Abstract
Introduction
In addition to glomerular lesion, renal vascular lesion is also an important prognostic marker of lupus nephritis (LN). Among patients with various vascular changes, individuals with thrombotic microangiopathy (TMA) present with severe clinical manifestations and have a high mortality. The aim of the present study was to assess the spectrum and impact of TMA on the outcomes of LN. In a prospective observational study of 2.5 years’ duration, clinical and renal histopathological data regarding biopsy-proven LN were noted, and evaluation for antiphospholipid syndrome (APS) as a cause of TMA in LN was also carried out.
Methods
Study subjects were followed up actively for 6 months, and various outcomes were noted. Cases were divided into 2 groups as LN with TMA and LN without TMA, and various features were compared between the 2 groups. Outcomes recorded were complete response (CR), partial response (PR), treatment failure, and death.
Results
Of the 197 patients with LN, 50 patients (25.4%) were diagnosed with co-existing renal TMA. Five patients (10%) were found to have concomitant APS. As compared to patients without TMA, those with TMA had significantly higher rates of oliguria (P = 0.035), advanced renal injury, that is, serum creatinine > 3mg/dl (P = 0.002), fibrocellular and fibrous crescents (P = 0.01), and tubular atrophy (P = 0.001). Outcomes included CR in 15 patients (30%), PR in 10 (20%), failure in 19 (38%), and death in 6 (12%). Patients with LN with TMA had higher rates of treatment failure (P = 0.02) compared to the group without TMA.
Discussion
The presence of TMA in patients with LN is associated with adverse clinicopathological presentation and poor outcome.
Keywords: antiphospholipid syndrome, lupus nephritis, thrombotic microangiopathy
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease of unknown etiology. In lupus nephritis (LN), apart from glomerulonephritis, involvement of renal vascular structures by SLE plays an important role in prognosis, and its presence can adversely affect the prognosis of renal disease. Among various vascular changes, the presence of thrombotic microangiopathy (TMA) is associated with the most severe clinical manifestations and high mortality.1
In patients with LN, the presence of antiphospholipid antibodies is considered to be a risk factor for TMA.2 Occurrence of TMA in patients with SLE is seen in 3% to 9% according to various studies.3 TMA can be renal limited or present with systemic symptoms. Patients with LN with TMA have higher proteinuria, serum creatinine, activity indices, and endocapillary proliferation, and chronicity indices compared to patients with LN without TMA. There are no standardized guidelines for the management of patients with LN with TMA, although the 2012 KDIGO Clinical Practice Guideline for Glomerulonephritis suggested that “patients with systemic lupus and thrombotic thrombocytopenic purpura (TTP) receive plasmapheresis as for patients with TTP without systemic lupus.”4 The renal outcome of patients with LN with TMA is poorer compared to that of patients without TMA.5 The aim of this study was to assess the spectrum and impact of TMA in patients with LN.
Materials and Methods
The present prospective study was carried out at the Department of Nephrology and Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. Patients >14 years of age with newly diagnosed SLE according to ACR criteria and with biopsy-proven LN (light microscopy, immunofluorescence, and electron microscopy, defined below) were included in the study. Patients who received prior immunosuppressive therapy or who had hepatitis B or C or HIV-I/II infection or diabetes mellitus were excluded from the study. The study was approved by the Institute Ethics Committee and was conducted in compliance with the Declaration of Helsinki.
At baseline, all patients underwent urine examination with 24-hour protein quantification, serum creatinine, liver function tests, antinuclear factor (ANF), antineutrophilic cytoplasmic antibody (ANCA), antibodies to double-stranded DNA (dsDNA), complement 3 and 4 levels, lupus anticoagulant, anticardiolipin antibody (ACA), antibody to β2 glycoprotein, and disease activity assessed by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). All the patients were followed up on a monthly basis for 6 months with urine protein, serum creatinine, albumin, and complete blood count. Anti-dsDNA, C3, and C4 tests were repeated at 6 months after starting therapy. All the patients were followed up for 6 months, and partial remission (PR) and complete remission (CR) were assessed at the end of 6 months after starting immunosuppressive therapy.
Definitions
The presence of LN was defined as SLE diagnosed based on The Systemic Lupus Collaborating Clinics (SLICC) criteria, with the presence of proteinuria (> 500 mg/d), with or without the presence of erythrocytes or leukocytes in urine, along with kidney biopsy results suggestive of LN as per the classification of International Society of Nephrology and the Renal Pathology Society (ISN/RPS) 2003 lupus nephritis classification system.6 Renal TMA was defined as interlobular artery, arteriole, and glomerular capillary lesions, including endothelial cell swelling, lumen narrowing or obliteration, and thrombi formation by light microscopy.4 Complete remission (CR) was defined as return of serum creatinine to the previous baseline value, plus a decline in the urine protein/creatinine ratio to <500 mg/g.4 Partial remission (PR) was defined as stabilization (±25%) or improvement of serum creatinine, but not to normal, plus a ≥ 50% decrease in the urine protein/creatinine ratio. If there was nephrotic-range proteinuria (urine protein/creatinine ratio ≥ 3000 mg/g), improvement required a ≥ 50% reduction in the urine protein/creatinine ratio, and a urine protein/creatinine ratio < 3000 mg/g.4 Treatment failure was defined as a sustained 25% increase in serum creatinine, an increase in proteinuria, or a reduction in proteinuria but not to the extent of complete or partial remission.4 Rapid progressive glomerulonephritis (RPGN) was defined as a rapid decline in GFR (as assessesd by the Modification of Diet in Renal Disease [MDRD] equation) to < 60 ml/min/1.73 m2 in days to weeks.
Assays
Serum ANF was detected using indirect immunofluorescence assay (EUROIMMUN, Lübeck, Germany) and anti-dsDNA antibodies were detected using Crithidia luciliae indirect immunofluorescence test. Serum C3 and C4 was determined using rate nephelometry assay. LA was done by a clot assay method using an automated analyzer, ACA, and β2 glycoprotein antibody. Both were performed by using the enzyme-linked immunosorbent assay (ELISA) method; if the test results were found to be abnormal at baseline, the test was repeated after 3 months and the results were declared as positive or negative.
Therapy
All the patients received induction therapy with oral prednisolone (1 mg/kg/d, with or without pulse methylprednisolone 1 g for 3 days, for 8 weeks, followed by a taper to 7.5 mg/d at the end of 6 months) and either mycophenolate mofetil (MMF) (1.5–3 g/d) or i.v. cyclophosphamide therapy. Treatments received by the patients were noted in detail, including the drug, dose, duration, frequency, and mode of administration. Patients were actively followed up for at least 6 months to assess outcome.
Statistical Analysis
Continuous variables are presented as the mean plus or minus the SD, and categorical variables are presented as frequencies and percentages. All the relevant data was recorded in an Excel worksheet (Microsoft Corporation, Redmond, WA) and analysed by SPSS 22.0 (IBM SPSS, Armonk, NY). Differences between the 2 groups were estimated by using the Student t test for unpaired continuous variables, χ2 test, or Fisher exact test for categorical variables. Multivariate analysis was used to assess the factors affecting the poor outcomes. A P value of < 0.05 was considered significant.
Results
A total of 197 patients with LN were enrolled in the study from July 2013 to December 2015. The mean age of the paients was 31.9 ± 10.2 years (range, 13–52 years). The study included 172 female (87.3%) and 25 male (12.7%) patients. The clinical presentations were nephrotic syndrome, nephritic syndrome, nephroto-nephritic, RPGN, and asymptomatic urinary abnormalities (AUA) in 60 (30.5%), 20 (10.2%), 30 (15.2%), 35 (17.8%), and 52 (26.4%) patients, respectively. Antibodies to dsDNA were positive in 185 cases (93.9%). Biopsy findings were suggestive of class II, III, IV, and class V in 8 (4.1%), 35 (17.8%), 119 (60.4%), and 35 (17.8%) patients, respectively.
Among 197 patients, 50 (25.4%) were found to have TMA. The mean age of the patients with TMA was 31.38 ± 11.63 years (range, 18–57 years). In patients with TMA, the clinical presentation was nephrotic syndrome, nephritic syndrome, nephroto-nephritic, RPGN, and AUA in 14 (28%), 5 (10%), 5 (10%), 18 (36%), and 8 (16%) patients, respectively. dsDNA antibody was positive in 48 (96%) cases, and serum hypocomplementemia was reported in 41 (82%). TMA was vascular in 40 cases (80%) and glomerular in 10 (20%). Demographic and baseline parameters of patients with and without TMA are listed in Table 1.
Table 1.
Clinical and laboratory parameters
| Symptom | Total (n = 197) | TMA (n = 50) | Non-TMA (n = 147) | P value |
|---|---|---|---|---|
| Pedal edema | 165 (83.8%) | 43 (86%) | 122 (83%) | 0.825 |
| Facial puffiness | 151 (76.6%) | 40 (80%) | 111 (75.5%) | 0.567 |
| Oliguria | 48 (24.4%) | 18 (36%) | 30 (20.4%) | 0.035 |
| Oral ulcer | 120 (60%) | 28 (56%) | 92 (62.6%) | 0.502 |
| Arthralgia | 147 (74.6%) | 42 (84%) | 105 (71.4%) | 0.092 |
| Fever | 116 (58.9%) | 32 (64%) | 84 (57%) | 0.241 |
| Malar rash | 131 (66.5%) | 36 (72%) | 95 (72.5%) | 0.381 |
| Anemia (Hb < 11 g/dl) | 151 (76.6%) | 42 (84%) | 109 (74.1%) | 0.179 |
| Thrombocytopenia (platelet count < 150,000) | 62 (31.5%) | 19 (38%) | 43 (29.3%) | 0.291 |
| Creatinine (1.2–3 mg/dl) | 47 (23.9%) | 16 (32%) | 31 (21.1%) | 0.128 |
| Creatinine (> 3 mg/dl) | 33 (16.8%) | 16 (32%) | 17 (11.6%) | 0.002 |
| Hypoalbuminemia (Alb 3–3.5 g/dl) | 39 (19.8%) | 10 (20%) | 29 (19.7%) | 0.835 |
| Hypoalbuminemia (Alb < 3 g/dl) | 132 (67%) | 31 (62%) | 101 (68.7%) | 0.299 |
| Proteinuria (g/d) | 2.92 ± 1.92 | 3.03 ± 1.76 | 2.89 ± 2.1 | 0.674 |
| Hematuria | 72 (36.5%) | 18 (36%) | 54 (36.7%) | 1 |
| Low C3 | 154 (78.2%) | 41 (82%) | 113 (76.9%) | 0.291 |
| Low C4 | 154 (78.2%) | 41 (82%) | 113 (76.9%) | 0.554 |
| ds DNA antibody | 185 (93.9%) | 48 (96%) | 137 (93.2%) | 0.734 |
| ANA | 196 (99.4) | 50 (100%) | 146 (99.3) | 0.771 |
| SLEDAI | 16.2 ± 3.74 | 16.4 ± 3.78 | 16.18 ± 3.73 | 0.643 |
Alb, albumin; ANA, antinuclear antibody; C3, complement factor 3; C4, complement factor 4; dsDNA, double-stranded DNA antibody; SLEDAI, System Lupus Erythematosus Disease Activity Index; TMA, thrombotic microangiopathy.
P value < 0.05 is significant, so the value less than 0.05 was kept in boldface.
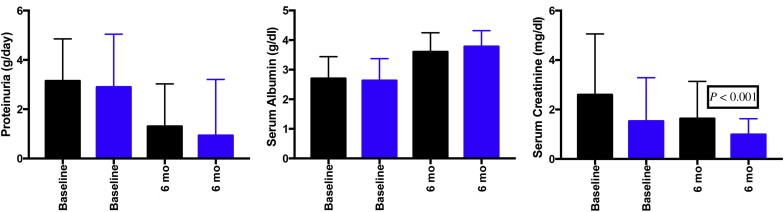
Except for oliguria (P = 0.035), serum creatinine >3 mg/dl (P = 0.002), tubular atrophy (P = 0.001), and endocapillary proliferation (P = 0.081), there was no significant difference between the TMA and non-TMA group (Tables 1 and 2). Five patients (25.4%) in the TMA group tested positive for antiphospholipid antibodies. Of these 5 patients, 2 patients showed only LA positivity, another 2 showed both ACA and β2 glycoprotein1 antibody positivity, and the fifth patient showed both LA and ACA positivity. Two patients had systemic APS syndrome, which was diagnosed based on revised classification criteria for APS.7 (One patient had a history of recurrent pregnancy loss, and the other had deep vein thrombosis.) At the end of 6 months of follow up, patients of the TMA group had higher proteinuria and serum creatinine and lower serum albumin compared to the non-TMA group (Figure 1).
Table 2.
Histopathological parameters in study groups
| Parameter | Total | TMA | Non-TMA | P value | |
|---|---|---|---|---|---|
| Class | II | 8 (4.1%) | 1 (2%) | 7 (4.8%) | |
| III | 35 (17.8%) | 8 (16%) | 27 (18.4%) | ||
| IV | 119 (60.4%) | 36 (72%) | 83 (56.5%) | 0.176 | |
| V | 35 (17.8%) | 5 (10%) | 30 (20.4%) | ||
| Crescents | Cellular | 66 (33.5%) | 20 (40%) | 46 (31.3%) | 0.299 |
| Fibrocellular+fibrous | 68 (34.5%) | 25 (50%) | 43 (29.25%) | 0.01 | |
| Endocapillary proliferation | 133 (67.5%) | 39 (78%) | 94 (63.9%) | 0.081 | |
| Interstitial inflammation | 107 (54.3%) | 32 (64%) | 75 (51%) | 0.139 | |
| Fibrinoid necrosis | 27 (13.7%) | 9 (18%) | 18 (12.2%) | 0.343 | |
| Karrhyorexis | 23 (11.7%) | 7 (14%) | 16 (10.9%) | 0.611 | |
| Hyaline thrombi | 39 (19.8%) | 13 (26%) | 26 (17.7%) | 0.221 | |
| Wireloop lesions | 68 (34.5%) | 22 (44%) | 46 (31.3%) | 0.122 | |
| Glomerular leukocytosis | 23 (11.7%) | 4 (8%) | 19 (12.9%) | 0.676 | |
| Tubular atrophy | 119 (60.4%) | 41 (82%) | 78 (53.1%) | 0.001 | |
| Interstitial fibrosis | 96 (48.7%) | 28 (56%) | 68 (46.3%) | 0.255 | |
| Glomerular sclerosis | 69 (35%) | 16 (32%) | 53 (36.1%) | 0.669 | |
TMA, thrombotic microangiopathy.
Figure 1.
Comparison of proteinuria, serum albumin, and serum creatinine between TMA and non-TMA groups at baseline and 6 months. Black bar, TMA; blue bar, non-TMA. TMA, thrombotic microangiopathy.
Treatment
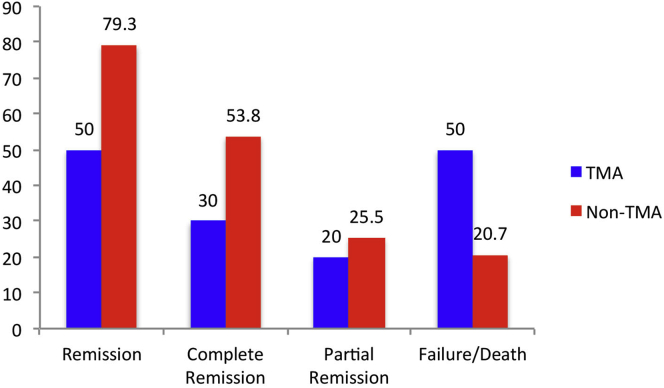
All patients included in study (both TMA and non-TMA) received oral prednisolone 1 mg/kg; 115 patients (58.4%) received pulse methylprednisolone. In the TMA group, 35 patients (70%) received cyclophosphamide and 15 (30%) received MMF. In the non-TMA group, 84 patients (57.1%) received cyclophosphamide and 59 (40.1%) received MMF. In addition to this, 8 patients (16%) from the TMA group received plasmapheresis (Supplementary Table S1). As compared to the non-TMA group, patients with TMA had higher treatment failure (P = 0.02) and lower CR (P = 0.005) rates (Figure 2); details regarding the patients and their respective outcomes are provided in Table 3.
Figure 2.
Comparison of outcomes between TMA and non-TMA groups of lupus nephritis. TMA, thrombotic microangiopathy.
Table 3.
Treatments received and outcomes in the 2 groups
| Drug | Both | TMA | Non-TMA | P value |
|---|---|---|---|---|
| Treatment | ||||
| Pulse steroid | 115 (58.4%) | 38 (76%) | 77 (52.4%) | 0.010 |
| Cyclophosphamide | 119 (60.4%) | 35 (70%) | 84 (57.1%) | 0.215 |
| MMF | 74 (37.5%) | 15 (30%) | 59 (40.1%) | 0.538 |
| Outcome | ||||
| Death | 13 (6.9%) | 6 (12%) | 7 (4.8%) | 0.100 |
| Failure | 42 (21.5%) | 19 (38%) | 23 (15.9%) | 0.020 |
| Complete response | 93 (47.7%) | 15 (30%) | 78 (53.8%) | 0.005 |
| Partial response | 47 (24.1%) | 10 (20%) | 37 (25.5%) | 0.565 |
MMF, mycophenolate mofetil; TMA, thrombotic microangiopathy.
On multivariate analysis oliguria (P < 0.001), photosensitivity (P = 0.020), malar rash (P = 0.024), serum creatinine (>3 mg/dl) at presentation (P < 0.001), TMA (P < 0.001), interstitial inflammation (P = 0.009) and fibrosis (P = 0.003) were associated with poorer treatment outcome in LN.
Discussion
In the present prospective observational study, we evaluated the clinical outcome of patients with LN and TMA. Patients with TMA had higher serum creatinine, adverse chronic tubulo-interstitial parameters, and higher treatment failure rates compared to those without TMA.
Song et al.5 evaluated TMA in 148 subjects with LN and reported its prevalence in 24% of the biopsy results. As compared to subjects without TMA, those with TMA had significantly higher serum creatinine, proteinuria, total activity scores, endocapillary hypercellularity, subendothelial hyaline deposits, interstitial inflammation, glomerular leukocyte infiltration, tubular atrophy, and interstitial fibrosis. Patients with TMA had higher treatment failure rates compared to those without TMA (44% vs. 15%, P < 0.001). The presence of TMA was also an independent risk factor for poorer clinical outcome in subjects with LN. Bridoux et al.8 studied 8 patients with LN with TMA retrospectively. All 8 patients presented with renal failure (mean serum creatinine 3.3 mg/dl); 6 patients had significant proteinuria (mean 2.5 g/d), and 4 patients (50%) had microscopic hematuria. Renal histology disclosed arterial and/or arteriolar thrombosis with parietal thickening without angiitis (8 patients), glomerular microthrombi (3 patients), and vascular fibrin deposits (5 of 6 patients). In 2 cases, vascular lesions were associated with a mesangial or a proliferative glomerulonephritis. Lupus anticoagulant was detected in 5 patients (62.5%). Treatment consisted of corticosteroids (8 patients), cytotoxic drugs (4 patients), plasma exchanges and/or i.v. Igs (4 patients), and antiplatelet and/or anticoagulant therapy (3 patients). Two patients recovered normal renal function, and 5 had persistent renal insufficiency. One patient started on hemodialysis died after 2 months due to infection. The prevalence of TMA in LN in the present study was 25%, which was similar to that reported by Song et al.5 but much higher compared to other studies9, 10; this could be explained partly by differences in definitions of TMA. The female-to-male ratio for TMA patients was 5.5:1, and age of presentation was most common in the third decade (age 21–30 years), followed by the fourth and fifth decades. Of the patients in the study, 11% had serological workup for APS, which was higher than reported by Bridoux et al. (5.5%).8 Antiphospholipid antibodies increase the incidence of capillary thrombi by upregulating the expression of adhesion molecules and tissue factor in endothelial cells, thereby enhancing the adhesion between blood vessel endothelial cells and platelets.11
In the present study, patients with TMA had higher serum creatinine and lower urine output at presentation compared to non-TMA patients. Similar to a previous report by Magil et al.9 (n = 8 patients), our study showed that patients with LN with TMA presented with more severe renal injury, as assessed by higher serum creatinine levels (>3 mg/dl) at presentation, fibrocelluar/fibrous crescents, tubular atrophy, and interstitial fibrosis (>50% of the biopsy area) compared to the group without TMA. Rapid progressive renal failure was more common in TMA patients (38%) compared to non-TMA patients (11.6%). Of the patients in the TMA group, 23% required dialysis at the time of their first presentation, which was significantly higher compared to the non-TMA group. Thus, patients with LN with TMA at presentation have worse clinical and pathological profiles compared to non-TMA patients. Class IV LN in TMA patients was more common in the present study (72%), which was comparable to previously reported studies.9 A summary of various studies of TMA in LN is provided in Table 4.
Table 4.
Reported studies of lupus nephritis with TMA
| Authors, reference, year | Study type | Patients | Control group | Treatment outcome |
|---|---|---|---|---|
| Song et al.,5 (2013) | Retrospective | Total 148 TMA 36 |
112 | CR 8/36 (22.2%) PR 12/36 (33.3%) |
| Bridoux et al.,8 (1997) | Retrospective, case series | Total 8 (all TMA) | No | Remission rate 2/8 (25%) |
| Chen et al.,12 (2010) | Retrospective | Total 2461 (TMA 25) | Control 2436 | Remission rate 11/25 (44%) |
| Kotb et al.,13 (2016) | Prospective | Total 50 (TMA 7) | Control 43 | Associated with worse renal prognosis |
| Present study | Prospective | Total 197 (TMA 50) | Control 147 | CR 15/50 (30%) PR 10/50 (20%) |
CR, complete response; PR, partial response; TMA, thrombotic microangiopathy.
In our study, both TMA and non-TMA groups received similar treatment. Only 8 patients (6.25%) in the TMA group received plasmapheresis due to the severe nature of the disease (dialysis-dependent renal failure; Supplementary Table S1). The treatment failure rates were significantly higher in the LN group with TMA compared to the group without TMA, which was similar to the study reported by Bridoux et al.,8 and also there was a significantly lower rate of complete response to treatment in the TMA group compared to the non-TMA group. Factors affecting the poor outcome in LN were oliguria, photosensitivity, malar rash, serum creatinine at presentation, TMA, interstitial inflammation, and interstitial fibrosis.
Our study is limited by the single-center experience, relatively short duration of follow-up to gauge the relapse rate, and lack of a systematic antiphospholipid antibodies workup in all the non-TMA patients.
To conclude, TMA in LN was seen in one-fourth of the cases. The presence of TMA in LN was associated with adverse clinicopathological presentation and poorer outcome.
Disclosure
All the authors declared no competing interests.
Footnotes
Table S1. Clinical details and outcome of patients with thrombotic microangiopathy
Supplementary material is linked to the online version of the paper at http://www.kireports.org.
Supplementary Material
Clinical details and outcome of patients with thrombotic microangiopathy
References
- 1.Lansigan F., Isufi I., Tagoe C. Microangiopathic haemolytic anaemia resembling thrombotic thrombocytopenic purpura in systemic lupus erythematosus: the role of ADAMTS13. Rheumatology. 2010;50:824–829. doi: 10.1093/rheumatology/keq395. [DOI] [PubMed] [Google Scholar]
- 2.Appel G.B., Pirani C.L., D’Agati V. Renal vascular complications of systemic lupus erythematosus. J Am Soc Nephrol. 1994;4:499–515. doi: 10.1681/ASN.V481499. [DOI] [PubMed] [Google Scholar]
- 3.Grishman E., Venkataseshan V.S. Vascular lesions in lupus nephritis. Mod Pathol. 1988;1:235–241. [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 5.Song D., Wu L., Wang F. The spectrum of renal thrombotic microangiopathy in lupus nephritis. Arthritis Res Ther. 2013;15:R12. doi: 10.1186/ar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hocherg M.C. Updating the American College of Rheumatology revised criteria for classification of systemic lupus nephritis. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 7.Garg N., Deodhar A. Sydney classification criteria for definite antiphospholipid syndrome. J Musculoskel Med. 2012;29:73–77. [Google Scholar]
- 8.Bridoux F., Vrtovsnik F., Noel C. Renal thrombotic microangiopathy in systemic lupus erythematosus: clinical correlations and long term renal survival. Nephrol Dial Transplant. 1998;13:298–304. doi: 10.1093/oxfordjournals.ndt.a027822. [DOI] [PubMed] [Google Scholar]
- 9.Magil A., McFadden D., Rae A. Lupus glomerulonephritis with thrombotic microangiopathy. Hum Pathol. 1986;17:192–194. doi: 10.1016/s0046-8177(86)80293-3. [DOI] [PubMed] [Google Scholar]
- 10.Fessler B. Thrombotic syndromes and autoimmune diseases. Rheum Dis Clin North Am. 1997;23:461–479. doi: 10.1016/s0889-857x(05)70340-9. [DOI] [PubMed] [Google Scholar]
- 11.Tang S., Lui S.L., Lai K.N. Pathogenesis of lupus nephritis: an update. Nephrology. 2005;10:174–179. doi: 10.1111/j.1440-1797.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen M.-H., Chen M.-H., Chen W.-S. Thrombotic microangiopathy in systemic lupus erythematosus: a cohort study in North Taiwan. Rheumatology. 2011;50:768–775. doi: 10.1093/rheumatology/keq311. [DOI] [PubMed] [Google Scholar]
- 13.Kotb H.A., Mokbel A.N., Elmaghraby A.A., Fadda S.A. Thrombotic microangiopathy in lupus nephritis patients. Kasr Al Ainy Med J. 2016;22:12–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical details and outcome of patients with thrombotic microangiopathy