Abstract
Objective: Strong evidence shows that 85% of women with chronic pelvic pain (CPP) have musculoskeletal disorders, such as abdominal myofascial pain syndrome (AMPS). The aim of this research was to assess the efficacy of local acupuncture treatment for women with CPP secondary to AMPS unresponsive to treatment with trigger-point injection.
Materials and Methods: This pilot study involved 17 women with moderate-to-severe AMPS-related CPP. Acupuncture treatments were given at abdominal-wall trigger points once per week for 10 consecutive weeks. Pain relief was assessed with a visual analogue scale (VAS), the McGill questionnaire, and pressure dynamometer. Quality of life and psychosocial function (risk for anxiety and depression) were evaluated using the Short-Form–36 questionnaire and the Hospital Anxiety and Depression scale. Assessments were performed at baseline and after 1, 3, and 6 months of treatment.
Results: Both the VAS and McGill pain questionnaire showed significantly decreased pain intensity (VAS, P < 0.001; and McGill, P 0.049), and the effects were sustained even at 6 months after treatment.
Conclusions: Acupuncture treatment was effective for the women who participated in this study, and the current authors believe that these preliminary results suffice to recommend performing randomized controlled trials.
Keywords: : acupuncture, chronic pelvic pain, myofascial syndrome
Introduction
Chronic pelvic pain (CPP) is a serious health problem for women, directly affecting their quality of life (QoL). The etiology of CPP is unclear.1 CPP might overlap with dyspareunia and dysmenorrhea as well as with gastrointestinal, genitourinary, neurologic, endocrine, and musculoskeletal disorders, and can be affected by psychologic and sociocultural factors.2,3 Research has shown that 60% of women with this disease have never received a specific diagnosis and 20% have never had any research to elucidate the cause of the pain.4 Several studies have reported the prevalence of CPP in women, but most use hospital patient samples that are considered incapable of providing accurate estimates of the actual prevalence of CPP in the general population.5
Approximately 35% of patients who undergo laparoscopic evaluation show no changes that could define the source of the pain.3 Some evidence indicates that up to 85% of patients with CPP have associated musculoskeletal disorders, with abdominal myofascial pain syndrome (AMPS) being one of the most common among them.6–9 At the current authors' institution, ∼30% of women with CPP at the CPP outpatient clinic have been diagnosed with AMPS.10
AMPS is characterized by deep and intense abdominal pain that occurs in association with hyperirritable myofascial trigger points.11 The trigger points may be classified as active or latent,12 and are able to cause muscle spasms and autonomic phenomena.12,13 Several pathophysiologic mechanisms have been proposed to clarify the development of these trigger points, albeit with limited scientific evidence. The diagnosis is clinical and is based on a history of acyclic and severe pain in the lower abdomen or pelvis lasting for at least 6 months and having the potential to interfere with normal activities of daily living (ADL), combined with the presence of active trigger points in the abdominal wall,14 which can be identified by Carnett's maneuver (Carnett's sign)15,16 and are accompanied by limited range of motion.17 A proximal nerve block is also useful for confirming the diagnosis.3
Treatment of AMPS requires a multidisciplinary approach, with the goals of interrupting the pain cycle, abolishing the myofascial trigger points, and restoring muscle flexibility by eliminating predisposing factors and perpetuation of the pain.18 In addition to symptomatic treatment with analgesics, muscle relaxants, antidepressants, and nonsteroidal anti-inflammatory drugs (NSAIDs),13 specific treatments, such as local anesthetic blockade in trigger points,19–22 ischemic compression,13,23,24 electrotherapy,25 and Botulinum toxin26–30 may be tried. Alternative or complementary therapies have also been used increasingly for musculoskeletal pain. These therapies include acupuncture,31–35 which is well-tolerated and has few severe adverse effects. Studies characterizing and assessing the role and treatment of musculoskeletal disorders in women with CPP are scarce, despite the importance of this issue. Thus, the present study was conducted to assess the efficacy of local acupuncture treatment in women with CPP secondary to AMPS who have not had relief with local anesthetic injections.
Materials and Methods
An uncontrolled prospective clinical trial was conducted wherein patients with the clinical diagnosis of CPP secondary to AMPS were recruited and treated at the Chronic Pelvic Pain Outpatient Clinic of the Hospital das Clínicas–Faculty of Medicine of Ribeirao Preto, University of São Paulo (HC-FMRP/USP), in São Paulo, Brasil. Those who had undergone local anesthetic blockade and reported no pain relief were included. The study was approved by the Research Ethics Committee of the HC-FMRP/USP on March 28, 2011, under the University Hospital of Ribeirao Preto (Hospital das Clínicas de Ribeirao Preto; HCRP) Process Number: HCRP 14301/2010. Written informed consent was obtained from all study participants.
The following were considered inclusion criteria: premenopausal women older than age 18 diagnosed with CPP and AMPS according to the diagnostic criteria of Carnett,15,16 Ferraz,6 and Tough et al.17; and women with only one active trigger point in the abdominal wall and experiencing no clinical relief after treatment with local lidocaine blockade (5 previous sessions); women with visual analogue scale (VAS) pain scores >4.4 (moderate pain) in accordance with Jensen and collaborators.36 Women with diagnoses of endometriosis, interstitial cystitis, irritable bowel syndrome, or other conditions causing or contributing to CPP were excluded. Endometriomas or hernias confirmed by abdominal-wall ultrasonography, abdominal-wall infections, and discontinuation of treatment before completion of the study duration were also considered exclusion criteria.
Baseline pain assessments were performed in eligible patients after diagnostic confirmation of AMPS by 2 of the study physicians (J.C. ReS and O.B. PN). Pain thresholds were measured on a linear 0–10 cm VAS on which patients were instructed to mark the point indicating pain severity, with 0 corresponding to no pain and 10 to the worst pain imaginable.36,37 The severity of acyclic pain was analyzed further, using the complete form of the McGill Multidimensional Questionnaire, which includes 78 descriptors (words) organized into 4 major groups of 20 subgroups describing sensory–discriminative, affective–motivational, cognitive–evaluative, and miscellaneous components of pain and arranged in such a way as to generate a pain index based on the sum of the values attributed to each descriptor.
Algometry was performed to determine the pain threshold with a Kratos brand DDK pressure dynamometer (Kratos Equipamentos Industriais, Cotia, São Paulo-SP, Brazil), capacity 20 kgf × 5 gf. An algometer is essentially a piston equipped with an electronic sensor that records the pressure applied to specific surfaces. It has four main parts: (1) a stimulation surface; (2) a control panel; (3) a body; and (4) an interruption cable. The stimulation surface of the DDK model has 3 stimulation probes (0.5-, 1-, and 2-cm diameters), which can be used according to study needs.38 A constant force is applied by the piston to determine the pain threshold, which is defined as the first instance of discomfort or pain. The pressure is interrupted immediately after the pain threshold is reached, and the pain score is recorded.
A total of 3 measurements were performed with this device; the measurements were separated by 1-minute intervals, and the mean value of the 3 scores was recorded. Each patient was informed that the same procedure would be repeated to assess pain-tolerance threshold, which is defined as the maximum tolerable pain during continuous application of pressure. The assessment was stopped immediately after the patient reached the point of maximum tolerable pain. This test was also performed with 3 repetitions to calculate the mean of measurements.
The Medical Outcomes Study Questionnaire Short Form–36 (SF-36) for assessment of QoL was also used. The SF-36 is a generic instrument of QoL assessment that is easy to use and understand. This instrument is a multidimensional questionnaire consisting of 36 items encompassed in 8 scales or components: (1) functional capacity; (2) physical aspects; (3) pain; (4) general health status; (5) vitality; (6) social aspects; (7) emotional aspects; and (8) mental health. The final score ranges from 0 to 100, wherein 0 corresponds to worst general health status and 100 to best health status.39–41
The Hospital Anxiety and Depression (HAD) scale was used to assess anxiety and depression symptoms. The scale consists of 7 well-defined items each for anxiety (HAD-A) and depression (HAD-D). Four alternatives exist for each item, with a score from 0 to 3. The sum of scores obtained for the items of each subscale provides a total score ranging from 0 to 21. A score of 8 is the cutoff point for anxiety and a score of 9 is the cutoff point for depression.42 All assessments were performed by the same rater.
Local acupuncture treatment (Ashi points in the abdominal area) was performed by a professionally qualified expert acupuncturist (A.M.deSM). Trigger points were identified by palpation, and needles were inserted blindly and remained in situ for 25 minutes. Each session was performed once per week over a total of 10 consecutive weeks. Filiform, cylindrical, high-grade stainless-steel needles, with reduced nickel content (to minimize possible allergic reactions) and thickness ranging from 0.25mm to 0.40 mm (DBC Brand; Dong Bang Acupuncture Manufacturer, Republic of Korea, 2014; Importer: XU LI Import and Export Trade Ltd., São Caetano do Sul, SP, Brazil) were used. Patients were reassessed with the same instruments that were used at baseline at 1 week and at 1, 3, and 6 months after completing the 10-week treatment course. All assessments and reassessments were conducted by a single researcher who was not the acupuncturist (A,P.MdeS).
Statistical analysis was performed using GraphPad Prism 4.0® 32-bit executable software (GraphPad Software Inc., San Diego, CA). A paired t-test and the Mann–Whitney-U test were used for analysis of continuous data with and without normal distribution, respectively. The analysis of variance (ANOVA) test for multiple variables with Bonferroni's post-test, repeated measures ANOVA, and pairwise comparison (Tukey's test) were also used. P < 0.05 was considered to be significant.
Results
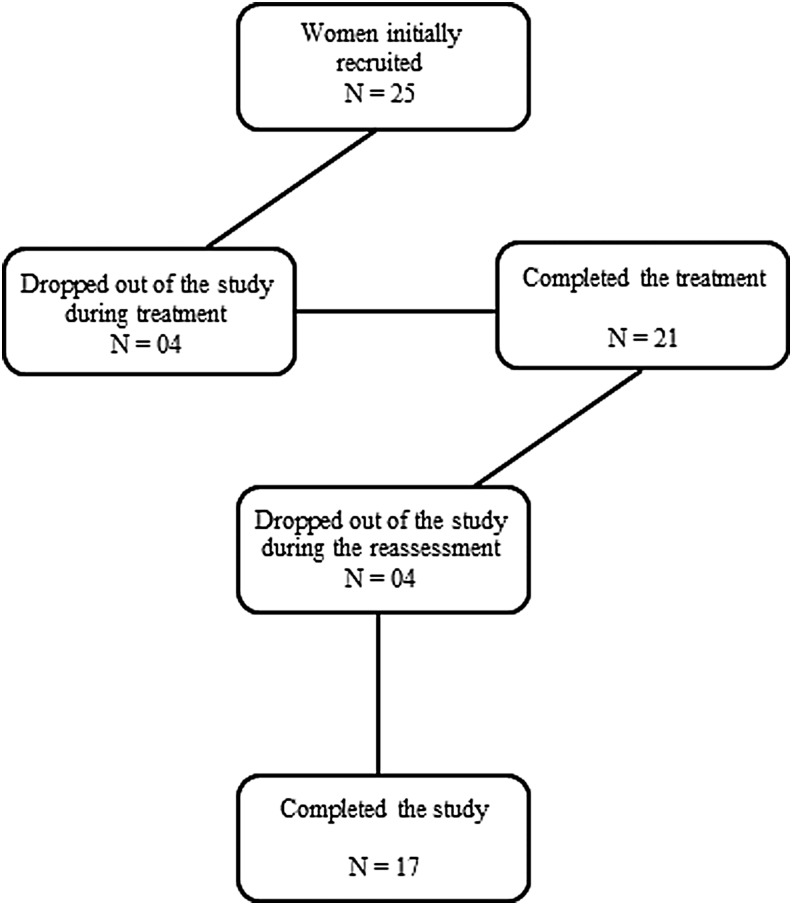
Twenty-five women with the clinical diagnosis of AMPS and having no relief with local anesthetic blockade treatment were initially included in the study. However, 4 patients discontinued the treatment for work or other reasons and 4 failed to return to perform the reassessments and did not respond to our attempts to contact them via telephone and email. Thus, 17 women completed the proposed treatment and all reassessments (Fig. 1). Patient characteristics are presented in detail in Table 1.
FIG. 1.
Recruitment flowchart.
Table 1.
Sample Characterization
| Variables | N = 17 | % |
|---|---|---|
| Age (mean/SD) | 40.64 ± 8.13 | — |
| Marital status | ||
| Single | 1 | 5.9% |
| Married/living common law | 13 | 76.5% |
| Divorced | 1 | 5.9% |
| Widow | 2 | 11.7% |
| Occupation | ||
| Housewife | 7 | 41.2% |
| Secretary | 2 | 11.76% |
| Domestic worker/housekeeper | 4 | 23.52% |
| Others | 4 | 23.52% |
| Physical activity | ||
| Yes | 2 | 11.7% |
| No | 15 | 88.3% |
| Parity | ||
| Pregnancies | 16 | 94.1% |
| Abortions | 6 | 37.5% |
| Only cesarean delivery | 8 | 50% |
| Only normal delivery | 4 | 25% |
| Cesarean and normal delivery | 4 | 25% |
SD, standard deviation.
The average age of the patients recruited for the acupuncture treatment was 40.64 ± 8.13 years. Characteristics for marital status were 76.5% (13) married/living in common law, 5.9 % (1) single, 5.9% (1) divorced, and 11.7% (2) widowed. With respect to level of physical activity, 88.3% (15) were sedentary with an average pain time of 78.56 ± 81.49 months. Regarding parity, 94.1% (16) were already mothers with an average of 2.64 children and 37.5% (6) had an average of 0.35 abortions. There were assessed rates of 50% (8) cesareans, 25% (4) normal deliveries, and 25% (4) had both cesarean and normal deliveries. Moreover, 75% (3) of the women who gave birth vaginally had some type of abdominal incision for other surgical indications and only 25 % (1) had no type of abdominal-wall incision (Table 1).
Overall, patients had significant pain relief following the acupuncture treatments, as indicated by both VAS scores (P < 0.001) and McGill questionnaire total pain scores (P = 0.049) as shown in Table 2. Conversely, also shown in Table 2, the HAD scale assessments showed no significant changes for either anxiety or depression. Neither the analysis of aspects regarding the number of descriptors and their sensory (P = 0.23), affective (P = 0.41), evaluative (P = 0.99), and miscellaneous (P = 0.59) components nor the pain index through its sensory (P = 0.44), affective (P = 0.48), evaluative (P = 0.97), and miscellaneous (P = 0.56) components showed significant changes. A significant decrease in pain intensity maintained for 6 months was noted when analyzing the mean data for different study periods. The absolute values also showed a sustained decrease in McGill scores, albeit nonsignificant, most likely because of the small number of patients. These findings are outlined in Tables 3 and 4, respectively.
Table 2.
Mean Pain Scores According to Visual Analogue Scale and Total McGill Score and Mean Risk for Anxiety and Depression According to the Hospital Anxiety and Depression Scale
| Item | T0 | Post-T | 1 month | 3 months | 6 months | P |
|---|---|---|---|---|---|---|
| VAS | 7.68 ± 1.40 | 3.55 ± 2.94 | 4.63 ± 3.16 | 4.53 ± 3.56 | 4.28 ± 3.15 | < 0.001 |
| McGill | 40.18 ± 18.29 | 24.65 ± 22.12 | 31.00 ± 20.78 | 29.59 ± 23.24 | 31.59 ± 24.98 | 0.0499 |
| HAD/A | 10.88 ± 4.04 | 9.70 ± 4.45 | 10.71 ± 5.35 | 10.35 ± 5.29 | 10.35 ± 5.23 | NS |
| HAD/D | 9.29 ± 5.92 | 7.88 ± 3.35 | 9.17 ± 3.91 | 8.76 ± 4.26 | 9.29 ± 4.67 | NS |
VAS, Visual analogue scale; McGill, McGill pain total score inventory; T0, baseline assessment; Post-T, immediately post-treatment; HAD/A, hospital anxiety and depression/anxiety scale; HAD/D, hospital anxiety and depression/depression scale; NS, not significant.
Table 3.
Mean Change in Pain Intensity by Visual Analogue Scale at 1 Week and at 1, 3, and 6 Months Post-Treatment
| Time period | Pretreatment | 1 week | 1 month | 3 months |
|---|---|---|---|---|
| Pretreatment | ||||
| 1 week | –4.13 (< 0.001) | |||
| 1 month | –3.05 (0.004) | 1.08 (NS) | ||
| 3 months | –3.15 (0.003) | 0.98 (NS) | –0.10 (NS) | |
| 6 months | –3.40 (0.001) | 0.73 (NS) | –0.35 (NS) | –0.25 (NS) |
Mean difference (P-value).
(NS), not significant.
Table 4.
Mean Change in McGill Scores at 1 Week and at 1, 3, and 6 Months Post-Treatment
| Time period | Pretreatment | 1 week | 1 month | 3 months |
|---|---|---|---|---|
| Pretreatment | ||||
| 1 week | –15.5 (0.023) | |||
| 1 month | –9.2 (NS) | 6.4 (NS) | ||
| 3 months | –10.6 (NS) | 4.9 (NS) | –1.4 (NS) | |
| 6 months | –8.6 (NS) | 6.9 (NS) | 0.6 (NS) | 2.0 (NS) |
Mean difference (P-value).
(NS), not significant.
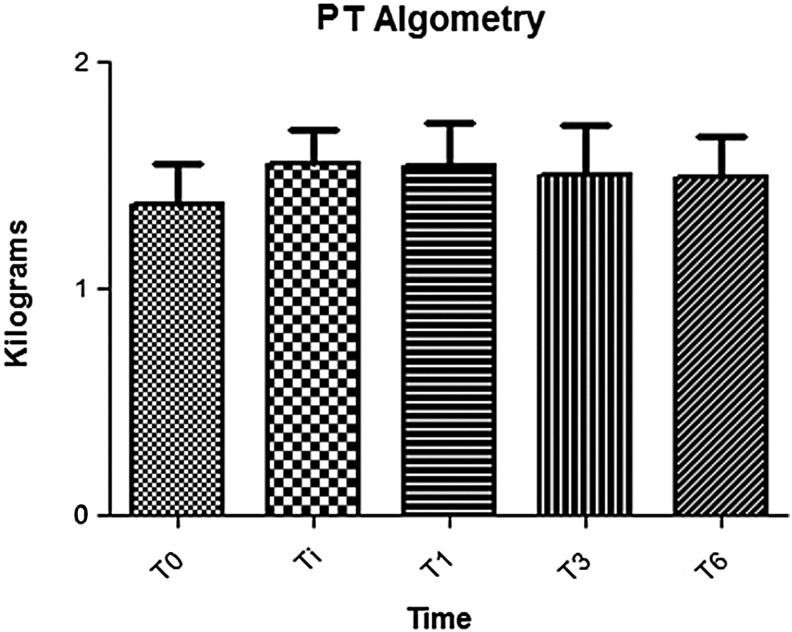
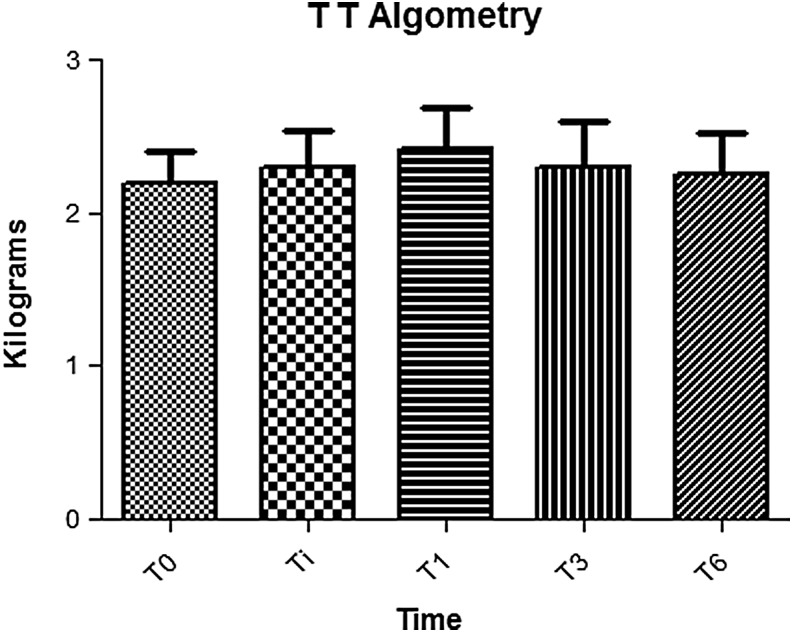
Changes in the pain threshold (P = 0.42) and pain-tolerance threshold (P = 0.81) at algometry are shown in Figures 2 and 3, respectively, and results of the SF-36 QoL assessments, which did not change significantly over the course of the study, are outlined in Table 5.
FIG. 2.
Pain algometry for pain threshold is ∼2.1–2.3 kg. PT, pain threshold; T0, baseline assessment (pretreatment); Ti, 1 week post-treatment; T1, 1 month post-treatment; T3, 3 months post-treatment; T6, 6 months post-treatment.
FIG. 3.
Pain tolerance: Maximum tolerable pain is ∼1.2-1.5 kg, which is lower than the threshold of pain. TT, pain tolerance threshold; T0, baseline assessment (pretreatment); Ti, 1 week post-treatment; T1, 1 month post-treatment; T3, 3 months post-treatment; T6, 6 months post-treatment.
Table 5.
Mean Quality of Life Scores During the Various Study Periods
| SF-36 | T0 | Post-T | 1 month | 3 months | 6 months | P |
|---|---|---|---|---|---|---|
| LPA | 22.92 ± 28.47 | 26.04 ± 33.36 | 37 ± 40.26 | 35 ± 40.82 | 37 ± 41.53 | 0.73 |
| PAIN | 19.17 ± 26.53 | 15.92 ± 21.95 | 14 ± 23.09 | 12 ± 14.72 | 11.64 ± 15.29 | 0.92 |
| GHS | 43.13 ± 10.71 | 40.92 ± 10.50 | 40.60 ± 12.77 | 40.48 ± 11.85 | 37.20 ± 15.55 | 0.58 |
| VIT | 48.54 ± 11.84 | 50 ± 11.80 | 49.40 ± 14.46 | 45.60 ± 10.34 | 49.80 ± 13.65 | 0.72 |
| SA | 49.48 ± 18.61 | 46.88 ± 12.90 | 49.50 ± 15.51 | 50.50 ± 17.49 | 48.50 ± 13.65 | 0.94 |
| EA | 44.45 ± 44.69 | 38.88 ± 38.91 | 48 ± 42.04 | 33.33 ± 39.68 | 49.34 ± 43.17 | 0.68 |
| MH | 52.17 ± 11.94 | 56.83 ± 13.50 | 57.12 ± 15.43 | 56 ± 13.22 | 54.56 ± 9.85 | 0.66 |
SF-36, quality of life questionnaire; T0, baseline (pretreatment); Post-T, immediately post-treatment; LPA, limitation due to physical aspects; GHS, general health status; VIT, vitality; SA, social aspects; EA, emotional aspects; MH, mental health.
Discussion
AMPS is one of the most common musculoskeletal disorders in patients with CPP,5 with an estimated prevalence of up to 74% among these patients. Such evidence should prompt more-comprehensive assessments of patients with CPP, covering not only gynecologic but also musculoskeletal and psychologic aspects, with the aim of formulating an interdisciplinary—and perhaps more-effective—treatment plan for these patients. Thus, in the present study, sector-standardized abdominal-pelvic and pain assessments were performed along with thorough evaluations of psychosocial aspects aimed at assessing the risks for anxiety and depression and the impacts of pain on the QoL of the women who participated in this study.
Treatment of CPP may include systemic pharmacologic therapy, including analgesics, muscle relaxants, antidepressants, and NSAIDs13 as well as targeted local treatments such as trigger-point injection, which is the most commonly used among the local therapies. However, there are potential complications of trigger-point injection, including vasovagal syncope, injection-site infection, injection-needle breakage, hematoma formation,12,13 and anesthetic-induced myotoxicity,43–46 which might worsen the pain symptoms. Trigger-point injection is also contraindicated in patients who are on anticoagulant medications or who have bleeding disorders; patients who have taken aspirin in the 72 hours prior to the procedure; and patients with local infections, allergies to anesthetic agents, acute muscle traumas, or extreme fear of needles.13
Ischemic compression is another local treatment option that has been proven to be effective for deactivating trigger points by temporarily suppressing skin perfusion at myofascial trigger point sites to promote increased oxygenation and nutrient and metabolite exchange upon reperfusion.13 Ischemic compression has the advantage of being a noninvasive and inexpensive therapy that should not increase anxiety levels of patients.22 However, although it is a relatively simple technique, if it is performed improperly—for example, without adequate analgesia—ischemic compression can exacerbate the patient's pain and perpetuate the pain cycle.
A 2015 clinical trial published by the researchers associated with the current authors' group—in which anesthetic blockade with lidocaine was compared with ischemic compression—showed that local anesthetic blockade failed to provide relief for ∼30% of women with AMPS, despite being recognized as the best treatment for this clinical condition.47
The analgesic efficacy of local treatments with electrical stimulation of myofascial trigger points has also been reported. The most commonly used methods of this type of treatment are transcutaneous electrical nerve stimulation (TENS) and interferential current therapy.12 TENS reduces pain effectively, compared with a placebo, and is an easy-to-apply, noninvasive, and low-cost method. However, TENS effects are apparently limited to the post-treatment period.12,48–50 The clinical applications of Botulinum toxin—including successful treatment of various myoinflammatory conditions—have also been increasingly reported during the past 20 years.29,30 The therapeutic action of Botulinum toxin begins within 3–10 days after injection and the effects last for ∼6 weeks to 6 months.26,27 Long-term recurrence of pain is reported in association with all proposed trigger-point treatments. In the current study, priority was given to an analysis of the responses to acupuncture treatment for women with AMPS-related CPP that had been unresponsive to anesthetic blockade treatment.
Clinical pain assessed using the VAS and McGill questionnaire showed that significant pain relief was sustained even at 6 months after acupuncture treatment. During the reassessment process, patients commonly reported pain relief accompanied by reduced impairment in performance of their ADL. There was a remarkable relief of pain after the first four treatment sessions, the treatments remained increasingly effective until the eighth session, and the benefits were sustained through the remainder of the treatment sessions and throughout the follow-up period. During the treatment period, the trigger-point examinations that were necessary to guide needle insertion during each acupuncture session showed that active trigger points were becoming less sensitive to palpation and that, in some cases, latent trigger points had disappeared. Patient reporting and rater–researcher analysis in these instances were based on subjective methods, precluding quantification. Improvements in health-related QoL associated with acupuncture treatments have been reported in other studies in conjunction with decreased pain intensity, improved capacity for ADL, regardless of the main complaint site.31,51 The current authors believe that such overall clinical improvement could be related to the systemic benefits of trigger-point treatments.
There is a close relationship between dry needling of myofascial trigger points and local acupuncture treatment. In general, there is evidence regarding the use of acupuncture as an adjuvant trigger-point therapy, although additional studies are required to assess treatment progression and specific needling procedures. In 2011, Chou et al. examined the remote effects of acupuncture on pain intensity and irritability by comparing placebo, simple dry needling, and modified acupuncture at trigger points in the upper trapezius muscle of 49 people.52 Subjects in the group treated with modified acupuncture reported greater reductions in pressure pain threshold at different pain points than subjects in the group treated with simple dry needling.
In 2008, a modified acupuncture or acupuncture-with-constant manipulations treatment similar to trigger point-injections was developed and proven to be effective in patients with fibromyalgia,53 and another recent study on the therapeutic efficacy of modified acupuncture technique, screwed in-and-out,54 showed that upper trapezius muscle trigger point irritability, measured as subjective pain intensity, pain threshold, and amplitude changes in endplate noise, could be suppressed after remote needling of acupuncture points. Nabeta and Kawakita54 have reported that the acupuncture technique known as sparrow pecking provided significant pain relief at pain points immediately after treatment, and that the pressure-pain threshold tended to increase after treatment with this type of acupuncture. Thus, studies assessing modified acupuncture methods and different modalities of simple acupuncture at pain points have shown significant relief, similar to that observed upon application of the local acupuncture method (using Ashi points) that was evaluated in the current study and proven to effective according to VAS and McGill Questionnaire data.
Conversely, in the current study, the algometry examination did not indicate significant pain relief, nor were there significant changes in psychosocial parameters analyzed with the HAD Scale and the SF-36 questionnaire. The current authors believe that this finding relates to the history of the unsuccessful “gold standard” local anesthetic–blockade treatments in these patients. Kurita and Pimenta55 reported that individuals with chronic pain mostly have a long history of pain, marked psychologic distress, physical and work compromise, and lack of confidence in treatment due to past experiences with unsatisfactory results. These findings were corroborated by the findings of the present study, specifically in terms of the aspects involving QoL and risk for anxiety and depression.
Furthermore, clinical and experimental findings reported by Chen-Yu et al56 have indicated that acupuncture may cease to be effective in an area supplied by a sensory nerve that has received local anesthetic blockade or altered by surgical sectioning, because sensory nerves are key components of acupuncture points. This could explain the results of the algometry examination in the present study, because all of the patients received acupuncture treatments at sites that had formerly been targets of local anesthetic trigger-point injections. The algometry examination depends directly on sensory receptor transduction at the skin site for assessment of the response to the tactile stimulus (pressure) applied during the examination.
Studies on acupuncture effects commonly face serious methodological limitations. Thus far, there is no universally accepted sham acupuncture method. Inert and indistinguishable control and placebo models are difficult to establish for nonpharmacologic interventions, and acupuncture is no exception. Although several studies on the potential usefulness of acupuncture have been published, most of them have inconclusive results because of issues including study design, sample size, use of inappropriate control groups, and other factors. Nevertheless, data supporting physiologic mechanisms for the benefits of acupuncture, regardless of the placebo effect, have been reported in the literature.57
However, despite the high incidence of CPP caused by AMPS, no studies addressing diagnosis and treatment associated with alternative therapies, including acupuncture, for these entities have been published. Thus, this initial research is based on studies that have addressed acupuncture treatment for patients with AMPS in other areas, primarily the trapezius muscle and the lumbar spine. The current lack of reproducible trials of acupuncture treatments for AMFS necessarily creates treatment protocols that are considered experimental. Implementation of such protocols as standard treatments is dependent directly on the success rates of such studies. Although the sample size in the present study was considered too small for statistical significance, these preliminary results should suffice to recommend performing randomized controlled trials of acupuncture treatment for women with CPP caused by AMPS, as data analysis using both VAS and the McGill Questionnaire showed clinical pain relief, even as the medical community continues to accept local anesthetic blockade as a standard treatment with a known failure rate.
The current authors believe that there is no single optimal therapy for every patient. It is crucial to perform a comprehensive assessment of any woman with CPP/AMPS, covering not only physical, but also psychologic aspects, and to use a multidisciplinary treatment approach, thereby limiting symptom recurrence and improving the quality of life of these patients.
Conclusions
The analysis of in the present study revealed that acupuncture treatment as an alternative method was feasible for women in the sample with the diagnosis of CPP secondary to AMPS refractory to local injection, particularly for pain relief and QoL improvement. Further studies conducting comprehensive assessments are necessary, and the current authors believe that RCTs are warranted for patients with CPP secondary to AMPS, with the aim to providing an effective complementary therapy that is less-invasive, less-costly, and of proven benefit for pain relief and improved QoL.
Acknowledgments
This research, as well as the free and informed consent term, were approved by the Ethics Committee of the Hospital das Clínicas of Ribeirao Preto under Process Number: HCRP 14301/2010. This research was registered with Rebec under number RBR-4Y8VD2, on June 19, 2017, at 09:52, recorded retrospectively.
Author Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Gelbaya TA, El-Halwagy HE. Focus on primary care: Chronic pelvic pain in women. Obstet Gynecol Surv. 2001;56(12):757–764 [DOI] [PubMed] [Google Scholar]
- 2.Butrick CW. Interstitial cystitis and chronic pelvic pain: New insights in neuropathology, diagnosis, and treatment. Clin Obstet Gynecol. 2003;46(4):811–823 [DOI] [PubMed] [Google Scholar]
- 3.Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101(3):594–611 [DOI] [PubMed] [Google Scholar]
- 4.Cheong Y, William Stones R. Chronic pelvic pain: Aetiology and therapy. Best Pract Res Clin Obstet Gynaecol. 2006;20(5):695–711 [DOI] [PubMed] [Google Scholar]
- 5.Ayorind AA, Macfarlane GJ, Saraswat L, Bhattacharya S. Chronic pelvic pain in women: An epidemiological perspective. Womens Health (Lond). 2015;11(6):851–864 [DOI] [PubMed] [Google Scholar]
- 6.Ferraz ED. Nerve entrapment syndrome not forgotten entity in laparoscopic era. Rev Bras Videocir. 2007;5(3):144–157 [Google Scholar]
- 7.Slocumb JC. Neurological factors in chronic pelvic pain: Trigger points and the abdominal pelvic pain syndrome. Am J Obstet Gynecol. 1984;149(5):536–543 [DOI] [PubMed] [Google Scholar]
- 8.Slocumb JC. Chronic somatic, myofascial, and neurogenic abdominal pelvic pain. Clin Obstet Gynecol. 1990;33(1):145–153 [DOI] [PubMed] [Google Scholar]
- 9.Baker PK. Musculoskeletal origins of chronic pelvic pain. Diagnosis and treatment. Obstet Gynecol Clin North Am. 1993;20(4):719–742 [PubMed] [Google Scholar]
- 10.Prendergast SA, Weiss JM. Screening for musculoskeletal causes of pelvic pain. Clin Obstet Gynecol. 2003;46(4):773–782 [DOI] [PubMed] [Google Scholar]
- 11.Montenegro ML, Gomide LB, Mateus-Vasconcelos EL, Rosa-e-Silva JC, Candido-dos-Reis FJ, Nogueira AA, Poli-Neto OB. Abdominal myofascial pain syndrome must be considered in the differential diagnosis of chronic pelvic pain. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):21–24 [DOI] [PubMed] [Google Scholar]
- 12.Farina S, Casaroto M, Benelle M, Tinazzi M, Fiaschi A, Goldoni M, Smania N. A randomized controlled study on the effect of two different treatments (FREMS AND TENS) in myofascial pain syndrome. Eura Medicophys. 2004;40(4):293–301 [PubMed] [Google Scholar]
- 13.Sharp HT. Myofascial pain syndrome of the abdominal wall for the busy clinician. Clin Obstet Gynecol. 2003;46(4):783–788 [DOI] [PubMed] [Google Scholar]
- 14.Alvarez DJ, Rockwell PG. Trigger points: Diagnosis and management. Am Fam Physician. 2002;65(4):653–660 [PubMed] [Google Scholar]
- 15.Carnett JB. Intercostal neuralgia as a cause of abdominal pain and tenderness. Surg Gynecol Obstet. 1926;42:625–632 [Google Scholar]
- 16.Carnett JB. The stimulation of gall-bladder disease by intercostal neuralgia of the abdominal wall. Ann Surg. 1927;86(5):747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tough EA, White AR, Richards S, Campbell J. Variability of criteria used to diagnose myofascial trigger point pain syndrome—evidence from a review of the literature. Clin J Pain. 2007;23(3):278–286 [DOI] [PubMed] [Google Scholar]
- 18.Yap EC. Myofascial pain—an overview. Ann Acad Med Singapore. 2007;36(1):43–48 [PubMed] [Google Scholar]
- 19.Ling FW, Slocumb JC. Use of trigger point injections in chronic pelvic pain. Obstet Gynecol Clin North Am. 1993;20(4):809–815 [PubMed] [Google Scholar]
- 20.Iwama H, Akama Y. The superiority of water-diluted 0.25% to neat 1% lidocaine for trigger-point injections in myofascial pain syndrome: A prospective, randomized, double-blinded trial. Anesth Analg. 2000;91(2):408–409 [DOI] [PubMed] [Google Scholar]
- 21.Nazareno J, Ponich T, Gregor J. Long-term follow-up of trigger point injections for abdominal wall pain. Can J Gastroenterol. 2005;19(9):561–565 [DOI] [PubMed] [Google Scholar]
- 22.Scott NA, Guo B, Barton PM, Gerwin RD. Trigger point injections for chronic non-malignant musculoskeletal pain: A systematic review. Pain Med. 2009;10(1):54–69 [DOI] [PubMed] [Google Scholar]
- 23.Sola AE, Bonica JJ. Myofascial pain syndromes. In: Febiger L, ed. The Management of Pain , 2d ed. Philadelphia: Bonica; 1990;352–367 [Google Scholar]
- 24.Bedard RJ, Kim KM, Grindstaff TL, Hart JM. Increased active hamstring stiffness after exercise in women with a history of low back pain. J Sport Rehabil. 2013;22(1):47–52 [DOI] [PubMed] [Google Scholar]
- 25.Rickards LD. The effectiveness of non-invasive treatments for active myofascial trigger point pain: A systematic review of the literature. Int J Osteopath Med. 2006;9(4):120–136 [Google Scholar]
- 26.Lang AM. Botulinum toxin type A therapy in chronic pain disorders. Arch Phys Med Rehabil. 2003;84(3[suppl1]):S69–S73 [DOI] [PubMed] [Google Scholar]
- 27.Royal MA. Botulinum toxins in pain management. Phys Med Rehabil Clin N Am. 2003;14(4):805–820 [DOI] [PubMed] [Google Scholar]
- 28.Issy AM, Sakata RK. Musculoskeletal pain. Bras J Med. Special General Clinic. 2005;10(67):3–11 [Google Scholar]
- 29.Cheng CM, Chen JS, Patel RP. Unlabeled uses of botulinum toxins: A review, part 1. Am J Health-Syst Pharm. 2006;63(2):145–153 [DOI] [PubMed] [Google Scholar]
- 30.Sinha D, Karri K, Arunkalaivanan AS. Applications of botulinum toxin in urogynaecology. Eur J Obstet Gynaecol Reprod Biol. 2007;133(1):4–11 [DOI] [PubMed] [Google Scholar]
- 31.Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point: The importance of the local twitch response. Am J Phys Med Rehabil. 1994;73(4):256–263 [DOI] [PubMed] [Google Scholar]
- 32.Kleinhenz J, Streitberger K, Windeler J, Gussbacher A, Mavridis G, Martin E. Randomised clinical trial comparing the effects of acupuncture and a newly designed placebo needle in rotator cuff tendinitis. Pain. 1999;83(2):235–241 [DOI] [PubMed] [Google Scholar]
- 33.Irnich D, Behrens N, Molzen H, et al. Randomised trial of acupuncture compared with conventional massage and “sham” laser acupuncture for treatment of chronic neck pain. BMJ. 2001;322(7302):1574–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White A; Editorial Board of Acupuncture in Medicine. Western medical acupuncture: A definition. Acupunct Med. 2009;27(1):33–35 [DOI] [PubMed] [Google Scholar]
- 35.Corujeira Rivera MC, Carregal Rañó A, Diz Gomez JC, Mayo Moldes M, Prieto Requeijo P, Arean González I. Evaluation of 2 invasive techniques for treating myofascial pain [in Spanish]. Rev Esp Anesthesiol Reanim. 2010;57(2):86–90 [DOI] [PubMed] [Google Scholar]
- 36.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4(7):407–414 [DOI] [PubMed] [Google Scholar]
- 37.Duleba AJ, Keltz MD, Olive DL. Evaluation and management of chronic pelvic pain. J Am Assoc Gynecol Laparosc. 1996;3(2):205–227 [DOI] [PubMed] [Google Scholar]
- 38.Piovesan EJ, Tatsui CE, Kowacs PA, Lange MC, Pacheco C, Werneck LC. Use of pressure algometry in determining painful trigeminal perception thresholds in healthy volunteers. Neuropsychol Arch. 2001;59(1):92–96 [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Sherbourne CD. The MOS 36 Item Short-form Health Survey (SF-36): Conceptual framework and item selection. Med Care. 1992;30(6):473–483 [PubMed] [Google Scholar]
- 40.Ciconelli RM, Ferraz MB, Santos W, Meinao I, Quaresma MR. Brazilian–Portuguese version of the SF-36: A reliable and valid quality of life outcome measure. Bras J Rheumatol. 1999;39(3):143–150 [Google Scholar]
- 41.Ware JE, Snow KK, Kosinski M, Grandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston: New England Medical Center; 1993 [Google Scholar]
- 42.Botega NJ, Bio MR, Zomignani MA, Garcia JR, Pereira WA. Mood disorders among inpatients in ambulatory and validation of the anxiety and depression scale HAD [in Portuguese]. Rev Saude Publica. 1995;29(5):355–363 [DOI] [PubMed] [Google Scholar]
- 43.Foster AH, Carlson BM. Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth Analg. 1980;59(10):727–736 [PubMed] [Google Scholar]
- 44.Zink W, Seif C, Bohl JR, et al. The acute myotoxic effects of bupivacaine and ropivacaine after continuous peripheral nerve blockades. Anesth Analg . 2003;97(4):1173–1179 [DOI] [PubMed] [Google Scholar]
- 45.Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth Pain Med. 2004;29(4):333–340 [DOI] [PubMed] [Google Scholar]
- 46.Zink W, Bohl JR, Hacke N, Sinner B, Martin E, Graf BM. The long term myotoxic effects of bupivacaine and ropivacaine after continuous peripheral nerve blocks. Anesth Analg. 2005;101(2):548–554 [DOI] [PubMed] [Google Scholar]
- 47.Montenegro ML, Braz CA, Rosa-e-Silva JC, Candido-dos-Reis FJ, Nogueira AA, Poli-Neto OB. Anaesthetic injection versus ischemic compression for the pain relief of abdominal wall trigger points in women with chronic pelvic pain. BMC Anesthesiol 2015;15:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graff-Radford SB, Reeves JL, Baker RL, Chiu D. Effects of transcutaneous electrical nerve stimulation on myofascial pain and trigger point sensitivity. Pain. 1989;37(1):1–5 [DOI] [PubMed] [Google Scholar]
- 49.Ardic F, Sarhus M, Topuz O. Comparison of two different techniques of electrotherapy on myofascial pain. J Back Musculoskelet Rehabil. 2002;16(1):11–16 [DOI] [PubMed] [Google Scholar]
- 50.Smania N, Corato E, Fiaschi A, Pietropoli P, Aglioti SM, Tinazzi M. Repetitive magnetic stimulation: A novel therapeutic approach for myofascial pain syndrome. J Neurol. 2005;252(3):307–314 [DOI] [PubMed] [Google Scholar]
- 51.MacDonald AJ, Macrae KD, Master BR, Rubin AP. Superficial acupuncture in the relief of chronic low back pain. Ann R Coll Surg 1983;65(1):44–46 [PMC free article] [PubMed] [Google Scholar]
- 52.Chou LW, Hsieh YL, Chen HS, Hong CZ, Kao MJ, Han TI. Remote therapeutic effectiveness of acupuncture in treating myofascial trigger point of the upper trapezius muscle. Am J Phys Med Rehabil. 2011;90(12):1036–1049 [DOI] [PubMed] [Google Scholar]
- 53.Chou LW, Hong JY, Hong CZ. A new technique for acupuncture therapy and its effectiveness in treating fibromyalgia syndrome: A case report. J Musculoskelet Pain. 2008;16(3):193–198 [Google Scholar]
- 54.Nabeta T, Kawakita K. Relief of chronic neck and shoulder pain by manual acupuncture to tender points: A sham-controlled randomized trial. Complement Ther Med. 2002;10(4):217–222 [DOI] [PubMed] [Google Scholar]
- 55.Kurita GP, Pimenta CA. Adherence to the treatment of chronic pain. Bras Arch Neuropsychiatry. 2003;61(2):416–425 [Google Scholar]
- 56.Chen-Yu C, Ching-Tsai C, Hsiu-Ling C, Lian-Fang Y. Peripheral afferent pathway for acupuncture analgesia. Scientia Sinica. 1973;16(1):210–217 [Google Scholar]
- 57.Medeiros R, Saad M. Acupuncture: Physiological effect beyond the placebo effect. Mundo Saude. 2009;33(1):69–72 [Google Scholar]