Conway DJ. Admixture in humans of two divergent Plasmodium knowlesi populations associated with different macaque host species. PLoS pathogens. 2015;11:e1004888. doi: 10.1371/journal.ppat.1004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.William T, Jelip J, Menon J, Anderios F, Mohammad R, Awang Mohammad TA, Grigg MJ, Yeo TW, Anstey NM, Barber BE. Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malaria journal. 2014;13:390. doi: 10.1186/1475-2875-13-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Divis PCS, Lin LC, Rovie-Ryan JJ, Kadir KA, Anderios F, Hisam S, Sharma RSK, Singh B, Conway DJ. Three divergent subpopulations of the malaria parasite plasmodium knowlesi. Emerging Infectious Diseases. 2017;23:616. doi: 10.3201/eid2304.161738. The authors genotype 182 P. knowlesi isolates at 10 microsatellite loci to reveal that human infections comprise three distinct clusters of, originating in pig-tailed macaques in Malaysian Borneo, long-tailed macaques in Malaysian Borneo, and long-tailed macaques in peninsular Malaysia, respecively. These analyses found no evidence of gene flow among clusters, indicating that these populations are reproductively isolated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh B, Sung LK, Matusop A, Radhakrishnan A, Shamsul SSG, Cox-singh J, Thomas A. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. The Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 41.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Laboratory investigation; a journal of technical methods and pathology. 2007;87:851–7. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **42.Dankwa S, Lim C, Bei AK, Jiang RHY, Abshire JR, Patel SD, Goldberg JM, Moreno Y, Kono M, Niles JC, et al. Ancient human sialic acid variant restricts an emerging zoonotic malaria parasite. Nature Communications. 2016;7:11187. doi: 10.1038/ncomms11187. The authors of this study expressed the chimpanzee CMAH gene in human hematopoietic stem cells and demonstrated that two P. knowlesi invasion ligands (PkDBPβ and PkDBPγ) bind to cells expressing Neu5Gc (the sialic acid of Old World monkeys), while PkDBPα binds cells in a sialic acid independent manner. These results suggest that the zoonotic emergence of P. knowlesi in the human population is attributable to the tolerance of PkDBPα for human sialic acid variant Neu5Ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim C, Hansen E, DeSimone TM, Moreno Y, Junker K, Bei A, Brugnara C, Buckee CO, Duraisingh MT. Expansion of host cellular niche can drive adaptation of a zoonotic malaria parasite to humans. Nature communications. 2013;4:1638. doi: 10.1038/ncomms2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitchen SF. The Infection of Reticulocytes by Plasmodium vivax. The American Journal of Tropical Medicine and Hygiene. 1938;1:347–359. [Google Scholar]
- 45.Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clinical microbiology reviews. 2005;18:570–581. doi: 10.1128/CMR.18.3.570-581.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitchen SF. The Infection of Mature and Immature Erythrocytes by Plasmodium falciparum and Plasmodium malariae. The American Journal of Tropical Medicine and Hygiene. 1939;1:47–62. [Google Scholar]
- 47.Chotivanich K, Udomsangpetch R, Simpson JA, Newton P, Pukrittayakamee S, Looareesuwan S, White NJ. Parasite multiplication potential and the severity of falciparum malaria. The Journal of infectious diseases. 2000;181:1206–1209. doi: 10.1086/315353. [DOI] [PubMed] [Google Scholar]
- *48.Moon RW, Sharaf H, Hastings CH, Shwen Y, Nair MB, Rchiad Z. Normocyte-binding protein required for human erythrocyte invasion by the zoonotic malaria parasite Plasmodium knowlesi. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:7231–7236. doi: 10.1073/pnas.1522469113. The authors of this article demonstrated that the deletion of the PkNBPXa invasion ligand facilitated long-term culture of P. knowlesi in cynomologous macaque red blood cells but restricted replication in human cells. This result suggests that the PkNBPXa gene is an essential determinant of P. knowlesi growth in humans and implicates invasion ligand deletion in host tropism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malaria journal. 2014;13:68. doi: 10.1186/1475-2875-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kosaisavee V, Suwanarusk R, Chua ACY, Kyle DE, Malleret B, Zhang R, Imwong M, Imerbsin R, Ubalee R, Sámano-Sánchez H, et al. Strict Tropism for CD71+/ CD234+ Human Reticulocytes Limits Plasmodium cynomolgi ’s Zoonotic Potential. Blood. 2017 doi: 10.1182/blood-2017-02-764787. [no volume] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mu J, Joy DA, Duan J, Huang Y, Carlton J, Walker J, Barnwell J, Beerli P, Charleston MA, Pybus OG, et al. Host Switch Leads to Emergence of Plasmodium vivax Malaria in Humans. Molecular Biology and Evolution. 2005;22:1686–1693. doi: 10.1093/molbev/msi160. [DOI] [PubMed] [Google Scholar]
- 52.Escalante AA, Cornejo OE, Freeland DE, Poe AC, Durrego E, Collins WE, Lal AA. A monkey’s tale: The origin of Plasmodium vivax as a human malaria parasite. Proceedings Nation Academy of Sciences USA. 2005;102:1980–1985. doi: 10.1073/pnas.0409652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camargos Costa D, Pereira de Assis GM, de Souza Silva FA, Araújo FC, de Souza Junior JC, Braga Hirano ZM, Satiko Kano F, Nóbrega de Sousa T, Carvalho LH, Ferreira Alves de Brito C. Plasmodium simium, a Plasmodium vivax-Related Malaria Parasite: Genetic Variability of Duffy Binding Protein II and the Duffy Antigen/Receptor for Chemokines. Plos One. 2015;10:e0131339. doi: 10.1371/journal.pone.0131339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tazi L, Ayala FJ. Unresolved direction of host transfer of Plasmodium vivax v. P. simium and P. malariae v. P. brasilianum. Infection, Genetics and Evolutin. 2011;11:209–221. doi: 10.1016/j.meegid.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Miller LH, McAuliffe FM, Mason SJ. Erythrocyte receptors for malaria merozoites. The American journal of tropical medicine and hygiene. 1977;26:204–208. doi: 10.4269/ajtmh.1977.26.204. [DOI] [PubMed] [Google Scholar]
- 56.Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, Miller LH. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- 57.Miller LH, Mason SJ, Clyde DF, McGinniss MH. The Resistance Factor to Plasmodium vivax in Blacks. New England Journal of Medicine. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 58.Hedrick PW. Resistance to malaria in humans: the impact of strong, recent selection. Malaria Journal. 2012;11:349. doi: 10.1186/1475-2875-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, Zimmerman PA, Barnadas C, Beall CM, Gebremedhin A, et al. The global distribution of the Duffy blood group. Nature communications. 2011;2:266. doi: 10.1038/ncomms1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan JR, Stoute JA, Amon J, Dunton RF, Mtalib R, Koros J, Owour B, Luckhart S, Wirtz RA, Barnwell JW, et al. Evidence for transmission of Plasmodium vivax among a Duffy antigen negative population in western Kenya. The American journal of tropical medicine and hygiene. 2006;75:575–581. [PubMed] [Google Scholar]
- 61.Cavasini CE, Mattos LC de, Couto ÁAD, Bonini-Domingos CR, Valencia SH, Neiras WC de S, Alves RT, Rossit ARB, Castilho L, Machado RLD. Plasmodium vivax infection among Duffy antigen-negative individuals from the Brazilian Amazon region: an exception? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:1042–1044. doi: 10.1016/j.trstmh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Ménard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, Thonier V, Carod JF, Domarle O, Colin Y, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5967–5971. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mendes C, Dias F, Figueiredo J, Mora VG, Cano J, de Sousa B, do Rosário VE, Benito A, Berzosa P, Arez AP. Duffy negative antigen is no longer a barrier to Plasmodium vivax - molecular evidences from the African West Coast (Angola and Equatorial Guinea) PLoS Neglected Tropical Diseases. 2011;5:2–7. doi: 10.1371/journal.pntd.0001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *64.Gunalan K, Lo E, Hostetler JB, Yewhalaw D, Mu J. Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proceedings Nation Academy of Sciences USA. 2016;113:6271–6276. doi: 10.1073/pnas.1606113113. This study describes an expansion of the PvDBP invasion ligand in P. vivax isolates from DARC-negative patients—traditionally thought to be refractory to this parasite—in Ethiopia. The authors suggest that this mechanism may facilitate the invasion of DARC-negative hosts via use of an alternative low-affinity receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **65.Hostetler JB, Lo E, Kanjee U, Amaratunga C, Suon S, Sreng S, Mao S, Yewhalaw D, Mascarenhas A, Kwiatkowski DP, et al. Independent Origin and Global Distribution of Distinct Plasmodium vivax Duffy Binding Protein Gene Duplications. PLoS Neglected Tropical Diseases. 2016 doi: 10.1371/journal.pntd.0005091. This study demonstrated that a copy number expansion of the PvDBP invasion ligand is present in Cambodian P. vivax isolates but is not associated with DARC genotype. This result suggests that P. vivax invades red blood cells of DARC-negative hosts via a different mechanism. The authors propose that an alternative invasion ligand-receptor interaction may govern the capacity of P. vivax to invade these hosts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hester J, Chan ER, Menard D, Mercereau-Puijalon O, Barnwell J, Zimmerman Pa, Serre D. De Novo Assembly of a Field Isolate Genome Reveals Novel Plasmodium vivax Erythrocyte Invasion Genes. PLoS Neglected Tropical Diseases. 2013;7 doi: 10.1371/journal.pntd.0002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–763. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hietanen J, Chim-ong A, Chiramanewong T, Gruszczyk J, Roobsoong W, Tham W-H, Sattabongkot J, Nguitragool W. Gene models, expression repertoire, and immune response of Plasmodium vivax reticulocyte binding proteins. Infection and immunity. 2016;84:677–685. doi: 10.1128/IAI.01117-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Araújo MS, Messias MR, Figueiró MR, Gil LHS, Probst CM, Vidal NM, Katsuragawa TH, Krieger Ma, Silva LHP Da, Ozaki LS. Natural Plasmodium infection in monkeys in the state of Rondônia (Brazilian Western Amazon) Malaria journal. 2013;12:180. doi: 10.1186/1475-2875-12-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Castro Duarte AMR, dos Santos Malafronte R, Cerutti C, Curado I, de Paiva BR, Maeda AY, Yamasaki T, Summa MEL, de Andrade D do VD, de Oliveira SG. Natural Plasmodium infections in Brazilian wild monkeys: reservoirs for human infections? Acta tropica. 2008;107:179–185. doi: 10.1016/j.actatropica.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 71.Springer SA, Diaz SL, Gagneux P. Parallel evolution of a self-signal: humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics. 2014;66:671–674. doi: 10.1007/s00251-014-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **72.Brasil P, Zalis MG, de Pina-Costa A, Siqueira AM, Júnior CB, Silva S, Areas ALL, Pelajo-Machado M, de Alvarenga DAM, da Silva Santelli ACF. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: a molecular epidemiological investigation. The Lancet Global Health. 2017 doi: 10.1016/S2214-109X(17)30333-9. This study analyzed blood samples collected from howler monkeys and symptomatic human patients with a history of travel within the Rio de Janeiro Atlantic Forest, Brazil. Mitochondrial DNA sequencing revealed that all 28 human samples analyzed were attributable to P. simium, the result of zoonotic monkey-to-human transmission. These results indicate that a subset of zoonotic P. simium cases have been misdiagnosed as P. vivax and that this parasite represents an underappreciated threat to malaria control efforts in this region. [DOI] [PubMed] [Google Scholar]
- 73.Golenda CF, Li J, Rosenberg R. Continuous in vitro propagation of the malaria parasite Plasmodium vivax. Proceedings Nation Academy of Sciences USA. 1997;94:6786–6791. doi: 10.1073/pnas.94.13.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurita R, Suda N, Sudo K, Miharada K, Hiroyama T, Miyoshi H, Tani K, Nakamura Y. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PloS one. 2013;8:e59890. doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trakarnsanga K, Griffiths RE, Wilson MC, Blair A, Satchwell TJ, Meinders M, Cogan N, Kupzig S, Kurita R, Nakamura Y, et al. An immortalized adult human erythroid line facilitates sustainable and scalable generation of functional red cells. Nature Communications. 2017;8:14750. doi: 10.1038/ncomms14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudson PJ, Grenfell BT. Epidemic dynamics at the human-animal interface. Science. 2009;326:1362–7. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lalremruata A, Magris M, Vivas-Martínez S, Koehler M, Esen M, Kempaiah P, Jeyaraj S, Perkins DJ, Mordmüller B, Metzger WG. Natural infection of Plasmodium brasilianum in humans: Man and monkey share quartan malaria parasites in the Venezuelan Amazon. EBioMedicine. 2015;2:1186–1192. doi: 10.1016/j.ebiom.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaiser M, Löwa A, Ulrich M, Ellerbrok H, Goffe AS, Blasse A, Zommers Z, Couacy-Hymann E, Babweteera F, Zuberbühler K, et al. Wild Chimpanzees Infected with 5 Plasmodium Species. Emerging Infectious Diseases. 2010;16:1956–1959. doi: 10.3201/eid1612.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mapua MI, Qablan Ma, Pomajbíková K, Petrželková KJ, Hůzová Z, Rádrová J, Votýpka J, Todd A, Jirků M, Leendertz FH, et al. Ecology of malaria infections in western lowland gorillas inhabiting Dzanga Sangha Protected Areas, Central African Republic. Parasitology. 2015 doi: 10.1017/S0031182015000086. [DOI] [PubMed] [Google Scholar]
- 80.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Weiss GE, Gilson PR, Taechalertpaisarn T, Tham WH, de Jong NWM, Harvey KL, Fowkes FJI, Barlow PN, Rayner JC, Wright GJ, et al. Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Plasmodium falciparum Invasion of Erythrocytes. PLoS Pathogens. 2015;11:1–25. doi: 10.1371/journal.ppat.1004670. [DOI] [PMC free article] [PubMed] [Google Scholar]