Abstract
A first-in-human trial of diaphragmatic gene therapy (AAV1-CMV-GAA) to treat respiratory and neural dysfunction in early-onset Pompe disease was conducted. The primary objective of this study was to assess the safety of rAAV1-CMV-hGAA vector delivered to the diaphragm muscle of Pompe disease subjects with ventilatory insufficiency. Safety was assessed by measurement of change in serum chemistries and hematology, urinalysis, and immune response to GAA and AAV, as well as change in level of health. The data demonstrate that the AAV treatment was safe and there were no adverse events related to the study agent. Adverse events related to the study procedure were observed in subjects with lower baseline neuromuscular function. All adverse events were resolved before the end of the study, except for one severe adverse event determined not to be related to either the study agent or the study procedure. In addition, an anti-capsid and anti-transgene antibody response was observed in all subjects who received rAAV1-CMV-hGAA, except for subjects who received concomitant immunomodulation to manage reaction to enzyme replacement therapy, as per their standard of care. This observation is significant for future gene therapy studies and serves to establish a clinically relevant approach to blocking immune responses to both the AAV capsid protein and transgene product.
Keywords: : AAV, safety, immune response, Pompe disease
Introduction
Pompe disease is a neuromuscular disease that results from mutations in the acid-α-glucosidase (Gaa) gene encoding an enzyme (GAA) responsible for the degradation of lysosomal glycogen.1,2 Lack of GAA activity results in pathologic glycogen accumulation that ultimately disrupts cardiac and skeletal muscle, as well as neural cellular architecture and function.1–4 The age of onset is determined by the severity of the genetic mutation, and the rate of progression is typically inversely correlated with residual GAA activity.5,6 Children with the most severe, early-onset form are usually diagnosed in the first few months of life with severe cardiomyopathy and cardiac hypertrophy.7 Pompe disease also significantly affects the respiratory system.8 The untreated natural history of early-onset disease reveals that affected infants succumb to cardiorespiratory failure in the first year of life.9
Pompe disease is an inherited myopathy with a Food and Drug Administration/European Medicines Agency (EMA)–approved cause-specific therapy, with 10 years of clinical experience following marketing approval in 2006. Enzyme replacement therapy (ERT) relies on biweekly infusions of human GAA (Lumizyme®). Clinical studies of ERT indicate that treatment extends patient survival and partially corrects the associated cardiomyopathy.10–15 However, it has also been observed that most of the early-onset patients on ERT continue to experience developmental delay and eventually require support for ventilatory dysfunction and ambulatory assistance. A new natural history has emerged in which 50% of early-onset patients on ERT ultimately progress to respiratory failure, full-time ventilatory dependence, and motor deficits in childhood rather than in infancy (4 years follow-up).9 Furthermore, murine and clinical studies have been done regarding the immunological response to the therapy; early-onset patients with little or no residual GAA activity typically have a poor response to ERT, which is influenced by anti-GAA antibody formation and the severity of the mutation, which contributes to ineffective treatment.16–21
One potential hypothesis for the limited efficacy of ERT on neuromuscular function is that GAA is not transported across the blood–brain barrier22,23 and therefore is unlikely to correct the progressive accumulation of glycogen in the central nervous system, especially in neurons. In fact, preclinical work in GAA-deficient knockout mice and autopsy reports on children treated with ERT confirm glycogen accumulation in motoneurons.24–26 In addition, studies in a murine Pompe model (the Gaa–/– mouse) demonstrate that delivery of rAAV1-GAA to the diaphragm improves muscle health and may improve phrenic neural output.27 Subsequent studies by Elmallah et al. show that intramuscular delivery of AAV can produce temporary correction of motoneuron histopathology in Gaa–/– mice via retrograde transport of the vector.8
Based on the results of preclinical work, the authors' team conducted the first-in-human trial of diaphragmatic gene therapy (AAV1-CMV-GAA) to treat respiratory and neural dysfunction in infantile-onset Pompe disease.28,29 Smith et al.28,30 demonstrated that intramuscular delivery of AAV1-CMV-GAA to the diaphragm improved dynamic respiratory motor function, possibly via retrograde transduction of phrenic motoneurons. Herein, the safety outcomes for the Phase I/II clinical trial of AAV1-CMV-GAA in children with Pompe disease are reported. Further, we discuss the potential benefits of the use of an immunomodulation regimen prior to AAV exposure to block immune response to AAV vectors and the transgene product.
Material and Methods
Study design and subjects
Details of the study are described in Byrne et al.29 and Smith et al.28 In summary, 11 subjects were enrolled in the study: nine participants completed the trial, and two were withdrawn. The participants were enrolled in an open-label, single-center, sequential two-arm, Phase I/II study evaluating the safety and potential activity of a single administration of rAAV1-CMV-hGAA vector injected intramuscularly into the diaphragm.
Vector administration was accomplished by video-assisted thoracoscopy conducted under general anesthesia at UF Health Shands Hospital. All study evaluations were conducted at the Clinical Research Center and UF Health Shands Hospital at the University of Florida, or at the study participant's home. Subjects were assigned to two dosing groups and received either 1 × 1012 vg (n = 3) or 5 × 1012 vg (n = 6) of rAAV1-CMV-hGAA (Tables 1 and 2). The selected doses were based on the preclinical dose-escalation studies and were 100 times lower than the maximal dose in murine biodistribution studies.24,27,31,32
Table 1.
Study design
| Groups # | 1 (n = 3) | 2 (n = 6) |
|---|---|---|
| Substance | rAAV1-CMV-GAA | rAAV1-CMV-GAA |
| Vector concentration (vg/mL) | 2.2 × 1011 vg/mL | 1.1 × 1012 vg/mL |
| Total dose | 1.0 × 1012 vg | 5.0 × 1012 vg |
| Total volume | 4.5 mL | 4.5 mL |
| Dosing regimen (one time dosing, two sites) | 3 injections (max 0.75 mL each) in each hemidiaphragm | 3 injections (max 0.75 mL each) in each hemidiaphragm |
| Route | IM—diaphragm muscle | IM—diaphragm muscle |
| Observation period (years) | 1 | 1 |
IM, intramuscular.
Table 2.
Study events
| Study day | Safety labs1 | Research labs2 |
|---|---|---|
| Day −120 to −28: screening | Yes | Yes |
| Day −1: baseline visit | N/A | Yes |
| Day 0: dosing | N/A | N/A |
| Day 1 | Yes | Yes |
| Day 2 | N/A | N/A |
| Day 3 | Yes | Yes |
| Day 14 (±4 days) | Yes | Yes |
| Day 30 (±7 days) | N/A | Yes |
| Day 60 (±7 days) | N/A | Yes |
| Day 90 (±7 days) | Yes | Yes |
| Day 180 (±10 days) | Yes | Yes |
| Day 270 (±10 days) | N/A | Yes |
| Day 365: end of study | N/A | Yes |
Safety labs: hematology including complete blood count (CBC); coagulation including PT/PTT; serum chemistry including sodium, potassium, chloride, total protein, albumin, calcium, phosphorous, glucose, blood urea nitrogen, creatinine, alkaline phosphatase, lactate dehydrogenase, serum aspartate aminotransferase, serum alanine aminotransferase and gamma-glutamyl transpeptidase, serum creatine kinase (CK) and urinalysis.
Research labs: AAV and GAA antibody levels by enzyme-linked immunosorbent assay, vector genome copy number by DNA-PCR, peripheral blood mononuclear cell (PBMC), Enzyme-Linked ImmunoSpot (ELISPOT).
All participants had a confirmed diagnosis of early-onset Pompe disease by whole-blood GAA assay and confirmatory sequence analysis. Inclusion criteria required chronic respiratory insufficiency despite long-term use of ERT. All subjects received standard of care ERT and had documented respiratory dysfunction requiring varying levels of ventilator assistance. Five subjects required 24 h mechanical ventilation through an invasive airway, and four subjects required partial-assisted ventilation. Three subjects were receiving concomitant standard of care medications to manage immune responses to ERT clinically, included rituximab and sirolimus, as described in Elder et al.33 and in Corti et al.34 These three subjects were CRIM-negative based on two severe mutations that were predicted to result in no residual GAA expression. Steroids prior and post administration of the study agent were used to minimize the inflammatory risk related to the surgical procedure.
Anti-AAV1 antibody measurement
Serum samples were assayed by enzyme-linked immunosorbent assay (ELISA) for circulating antibodies to the AAV1 capsid proteins at baseline and on days 14, 90, 180, and 365, and recombinant human GAA at baseline and on days 14, 30, 60, 90, 180, 270, and 365. Ninety-six-well plates were coated with 1.2 × 109 AAV1 particles/well overnight at 4°C. A wash with phosphate-buffered saline (PBS)–Tween was followed by blocking for 2 h at 37°C with 10% fetal bovine serum (Cellgro; Mediatech). After a wash with PBS–Tween, samples and a known positive human standard were diluted between 1:10 and 1:10,240 and allowed to bind overnight at 4°C. Washing was followed by the addition of the detection antibody at a dilution of 1:50,000 (goat anti-human immunoglobulin, conjugated with horseradish peroxidase [HRP; BioSource International]) for 2 h at 37°C. Finally, the plate was washed and exposed to tetramethylbenzidine peroxidase detection substrate (KPL) and read at 450 nm with an ELISA plate reader (BioTekQuant Plate Reader). Sample anti-AAV1 titers were then read relative to a human standard curve derived from the same plate.
Anti-hGAA antibody measurements
Immulon microtiter plates were coated overnight at 4°C with 100 μL of 5 μg/mL hGAA in PBS 0.1 M NaHCO3, pH 8.4. Wells were washed three times with 300 μL of PBS containing 0.05% Tween 20 (PBS/T) and blocked with 300 μL of 10% fetal bovine serum in PBS for 2 h at room temperature. Wells were washed three times, and serum samples (diluted 1:10 in blocking reagent) were added to the wells in a total volume of 100 μL and serially diluted. Serial dilutions of an anti-GAA antibody standard were used to derive the standard curve. Samples and standards were incubated overnight at 4°C. Washing was repeated, and 100 μL of sheep anti-human IgG-HRP conjugate (Amersham RPN 4101) diluted 1:20,000 in blocking buffer was added to all wells and incubated for 2 h at room temperature. After incubation, washing was repeated, and 100 μL of tetramethylbenzidine was added to the wells for 3 min. The reaction was stopped with 100 μL of 0.5 M H2SO4 and absorbance was measured at 450 nm (BioTekQuant Plate Reader).
Results
Toxicity events
Several laboratory tests were used to evaluate the toxicity of the treatment (Table 3). Since most of the patients had abnormal aspartate transaminase/alanine transaminase during the screening visit, the toxicity grade was calculated based on each subject's baseline values. Table 3 reports the cumulative number of times that the values exceeded the baseline value for each subject and the grade of toxicity.
Table 3.
Modified toxicity scale
| Labs | Normal range | Toxicity grade | First cohort | Second cohort | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 101 | 102 | 103 | 201 | 204 | 206 | 301 | 302 | 304 | ||||
| Total bilirubin | 0–1 mg/dL | Grade 1: 1.2–2.0 × subject's baseline | 1 | 2 | 0 | 1 | 4 | 0 | 2 | 5 | 0 | 15 |
| Grade 2: 2.1–3.0 × subject's baseline | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | ||
| Grade 3: 3.1–5.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 4: >5.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Gamma-GT | 5–61 IU/L | Grade 1: 1.3–2.0 × subject's baseline | 0 | 1 | 0 | 3 | 0 | 2 | 1 | 0 | 0 | 7 |
| Grade 2: 2.1–3.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 3: 3.1–5.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 4: >7.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Alkaline phosphatase | 75–271 IU/L | Grade 1: 1.3–3.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 |
| Grade 2: 3.1–4.9 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 3: 5.0–6.9 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 4: >7.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Aspartate transaminase | 0–37 IU/L | Grade 1: 2.5–3.4 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 2: 3.5–5.4 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 3: 5.5–10.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 4: >10.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Alanine aminotransferase | 0–41 IU/L | Grade 1: 2.5–3.4 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 2: 3.5–5.4 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 3: 5.5–10.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 4: >10.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| White blood cells | 4.5–13.5 thou/cu mm | Grade 1: 1.4–2.0 × subject's baseline | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Grade 2: 2.1–3.0 × subject's baseline | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Grade 3: 3.1–5.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 4: >5.0 × subject's baseline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Hemaglobin | 11.5-13.5 g/dL | Grade 1: 10.0–normal | 0 | 0 | 0 | 1 | 3 | 6 | 2 | 0 | 2 | 14 |
| Grade 2: 8.0–10.0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 3 | ||
| Grade 3: 6.5–7.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Grade 4: <6.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
The numbers reported in the table reflect the occurrence of the toxicity events for each toxicity grade.
Other pathology tests, including comprehensive metabolic panel (CMP), complete blood count (CBC) with differential, gamma-glutamyl transferase (GGT), lactate dehydrogenase (LDH), total creatine kinase (CK), urinalysis, prothrombin time (PT), partial thromboplastin time (PTT), and international normalized ratio (INR), are not included in Table 3. These laboratory studies were evaluated throughout the study and did not show any significant change. Moreover, none of the elements reported below were considered as an adverse event (AE) after an independent safety monitor review.
AEs
At the end of the study, no AEs related to the study agent were identified. Seven serious AEs (SAEs) were found to be related (or possibly related) to the study procedure (Table 4). Among these, half were considered as anticipated occurrences. Moreover, other non-serious AEs, including fever, diarrhea, tracheitis, and urinary tract infection, occurred but were not considered to be related to the study agent or the study procedure. The most frequent anticipated AE was pain within 1–2 days post surgery, with a grade level ranging from 1 to 2, except for one patient who experienced grade 3 AE-related to pain. The localization of the discomfort was typically in the shoulders and hips. In all cases, the pain was managed and resolved with an analgesic treatment (morphine or not narcotic).
Table 4.
Adverse events
| Adverse events | # of subject | Related to study agent | Related to study procedure | Serious | Resolved | Treatment |
|---|---|---|---|---|---|---|
| Pain (shoulder/hip) | 6 | No | Yes/possible | No | Yes | Analgesic medication |
| Capnothorax/pneumothorax | 6 | No | Yes | No | Yes | Supplemental oxygen/chest tube placement/none |
| Lung contusion | 2 | No | Yes | No | Yes | Chest tube placement/none |
| Pleural effusion | 1 | No | Possible | Yes | Yes | Effusion evacuated, medication-steroid, hospitalization |
Six subjects (subjects 101, 102, 103, 301, 302, and 304) had anticipated procedure-related capnothorax/pneumothorax that was resolved by a chest tube insertion or with active surveillance due to the low severity (first- and second-degree toxicity). Subject 302 also experienced a mild left-lung contusion, which resolved with active surveillance. Subject 101 had a mild bilateral pneumothorax, which resolved with supplemental oxygen.
Subject 201 had an acute episode of pericardial and pleural effusion requiring hospitalization approximately 3 months post study agent dosing. This unanticipated SAE was managed with chest tube drainage and steroid medication. The cause of the pleural effusion was determined to be caused by partial obstruction of the thoracic duct secondary to transient use of a PICC catheter for administration of standard-of-care ERT. The temporary catheter was inserted following Infusaport line fracture. The subject recovered without sequelae once the Infusaport was replaced.
Subject 102 experienced a mild right upper-lobe lung contusion, related to preexisting lung adhesion at the site of thoracospy port placement at the time of study agent dosing. Direct visualization revealed lung adhesions not identified preoperatively. This unanticipated AE was related to the study procedure and was managed with conservatively with chest tub insertion. Also at the time of study agent dosing, subject 102 experienced bilateral pneumothorax, which was considered procedure related and was resolved with a chest tube. At 6 months post study agent dosing, subject 102 experienced acute right-side spontaneous pneumothorax (considered not related to the study agent or study procedure) while on positive pressure ventilation in the home setting that required emergency evaluation and hospitalization. Subject 102 also had other SAEs not related to either the study agent or the study procedure that led to hospitalization and death 1 year after the study participation was concluded. A summary of the death report is included below.
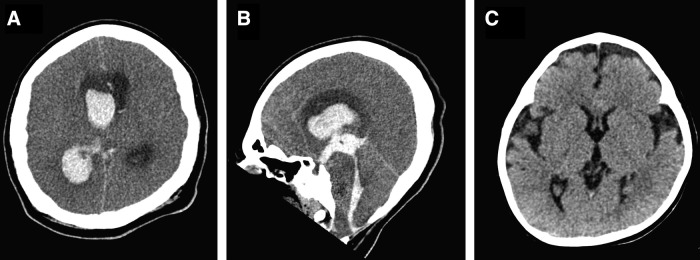
SAE summary
Subject 102 died at 12 years of age, 44 months post study agent dosing, from causes that were considered not related to the drug administration or study agent. The subject was a quadriplegic 12-year-old male with a tracheostomy and ventilator dependency since infancy. He also had a history of dilated cardiomyopathy and developmental delay, including mild cognitive impairment. He had received enzyme replacement therapy since he was 15 months of age. The ERT dose at the time of study enrollment was 40 mg/kg administered intravenously, every 2 weeks. He enrolled in the study and received AAV dosing via bilateral diaphragmatic injections at 9 years of age. At 12 years of age, he developed acute irritability, which was thought to be related to a urinary tract infection. He was later found to have unilateral pupillary dilation and was brought to the Emergency Department for further evaluation. A computed tomography (CT) scan disclosed a large acute intraventricular hemorrhage centered in the right lateral ventricle, which is likely secondary to right thalamus parenchymal or ependymal hemorrhage with rupture into the ventricle. There was also scattered acute subarachnoid hemorrhage likely due to redistribution of intraventricular bleeding. Extensive intraventricular hemorrhage resulted in obstructive hydrocephalus with mild leftward shift of the midline structures, effacement of basal cisterns and cerebral sulci, causing tonsillar herniation as well as brain-stem compression and diffuse cerebral edema (Fig. 1). The criteria for brain death were met, and the ventilator was discontinued. The subject died of cardiopulmonary arrest secondary to intraventricular hemorrhage and obstructive hydrocephalus, resulting in tonsillar herniation and brain-stem compression. The etiology of the massive intracranial hemorrhage was believed to be secondary to glycogenosis of intracranial vascular smooth muscle, which resulted in arteriopathy and hypertension.
Figure 1.
Brain computed tomography (CT) scan. Axial (A) and sagittal (B) unenhanced CT head images obtained when the patient was 12 years old during hospital admission demonstrates extensive acute intraventricular hemorrhage centered in the right lateral ventricle secondary to right thalamus parenchymal hemorrhage with rupture to the right lateral ventricle. There is obstructive hydrocephalus with mild leftward shift of midline structures, effacement of basal cisterns and cerebral sulci causing tonsillar herniation as well as brain-stem compression and diffuse cerebral edema. The head CT obtained when the patient was 15 months old (C) demonstrates prominent bifrontotemporal extra-axial cerebrospinal fluid spaces with no acute abnormality or hydrocephalus.
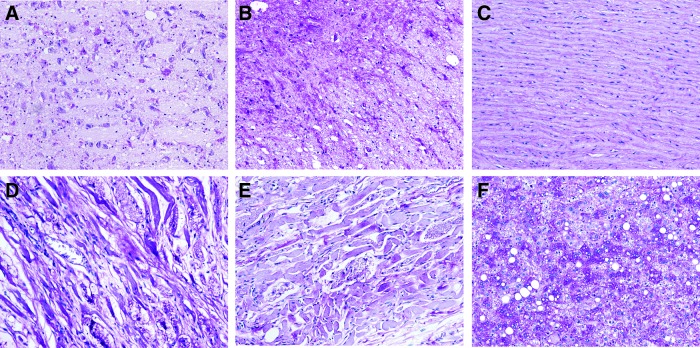
The postmortem examination confirmed marked glycogenosis and widespread degenerative changes of skeletal muscle with atrophy and extensive fatty infiltration, free cytosolic glycogen. Muscle fibers contained abundant lysosomal glycogen, typical of Pompe disease (Fig. 2). The brain and spinal cord (Fig. 2A and B) showed abnormal glycogen accumulation, predominantly detected in neurons. The diaphragm, quadriceps, and psoas (Fig. 2C, D, and E) muscles also demonstrated abnormal glycogen accumulation with compromised morphology and extensive fat replacement with small residual bundles of muscle fibers. The fibers varied in size and many had marked cytoplasmic vacuolization, consistent with glycogen storage. The liver also showed abnormal glycogen accumulation and vacuolization (Fig. 2E).
Figure 2.
Histopathology of tissues collected during autopsy and stained for glycogen (periodic acid–Schiff). (A and B) Abnormal glycogen accumulation in the brain and spinal cord, respectively, predominantly detected in the neurons. (C) Diaphragm, (D) quadriceps, and (E) psoas, all exhibiting an abnormal accumulation of glycogen with compromised morphology. (F) Abnormal glycogen accumulation and vacuolization in the liver. Images were taken at 20 × magnification.
Anti-AAV1 and anti-hGAA circulating antibody
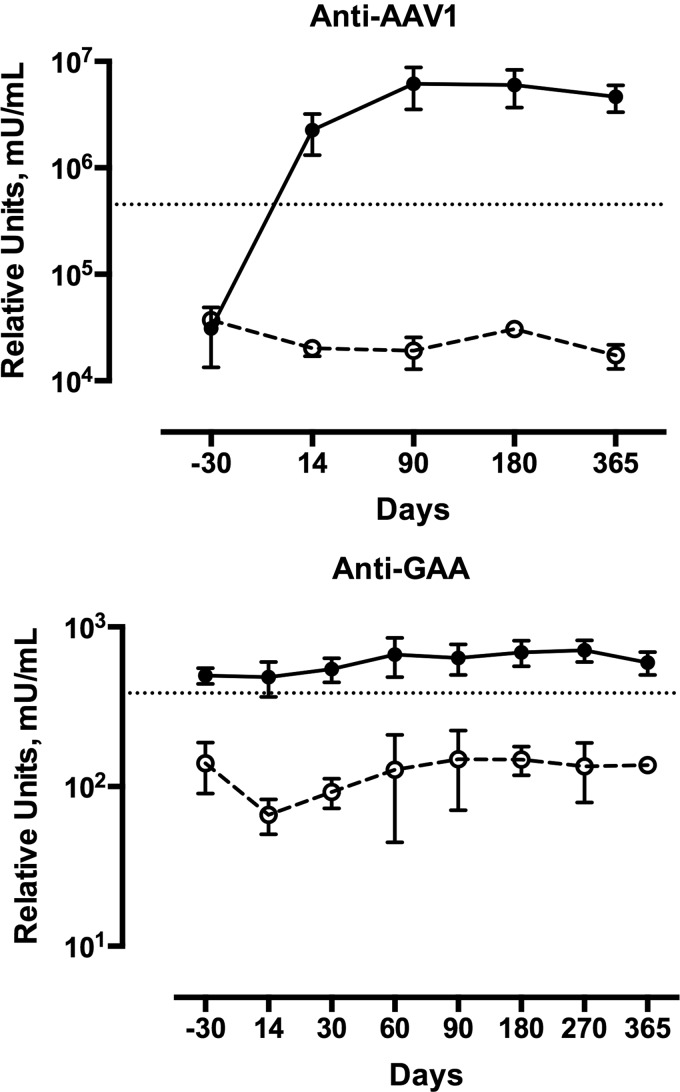
Serum samples were assayed by ELISA for circulating antibodies to AAV1 capsid proteins and alpha-glucosidase. Three subjects receiving immune modulation were compared to five subjects who did not receive immunomodulation. The group that did not receive immunomodulation demonstrated an average 150-fold increase in anti-AAV1 titer after exposure to AAV1. However, the three subjects who received immunomodulation had no response to AAV capsid proteins through day 365 of the study (Fig. 3).
Figure 3.
Circulating antibodies against adeno-associated virus serotype 1 (AAV1) in three subjects with Pompe disease who received rituximab and sirolimus. Data from one subject was published in Corti et al. (34). Three children with Pompe disease (dashed lines, empty circles) received administration of rituximab along with 10 mg/kg of methylprednisolone prior to dosing with 5 × 1012 vg/kg of rAAV1-CMV-hGAA. Total rituximab dose administered ranged from 1125 to 1500 mg/m2 in the three subjects. In two subjects, the total induction dose was divided into two doses (750 mg/m2) separated by 10–14 days. To limit the volume of the infusion, one subject received three divided doses (375 mg/m2) separated by 1 week. All subjects also received daily oral administration of 0.06–1 mg/m2 of sirolimus through the study and continued B-cell depletion with rituximab every 12 weeks. Six Pompe subjects (full lines, full circles) received 1–5 × 1012 vg/kg of rAAV1-CMV-hGAA. Serum samples were assayed by enzyme-linked immunosorbent assay for circulating antibodies to the AAV1 capsid proteins. The horizontal line represents +2SD population level. (A) Circulating antibodies against AAV1. (B) Circulating antibodies against hGAA.
Discussion
Safety of AAV1-CMV-hGAA
The safety of the study agent was confirmed by the absence of laboratory or clinical findings suggesting an adverse response to the AAV vector used at the two doses in this study. These findings are consistent with the non-clinical studies, which evaluated the study agent by the planned route of delivery and established 10-fold dose safety margin for the clinical dose.27,32
The intent to study just the diaphragmatic response required an invasive delivery strategy, which was adapted from the non-clinical studies that showed positive effects of this regional delivery strategy on respiratory function. There were AEs related to the delivery procedure, and in recent studies, we have pursued systemic delivery in lieu of the regional approach used in the current study with AAV1.
Intracranial hemorrhage
Patient 102 died secondary to a large intracranial hemorrhage resulting in brain herniation and brain-stem compression. In the literature, it has been confirmed by autopsy studies in late-onset Pompe disease (LOPD) patients, who also demonstrate glycogen deposition in the smooth muscle cells of intracranial arteries, particularly involving the small- and medium-sized vessels,35,36 resulting in arteriopathy. Involvement of the cerebrovascular system in LOPD, including increased incidence of dolicoectasia, intracranial aneurysms, micro-hemorrhages, and intracranial hemorrhages, have been found in sporadic cases36–53 and are summarized in Pichiecchio et al.54 and Montagnese et al.55 Evidence of vertebrobasilar dolichoectasia was found in 48% of patients, and 43% of patients had a normal CT angiogram. Signs of lacunar encephalopathy were detected in 62% of patients, two of whom showed a former parietal and temporal ischemic stroke. Sandhu52 reported a case with LOPD, who presented mild sensory symptoms and high blood pressure, with a subsequent head CT demonstrating small acute left thalamic hemorrhage. However, follow-up magnetic resonance imaging (MRI) and magnetic resonance angiogram (MRA) demonstrated extensive micro-hemorrhages, particularly in the central and posterior vascular territory with dolicoectesia of the vertebrobasilar system, which is thought to be secondary to small- and middle-vessel intracranial arteriopathy. Similarly, patient 102 in the present study likely had a superficial thalamic or ependymal hemorrhage, which resulted in extensive intraventricular extension that subsequently resulted in obstructive hydrocephalus and herniation. Patient 102 had no MRI or MRA/CTA imaging to confirm possible other micro-hemorrhages or dolicoectasia.
Glycogen accumulation is also believed to contribute to diminished aortic compliance and hypertension in non-classic infantile Pompe disease.56 Previous studies showed that more patients with Pompe disease than volunteers had been diagnosed with hypertension. Also, despite treatment with antihypertensive medication, blood pressure continued to be high in 52% of the Pompe patients who were treated for hypertension and in 65% of the volunteers.56 Based on sporadic case reports36,37,39–53 and the Montagnese study,54,55 a causal relation of cerebrovascular abnormalities to Pompe disease is very likely. Shüller et al.57 presented cases with cardiac and cerebrovascular alterations and proposed the term “cardio-cerebrovascular pattern” to define aortic stiffness, dilatation of thoracic aorta, and dolichoectasias in cerebral arteries caused by changes in the smooth-muscle cells associated with glycogen accumulation. Therefore, screening for intracranial parenchymal and vascular imaging may be beneficial in patients with Pompe disease to facilitate early diagnosis of cerebral pathology such as dolicoectasia, micro-hemorrhages, or aneurysm formation. To reduce the potential risk of cardiovascular and neurovascular diseases, blood pressure and other common risk factors should be monitored closely. Neurologic symptoms should prompt urgent evaluation.
Further, there is limited non-clinical evidence to evaluate the impact of ERT on the vascular smooth muscle. Despite extensive exposure to the vasculature during biweekly infusion, it seems the vascular smooth muscle is not impacted by ERT. The limited effect of ERT on the smooth muscle of large conducting arteries and arterioles may be related to the integrity of the endothelia or possibly low M6P receptor density in vascular smooth muscle.
Immune response to gene therapy and immunomodulation
The development of antibodies through natural exposure to AAV is frequent early in life and may influence the use of AAV as a gene therapy vector.58,59 In humans, it has been shown consistently that administration of AAV in the dose range required for regional or systemic exposure leads to development of anti-AAV antibodies.28,34,60 For example, following intramuscular administration of alipogene tiparvovec, all patients (n = 27) showed treatment-emergent antibody responses to AAV1.61 Furthermore, the development of relatively low neutralizing antibody (NAb) titers can completely block transduction by AAV vectors.60 For example, in mice and nonhuman primates (NHPs), NAb titers as low as 1:5 can block AAV vector transduction of liver completely.62–64 In addition, in an early clinical trial of liver gene transfer with AAV2 encoding coagulation Factor IX, no circulating Factor IX was detected in a patient with a NAb titer of 1:17, whereas the other subject in the same dose cohort expressed peak levels of Factor IX at 11% and had a NAb titer of 1:2.65 Therefore, management of the host's immune response to both the vector capsid and transgene product remains a critical challenge for the success of gene therapy.
It is possible that repeat AAV administration may be required in some patients. For example, if a subject was administered a dose below the optimal therapeutic threshold in early-phase studies, re-administration may be desirable. In addition, many subjects may require re-dosing later in life, as increasing muscle mass or loss of copy number with age may reduce transgene expression to below the effective levels. In Pompe disease, the increasing frequency of early diagnosis by newborn screening may necessitate early dosing that would certainly require repeat exposure later in life. There is strong evidence that potent humoral and cellular memory responses to AAV may compromise the subsequent use of the same vector and may influence duration of gene transfer.60,66
The effect of immune response on re-dosing with gene therapy has been demonstrated in non-clinical studies with AAV-mediated gene transfer, where pre-immunization of mice and rabbits with AAV5, or pre-immunization of mice with AAV2/8, substantially diminished the efficacy of a second vector administration.67,68 The current strategy in many gene therapy clinical trials is to exclude seropositive subjects from study participation. However, this approach prevents a large proportion of subjects from receiving potentially beneficial therapies and does not address vector re-administration. Therefore, it is essential to develop strategies to manage immune response as a sustainable approach to deliver safe and long-term expression of the therapeutic gene by AAV-mediated gene therapy. One of the strategies to overcome the presence of NAbs in rAAV-mediated gene therapy is pharmacological modulation of the humoral immune response.60 In this context, two approved drugs, Rituxan® (rituximab) and Rapamune® (sirolimus), have both shown promising results in preventing immune responses to gene therapy in both non-clinical and clinical studies. Furthermore, the combination of rituximab and sirolimus has been demonstrated to prevent immune responses to AAV-GAA gene therapy in the current AAV1 study and previously reported in a single subject.34
Rituximab is a monoclonal antibody that induces B-cell depletion by binding CD20 found on the surface of B cells. Due to its safety and efficacy profile, rituximab has gained wide acceptance in the clinic for the treatment of autoimmune disease. In addition, rituximab has been investigated as a potential immune modulation therapy for use in gene therapy.69–72 Overall, B-cell depletion has some effect in modulating a preexisting immune response, and anti-CD20 treatment may facilitate both tolerance to the transgene product and re-administration of vector by preventing both binding and NAbs against the vector.
Sirolimus targets the mammalian target of rapamycin (mTOR), which is responsible for regulating cell survival, growth, and proliferation of lymphocytes. In addition to its effect on T-cell function, sirolimus is also an inhibitor of B-cell proliferation.73 Sirolimus also selectively promotes the survival and expansion of regulatory T cells while allowing for programmed cell death of activated effector T lymphocytes.74–77 In the context of gene therapy, sirolimus has been shown to be an effective immunomodulator in non-clinical studies.78 Therefore, sirolimus represents an additional immunomodulatory drug with a mechanism of action complementary to rituximab.
As described above, rituximab and sirolimus are immunomodulatory agents with complementary mechanisms of action. To this end, non-clinical studies and clinical studies have investigated the safety and effectiveness of CD20 mediated B-cell ablation combined with inhibition of mTOR. The combination of B-cell ablation with sirolimus has been investigated in hemophilia A mice following non-viral gene transfer with pBS-HCRHPI-FVIIIA.79 This study demonstrated that treatment with interleukin (IL)-2/IL-2mAb complexes plus sirolimus combined with anti-CD20 antibody had a synergistic effect to reduce NAb titers. Furthermore, no increase in NAb titer was observed following a second gene transfer challenge. It was demonstrated that anti-CD20 predominantly depleted hFVIII-specific memory B cells, and IL-2/IL-2mAb complexes + rapamycin helped reduce hFVIII-specific antibody secreting cells.
Non-clinical studies in mice and rhesus macaques were also conducted by our group to: (1) assess the safety and biodistribution of the vector following single and repeated intramuscular administration, and (2) assess the efficacy these two immunosuppressive agents, administered alone and in combination, to attenuate neutralizing antibody response to AAV9-Des-GAA and facilitate effective cell transduction by a second dose of vector.66 The NHP and mouse studies enable a human clinical study to test the ability to re-administer AAV9 (NCT02240407) directly.
In summary, the primary outcomes of safety and tolerability of AAV1-GAA in the current study have been established, since there were no AEs related or possibly related to the investigational product. Procedure-related AEs warrant the transition from regional to systemic therapy with AAV vectors expressing GAA. The clinical outcomes, which we have previously reported,28,30 support the continued development of AAV-mediated gene therapy for Pompe disease.
Additional non-clinical studies have established the need for systemic therapy to address deficits in motor units, comprised of the lower motor neuron, neuromuscular junction, and myofiber. Therefore, clinical development of systemic delivery of AAV-GAA has continued to include new developments in neurotrophic capsids, as well as modification of the expression cassette. To enhance safety of the vector following systemic exposure further, the vector expression cassette has been modified to include tissue-restricted expression in neurons and striated muscle using a modified desmin promoter. Ongoing studies will use systemic administration of AAV9-GAA in early-onset Pompe patients.
Intravenous administration of AAV could be considered a greater risk then intramuscular administration. However, the risk–benefit ratio should be considered. The first study to use systemic delivery of AAV9 has recently been published in a Phase I/II study of spinal muscular atrophy (NCT02122952). The study has confirmed a favorable safety profile, with no treatment-related safety or tolerability concerns identified, as well as important findings of efficacy.80 An additional study of systemic AAV9 for MSP IIIA is underway with favorable results thus far (NCT02716246). Further, two ongoing trials are evaluating the safety of intrathecal administration of AAV (in giant axonal neuropathy, NCT02362438; and infantile Batten disease, NCT02725580). To date, none of the studies has shown treatment-related safety or tolerability concerns.
One of the most important challenges of systemic AAV exposure is the potential for immune responses to the experimental agent, especially in subjects who were previously exposed to AAV. In order to establish strategies that will allow all candidates for AAV-medicated gene therapy to benefit from this approach, the hypothesis is that pharmacological modulation of the humoral immune response will increase safety and long-term expression of the therapeutic gene.
Acknowledgments
The UF Human Applications Laboratory manufactured rAAV vectors for the clinical trial. This study was supported by grants from the National Institute of Health (NHLBI P01 HL59412-06, the NHLBI Gene Therapy Resource Program GTRP—NHLBI and NICHD-K12HD055929-02 [B.K.S.]).
Author Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hers HG. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J 1963;86:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschhorn R, Reuser AJ. Glycogen storage disease type II: acid α-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Sly W, et al., eds. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw Hill, 2001:3389–3420 [Google Scholar]
- 3.Martin JJ, de Barsy T, van Hoof F, et al. Pompe's disease: an inborn lysosomal disorder with storage of glycogen. A study of brain and striated muscle. Acta Neuropathol 1973;23:229–244 [DOI] [PubMed] [Google Scholar]
- 4.Raben N, Plotz P, Byrne BJ. Acid alpha-glucosidase deficiency (glycogenosis type II, Pompe disease). Curr Mol Med 2002;2:145–166 [DOI] [PubMed] [Google Scholar]
- 5.Kroos M, Hoogeveen-Westerveld M, van der Ploeg A, et al. The genotype–phenotype correlation in Pompe disease. Am J Med Genet C Semin Med Genet 2012;160C:59–68 [DOI] [PubMed] [Google Scholar]
- 6.ACMG Work Group on Management of Pompe Disease; Kishnani PS, Steiner RD, et al. Pompe disease diagnosis and management guideline. Genet Med 2006;8:267–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slonim AE, Bulone L, Ritz S, et al. Identification of two subtypes of infantile acid maltase deficiency. J Pediatr 2000;137:283–285 [DOI] [PubMed] [Google Scholar]
- 8.Elmallah MK, Falk DJ, Nayak S, et al. Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in pompe mice. Mol Ther 2014;22:702–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J, Desai AK, Kazi ZB, et al. The emerging phenotype of late-onset Pompe disease: a systematic literature review. Mol Genet Metab 2017;120:163–172 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez C, Legido A, Jethva R, et al. Correction of a short cardiac PR interval in a 12-year-old girl with late-onset Pompe disease following enzyme replacement therapy. Genet Med 2012;14:757–758 [DOI] [PubMed] [Google Scholar]
- 11.van Capelle CI, van der Beek NA, Hagemans ML, et al. Effect of enzyme therapy in juvenile patients with Pompe disease: a three-year open-label study. Neuromuscul Disord 2010;20:775–782 [DOI] [PubMed] [Google Scholar]
- 12.Jurecka A, Zuberuber Z, Opoka-Winiarska V, et al. Effect of rapid cessation of enzyme replacement therapy: a report of 5 cases and a review of the literature. Mol Genet Metab 2012;107:508–512 [DOI] [PubMed] [Google Scholar]
- 13.Toscano A, Schoser B. Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. J Neurol 2013;260:951–959 [DOI] [PubMed] [Google Scholar]
- 14.Chen CA, Chien YH, Hwu WL, et al. Left ventricular geometry, global function, and dyssynchrony in infants and children with pompe cardiomyopathy undergoing enzyme replacement therapy. J Card Fail 2011;17:930–936 [DOI] [PubMed] [Google Scholar]
- 15.Byrne BJ, Kishnani PS, Case LE, et al. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol Genet Metab 2011;103:1–11 [DOI] [PubMed] [Google Scholar]
- 16.Ohashi T, Iizuka S, Shimada Y, et al. Administration of anti-CD3 antibodies modulates the immune response to an infusion of alpha-glucosidase in mice. Mol Ther 2012;20:1924–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries JM, van der Beek NA, Kroos MA, et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab 2010;101:338–345 [DOI] [PubMed] [Google Scholar]
- 18.Kishnani PS, Dickson PI, Muldowney L, et al. Immune response to enzyme replacement therapies in lysosomal storage diseases and the role of immune tolerance induction. Mol Genet Metab 2016;117:66–83 [DOI] [PubMed] [Google Scholar]
- 19.Nayak S, Sivakumar R, Cao O, et al. Mapping the T helper cell response to acid alpha-glucosidase in Pompe mice. Mol Genet Metab 2012;106:189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne BJ, Falk DJ, Pacak CA, et al. Pompe disease gene therapy. Hum Mol Genet 2011;20:R61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel TT, Banugaria SG, Case LE, et al. The impact of antibodies in late-onset Pompe disease: a case series and literature review. Mol Genet Metab 2012;106:301–309 [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi T, Yang HW, Pennybacker M, et al. Clinical and metabolic correction of Pompe disease by enzyme therapy in acid maltase-deficient quail. J Clin Invest 1998;101:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raben N, Danon M, Gilbert AL, et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab 2003;80:159–169 [DOI] [PubMed] [Google Scholar]
- 24.DeRuisseau LR, Fuller DD, Qiu K, et al. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci U S A 2009;106:9419–9424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller DD, ElMallah MK, Smith BK, et al. The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol 2013;189:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todd AG, McElroy JA, Grange RW, et al. Correcting neuromuscular deficits with gene therapy in Pompe disease. Ann Neurol 2015;78:222–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mah CS, Falk DJ, Germain SA, et al. Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease. Mol Ther 2010;18:502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith BK, Collins SW, Conlon TJ, et al. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther 2013;24:630–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne PI, Collins S, Mah CC, et al. Phase I/II trial of diaphragm delivery of recombinant adeno-associated virus acid alpha-glucosidase (rAAaV1-CMV-GAA) gene vector in patients with Pompe disease. Hum Gene Ther Clin Dev 2014;25:134–163 [DOI] [PubMed] [Google Scholar]
- 30.Smith BK, Martin AD, Lawson LA, et al. Inspiratory muscle conditioning exercise and diaphragm gene therapy in Pompe disease: clinical evidence of respiratory plasticity. Exp Neurol 2017;287:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mah C, Fraites TJ, Jr, Cresawn KO, et al. A new method for recombinant adeno-associated virus vector delivery to murine diaphragm. Mol Ther 2004;9:458–463 [DOI] [PubMed] [Google Scholar]
- 32.Conlon TJ, Erger K, Porvasnik S, et al. Preclinical toxicology and biodistribution studies of recombinant adeno-associated virus 1 human acid alpha-glucosidase. Hum Gene Ther Clin Dev 2013;24:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elder ME, Nayak S, Collins SW, et al. B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 2013;163:847–854.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corti M, Elder M, Falk D, et al. B-Cell depletion is protective against anti-AAV capsid immune response: a human subject case study. Mol Ther Methods Clin Dev 2014;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hobson-Webb LD, Proia AD, Thurberg BL, et al. Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol Genet Metab 2012;106:462–469 [DOI] [PubMed] [Google Scholar]
- 36.Kretzschmar HA, Wagner H, Hubner G, et al. Aneurysms and vacuolar degeneration of cerebral arteries in late-onset acid maltase deficiency. J Neurol Sci 1990;98:169–183 [DOI] [PubMed] [Google Scholar]
- 37.Anneser JM, Pongratz DE, Podskarbi T, et al. Mutations in the acid alpha-glucosidase gene (M. Pompe) in a patient with an unusual phenotype. Neurology 2005;64:368–370 [DOI] [PubMed] [Google Scholar]
- 38.McIntosh PT, Hobson-Webb LD, Kazi ZB, et al. Neuroimaging findings in infantile Pompe patients treated with enzyme replacement therapy. Mol Genet Metab 2017. October 13 [Epub ahead of print]; DOI: 10.1016/j.ymgme.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braunsdorf WE. Fusiform aneurysm of basilar artery and ectatic internal carotid arteries associated with glycogenosis type 2 (Pompe's disease). Neurosurgery 1987;21:748–749 [DOI] [PubMed] [Google Scholar]
- 40.Brettschneider J, Sperfeld AD, Ludolph AC, et al. Intracerebral hemorrhage in a patient with glycogenosis type II (Pompe disease): is there a pathophysiological relationship? Muscle Nerve 2008;38:1211–1212 [DOI] [PubMed] [Google Scholar]
- 41.Garibaldi M, Sacconi S, Antonini G, et al. Long term follow-up of cerebrovascular abnormalities in late onset Pompe disease (LOPD). J Neurol 2017;264:589–590 [DOI] [PubMed] [Google Scholar]
- 42.Hensel O, Hanisch F, Stock K, et al. Morphology and function of cerebral arteries in adults with pompe disease. JIMD Rep 2015;20:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laforet P, Petiot P, Nicolino M, et al. Dilative arteriopathy and basilar artery dolichoectasia complicating late-onset Pompe disease. Neurology 2008;70:2063–2066 [DOI] [PubMed] [Google Scholar]
- 44.Makos MM, McComb RD, Hart MN, et al. Alpha-glucosidase deficiency and basilar artery aneurysm: report of a sibship. Ann Neurol 1987;22:629–633 [DOI] [PubMed] [Google Scholar]
- 45.Matsuoka Y, Senda Y, Hirayama M, et al. Late-onset acid maltase deficiency associated with intracranial aneurysm. J Neurol 1988;235:371–373 [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto Y, Etoh Y, Joh R, et al. Adult-onset acid maltase deficiency in siblings. Acta Pathol Jpn 1985;35:1533–1542 [DOI] [PubMed] [Google Scholar]
- 47.Peric S, Fumic K, Bilic K, et al. Rupture of the middle cerebral artery aneurysm as a presenting symptom of late-onset Pompe disease in an adult with a novel GAA gene mutation. Acta Neurol Belg 2014;114:165–166 [DOI] [PubMed] [Google Scholar]
- 48.Quenardelle V, Bataillard M, Bazin D, et al. Pompe disease presenting as an isolated generalized dilative arteriopathy with repeated brain and kidney infarcts. J Neurol 2015;262:473–475 [DOI] [PubMed] [Google Scholar]
- 49.Refai D, Lev R, Cross DT, et al. Thrombotic complications of a basilar artery aneurysm in a young adult with Pompe disease. Surg Neurol 2008;70:518–520 [DOI] [PubMed] [Google Scholar]
- 50.Renard D, Labauge P. Neurological picture: cerebral microbleeds in Pompe disease. J Neurol Neurosurg Psychiatry 2010;81:1217. [DOI] [PubMed] [Google Scholar]
- 51.Sacconi S, Bocquet JD, Chanalet S, et al. Abnormalities of cerebral arteries are frequent in patients with late-onset Pompe disease. J Neurol 2010;257:1730–1733 [DOI] [PubMed] [Google Scholar]
- 52.Sandhu D, Rizvi A, Kim J, et al. Diffuse cerebral microhemorrhages in a patient with adult-onset Pompe's disease: a case report. J Vasc Interv Neurol 2014;7:82–85 [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang B, Zhao Y, Liu J, et al. Late-onset Pompe disease with complicated intracranial aneurysm: a Chinese case report. Neuropsychiatr Dis Treat 2016;12:713–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pichiecchio A, Sacco S, De Filippi P, et al. Late-onset Pompe disease: a genetic-radiological correlation on cerebral vascular anomalies. J Neurol 2017. October;264:2110–2118 [DOI] [PubMed] [Google Scholar]
- 55.Montagnese F, Barca E, Musumeci O, et al. Clinical and molecular aspects of 30 patients with late-onset Pompe disease (LOPD): unusual features and response to treatment. J Neurol 2015;262:968–978 [DOI] [PubMed] [Google Scholar]
- 56.Wens SC, Kuperus E, Mattace-Raso FU, Kruijshaar ME, Brusse E, van Montfort KC, de Boer MS, Sijbrands EJ, van der Ploeg AT, van Doorn PA. Increased aortic stiffness and blood pressure in non-classic Pompe disease. J Inherit Metab Dis 2014;37:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuller A, Wenninger S, Strigl-Pill N, et al. Toward deconstructing the phenotype of late-onset Pompe disease. Am J Med Genet C Semin Med Genet 2012;160C:80–88 [DOI] [PubMed] [Google Scholar]
- 58.Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 2013;4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masat E, Pavani G, Mingozzi F. Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discov Med 2013;15:379–389 [PubMed] [Google Scholar]
- 60.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 2013;122:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferreira V, Twisk J, Kwikkers K, et al. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a Phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther 2014;25:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scallan CD, Jiang H, Liu T, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 2006;107:1810–1817 [DOI] [PubMed] [Google Scholar]
- 63.Murphy SL, Li H, Zhou S, et al. Prolonged susceptibility to antibody-mediated neutralization for adeno-associated vectors targeted to the liver. Mol Ther 2008;16:138–145 [DOI] [PubMed] [Google Scholar]
- 64.Jiang H, Couto LB, Patarroyo-White S, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 2006;108:3321–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 66.Corti M, Cleaver B, Clement N, et al. Evaluation of readministration of a recombinant adeno-associated virus vector expressing acid alpha-glucosidase in Pompe disease: preclinical to clinical planning. Hum Gene Ther Clin Dev 2015;26:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kozarsky KF, McKinley DR, Austin LL, et al. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J Biol Chem 1994;269:13695–13702 [PubMed] [Google Scholar]
- 68.Gao GP, Lu F, Sanmiguel JC, et al. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol Ther 2002;5:644–649 [DOI] [PubMed] [Google Scholar]
- 69.Unzu C, Hervas-Stubbs S, Sampedro A, et al. Transient and intensive pharmacological immunosuppression fails to improve AAV-based liver gene transfer in non-human primates. J Transl Med 2012;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sack BK, Merchant S, Markusic DM, et al. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One 2012;7:e37671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mingozzi F, Chen Y, Murphy SL, et al. Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol Ther 2012;20:1410–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fontanellas A, Hervas-Stubbs S, Mauleon I, et al. Intensive pharmacological immunosuppression allows for repetitive liver gene transfer with recombinant adenovirus in nonhuman primates. Mol Ther 2010;18:754–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Limon JJ, Fruman DA. Akt and mTOR in B cell activation and differentiation. Front Immunol 2012;3:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nayak S, Cao O, Hoffman BE, et al. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. J Thromb Haemost 2009;7:1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moghimi B, Sack BK, Nayak S, et al. Induction of tolerance to factor VIII by transient co-administration with rapamycin. J Thromb Haemost 2011;9:1524–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bestard O, Cassis L, Cruzado JM, et al. Costimulatory blockade with mTor inhibition abrogates effector T-cell responses allowing regulatory T-cell survival in renal transplantation. Transpl Int 2011;24:451–460 [DOI] [PubMed] [Google Scholar]
- 77.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 2005;105:4743–4748 [DOI] [PubMed] [Google Scholar]
- 78.Nayak S, Sarkar D, Perrin GQ, et al. Prevention and reversal of antibody responses against Factor IX in gene therapy for hemophilia B. Front Microbiol 2011;2:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu CL, Ye P, Lin J, et al. Anti-CD20 as the B-cell targeting agent in a combined therapy to modulate anti-Factor VIII immune responses in hemophilia A inhibitor mice. Front Immunol 2014;4:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 2017;377:1713–1722 [DOI] [PubMed] [Google Scholar]