Abstract
Background: Among low-income women with and without HIV, it is a priority to reduce age-related comorbidities, including hypertension and its sequelae. Because consistent health insurance access has been identified as an important factor in controlling many chronic diseases, we estimated the effects of coverage interruption on loss of hypertension control in a cohort of women in the United States.
Methods: We analyzed prospective, longitudinal data from the Women's Interagency HIV Study. HIV-infected and HIV-uninfected women were included between 2005 and 2014 when they reported health insurance at consecutive biannual visits and had controlled hypertension, and were followed for any insurance break and loss of hypertension control. We estimated hazard ratios (HRs) by Cox proportional hazards regression with inverse-probability-of-treatment-and censoring weights (marginal structural models), and plotted the cumulative incidence of hypertension control loss.
Results: Among 890 HIV-infected women, the weighted HR for hypertension control loss comparing health insurance interruption to uninterrupted coverage was 1.37 (95% confidence interval [CI], 0.99–1.91). Inclusion of AIDS Drug Assistance Program (ADAP) participation with health insurance modestly increased the HR (1.47; 95% CI, 1.04–2.07). Analysis of 272 HIV-uninfected women yielded a similar HR (1.39; 95% CI, 0.88–2.21). Additionally, there were indications of uninterrupted coverage having a protective effect on hypertension when compared with the natural course in HIV-infected (HR, 0.82; 95% CI, 0.61–1.11) and HIV-uninfected (HR, 0.78; 95% CI, 0.52–1.19) women.
Conclusions: This study provides evidence that health insurance continuity promotes hypertension control in key populations. Interventions that ensure coverage stability and ADAP access should be a policy priority.
Keywords: : health insurance, HIV/AIDS, hypertension, women
Introduction
As women with HIV are living longer,1 it is a priority to determine ways to reduce their age-related comorbidities, including hypertension and its sequelae. For low-income HIV-uninfected women, who have poorer health than higher income women,2 the consequences of these comorbidities may be even greater. One method of improving health is increasing the proportions of populations with health insurance, which is associated with positive health-related outcomes among both HIV-infected and low-income individuals in the United States (US).3–5
Among persons with HIV, lack of health insurance has been associated with delayed HIV care6 and failure to enter care,7 and having insurance has been related to decreases in HIV-related mortality.3 Continuous health insurance coverage has strong, positive effects on antiretroviral therapy (ART) use and ambulatory care.5 Interruptions in coverage have been associated with ART discontinuation,8 and changes in insurance have been linked to decreased ART use.9 Despite increases in health insurance access, ∼30% of HIV-infected persons in the US were uninsured in 2013.10
Low-income HIV-uninfected women may be even less likely to have coverage and receive healthcare than HIV-infected women.11 This is likely due, in part, to not having access to specialized programs such as the Ryan White HIV/AIDS Program, which supports services, including medication assistance, through the AIDS Drug Assistance Program (ADAP).12 Millions of low-income individuals lack coverage because their incomes are above the Medicaid eligibility limit but below the limit for Marketplace premium tax credits.13
Being low-income and uninsured are each linked to not having a regular provider, cost-related access problems, underutilization of preventive services, and lower healthcare quality.14 Although expanding public health insurance access for low-income adults has beneficial effects on healthcare access and use, financial strain, and health,4 Medicaid interruptions (e.g., dropout or eligibility loss) are frequent15,16 and linked to higher hospitalization rates for outpatient-manageable conditions.17
High blood pressure (BP) can lead to coronary heart disease, heart failure, kidney failure, and stroke.18 The co-occurrence of hypertension and HIV is of growing concern not only because of ART-driven increasing life expectancy on a population level but also because of high cardiovascular risk possibly due to HIV and ART19 and evidence of higher myocardial infarction rates in HIV-infected persons.20,21 Poor hypertension control has been noted in HIV-infected cohorts,22,23 with increased BP associated with cardiovascular events.24 Hypertension is also a crucial issue for low-income HIV-uninfected women, given the association between low socioeconomic status and hypertension25 as well as poverty's associations with lack of access to healthcare14 and insurance,26,27 both of which are essential to obtain antihypertensive medications and avert negative outcomes.
We prospectively evaluated the effects of health insurance interruption on loss of hypertension control among low-income US women with or at risk for HIV infection in the Women's Interagency HIV Study (WIHS), as to our knowledge there has been no prior research on this relationship among either HIV-infected or HIV-uninfected low-income women.
Materials and Methods
Study population
Our data source was the WIHS, a multisite US cohort, including both HIV-infected women and HIV-uninfected women at risk for HIV infection, specifically designed to allow the evaluation of topical research questions across and within these populations. The presence of HIV-infected and HIV-uninfected women in the cohort was a reason it was selected to pursue the study aims. WIHS visits are conducted biannually and consist of a clinical examination, specimen collection, and an interview to obtain self-reported medical history, medication use, and sociodemographic information.
While age and other eligibility criteria for the WIHS have evolved across four waves of enrollment, women “at risk” for HIV have generally been defined as reporting at least one of the following criteria during the past year: having sex with a man with known HIV infection; having sex with six or more men, having condomless sex with three or more men; having sex for drugs, money, or shelter; diagnosis by a healthcare provider with a sexually transmitted disease; using crack, cocaine, heroin, methamphetamine, or injection drugs.28 Further details on participant recruitment, retention, and characteristics have been previously reported.29,30
HIV-infected and HIV-uninfected women were analyzed separately and included at their first study visit (from October 2005 to March 2006 WIHS visit window or during a later 6-month window, but no later than the October 2013 to March 2014 window) with stable health insurance and controlled hypertension, if they attended at least one visit after this “analysis baseline.” This period was chosen as the earliest possible baseline because it corresponds approximately to the beginning of the modern ART era, examined in a recent WIHS of health insurance and HIV suppression,31 and shortly follows the release and implementation of updated hypertension definition and treatment guidelines.32
Follow-up began at the “analysis baseline” and ended at the first occurrence of uncontrolled hypertension (systolic BP ≥140 [mmHg] or diastolic BP ≥90), censoring (death date or two consecutive missed visits), or administrative censoring after the April 2014 to September 2014 visit. All women meeting the inclusion criteria were analyzed; no sampling scheme was used to select study participants.
Specification of health insurance
Stable health insurance at baseline was defined as self-reported health insurance for at least two consecutive attended study visits. Health insurance was classified into four mutually exclusive groups similar to those described by the Kaiser Family Foundation,33 with categories assigned in this order: Medicaid, private or student, Medicare or other public (including Tricare/CHAMPUS, Veteran's Administration, and city/county), and no health insurance. Categories were then collapsed into any or no coverage.
Group A included HIV-infected women with stable health insurance coverage at baseline who may or may not also have participated in ADAP. For Group A, loss of coverage (the exposure) was defined solely as loss of insurance, regardless of ADAP participation.
Because HIV-infected individuals who lack health insurance but report ADAP participation may be closer to functionally insured than uninsured, we also developed an additional specification, Group B, which included HIV-infected women who at baseline had stable health insurance alone, ADAP coverage alone, or both health insurance and ADAP coverage. Loss of coverage for Group B was defined having neither health insurance nor ADAP protection after the baseline visit.
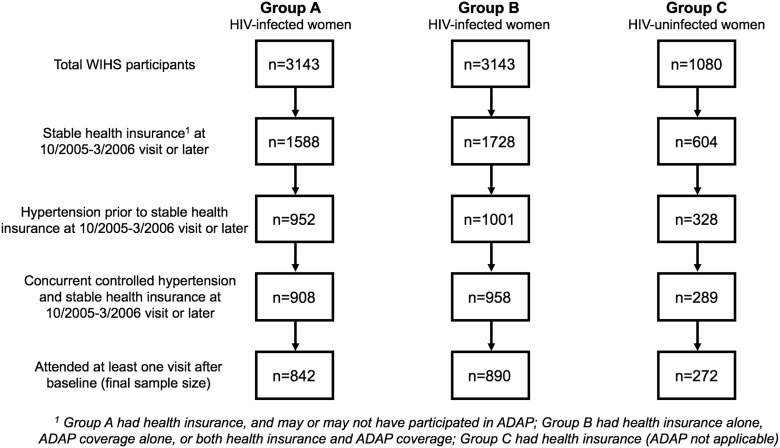
Group C included HIV-uninfected women with stable health insurance coverage at baseline; this population is not eligible for ADAP, so loss of coverage for them was simply defined as loss of health insurance after the baseline visit. A flowchart detailing participant selection is depicted in Figure 1.
FIG. 1.
Participant selection flowchart: Groups A, B, and C.
Ascertainment of hypertension
Controlled hypertension was defined as systolic BP <140 mmHg and diastolic BP <90 mmHg following previous documentation of hypertension, defined as antihypertensive medication use or at least two WIHS visits with systolic BP ≥140 or diastolic BP ≥90. One visit with systolic BP <140 and diastolic BP <90 was sufficient to establish controlled hypertension.
Starting in 2004, three BP measurements per visit were taken, with the mean of the second and third measurements used. Between 2001 and 2004, staff measured each participant's BP twice; the second measurement was used unless the readings differed in their systolic or diastolic values by more than 80 and 45 mmHg, respectively, in which case the higher measurement was used. Before 2001, BP was measured only once. BP measurements were collected from the right arm of a seated participant using an automated Dinamap blood pressure monitor (DinaMap ProCare Series; GE Medical Systems, Chicago, IL).28 The monitor automatically measured BP at 1-minute intervals following a 5-minute period of sitting quietly without talking. The clinician was in the room during BP measurement and passively raised the participant's arm overhead for the first 5 seconds between each measurement.
Covariates and definitions
To identify possible confounders, we constructed causal diagrams.34 Baseline confounders (reference levels marked by asterisk) were site (Brooklyn or Bronx,* Chicago, San Francisco, Los Angeles, Washington, D.C.), race (African American,* white, other), age, CD4 count, and HIV viral load. Time-varying confounders affected by prior exposure were annual household income (<$6,000, $6,001–$12,000,* $12,001–$18,000, >$18,000), body mass index, and in HIV-infected women only, CD4 count and HIV viral load. Other considered confounders (e.g., depression, employment, drug/alcohol/tobacco use) that decreased precision while negligibly changing parameter estimates were not included.
Nominal covariates were coded as disjoint indicator variables, with continuous variables specified as splines. Undetectable HIV viral load measurements were assigned half the lower detection limit.35 If data were missing, prior visit values were used; no baseline confounder had >1.5% of missing values and no time-varying confounder was missing at >5.7% of observed visits. The definition of ART followed DHHS guidelines.36
Statistical analysis
Proportions were compared using the chi-square test, with a Wilcoxon nonparametric test for location used to compare medians.37 For modeling analyses, the exposure variable switched from “coverage” (i.e., unexposed; by definition, women had coverage at baseline) to “no coverage” (i.e., exposed) at the first postbaseline visit that a woman reported loss of coverage. This status change is synonymous with a coverage break/interruption; once a woman's status switched to “no coverage,” she was classified as having a break/interruption until the end of follow-up. Because we hypothesized that the consequences of coverage interruption may be temporally limited, we did a sensitivity analysis where follow-up was censored 3 years following a break.
Our primary aim was to estimate the total effect of coverage interruption on loss of hypertension control, which requires adjustment not only for baseline confounders but also for time-varying confounders affected by prior exposure (causal intermediates between coverage status and loss of hypertension control). This was accomplished by using logistic regression to predict probabilities of (a) treatment (i.e., exposure) and (b) censoring based on covariate histories (i.e., past and current data), and constructing stabilized inverse-probability-of-treatment-and-censoring weights.38 We weighted by baseline and time-varying confounders affected by prior exposure and stabilized by baseline confounders. Models for the censoring weights additionally included time-varying health insurance, and all models included time specified as a restricted cubic spline with four knots at the 5th, 35th, 65th, and 95th percentiles.39
Assuming positivity, consistency, and no model misspecification, unmeasured confounding, or informative censoring, weighting creates a pseudo population in which exposure is independent from measured confounders.40 Therefore, if women had continuous coverage before interruption was reported (reasonable as status was assessed biannually), the weighted hazard ratio (HR) estimates the parameter from a randomized trial, contrasting the hazard of hypertension control loss had all women experienced a coverage interruption at baseline to the hazard had all women had continuous coverage throughout follow-up. Our use of stabilized weights necessitated inclusion of baseline confounders in the marginal structural model.41
Proportionality of hazards was verified by visual inspection of log-negative-log survival estimates. In addition, the complements of weighted survivor functions were plotted as estimates of the cumulative incidence of hypertension control loss for (a) uninterrupted coverage and (b) coverage interruption, since baseline. We also compared uninterrupted coverage to the natural course (i.e., what was actually observed),42 the contrast most relevant to policies or interventions that prevent coverage breaks.
Analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC). All women consented to WIHS participation, with consent obtained by trained study personnel at clinic sites, and agreed to contribute their data to research. Approval was granted by relevant institutional review boards.
Results
Of the total 3,143 HIV-infected WIHS participants, 1,558 (49.6%) had stable health insurance at the baseline visit, of whom 952 (61.1%) had prior hypertension, of whom 908 (95.4%) had concurrent controlled hypertension and stable health insurance, of whom 842 (92.7%) attended at least one visit after baseline (Fig. 1). These 842 women comprised Group A. Most enrolled in either 1994–1995 (n = 534, 63.4%) or 2001–2002 (n = 224, 26.6%) (Table 1). The proportion of the population contributed from any WIHS site ranged from 11.3% (Los Angeles) to 21.5% (Bronx). Over two-thirds of women were African American (n = 575, 68.3%), with 145 (17.2%) white and 122 (14.5%) other. The highest level of educational attainment was roughly split between less than (n = 318, 37.8%), equal to (n = 253, 30.1%), and greater than high school (n = 270, 32.1%).
Table 1.
Characteristics and Outcomes of Women with Controlled Hypertension and Stable Health Insurance in the Women's Interagency HIV Study, 2005–2014
| Group A: HIV-infected, health insurance n = 842 | Group B: HIV-infected, health insurance or ADAP n = 842 | Group C: HIV-uninfected n = 842 | |
|---|---|---|---|
| Baseline | |||
| Age in years, median (IQR) | 47.7 (42.4–52.4) | 47.6 (42.0–52.3) | 47.3 (41.6–52.8) |
| Race, n (%) | |||
| African American | 575 (68.3) | 587 (66.0) | 193 (71.0) |
| White | 145 (17.2) | 162 (18.2) | 38 (14.0) |
| Other | 122 (14.5) | 141 (15.8) | 41 (15.1) |
| Site, n (%) | |||
| Bronx | 181 (21.5) | 181 (20.3) | 81 (29.8) |
| Brooklyn | 164 (19.5) | 168 (18.9) | 44 (16.2) |
| Washington, D.C. | 126 (15.0) | 129 (14.5) | 39 (14.3) |
| Los Angeles | 95 (11.3) | 127 (14.3) | 32 (11.8) |
| San Francisco | 138 (16.4) | 140 (15.7) | 47 (17.3) |
| Chicago | 138 (13.4) | 145 (16.6) | 29 (10.7) |
| CD4 count, cells/mm3, median (IQR) | 497 (308–715) | 495 (307–714) | N/A |
| HIV viral load, copies/mL, median (IQR) | 40 (40–1,700) | 40 (40–1,300) | N/A |
| Receiving ART, n (%) | 638 (75.8) | 684 (76.9) | N/A |
| Prior AIDS, n (%) | 369 (43.8) | 381 (42.8) | N/A |
| Systolic blood pressure, mmHg, median (IQR) | 119 (111–129) | 119 (111–129) | 124 (114–131.5) |
| Diastolic blood pressure, mmHg, median (IQR) | 75 (69–81) | 74 (68–81) | 75 (69–82) |
| BMI, median (IQR) | 28.8 (24.2–34.7) | 29.1 (24.5–34.8) | 33.3 (28.0–39.3) |
| Annual household income, n (%) | |||
| <$6,000 | 117 (13.9) | 131 (14.7) | 58 (21.3) |
| $6,001–$12,000 | 340 (40.4) | 349 (39.2) | 83 (30.5) |
| $12,001–$18,000 | 118 (14.0) | 130 (14.6) | 37 (13.6) |
| >$18,000 | 267 (31.7) | 280 (31.5) | 94 (34.6) |
| Education, n (%)a | |||
| Less than high school | 318 (37.8) | 343 (38.6) | 98 (36.2) |
| High school | 253 (30.1) | 266 (29.9) | 93 (34.3) |
| More than high school | 270 (32.1) | 280 (31.5) | 80 (29.5) |
| Health insurance type, n (%) | |||
| Medicaid | 647 (76.8) | 632 (71.0) | 183 (67.3) |
| Private | 155 (18.4) | 149 (16.7) | 63 (23.2) |
| Medicare | 25 (3.0) | 25 (2.8) | 11 (4.0) |
| Other public | 13 (1.5) | 12 (1.3) | 10 (3.7) |
| Not categorizable | 2 (0.2) | 2 (0.2) | 5 (1.8) |
| ADAP only | 0 (0.0) | 70 (7.9) | 0 (0.0) |
| ADAP, n (%) | 111 (13.2) | 174 (19.6) | N/A |
| Follow-up | |||
| Visits, n; median (IQR) | 5,030; 4 (2–8) | 5,362; 4 (2–8) | 1,498; 4 (2–7) |
| Systolic blood pressure, mmHg, median (IQR) | 119 (110–131) | 119 (110–131) | 122 (112–133) |
| Diastolic blood pressure, mmHg, median (IQR) | 73 (68–80) | 73 (68–80) | 75 (69–81) |
| Health insurance interruption, n (%)b | 73 (8.7) | 58 (6.5) | 40 (14.7) |
| Person-years, n | 2125.5 | 2270.3 | 620.4 |
| Median (IQR) | 1.6 (0.8–3.6) | 1.6 (0.8–3.6) | 1.5 (0.6–3.1) |
| Before interruption (%) | 1949.1 (91.7) | 2129.6 (93.8) | 528.1 (85.1) |
| After interruption (%) | 176.4 (8.3) | 140.7 (6.2) | 92.3 (14.9) |
| Outcome, n (%) | |||
| Censored due to missed visits or death | 89 (10.6) | 101 (11.3) | 22 (8.1) |
| Active at end of follow-up | 184 (21.9) | 189 (21.2) | 45 (16.5) |
| Loss of hypertension control | 569 (67.6) | 600 (67.4) | 205 (75.4) |
| Before interruption (%) | 523 (91.9) | 561 (93.5) | 176 (85.9) |
| After interruption (%) | 46 (8.1) | 39 (6.5) | 29 (14.1) |
Data missing on one woman in each HIV-infected group and on one HIV-uninfected woman.
Defined as absence of health insurance and absence of ADAP for “HIV-infected, health insurance, or ADAP” group.
ADAP, AIDS Drug Assistance Program; ART, antiretroviral therapy; BMI, body mass index; IQR, interquartile range; N/A, not applicable; WIHS, Women's Interagency HIV Study.
At baseline, the median age was 47.7 years with an interquartile range (IQR) of 42.4–52.4. Overall, the women were low-income; approximately half had annual household incomes of $12,000 or less (n = 457, 53.4%) with less than a third exceeding $18,000 (n = 267, 31.7%). Accordingly, most women received Medicaid (n = 647, 76.8%) with a minority having private health insurance (n = 155, 18.4%); few had Medicare or other public coverage (n = 38, 4.5%). Almost half had a prior AIDS diagnosis (n = 369, 43.8%). Most were receiving ART (n = 638, 75.8%), and about half had an undetectable HIV viral load (n = 448, 53.2%). Median CD4 count was 497 cells/mm3 (IQR, 308–715).
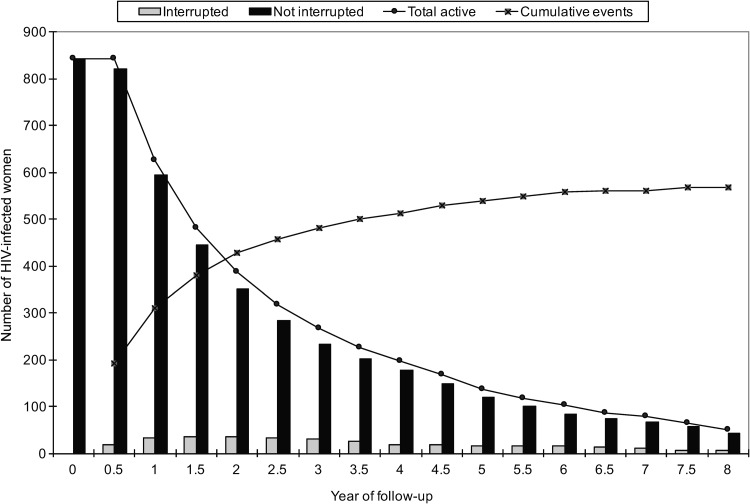
These 842 women contributed 2125.5 person-years (median, 1.6 years; IQR, 0.8–3.6) and attended 5,030 study visits (median, four visits; IQR, 2–8), approximately two per person-year reflecting the biannual visit schedule (Table 1). The relatively short median duration of follow-up per woman was a consequence of most women (n = 569, 67.6%) experiencing hypertension control loss (Fig. 2); 89 women (10.6%) were censored due to missed visits or death and 184 (21.9%) maintained controlled hypertension. Therefore, the overall rate of hypertension control loss was high at 26.8 per 100 person-years, with a 95% confidence interval (CI) of 24.6–29.1. Few women (n = 73, 8.7%) experienced coverage interruption (Fig. 2), with similar proportions of total person-time (8.3%) and outcomes (n = 46, 8.1%) occurring postinterruption.
FIG. 2.
Number of HIV-infected women by year of follow-up and health insurance status, and cumulative events (instances of hypertension control loss) over time.
Of the postinterruption person-time, 35.9% were uninsured (i.e., some women regained coverage following the break). The median systolic and diastolic BP at visits where insurance was reported were 119 (IQR, 110–130) and 74 (IQR, 68–80), respectively, similar to the median systolic (121.5; IQR, 112–134) and diastolic (76; IQR, 68–84) values at visits where no insurance was reported. When the “health insurance or ADAP” definition of coverage was used (Group B), a similar number of women (n = 890) were eligible (Fig. 1). This minor change to the inclusion criteria resulted in no notable changes to the baseline composition of the study population or its experiences during follow-up; only 70 women (7.9%) had no coverage besides ADAP at baseline.
Among HIV-infected women when the coverage definition did not include ADAP (Group A), the crude Cox proportional hazards model suggested a greater hazard of hypertension control loss (HR, 1.29, 95% CI, 0.95–1.75), given coverage interruption compared to uninterrupted coverage (Table 2). The HRs were slightly further from the null in the weighted model (HR, 1.37, 95% CI, 0.99–1.91) and when follow-up was censored 3 years postinterruption (HR, 1.42, 95% CI, 1.00–2.02). Among HIV-infected women when coverage included ADAP (Group B), the trend across the three models was the same although each of the measures was modestly stronger, increasing from the crude (HR, 1.40; 95% CI, 1.01–1.94) to the weighted (HR, 1.47; 95% CI, 1.04–2.07) to the weighted with follow-up censored (HR, 1.53; 95% CI, 1.04–2.23).
Table 2.
Estimated Effects of Health Insurance Interruption on Loss of Hypertension Control Among Women's Interagency HIV Study Women, 2005–2014
| Group A: HIV-infected, health insurance | Group B: HIV-infected, health insurance or ADAP | Group C: HIV-uninfected | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Unweighted, unadjusted | 1.29 | 0.95–1.75 | 1.40 | 1.01–1.94 | 1.24 | 0.83–1.86 |
| Weighted (marginal structural model)a | 1.37 | 0.99–1.91 | 1.47 | 1.04–2.09 | 1.39 | 0.88–2.21 |
| Weighted (marginal structural model),a censor 3 years postbreak | 1.42 | 1.00–2.02 | 1.53 | 1.04–2.23 | 1.44 | 0.91–2.29 |
Accounting for baseline confounders: site, race, age, and in HIV-infected women only, CD4 count and HIV viral load, as well as time-varying confounders affected by prior exposure: annual household income, body mass index, and in HIV-infected women only, CD4 count and HIV viral load.
HR, hazard ratio; CI, confidence interval.
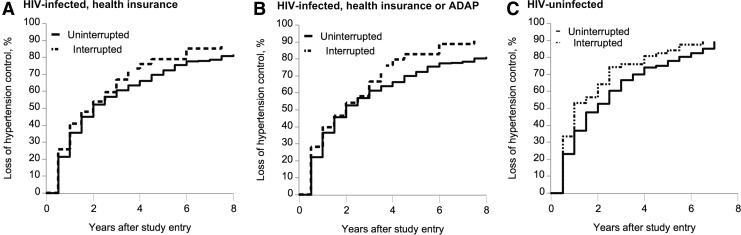
The cumulative incidences of hypertension control loss were estimated given the scenarios of (a) uninterrupted coverage and (b) coverage interruption, since baseline (Fig. 3). In Groups A and B, the cumulative incidence of hypertension control loss given coverage interruption at baseline was higher than given the natural course (not shown because curves were nearly coincident with, although slightly higher than, uninterrupted coverage). For scenarios (a) and (b), the 6-year cumulative incidences were 77.6% (95% CI, 71.6–82.4) and 85.3% (95% CI, 76.3–90.8) in Group A, and 77.4% (95% CI, 71.5–82.1) and 89.0% (95% CI, 80.1–94.0) in Group B. Comparing uninterrupted coverage to the natural course, the HRs (95% CI) in Groups A and B were 0.82 (0.61–1.11) and 0.74 (0.53–1.03), respectively.
FIG. 3.
Estimates of the cumulative incidences of loss of hypertension control given uninterrupted health insurance and interruption at baseline among women with stable health insurance in the WIHS, 2005–2014. Estimates are at the reference level of nominal covariates (site: Brooklyn or Bronx; race: African American) and the average level of continuous covariates: age; CD4 count, and HIV viral load (Groups A and B only; there are no covariates specific to Group C). ADAP, AIDS Drug Assistance Program; WIHS, Women's Interagency HIV Study.
Of the total 1,080 HIV-uninfected WIHS participants, 604 (55.9%) had stable health insurance at the baseline visit, of whom 328 (54.3%) had prior hypertension, of whom 289 (88.1%) had concurrent controlled hypertension and stable health insurance, of whom 272 (94.1%) attended at least one visit after baseline (Fig. 1). These 272 women comprised Group C, and accrued 620.4 person-years of follow-up and 1,498 study appointments (Table 1). These women did not markedly differ from the 842 HIV-infected women by race, education, age at baseline, or type of health insurance (public versus private) at baseline (all p > 0.05). However, HIV-uninfected women had lower annual household incomes at baseline (p < 0.01), with 21.3% of women in the <$6,000 category compared to 13.9%.
As in the HIV-infected women, the rate of hypertension control loss was high (33.0 per 100 person-years; 95% CI, 29.3–37.1), with most women (n = 205, 75.4%) experiencing this outcome. Few women were censored due to missed visits or death (n = 22, 8.1%) or maintained controlled hypertension (n = 45, 16.5%). Coverage interruption occurred in 14.6% of HIV-uninfected women (n = 40) at a rate of 7.6 per 100 person-years (95% CI, 5.4–10.3), compared to 8.7% of HIV-infected women at 3.7 per 100 person-years (95% CI, 2.9–4.7). This resulted in a larger proportion of follow-up occurring postinterruption (14.9%, 92.3 person-years); 46.7% of postinterruption person-time was uninsured. The median systolic and diastolic BP at visits where insurance was reported was 122 mmHg (IQR, 112–132) and 75 mmHg (IQR, 69–81), respectively, similar to the median systolic (123.5 mmHg; IQR, 113–132) and diastolic (77 mmHg; IQR, 70–81) values at visits where no insurance was reported.
Estimates of the effect of coverage interruption on loss of hypertension control in HIV-uninfected women were similar to those from HIV-infected women, although HRs were less precise due to the smaller sample (Table 2). In Group C, as in Groups A and B, the cumulative incidence of hypertension control loss given coverage interruption at baseline was higher than given the natural course. In this HIV-uninfected group, the 6-year cumulative incidences of hypertension control loss were 82.5% (95% CI, 72.5–88.9) given uninterrupted coverage and 87.6% (95% CI, 74.9–93.9) given coverage interruption, since baseline (Fig. 3). The HR comparing uninterrupted coverage to the natural course was 0.78 (95% CI, 0.52–1.19).
Discussion
Our analyses demonstrate that health insurance interruption increases the hazard of hypertension control loss in both HIV-infected (HR, 1.37, 95% CI, 0.99–1.91) and HIV-uninfected women at risk for HIV infection (HR, 1.39; 95% CI, 0.88–2.21) in the US. These HRs support the notion that insurance continuity maintains lower BP levels, which would be expected to prevent downstream morbidity and mortality due to hypertension,43,44 and encourage policy efforts to expand and strengthen coverage. In tandem with other BP-lowering interventions such as self-monitoring and patient/provider education,45 needed to minimize the individual and public health burdens of elevated BP given the observed high incidences of hypertension control loss even with insurance, our findings advocate continuous insurance as a strategy for BP maintenance.
The substantial proportion of women who experienced hypertension control loss is consistent with a national estimate that only 52% of hypertensive patients exhibit BP control.46 Our results are also consistent with a recent report of high prevalence of uncontrolled hypertension among hypertensive HIV patients, in which 41% of hypertensive HIV-infected men lacked hypertension control after a median of 3.4 years of follow-up.47
A stronger effect estimate was noted when the coverage definition included ADAP, likely reflecting that ADAP is a source of antihypertensive medications for many uninsured HIV patients; a break had greater consequences when women were uninsured and also without ADAP coverage. This highlights another reason beyond ART access (and subsequent HIV suppression) for policymakers to support ADAP financially–over 6 years there would be 116 fewer instances of hypertension control loss in 1,000 HIV-infected women with continuous health insurance or ADAP than in women without either.
When follow-up was censored, the HR slightly increased, suggesting a negative impact on hypertension control that is temporally proximate to coverage interruption, and heightened vulnerability to losing control in the period immediately following a break. This is consistent with losing access to hypertension medications as a causal mechanism, and underscores the need to remove administrative barriers contributing to disruptions in plans providing drugs (e.g., states requiring biannual ADAP recertification48).16
Our findings underscore a potential and previously unknown population-level benefit on hypertension control if additional states were to expand Medicaid, given these areas' high numbers of low-income13 and HIV-infected49 individuals, and encourage such action. This study dovetails with a projection that health insurance expansions will increase the proportion of treated hypertensive patients by 5.1% by 2016, with longer term reductions in cardiovascular-related events.50
HIV-uninfected women in our sample had lower household incomes while also experiencing higher crude rates of coverage interruption and hypertension control loss; suggestions of higher cumulative incidences were limited by precision. While differences may be partly due to enrollment criteria that are unique to HIV-uninfected women (e.g., high HIV-risk sex or drug use) or poorer healthcare access, they also illustrate the population's vulnerability to insurance coverage interruptions and loss of hypertension control, as well as the importance of targeting interventions to this group.
Study limitations include self-reported health insurance data and 6-monthly interval measurement that precluded more precise information on exactly when coverage interruption and hypertension outcomes occurred, although resultant biases were likely mitigated by the frequent visit schedule. We also incorporated the unverifiable assumption of no unmeasured confounding and assumed homogeneity across insurance types (i.e., collapsing coverage into yes or no), an oversimplification given WIHS evidence that HIV suppression differs by insurance type.31 Exposure dichotomization was necessary due to limited sample size. Although our HRs suggested meaningful effects, larger studies would allow for closer investigation of coverage continuity's impacts on hypertension control and other outcomes.
It is also worth noting that while the prognostic significance of a point-in-time loss of hypertension control remains largely unknown, the relatively small number of women eligible for the analysis coupled with a low incidence of cardiovascular episodes precluded evaluation of a clinical event outcome. Given strong evidence that poorly controlled hypertension imparts cardiovascular risk,43,44 it was still worthwhile to examine loss of hypertension control, especially since the multiple BP measurements at a single time point provided a high degree of confidence in outcome classification. While it would have been useful to describe the duration of hypertension control loss in a more detailed manner, this was not possible because the interval cohort design of the WIHS only allows BP measurement twice annually. Additional studies of insurance continuity should evaluate cardiovascular events as an outcome, as well as cardiovascular events downstream of uncontrolled hypertension.
Utilization of a different threshold for hypertension control, such as the 120 mmHg target for systolic BP investigated in the Systolic Blood Pressure Intervention Trial (SPRINT),51 may have yielded substantially different results. Along with highlighting that more nuanced treatment of definitions for hypertension and its control will be required in future similar analyses if the trend continues toward unique BP targets for different age and risk factor groups,52–55 the higher rates of cardiovascular events and death at the 140 mmHg target (vs. 120) revealed by SPRINT further emphasize the individual and public health significance of our findings. We used a cut point of 140 mmHg systolic, which may be associated with higher morbidity and mortality risk than lower thresholds.51,56,57
Study strengths include minimal missing data, rigorous quality control of laboratory and outcome ascertainment, and inclusion of HIV-infected and HIV-uninfected women. Notably, HIV-infected WIHS women represent the greater population of HIV-infected women in the US.29 However, because data were from study participants who had contact with healthcare, our results could be muted compared to those from non-WIHS populations.
This work also responds to a call to report not just “always versus never” contrasts,58 that is, continuously insured versus interrupted since baseline, as it is difficult to imagine an entire population losing its coverage. Specifically, we contrasted continuous insurance—a hypothetical scenario attainable if policy changes establish universal coverage—to the natural course, revealing a HR of 0.74 (95% CI, 0.53–1.03) in HIV-infected women when coverage included ADAP. Assuming the continuously insured population represents the population continuously insured under universal coverage, the contrast reflects and informs an intervention that instates universal coverage.
Conclusions
This study indicates that health insurance continuity prevents loss of hypertension control in key populations in the US, an impact that may have been surmised but was heretofore unquantified. Interventions targeted at low-income and HIV-infected individuals to ensure coverage stability and access, including for ADAP, should be a policy priority.
Acknowledgments
Data in this article were collected by the WIHS. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (M.H.C. and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young and Seble Kassaye), U01-AI-034994; Miami WIHS (M.F. and Lisa Metsch), U01-AI-103397; UNC WIHS (A.A.A.), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (J.M.), U01-HD-032632 (WIHS I–WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). M.O.J. is supported by K24DA037034 from NIDA.
Author Disclosure Statement
J.J.E. Jr. receives research support and is a consultant to several pharmaceutical companies that make antiretroviral agents. D.M. has been an expert witness for probiotic cases for General Mills, Procter & Gamble, Nestle, and Bayer Health. A.A.A. has a consultancy with ViiV Healthcare and a research grant from Gilead. No other competing financial interests exist.
References
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinson ML. Income inequality in health at all ages: A comparison of the United States and England. Am J Public Health 2012;102:2049–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya J, Goldman D, Sood N. The link between public and private insurance and HIV-related mortality. J Health Econ 2003;22:1105–1122 [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein A, Taubman S, Wright B, et al. The Oregon health insurance experiment: Evidence from the first year. Q J Econ 2012;127:1057–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley ED, Moore KL, Haber S, Neilands TB, Cohen J, Kral AH. Population-level effects of uninterrupted health insurance on services use among HIV-positive unstably housed adults. AIDS Care 2011;23:822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan PS, Juhasz M, McNaghten AD, Frankel M, Bozzette S, Shapiro M. Time to first annual HIV care visit and associated factors for patients in care for HIV infection in 10 US cities. AIDS Care 2011;23:1314–1320 [DOI] [PubMed] [Google Scholar]
- 7.Muthulingam D, Chin J, Hsu L, Scheer S, Schwarcz S. Disparities in engagement in care and viral suppression among persons with HIV. J Acquir Immune Defic Syndr 2013;63:112–119 [DOI] [PubMed] [Google Scholar]
- 8.Hughes AJ, Mattson CL, Scheer S, Beer L, Skarbinski J. Discontinuation of antiretroviral therapy among adults receiving HIV care in the United States. J Acquir Immune Defic Syndr 2014;66:80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SR, Kirking DM. The effect of insurance coverage changes on drug utilization in HIV disease. J Acquir Immune Defic Syndr 2001;28:140–149 [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services. The affordable care act helps people with HIV, 2013. Available at: https://aids.gov/pdf/how-does-the-aca-help-plwh.pdf Accessed December8, 2016
- 11.Solomon L, Stein M, Flynn C, et al. Health services use by urban women with or at risk for HIV-1 infection: The HIV Epidemiology Research Study (HERS). J Acquir Immune Defic Syndr Hum Retrovirol 1998;17:253–261 [DOI] [PubMed] [Google Scholar]
- 12.Health Resources and Services Administration. About the Ryan White HIV/AIDS program, 2016. Available at: http://hab.hrsa.gov/abouthab/aboutprogram.html Accessed December8, 2016
- 13.Garfield R, Damico A. The coverage gap: Uninsured poor adults in states that do not expand medicaid—an update, 2016. Available at: https://kaiserfamilyfoundation.files.wordpress.com/2016/01/8659-04-the-coverage-gap-uninsured-poor-adults-in-states-that-do-not-expand-medicaid.pdf Accessed December8, 2016
- 14.Berenson J, Doty M, Abrams M, Shih A. Achieving better quality of care for low-income populations: The role of health insurance and the medical home for reducing health inequities, 2012. Available at: www.commonwealthfund.org/∼/media/files/publications/issue-brief/2012/may/1600_berenson_achieving_better_quality_care_low_income_v2.pdf Accessed December8, 2016 [PubMed]
- 15.Sommers BD. Loss of health insurance among non-elderly adults in medicaid. J Gen Intern Med 2009;24:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommers BD, Graves JA, Swartz K, Rosenbaum S. Medicaid and marketplace eligibility changes will occur often in all states; policy options can ease impact. Health Aff (Millwood) 2014;33:700–707 [DOI] [PubMed] [Google Scholar]
- 17.Bindman AB, Chattopadhyay A, Auerback GM. Interruptions in medicaid coverage and risk for hospitalization for ambulatory care-sensitive conditions. Ann Intern Med 2008;149:854–860 [DOI] [PubMed] [Google Scholar]
- 18.National Heart, Lung, and Blood Institute. Description of high blood pressure, 2015. Available at: www.nhlbi.nih.gov/health/health-topics/topics/hbp Accessed December8, 2016
- 19.Calo LA, Caielli P, Maiolino G, Rossi G. Arterial hypertension and cardiovascular risk in HIV-infected patients. J Cardiovasc Med (Hagerstown) 2013;14:553–558 [DOI] [PubMed] [Google Scholar]
- 20.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: A cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr 2011;57:245–253 [DOI] [PubMed] [Google Scholar]
- 21.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Socio GV, Ricci E, Maggi P, et al. Prevalence, awareness, treatment, and control rate of hypertension in HIV-infected patients: The HIV-HY study. Am J Hypertens 2014;27:222–228 [DOI] [PubMed] [Google Scholar]
- 23.Myerson M, Poltavskiy E, Armstrong EJ, Kim S, Sharp V, Bang H. Prevalence, treatment, and control of dyslipidemia and hypertension in 4278 HIV outpatients. J Acquir Immune Defic Syndr 2014;66:370–377 [DOI] [PubMed] [Google Scholar]
- 24.Nuesch R, Wang Q, Elzi L, et al. Risk of cardiovascular events and blood pressure control in hypertensive HIV-infected patients: Swiss HIV Cohort Study (SHCS). J Acquir Immune Defic Syndr 2013;62:396–404 [DOI] [PubMed] [Google Scholar]
- 25.Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: A meta-analysis. J Hypertens 2015;33:221–229 [DOI] [PubMed] [Google Scholar]
- 26.Short PF, Graefe DR, Swartz K, Uberoi N. New estimates of gaps and transitions in health insurance. Med Care Res Rev 2012;69:721–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins S, Robertson R, Garber T, Doty M. The income divide in health care: How the affordable care act will help restore fairness to the U.S. health system, 2012. Available at: www.commonwealthfund.org/∼/media/files/publications/issue-brief/2012/feb/1579_collins_income_divide_tracking_brief.pdf Accessed December8, 2016 [PubMed]
- 28.Women's Interagency HIV Study. Visit 46 manual of operations. Version: April 1, 2017, 2017. Available at: https://statepi.jhsph.edu/wihs/wordpress/wihs-manual-of-operations/moo-v46 Accessed March8, 2017
- 29.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's interagency HIV study. WIHS collaborative study group. Epidemiology 1998;9:117–125 [PubMed] [Google Scholar]
- 30.Hessol NA, Weber KM, Holman S, et al. Retention and attendance of women enrolled in a large prospective study of HIV-1 in the United States. J Womens Health 2009;18:1627–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludema C, Cole SR, Eron JJ, et al. Impact of health insurance, ADAP, and income on HIV viral suppression among US women in the Women's Interagency HIV Study, 2006–2009. J Acquir Immune Defic Syndr 2016;73:307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003;42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 33.Kates J, Garfield R, Young K, Quinn K, Frazier E, Skarbinski J. Assessing the impact of the affordable care act on health insurance coverage of people with HIV, 2014. Available at: https://kaiserfamilyfoundation.files.wordpress.com/2013/12/8535-assessing-the-impact-of-the-affordable-care-act-on-health-insurance-coverage.pdf Accessed December8, 2016
- 34.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48 [PubMed] [Google Scholar]
- 35.Cates JE, Westreich D, Edmonds A, et al. The effects of viral load burden on pregnancy loss among HIV-infected women in the United States. Infect Dis Obstet Gynecol 2015;2015:362357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, 2008. Available at: https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL001226.pdf Accessed December8, 2016
- 37.SAS Institute, Inc. The NPAR1WAY procedure. Example 71.1 Two-sample location tests and plots, 2014. Available at: http://support.sas.com/documentation/cdl/en/statug/67523/HTML/default/viewer.htm-statug_npar1way_examples01.htm Accessed December8, 2016
- 38.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570 [DOI] [PubMed] [Google Scholar]
- 39.Harrell FE., Jr. DASPLINE macro, 1991. Available at: http://biostat.mc.vanderbilt.edu/wiki/pub/Main/SasMacros/survrisk.txt Accessed December8, 2016
- 40.Hernan MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health 2006;60:578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keil AP, Edwards JK, Richardson DB, Naimi AI, Cole SR. The parametric g-formula for time-to-event data: Intuition and a worked example. Epidemiology 2014;25:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cushman WC. The burden of uncontrolled hypertension: Morbidity and mortality associated with disease progression. J Clin Hypertens (Greenwich) 2003;5:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu Q, Burt VL, Paulose-Ram R, Yoon S, Gillum RF. High blood pressure and cardiovascular disease mortality risk among U.S. adults: The third National Health and Nutrition Examination Survey mortality follow-up study. Ann Epidemiol 2008;18:302–309 [DOI] [PubMed] [Google Scholar]
- 45.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev 2010:CD005182. [DOI] [PubMed] [Google Scholar]
- 46.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013;133:1–8 [PubMed] [Google Scholar]
- 47.De Socio GV, Ricci E, Maggi P, et al. Time trend in hypertension prevalence, awareness, treatment, and control in a contemporary cohort of HIV-infected patients: The HIV and hypertension study. J Hypertens 2017;35:409–416 [DOI] [PubMed] [Google Scholar]
- 48.Colorado Department of Public Health & Environment. Colorado AIDS drug assistance program (ADAP), 2016. Available at: www.colorado.gov/pacific/cdphe/colorado-aids-drug-assistance-program-adap Accessed December8, 2016
- 49.Snider JT, Juday T, Romley JA, et al. Nearly 60,000 uninsured and low-income people with HIV/AIDS live in states that are not expanding Medicaid. Health Aff (Millwood) 2014;33:386–393 [DOI] [PubMed] [Google Scholar]
- 50.Li S, Bruen BK, Lantz PM, Mendez D. Impact of health insurance expansions on nonelderly adults with hypertension. Prev Chronic Dis 2015;12:E105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.SPRINT Research Group, Wright JT, Jr., Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chobanian AV. Hypertension in 2017-what is the right target? JAMA 2017;317:579–580 [DOI] [PubMed] [Google Scholar]
- 53.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–520 [DOI] [PubMed] [Google Scholar]
- 54.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–1357 [DOI] [PubMed] [Google Scholar]
- 55.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 2014;32:3–15 [DOI] [PubMed] [Google Scholar]
- 56.Taylor BC, Wilt TJ, Welch HG. Impact of diastolic and systolic blood pressure on mortality: Implications for the definition of “normal”. J Gen Intern Med 2011;26:685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber MA, Bloch M, Bakris GL, et al. Cardiovascular outcomes According to systolic blood pressure in patients with and without diabetes: An ACCOMPLISH Substudy. J Clin Hypertens (Greenwich) 2016;18:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westreich D. From exposures to population interventions: Pregnancy and response to HIV therapy. Am J Epidemiol 2014;179:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]