Abstract
Achromatopsia is an inherited retinal disorder of cone photoreceptors characterized by markedly reduced visual acuity, extreme light sensitivity, and absence of color discrimination. Approximately 50% of cases are caused by mutations in the cone photoreceptor-specific cyclic nucleotide gated channel beta subunit (CNGB3) gene. Studies in CNGB3-mutant dogs showed that subretinal injection of an AAV vector expressing human CNGB3, which has 76% amino acid identity with canine CNGB3, driven by a 2.1 kb human red cone opsin promoter (PR2.1) and packaged in AAV5 capsids (AAV5-PR2.1-hCNGB3) rescued cone photoreceptor function, but at high doses was associated with an inflammatory response (focal chorioretinitis) consistent with immune-mediated toxicity. AAV vectors containing the PR2.1 promoter packaged in AAV5 capsids and expressing either the native canine CNGB3 (AAV5-PR2.1-cCNGB3) or the human CNGB3 (AAV5-PR2.1-hCNGB3) were evaluated at different dose levels in CNGB3-mutant dogs. The vector expressing canine CNGB3 achieved somewhat better rescue of cone function but unexpectedly was associated with a greater degree of retinal toxicity than the vector expressing human CNGB3. Very low-level T-cell immune responses to some AAV or CNGB3 peptides were observed in animals that received the higher vector dose. There was a more than twofold increase in serum neutralizing antibodies to AAV in one of three animals in the low-dose group and in two of three animals in the high-dose group. No serum anti-hCNGB3 antibodies were detected in any animal. The results of this study do not support the hypothesis that the focal chorioretinitis seen with high doses of AAV5-PR2.1-hCNGB3 in the initial studies was due to an immune response to human CNGB3.
Keywords: : achromatopsia, AAV, gene therapy, chorioretinitis
Introduction
Achromatopsia, also known as rod monochromacy, is a rare, autosomal recessive congenital retinal disorder characterized by severely reduced visual acuity, pendular nystagmus, severe photophobia, a small central scotoma, eccentric fixation, and reduced or complete loss of color discrimination.1 Complete achromatopsia is an orphan disease, with an estimated prevalence of approximately 1 in 30,000.2 Unlike the usual forms of so-called “red/green color blindness” in which patients have difficulty distinguishing color differences but have normal visual acuity because S-cones and either M- or L-cones are still present, patients with complete achromatopsia have profoundly impaired visual function. Because the only functioning photoreceptors in achromatopsia are rods, which are not functional under photopic conditions, these patients experience extreme light sensitivity and daytime blindness. Best corrected visual acuity even under subdued light conditions is usually 20/200 or worse. Electroretinogram (ERG) recordings show absent or markedly diminished cone responses.1
Current management of patients with achromatopsia consists of the use of heavily tinted lenses, which reduces the photophobia and photobleaching of rhodopsin that occurs in normally illuminated public spaces, but does not address the underlying defects in visual acuity, color discrimination, or daytime blindness. Low vision aids such as high-powered magnifiers for reading are recommended and needed, even in low-light environments. There is currently no specific therapy for this disease.
The genetic basis of the disease can be established in a majority of individuals. Approximately 50% and 25% of cases, respectively, are caused by mutations in the cone photoreceptor-specific cyclic nucleotide-gated channel beta (CNGB3) or alpha (CNGA3) subunit genes,3,4 and a small number are caused by mutations in other genes involved in cone photoreceptor function.5–8 CNGA3 and CNGB3 mutations result in a loss of cone photoreceptor function in humans and in animals with mutations in the homologous genes.3,4,9–13
Studies in dog and mouse models of CNGB3-achromatopsia indicate that gene therapy using a recombinant adeno-associated virus (AAV) vector expressing human CNGB3, which has 76% amino acid identity with canine CNGB3, can correct the genetic defect and restore cone photoreceptor function.14,15 However, dogs treated with high doses of an AAV vector expressing human CNGB3 driven by a 2.1 kb human red opsin promoter and packaged in AAV5 capsids (AAV5-PR2.1-hCNGB3) developed focal chorioretinitis, an inflammatory response consistent with immune-mediated toxicity. Chorioretinitis developed only at higher vector concentrations, was easily controlled with systemic steroid treatment, and did not occur with lower vector doses.14 Similar inflammatory changes (retinitis, chorioretinitis, or vasculitis) have also been reported in dogs administered high doses of AAV vectors expressing green fluorescent protein.16
The frequent late-onset of these retinal alterations (∼6 weeks post injection) in those studies suggested that they may have been triggered by an immune-mediated response to the expressed foreign protein. To confirm and extend these findings and to evaluate the possibility that the chorioretinitis associated with high doses of AAV5-hCNGB3 vectors might be related to an adaptive immune response to human CNGB3, AAV vectors expressing either human or canine CNGB3 were constructed, and a range of dose levels of these vectors was evaluated in phenotypically normal and CNGB3-mutant dogs. Surprisingly, chorioretinitis developed in two of three eyes that received the higher dose of vector expressing canine CNGB3 and in none of three eyes that received the higher dose of vector expressing human CNGB3.
Results and Discussion
Relevance for clinical trials
Previous studies have indicated that high-dose levels of AAV5-hCNGB3 vectors can result in retinal toxicity, characterized by chorioretinitis and retinal thinning. The objectives of the current studies were to confirm and extend these findings by evaluating a range of dose levels of AAV5-CNGB3 vectors, and to evaluate the potential role of adaptive immune responses to a foreign protein (human CNGB3 in dogs) by comparing AAV vectors expressing human or canine CNGB in a dog model of CNGB3-related achromatopsia. Results of these studies will help to understand better the safety and efficacy profile of AAV vectors expressing human therapeutic genes obtained from IND enabling studies using animal models and therefore are relevant to the design and conduct of ongoing clinical trials of AAV vectors expressing CNGB3 or CNGA3 in patients with achromatopsia. Details of the clinical trials are available at https://www.clinicaltrials.gov (NCT02599922 and NCT02935517).
Objectives and study design
To help define the relationship between vector dose and development of multifocal chorioretinitis seen in a previous study,14 two studies were conducted that evaluated subretinally injected AAV5-PR2.1-hCNGB3 at a range of doses in non-affected or CNGB3-mutant dogs. In the first study (Table 1, study 1), phenotypically normal dogs heterozygous for a CNGB3 D262N missense mutation (CNGB3*/+; n = 4 females, 30 weeks of age at treatment) received an injection in each eye in a volume of 0.13–0.14 mL with a vector concentration of 8 × 1010–8 × 1013 vg/mL. In the second study (Table 1, study 2), achromatopsia-affected dogs homozygous for a CNGB3 D262N missense mutation (CNGB3*/*; n = 5, three males and two females, 15–22 weeks of age at treatment) received an injection in each eye in a volume of 0.15 mL with a vector concentration of 5 × 1010–5 × 1012 vg/mL.
Table 1.
Design of studies evaluating AAV vectors expressing human or canine CNGB3 in phenotypically normal or CNGB3-mutant dogs
| Dose level | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Group | Number of animals (eyes) | Animal ID numbers (sex) | Genotype | Vectors | vg/mL | Volume (mL) | vg/eye |
| 1 | 1 | 1 (2) | GS135 (F) | CNGB3*/+ | AAV5-PR2.1-hCNGB3 | 8 × 1010 | 0.13 | 1.0 × 1010 |
| 2 | 1 (2) | GS137 (F) | CNGB3*/+ | AAV5-PR2.1-hCNGB3 | 8 × 1011 | 0.13 | 1.0 × 1011 | |
| 3 | 1 (2) | GS138 (F) | CNGB3*/+ | AAV5-PR2.1-hCNGB3 | 8 × 1012 | 0.14 | 1.1 × 1012 | |
| 4 | 1 (2) | GS139 (F) | CNGB3*/+ | AAV5-PR2.1-hCNGB3 | 8 × 1013 | 0.14 | 1.1 × 1013 | |
| 2 | 1 | 1 (2) | GS225 (M) | CNGB3*/* | AAV5-PR2.1-hCNGB3 | 5 × 1010 | 0.15 | 7.5 × 109 |
| 2 | 2 (4) | GS226 (M) GS229 (M) | CNGB3*/* | AAV5-PR2.1-hCNGB3 | 5 × 1011 | 0.15 | 7.5 × 1010 | |
| 3 | 2 (4) | GS228 (F) GS233 (F) | CNGB3*/* | AAV5-PR2.1-hCNGB3 | 5 × 1012 | 0.15 | 7.5 × 1011 | |
| 3 | 1 | 3 (6) | M731 (F) M732 (F) M733 (F) | CNGB3*/del | AAV5-PR2.1-hCNGB3 and AAV5-PR2.1-cCNGB3 | 5 × 1011 | 0.1 | 5 × 1010 |
| 2 | 3 (6) | M728 (M) M729 (M) M730 (F) | CNGB3*/del | AAV5-PR2.1-hCNGB3 and AAV5-PR2.1-cCNGB3 | 5 × 1012 | 0.1 | 5 × 1011 | |
In studies 1 and 2, each animal received a subretinal injection of the same dose of the same vector in both eyes on study day 1. In study 3, each animal received a subretinal injection of AAV5-PR2.1-hCNGB3 in the left eye and AAV5-PR2.1-cCNGB3 in the right eye. Animals in study 1 were unaffected (heterozygous for a CNGB3 D262N missense mutation; */+) and animals in study 2 were affected (homozygous for a CNGB3 D262N missense mutation; */*). Study 3 animals were affected (compound heterozygous for two CNGB3 mutations, the D262N missense mutation and a genomic deletion, */del).
AAV, adeno-associated virus; hCNGB3, human CNGB3; cCNGB3, canine CNGB3.
In order to evaluate the possible role of adaptive immune responses to a foreign protein (human CNGB3) in the development of multifocal chorioretinitis in dogs treated with AAV-hCNGB3 vectors, a third study was conducted that evaluated subretinally injected AAV5-PR2.1-hCNGB3, and this was compared with subretinally injected AAV5-PR2.1-cCNGB3 at each of two dose levels in CNGB3-mutant dogs (Table 1, study 3). Because human CNGB3 has only 76% amino acid identity with canine CNGB3, it was hypothesized that adaptive immune responses to human CNGB3 might be involved in the development of chorioretinitis after administration of AAV5-PR2.1-hCNGB3, and that the frequency or severity of chorioretinitis might be reduced after administration of AAV5-PR2.1-cCNGB3. The study included six CNGB3-mutant dogs (two males and four females, 16 weeks of age at treatment) that were compound heterozygous for two CNGB3 mutations: the D262N missense mutation and a genomic deletion of CNGB3. Each animal received AAV5-PR2.1-hCNGB3 in one eye and AAV5-PR2.1-cCNGB3 in the other eye by subretinal injection in a volume of 0.10 mL at a vector concentration of 5 × 1011 or 5 × 1012 vg/mL.
Animals were observed for 12–14 weeks after treatment. Ophthalmic exams (slit lamp biomicroscopy and indirect ophthalmoscopy) were performed before treatment and at 1, 3, and 7 days after treatment and weekly thereafter. Rescue of cone function was evaluated by electroretinography before and 6 and 12 weeks after treatment for the achromatopsia-affected animals. Animals were sacrificed 3 months after treatment, and retinal tissue was obtained for histopathology assessment.
Within each study, both eyes were surgically treated on the same day for each animal. In all studies, vectors packaged in AAV5 capsids were used because this capsid had been used in prior studies demonstrating efficacy of subretinally injected AAV-PR2.1-hCNGB3 vectors in achromatopsia dogs. More recently, an AAV2 capsid with tyrosine to phenylalanine mutations in three surface-exposed tyrosine residues, designated AAV2tYF, has been shown to be more efficient than AAV5 for transduction of primate photoreceptors after subretinal injection,17 and the AAV2tYF capsid is being used in clinical trials in patients with ACHM caused by mutations in CNGB3 or CNGA3 (ClinicalTrials.gov NCT02935517 and NCT02599922). Different batches of the AAV-PR2.1-hCNGB3 vector were used for each study.
Summary of data
In all three studies, subretinal injections were well tolerated, with mild postoperative uveitis during the first few days following surgery and no signs of systemic side effects. This type of inflammation has been routinely seen following subretinal AAV injections in dogs and is easily controlled by routine topical and systemic anti-inflammatory therapy.14,18,19
Study 1
In unaffected dogs heterozygous for the CNGB3 missense mutation, signs of multifocal chorioretinitis (“black foci” in area of treatment, i.e., bleb area) and retinal thinning in the treated retinal regions were clinically observed in all eyes treated with the higher two dose levels (1.1 × 1012 and 1.1 × 1013 vg/eye) of the AAV5-PR2.1-hCNGB3 vector and confirmed by routine histopathologic examination (Table 2 and Fig. 1).
Table 2.
Retinal findings in study 1 after subretinal injection of AAV5-PR2.1-hCNGB3 in phenotypically normal dogs
| Group | Animal ID | Eye | Vector dose (vg/eye) | Clinical ophthalmic examination | Histopathologic findings in retina |
|---|---|---|---|---|---|
| 1 | GS135 | OD | 1 × 1010 | Normala | No product-related abnormalities |
| OS | 1 × 1010 | Normala | No product-related abnormalities | ||
| 2 | GS137 | OD | 1 × 1011 | Normala | No product-related abnormalities |
| OS | 1 × 1011 | Normala | No product-related abnormalities | ||
| 3 | GS138 | OD | 1.1 × 1012 | Multifocal retinitis (“black foci,” started at week 5) and focal retinal thinning (hyper-reflective areas by week 14) in bleb region | “Black foci” confirmed as subretinal aggregates of inflammatory cells with loss of photoreceptor inner and outer segments (retinal thinning) |
| OS | 1.1 × 1012 | Same as OD | Same as OD | ||
| 4 | GS139 | OD | 1.1 × 1013 | Multifocal retinitis (“black foci”) and diffuse retinal thinning (hyperreflective areas) in bleb regions started at week 3 | “Black foci” confirmed as subretinal aggregates of inflammatory cells. Retinal thinning due to atrophy of outer nuclear layer. |
| OS | 1.1 × 1013 | Same as OD | Same as OD |
Normal except for retinotomy scar, which was seen in all eyes.
OS, left eye; OD, right eye.
Figure 1.
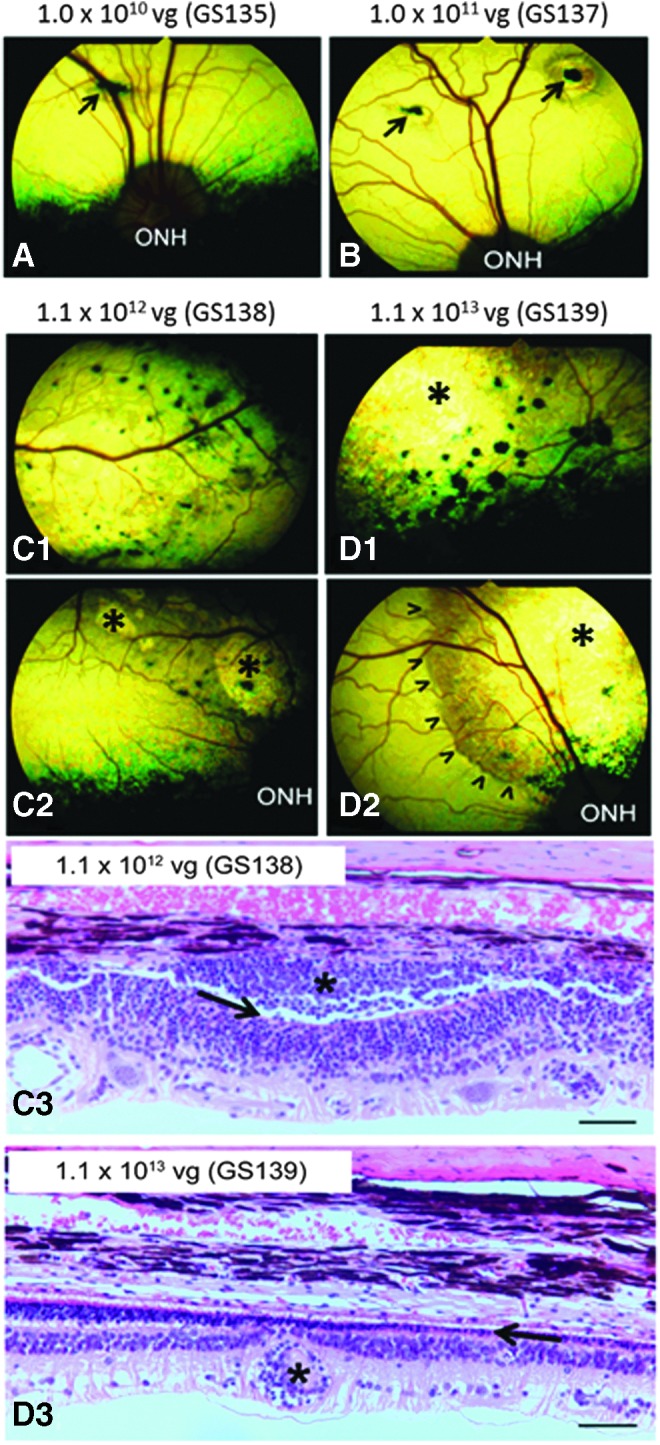
Representative fundus photographs and histologic images taken 14 weeks after subretinal injection of AAV5-PR2.1-hCNGB3 in phenotypically normal dogs from study 1. The vector dose level (vg/eye) and animal number are indicated above the images. (A and B) Other than the retinotomy scars (arrows), no abnormalities could be observed clinically at the 1.0 × 1010 and 1.0 × 1011 vg doses. Multifocal chorioretinitis within the treated bleb areas developed at the 1.1 × 1012 and 1.1 × 1013 vg doses (“black foci” in C1, C2, D1, and D2). Focal (* in C2) and diffuse (* in D1 and D2) retinal thinning could be recognized as hyper-reflective (brighter than normal) tapetal reflection. Arrowheads (D2) mark the edge of the original bleb area. Examination of hematoxylin and eosin stained sections confirmed the “black foci” as focal aggregates of inflammatory cells within the subretinal space (* in C3) associated with complete loss of photoreceptor inner and outer segments (arrow in C3). The retinal thinning was mainly caused by a severe loss of the outer nuclear layer and photoreceptors (arrow in D3). Perivascular aggregates of inflammatory cells were also observed (* in D3). ONH, optic nerve head. Bars = 50 μm.
Study 2
In affected dogs homozygous for the missense mutation in CNGB3, signs of multifocal retinitis and retinal thinning in the bleb region were observed in all four eyes treated with the high dose (7.5 × 1011 vg/eye) of AAV5-PR2.1-hCNGB3 vector (Table 3). ERG testing at 6 and 12 weeks after treatment demonstrated rescue of cone function in none of two eyes treated with the lowest dose, four of four eyes treated with the middle dose, and two of four eyes treated with the highest dose (Table 3). The presence of multifocal retinitis in eyes treated with the highest dose of vector did not preclude rescue of ERG cone responses; moderate cone ERG responses (3.4 and 2.7 μV) were observed in two of the four eyes treated with the highest vector dose (animal GS233 in Table 3). Histopathologic examination demonstrated predominantly perivascular inner retinal inflammation composed of lymphocytes, macrophages, and fewer plasma cells at all three dose levels that was most severe at the highest dose level. Areas of focal to multifocal retinal atrophy, up to 2 mm in length in the bleb area, were also observed in eyes that received the highest dose.
Table 3.
ERG responses and retinal findings in study 2 after subretinal injection of AAV5-PR2.1-hCNGB3 in CNGB3-mutant dogs
| Group | Animal ID | Eye | Vector dose (vg/eye) | Cone ERG (μV) | Clinical ophthalmic examination | Histopathologic findings in retina |
|---|---|---|---|---|---|---|
| 1 | GS225 | OD | 7.5 × 109 | 0 | Focal retina thinning | Focal inner and outer nuclear layer attenuation |
| OS | 7.5 × 109 | 0 | Normala | Focal disruption of retinal architecture; minimal neuroretinal accumulation of mononuclear inflammatory cells | ||
| 2 | GS226 | OD | 7.5 × 1010 | 2.12 | Retinal fold; focal retinal thinning | Focal detachment with associated focal neuroretinal (predominantly perivascular) accumulation of mononuclear inflammatory cells; rare vitreal inflammatory cells |
| OS | 7.5 × 1010 | 2.90 | Retinal fold; focal retinal thinning | Moderate neuroretinal (predominantly perivascular) accumulation of mononuclear inflammatory cells; rare vitreal inflammatory cells | ||
| GS229 | OD | 7.5 × 1010 | 13.00 | Normala | Multifocal, predominantly perivascular mononuclear inflammation of the neuroretina | |
| OS | 7.5 × 1010 | 19.10 | Discoloration at injection site | Focal neuroretinal atrophy (retinotomy scar) | ||
| 3 | GS228 | OD | 7.5 × 1011 | 0 | Multifocal chorioretinitis started at week 7 | No significant lesions |
| OS | 7.5 × 1011 | 0 | Multifocal chorioretinitis started at week 4 | Locally extensive retinal attenuation with neuroretinal parenchymal and perivascular accumulation of mononuclear inflammatory cells; rare vitreal inflammatory cells | ||
| GS233 | OD | 7.5 × 1011 | 3.41 | Multifocal chorioretinitis started at week 6 | Focal retinal detachment with neuroretinal parenchymal and perivascular mononuclear inflammation; retinal pigment epithelium loss and associated inflammation | |
| OS | 7.5 × 1011 | 2.73 | Multifocal chorioretinitis started at week 6 | Locally extensive marked retinal atrophy; perivascular mononuclear inflammation |
Each animal received a 0.15 mL subretinal injection of AAV5-PR2.1-hCNGB3 in both eyes (OD and OS).
Normal except for retinotomy scar which was seen in all eyes.
ERG, electroretinogram.
Histopathologic abnormalities occurred in study 2 at a dose of 7.5 × 1010 vg/eye but not in study 1 at a slightly higher dose of 1 × 1011 vg/eye. However, the vector concentration was determined in different laboratories using slightly different methods, and variability in determination of vector concentration in different laboratories, even when using the same method, has been reported previously.20
Study 3
In affected dogs compound heterozygous for missense and genomic deletion mutations in CNGB3, animal M728 had a total of 200 μL injected in the left eye, most of which was delivered into the vitreous with only a small subretinal bleb achieved. There was delayed absorption of subretinal fluid in the right eye of this animal and the right eye of animal M731.
Clinical ophthalmic examination showed no evidence of ocular toxicity in 8/12 eyes (Table 4). Multifocal chorioretinitis developed in the bleb area in two eyes treated with the higher dose of the vector expressing canine CNGB3, and severe thinning of the retina in the bleb area was seen in two eyes: one treated with the lower dose of the vector expressing canine CNGB3, and one treated with the higher dose of the vector expressing human CNGB3. In both of these eyes, there was delayed absorption of the subretinally injected vector.
Table 4.
ERG responses and retinal findings in study 3 after subretinal injection of AAV5-PR2.1-hCNGB3 or AAV5-PR2.1-cCNGB3 in CNGB3-mutant dogs
| Group | Animal ID | Eye | Vector dose (vg/eye) | Cone ERG (μV) | Clinical ophthalmic examination | Histopathologic findings in retina |
|---|---|---|---|---|---|---|
| 1 | M731 | OD | 5 × 1010 | 3.94 | Retinal thinning | Variably severe retinal atrophy and loss of retinal pigment epithelium (70% of the superior retina) |
| OS | 5 × 1010 | 1.54 | Normala | Retinotomy site | ||
| M732 | OD | 5 × 1010 | 7.66 | Normala | No significant lesions | |
| OS | 5 × 1010 | 3.80 | Normala | No significant lesions | ||
| M733 | OD | 5 × 1010 | 3.17 | Normala | No significant lesions | |
| OS | 5 × 1010 | 1.57 | Normala | No significant lesions | ||
| 2 | M728 | OD | 5 × 1011 | 3.80 | Multifocal chorioretinitis and retinal thinning | Locally extensive retinal atrophy (superior retina); scattered foci of perivascular lymphoplasmacytic chorioretinitis |
| OS | 5 × 1011 | 3.16 | Retinal thinning | Locally extensive retinal atrophy (superior retina); adjacent to retinotomy site | ||
| M729 | OD | 5 × 1011 | 6.80 | Normala | Variably severe predominantly perivascular mononuclear retinitis (superior retina) | |
| OS | 5 × 1011 | 1.01 | Normala | Variably severe predominantly perivascular mononuclear retinitis (superior retina); gliotic retinotomy site | ||
| M730 | OD | 5 × 1011 | 4.42 | Multifocal chorioretinitis | Variably severe mononuclear retinitis, parenchymal and perivascular, involving most of the superior retina, and extending into the choroid with areas of RPE loss | |
| OS | 5 × 1011 | 5.22 | Normala | Several perivascular inflammatory nodules, single region of choroidal inflammation, and a focus of outer nuclear atrophy and RPE loss |
Each animal received a 0.1 mL subretinal injection of AAV5-PR2.1-hCNGB3 in the left eye (OS) and AAV5-PR2.1-cCNGB3 in the right eye (OD). The vector concentration was 5 × 1012 vg/mL in group 1 and 5 × 1011 vg/mL in group 2. Cone ERG values are the 30 Hz flicker amplitude at week 12. In animal M728, a total of 200 μL was injected in the left eye, mostly intravitreal, with only a small subretinal bleb, and there was delayed absorption of subretinal fluid in the right eye. In animal M731, there was delayed absorption of subretinal fluid in the right eye.
No visible lesions except for retinotomy scar, which was seen in all eyes.
Histologically, there was evidence of ocular inflammation in five of six eyes that received the higher dose of either vector, which was most severe and involved the choroid in the two eyes with clinical evidence of chorioretinitis (Table 4). Retinal atrophy was seen in three eyes: one injected with a low dose of the vector expressing canine CNGB3, and two injected with a high dose of vector expressing either canine or human CNGB3. Each of these eyes had retinal thinning observed clinically.
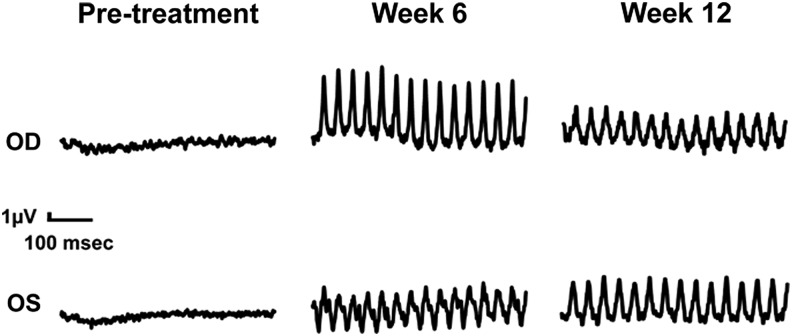
Rescue of cone ERG function was observed in all 12 treated eyes (Table 4 and Fig. 2), and the magnitude of restored cone ERG responses tended to be higher in eyes treated with the vector expressing the canine CNGB3 than in eyes treated with the vector expressing the human CNGB3. For eyes treated with the vector expressing canine CNGB3, the mean ± standard deviation (SD) cone b-wave amplitude was 5.01 ± 1.58 μV at the higher dose and 4.92 ± 2.40 μV at the lower dose. For eyes treated with the vector expressing human CNGB3, the mean ± SD cone b-wave amplitude was 3.13 ± 2.11 μV at the higher dose and 2.30 ± 1.30 μV at the lower dose. Cone ERG responses were similar in the lower-dose and higher-dose groups, suggesting that the doses used may have been at the upper end of a dose–response relationship.
Figure 2.
Representative cone flicker electroretinogram (ERG) responses 6 and 12 weeks after subretinal injection of AAV5-PR2.1-hCNGB3 or AAV5-PR2.1-cCNGB3 in CNGB3-mutant dogs (study 3). Animal M730 received the vector expressing human CNGB3 in the left eye (OS) and the vector expressing canine CNGB3 in the right eye (OD).
Immune responses to AAV capsid (a more than twofold increase in AAV neutralizing antibodies) occurred in three of six dogs, but no animal developed antibodies to human CNGB3 protein (Supplementary Table S1). Results of Enzyme-Linked ImmunoSpot (ELISPOT) testing were available for all animals at 12 weeks after vector administration and for one animal at 6 weeks after vector administration (Supplementary Table S2). Responses to AAV5 and human and canine CNGB3 peptides were negative for all animals receiving the lower dose of the AAV vectors. In the higher-dose group, very low positive responses to one or more CNGB3 peptide pools were observed in all three animals. Animal M728 responded to one human CNGB3 peptide pool, and animal M729 responded to one human and two canine CNGB3 peptide pools. Animal M730 responded to one human CNGB3 peptide pool at week 6 and to one canine CNGB3 peptide pool at week 12. All positive responses were very low and close to the limit of detection.
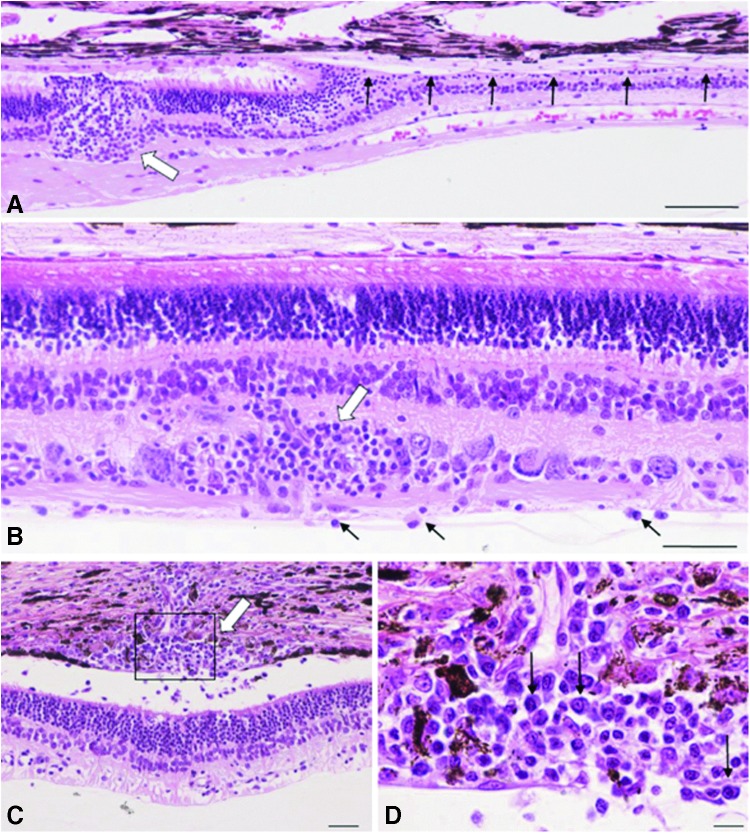
Histopathologic examination of animals in study 3 demonstrated abnormalities in all eyes at the higher dose, and in one of six eyes at the lower dose (Table 4 and Fig. 3). The two eyes that had delayed resorption of the injected vector had focal retinal atrophy and detachment with associated retinal pigment epithelial loss in the bleb area, unaccompanied by inflammation. All three eyes treated with the higher dose of vector expressing canine CNGB3 and two of three eyes treated with the higher dose of vector expressing human CNGB3 displayed variable combinations and degrees of the following lesions: inflammation of retina and choroid, characterized by predominantly perivascular inner retinal inflammation composed of lymphocytes, macrophages, and fewer plasma cells, with extension to the choroid in three eyes. Overall, inflammation was typically focal, was limited to the retinas treated with the higher vector dose, and was seen in eyes treated with vectors expressing either human or canine CNGB3. Except for focal retinal atrophy in the right eye of animal M731, who received the lower dose of the vector expressing canine CNGB3, the pathologic changes were minimal or non-existent in the eyes treated with the lower vector dose.
Figure 3.
Histologic changes in animals receiving the highest vector dose (5 × 1011 vg/eye) of AAV5-PR2.1-hCNGB3 or AAV5-PR2.1-cCNGB3 in study 3. (A) M728 left eye, AAV5-PR2.1-hCNGB3. A retinotomy lesion (thick white arrow) is characterized by focal disruption of outer and inner nuclear layers with prolapse of photoreceptor nuclei into the subretinal space of locally detached retina. The retinal pigment epithelium is intact. Abrupt transition to a zone of marked outer nuclear layer atrophy accompanied by loss of retinal pigment epithelium is apparent (row of black arrows). (B) M729 right eye, AAV5-PR2.1-cCNGB3. Perivascular aggregates of mononuclear inflammatory cells are evident around inner retinal vessels (white arrow). A few inflammatory cells within the vitreous chamber cling to the inner limiting membrane (black arrows). (C) M730 left eye, AAV5-PR2.1-hCNGB3. Inflammatory cells surrounding a choroidal vessel (white arrow) extend into the subretinal space. There is accompanying retinal detachment, atrophy of inner and outer segments, and retinal pigment epithelial attenuation. (D) Boxed region in (C) reveals an inflammatory infiltrate (lymphocytes and plasma cells, black arrows) consistent with an adaptive immune response. Hematoxylin and eosin, bars = 100 μm (A), 50 μm (B and C), and 10 μm (D).
The dose-related inflammation seen in these studies is consistent with results seen in other studies of gene therapy vectors delivered by intraocular injection in a variety of species,17,21–23 but an explanation for the unexpected development of more severe chorioretinitis with the canine CNGB3 vector than with the human CNGB3 vector in study 3 is not apparent. It was not due to a mix-up in labeling of the vectors that were administered, since residual samples of vector collected after subretinal administration were tested by polymerase chain reaction (PCR) and determined to have the correct sequence of the canine or human CNGB3 cDNA specified in the protocol (data not shown). T-cell responses to AAV5 and human or canine CNGB3 peptides were infrequent, barely above the limits of detection when they occurred, and were not correlated with the presence or absence of chorioretinitis (Supplementary Table S2). The two animals with chorioretinitis developed the highest anti-AAV antibody titers (Supplementary Table S1), but if chorioretinitis were caused by an immune response to the AAV capsid, one would expect to see similar changes in both eyes, since both eyes received the same total vector dose. None of the animals developed antibodies to human CNGB3 protein. Although it is theoretically possible that testing using canine CNGB3 protein might have yielded different results, it seems unlikely that antibodies to canine CNGB3 but not human CNGB3 would be induced in these animals. The same method was used to quantify the vectors expressing human and canine CNGB3 used in study 3, but it is possible that there were small differences in the actual vector concentrations administered that could have contributed to the observed results, since a higher vector concentration of AAV5-PR2.1-hCNGB3 has been shown in these and other studies to be associated with greater inflammation.
Conclusions
Results of these studies confirmed previous findings that in the dog model of achromatopsia, high doses of vectors expressing human CNGB3 administered by subretinal injection are associated with chorioretinitis in the area of the subretinal bleb, and also demonstrated that chorioretinitis and retinal thinning in the area of the subretinal bleb after administration of high doses of an AAV-hCNGB3 vector occurs in phenotypically normal dogs that are heterozygous for the achromatopsia gene defect. However, the results did not support the hypothesis that this toxicity was due to an immune response to human CNGB3, as the vector expressing the canine CNGB3 transgene at the same dose resulted in a higher frequency of chorioretinitis.
Some of the objectives of conducting studies in animal models are to identify dose levels that are effective and to identify safety signals that help to guide selection of clinical parameters to be evaluated in human clinical trials. The studies reported here demonstrated that vector concentrations of 5 × 1010 vg/mL or higher were effective, and higher dose levels were associated with evidence of ocular inflammation. Based on these results, it is concluded that administration of AAV-hCNGB3 vectors in clinical trials should proceed cautiously, especially as the vector dose is increased. Additional studies are being conducted to evaluate the vector components and mechanisms involved in development of inflammation after ocular administration of AAV vectors.
Materials and Methods
Study designs and animals
The animals were purpose-bred mongrel dogs that had a common mixed-breed genetic background24 but segregated independently the CNGB3 missense and genomic deletions causing achromatopsia, and were related with the same genetic background influenced by laboratory beagles; they only differed in their CNGB3 genotype. These animals were part of the same research colony maintained independently at the Retinal Disease Studies Facility (Kennett Square, PA) and at the Michigan State University College of Veterinary Medicine Vivarium (East Lansing, MI). All studies were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by Michigan State University and the University of Pennsylvania Institutional Animal Care and Use Committees.
Canine CNGB3 cDNA cloning
Although the sequence of the canine CNGB3 gene was previously reported,12 previous attempts to clone the gene into a eukaryotic expression vector have been unsuccessful.13 This may due to production of a toxic gene product from cCNGB3 that is not tolerated by the Escherichia coli cells used to propagate the vector expression cassette, possibly by using an internal promoter for mRNA transcription, an assumption based on findings during cloning of mouse CNGB3.25 Successful cloning of the mouse CNGB3 cDNA expression cassette required a methionine to leucine (M337L) mutation at a potential internal start codon and introduction of several silent mutations in a region upstream of this codon to suppress a possible cryptic internal promoter. The approach taken to clone a stable cCNGB3 cDNA included codon optimization of the entire dog CNGB3 open reading frame in order to prevent transcription and protein translation in E. coli. Codons of the cCNGB3 cDNA were modified based on transfer RNA frequencies in a way that they favor gene expression in humans but are rarely utilized in E. coli.26,27 GC content was also increased to promote RNA stability. This modified cCNGB3 cDNA, including an optimized Kozak sequence, was synthesized and cloned into a conventional cloning vector, and its genetic stability in propagation in E. coli was confirmed. The cCNGB3 cDNA was then cloned into an AAV proviral plasmid to generate pTR-PR2.1-cCNGB3, in which the expression of cCNGB3 is driven by 2.1 kb human red cone opsin promoter (PR2.1). The cCNGB3 cDNA sequence is available at GenBank (access number KY354293).
Vector production and characterization
AAV5 vectors expressing either hCNGB3 or cCNGB3 were produced using a plasmid transient transfection method and purified by iodixanol gradient centrifugation followed by Q HP column chromatography.28 The rAAV5-PR2.1-hCNGB3 vector used for study 1 was produced and purified in the Hauswirth laboratory at the University of Florida (UF), assayed for purity, sterility, and endotoxin, and determination of vector concentration by SYBR green-based quantitative real-time PCR (qRT-PCR), as previously described.14 The rAAV5-PR2.1-hCNGB3 vector used for studies 2 and 3 and the rAAV5-PR2.1-cCNGB3 used for study 3 were produced and purified at the UF vector core and assayed at AGTC for identity, purity, sterility, and endotoxin level, and determination of vector concentration by Taqman probe–based qRT-PCR, as previously described.29 Each batch of vector had endotoxin concentrations below the level of detection, which was <1.25 EU/mL for the vector used for study 1 and <0.3 EU/mL for studies 2 and 3. Final purified AAV vectors were dispensed in small aliquots that were stored at ≤-65°C until used.
Subretinal injections
The AAV vector was injected using a RetinaJect™ subretinal injector (SurModics, Eden Prairie, MN) through a trans-vitreal approach, as previously described.14,18,19 The blebs covered ∼25% of the photoreceptor surface area. Whenever possible, the area centralis was targeted because it has the highest cone density.30,31 Immediately following surgery, the retinal location and extent of the subretinal blebs were documented by fundus drawings or photography (Kowa Genesis-D or RetCamII) for reference in the morphologic studies. In general, flattening of the subretinal bleb and retinal reattachment occurred within 24–36 h.
Medical therapy following subretinal injections included a combination of subconjunctival steroids, topical antibiotic-steroids and mydriatics, and short-term systemic steroids and antibiotics, as described previously.14,18,32
Routine clinical examination
Complete ophthalmic examinations were performed on awake dogs by slit lamp biomicroscopy (SL15; Kowa Optimed, Inc., Torrance, CA), indirect ophthalmoscopy (All Pupil II; Keeler Instruments, Inc., Broomall, PA), and retinal photography (Kowa Genesis-D, Nagoya, Japan; RetCamII; Clarity Medical Systems, Pleasanton, CA).
General anesthesia
Dogs were anesthetized for intraocular injections and electroretinography. They were first pre-medicated with intravenous acepromazine at a dose of 0.02–0.5 mg/kg (AceproJect; Henry Schein, Dublin, OH) and induced with intravenous propofol (Propoflo™ 28; Abbott; Abbott Park, IL) to effect (starting dose 4 mg/kg). The dogs were then intubated, and general anesthesia was maintained with isoflurane (2–3% vaporizer setting; Isothesia™; Henry Schein) in oxygen. Heart rate, respiratory rate, and body temperature were monitored throughout the procedure. A portable multi-parameter veterinary monitor (PM-9000Vet; Shenzhen Mindray Bio-Medical Electronics Co. Ltd., Nanshan, P.R. China) was used to assess blood pressure, oxygen saturation, and end-tidal CO2. Anesthesia level was evaluated by monitoring changes in respiration or heart rate.
ERG
Standard Ganzfeld scotopic and photopic ERGs were recorded from anesthetized dogs using either a modified Ganzfeld dome fitted with the LED stimuli of a ColorDome stimulator (Diagnosys LLC, Lowell, MA) or RETIport system with a Ganzfeld dome (Roland Consult, Brandenburg, Germany).14,19,33 Rod and mixed rod-cone mediated responses were recorded after 20 min of dark adaptation with single white flash stimuli of increasing intensities (from 0.0003 to 10.26 cd.s/m2. Following 10 min of light adaptation (34.26 cd/m2), cone-mediated signals were recorded to 1 Hz single flash (from 0.00577 to 10.26 cd.s/m2) and 29.41 Hz flicker stimuli of increasing intensities (from 0.00577 to 5.77 cd.s/m2. The combination of light-adaptation with short inter-flash intervals led to saturation of rods and isolation of pure cone-mediated responses. Except for the brighter scotopic light stimuli (≥0.577 cd.s/m2), multiple responses were averaged.
Histopathology
For terminal procedures, the dogs were euthanatized with an overdose of pentobarbital sodium (173–200 mg/kg; Euthasol; Virbac, Fort Worth, TX; or Fatal Plus; Vortech Pharmaceuticals Ltd., Dearborn, MI), and the eyes were enucleated for routine histopathologic examinations. Globes were fixed in Davidson's Solution and processed for routine paraffin embedding and hematoxylin and eosin staining. All sections were made through the optic nerve head and the subretinal bleb area.
Immune responses to AAV and hCNGB3
In study 3, sera for measurement of antibodies to AAV and hCNGB3 were obtained before dosing and at sacrifice, and peripheral blood mononuclear cells (PBMC) for measurement of cell-mediated immune responses to hCNGB3 and AAV were obtained before dosing and 12 weeks after vector administration. Antibodies to AAV capsid were measured using a neutralization assay, as previously described.34 Antibodies to hCNGB3 protein were measured using an enzyme-linked immunosorbent assay developed to detect anti-CNGB3 antibodies in dog serum. In brief, 96-well microtiter plates were coated with recombinant human CNGB3 protein or with purified dog immunoglobulin G (IgG). After incubation, dog sera (test samples and negative controls) or serial dilutions of rabbit anti-hCNGB3 reference standard and positive controls were added to the wells coated with hCNGB3 antigen. Blocking buffer was added to the selected wells coated with dog IgG. Antibodies in the sera that bind to the hCNGB3 capture antigen are then detected using horseradish peroxidase (HRP)-labeled secondary antibody cocktail (goat anti-rabbit IgG and goat anti-dog IgG) followed by addition of an enzyme substrate (TMB) that changes color in the presence of HRP. The level of antibody in serum is quantified by comparison to the standard curve generated from dilutions of the reference standard. Canine CNGB3 protein was not available to use in antibody testing. Cell-mediated immune responses to AAV capsid and hCNGB3 peptides were evaluated by measuring interferon gamma ELISPOT responses in PBMC, as previously described.35
Supplementary Material
Acknowledgments
This study was supported by a grant from the Foundation Fighting Blindness and by grants R01-EY019304, K12-EY015398, R01-006855, R01-17549, R42EY023123, R24-EY022023, and P30-EY021721 from the National Eye Institute, National Institutes of Health. We thank Jun Xie and Guangping Gao at University of Massachusetts Medical School for performing AAV neutralizing antibody testing, Jixiang Seaney for performing CNGB3 antibody testing, the veterinary staff at the University of Pennsylvania and Michigan State University for excellent animal care, Excalibur Pathology Inc., Norman, OK, for tissue processing, Mark Sherman for critical review of the manuscript, and Lydia Melnyk for administrative support.
Author Disclosure
G.Y. and J.D.C. are employees and shareholders of AGTC and have a conflict of interest to the extent that this work potentially increases their financial interests. W.W.H. and the University of Florida have a financial interest in the use of AAV therapies. W.W.H. owns equity in and is a consultant for AGTC and has a conflict of interest to the extent that this work potentially increases his financial interests. No competing financial interests exist for the remaining authors.
References
- 1.Kohl S, Jagle H, Sharpe LT, et al. . Achromatopsia. In: Pagon RA, Bird TC, Dolan CR, Stephens K, eds. Gene Reviews [Internet]. Seattle, WA: University of Washington [Google Scholar]
- 2.Sharpe LT, Stockman A, Jagle H, et al. . Opsin genes, cone photopigments, color vision, and color blindness: rod monochromacy. In: Gegenfurtner K, Sharpe LT, eds. Color Vision: From Genes to Perception. Cambridge, United Kingdom: Cambridge University Press, 1999:48–51 [Google Scholar]
- 3.Kohl S, Varsanyi B, Antunes GA, et al. . CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet 2005;13:302–308 [DOI] [PubMed] [Google Scholar]
- 4.Wissinger B, Gamer D, Jagle H, et al. . CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet 2001;69:722–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohl S, Baumann B, Rosenberg T, et al. . Mutations in the cone photoreceptor G-protein alpha-subunit gene GNAT2 in patients with achromatopsia. Am J Hum Genet 2002;71:422–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang B, Grau T, Dangel S, et al. . A homologous genetic basis of the murine cpfl1 mutant and human achromatopsia linked to mutations in the PDE6C gene. Proc Natl Acad Sci U S A 2009;106:19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohl S, Coppieters F, Meire F, et al. . A nonsense mutation in PDE6H causes autosomal-recessive incomplete achromatopsia. Am J Hum Genet 2012;91:527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohl S, Zobor D, Chiang WC, et al. . Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat Genet 2015;47:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding XQ, Harry CS, Umino Y, et al. . Impaired cone function and cone degeneration resulting from CNGB3 deficiency: down-regulation of CNGA3 biosynthesis as a potential mechanism. Hum Mol Genet 2009;18:4770–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michalakis S, Geiger H, Haverkamp S, et al. . Impaired opsin targeting and cone photoreceptor migration in the retina of mice lacking the cyclic nucleotide-gated channel CNGA3. Invest Ophthalmol Vis Sci 2005;46:1516–1524 [DOI] [PubMed] [Google Scholar]
- 11.Reicher S, Seroussi E, Gootwine E. A mutation in gene CNGA3 is associated with day blindness in sheep. Genomics 2010;95:101–104 [DOI] [PubMed] [Google Scholar]
- 12.Sidjanin DJ, Lowe JK, McElwee JL, et al. . Canine CNGB3 mutations establish cone degeneration as orthologous to the human achromatopsia locus ACHM3. Hum Mol Genet 2002;11:1823–1833 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N, Dutrow EV, Miyadera K, et al. . Canine CNGA3 gene mutations provide novel insights into human achromatopsia-associated channelopathies and treatment. PloS One 2015;10:e0138943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komaromy AM, Alexander JJ, Rowlan JS, et al. . Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet 2010;19:2581–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho LS, Xu J, Pearson RA, et al. . Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet 2011;20:3161–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltran WA, Boye SL, Boye SE, et al. . rAAV2/5 gene-targeting to rods: dose-dependent efficiency and complications associated with different promoters. Gene Ther 2010;17:1162–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye GJ, Budzynski E, Sonnentag P, et al. . Safety and biodistribution evaluation in cynomolgus macaques of rAAV2tYF-PR1.7-hCNGB3, a recombinant AAV vector for treatment of achromatopsia. Hum Gene Ther Clin Dev 2016;27:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komaromy AM, Alexander JJ, Cooper AE, et al. . Targeting gene expression to cones with human cone opsin promoters in recombinant AAV. Gene Ther 2008;15:1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komaromy AM, Rowlan JS, Corr AT, et al. . Transient photoreceptor deconstruction by CNTF enhances rAAV-mediated cone functional rescue in late stage CNGB3-achromatopsia. Mol Ther 2013;21:1131–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lock M, McGorray S, Auricchio A, et al. . Characterization of a recombinant adeno-associated virus type 2 Reference Standard Material. Hum Gene Ther 2010;21:1273–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beltran WA, Boye SL, Boye SE, et al. . rAAV2/5 gene-targeting to rods: dose-dependent efficiency and complications associated with different promoters. Gene Ther 2010;17:1162–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gootwine E, Ofri R, Banin E, et al. . Safety and efficacy evaluation of rAAV2tYF-PR1.7-hCNGA3 vector delivered by subretinal injection in CNGA3 mutant achromatopsia sheep. Hum Gene Ther Clin Dev 2017;28:96–107 [DOI] [PubMed] [Google Scholar]
- 23.Ye GJ, Budzynski E, Sonnentag P, et al. . Safety and biodistribution evaluation in cynomolgus macaques of rAAV2tYF-CB-hRS1, a recombinant adeno-associated virus vector expressing retinoschisin. Hum Gene Ther Clin Dev 2015;26:165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyadera K, Acland GM, Aguirre GD. Genetic and phenotypic variations of inherited retinal diseases in dogs: the power of within- and across-breed studies. Mamm Genome 2012;23:40–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstner A, Zong X, Hofmann F, et al. . Molecular cloning and functional characterization of a new modulatory cyclic nucleotide-gated channel subunit from mouse retina. J Neurosci 2000;20:1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicola A, Brown B, Sharma S, et al. . Codon optimization can improve expression of human genes in Escherichia coli: a multi-gene study. Protein Expr Purif 2008;59:94–102 [DOI] [PubMed] [Google Scholar]
- 27.Sharp PM, Cowe E, Higgins DG, et al. . Codon usage patterns in Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae, Schizosaccharomyces pombe, Drosophila melanogaster and Homo sapiens: a review of the considerable within-species diversity. Nucleic Acids Res 1988;16:8207–8211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolotukhin S, Potter M, Zolotukhin I, et al. . Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 2002;28:158–167 [DOI] [PubMed] [Google Scholar]
- 29.Chulay JD, Ye GJ, Thomas DL, et al. . Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method. Hum Gene Ther 2011;22:155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beltran WA, Cideciyan AV, Guziewicz KE, et al. . Canine retina has a primate fovea-like bouquet of cone photoreceptors which is affected by inherited macular degenerations. PloS One 2014;9:e90390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mowat FM, Petersen-Jones SM, Williamson H, et al. . Topographical characterization of cone photoreceptors and the area centralis of the canine retina. Mole Vis 2008;14:2518–2527 [PMC free article] [PubMed] [Google Scholar]
- 32.Komaromy AM, Varner SE, de Juan E, et al. . Application of a new subretinal injection device in the dog. Cell Transplant 2006;15:511–519 [DOI] [PubMed] [Google Scholar]
- 33.Yeh CY, Koehl KL, Harman CD, et al. . Assessment of rod, cone, and intrinsically photosensitive retinal ganglion cell contributions to the canine chromatic pupillary response. Invest Ophthalmol Vis Sci 2017;58:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calcedo R, Vandenberghe LH, Gao G, et al. . Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brantly ML, Chulay JD, Wang L, et al. . Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A 2009;106:16363–16368 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.