Abstract
Introduction
Measuring the chemokine CXCL9 in urine by enzyme-linked immunosorbent assay (ELISA) can diagnose acute cellular rejection (ACR) noninvasively after kidney transplantation, but the required 12- to 24-hour turnaround time is not ideal for rapid, clinical decision-making.
Methods
We developed a biolayer interferometry (BLI)−based assay to rapidly measure urinary CXCL9 in <1 hour. We validated this new assay versus standard ELISA in 86 urine samples from kidney transplantation recipients with various diagnoses. We then used BLI to analyze samples from 56 kidney transplantation recipients, including 46 subjects who experienced an acute rise in serum creatinine associated with biopsy-proven ACR (n = 22), subclinical rejection (n = 15), or no infiltrates (n = 9), and 10 stable kidney transplantation recipients with surveillance biopsies. To assess its usefulness in detecting adequacy of therapy we serially measured serum creatinine and urinary CXCL9 in 6 subjects after treatment for ACR, and correlated the results with histological diagnoses on follow-up biopsies.
Results
BLI accurately and reproducibly detected urinary CXCL9 in <1 hour. BLI-based results showed that urinary CXCL9 was >200 pg/ml in subjects with ACR and ≤100 pg/ml in subjects with stable kidney function without cellular infiltrates. In samples obtained after treatment for ACR, BLI CXCL9 measurements detected biopsy-proven intragraft infiltrates despite treatment-induced reduction in serum creatinine.
Discussion
Together, our proof-of-principle results demonstrate that BLI-based urinary CXCL9 detection has potential as a point-of-care noninvasive biomarker to diagnose and guide therapy for ACR in kidney transplantation recipients.
Keywords: acute rejection, biomarker, chemokines, CXCL9, kidney transplantation
Despite reduction of acute cellular rejection (ACR) rates since the 1990s,1, 2 ACR continues to affect long-term kidney allograft survival negatively.3, 4 A few experienced centers perform follow-up biopsies to assess efficacy of antirejection therapy, but this practice is impractical, risky, and therefore not done routinely in most transplantation centers. Noninvasive monitoring tools capable of rapidly assessing intragraft inflammation could guide therapeutic decision-making following treatment for rejection and thereby potentially improve graft function and patient health.
Urinary chemokines are among candidate biomarkers for detecting kidney allograft inflammation.5, 6 CXCL9 is an interferon-γ−induced, T-cell chemoattractant chemokine produced by monocyte/macrophages, endothelial cells, and renal parenchymal cells.7 Results of single-center studies8, 9, 10, 11, 12, 13, 14 showed that measurements of urinary CXCL9 (uCXCL9) can differentiate ACR from most other causes of acute post-transplantation kidney dysfunction in the absence of infection. Findings from Clinical Trials in Organ Transplantation (CTOT)-01 (NCT01974999), a prospective, multicenter, observational study of 280 kidney transplantation recipients (KTRs) showed that uCXCL9 (by enzyme-linked immunosorbent assay [ELISA]) at a threshold of ≥200 pg/ml, diagnosed ACR at the time of an acute elevation of serum creatinine (negative predictive value: 92%, positive predictive value: 67%).12 Excluding subjects with BK virus (BKV) or urinary tract infection increased the positive predictive value to >80%.12 In a follow-up tacrolimus withdrawal trial (CTOT-09, NCT01517984), serial ELISA uCXCL9 measurements detected ACR 3 to 30 days before clinical presentation.14 The 12- to 24-hour requisite turnaround time for ELISAs is not ideal for “real-time” implementation of therapeutic changes based on assay results.
Herein we report an alternative, automated, point-of-care uCXCL9 assay that can be performed in <1 hour. In addition to confirming that this innovative technology can diagnose ACR noninvasively, we provide proof of concept that serial uCXCL9 measurements following therapy for ACR could be used to guide subsequent clinical decision-making.
Materials and Methods
Samples and Patients
Aliquots of stored BKV-negative urine samples were obtained from 2 multicenter, prospective, observational kidney transplantation studies, CTOT-0112 and CTOT-08 (www.ctot.org, NCT01289717). These samples (n = 86) were used to compare a biolayer interferometry (BLI) assay and ELISA (Figure 1).
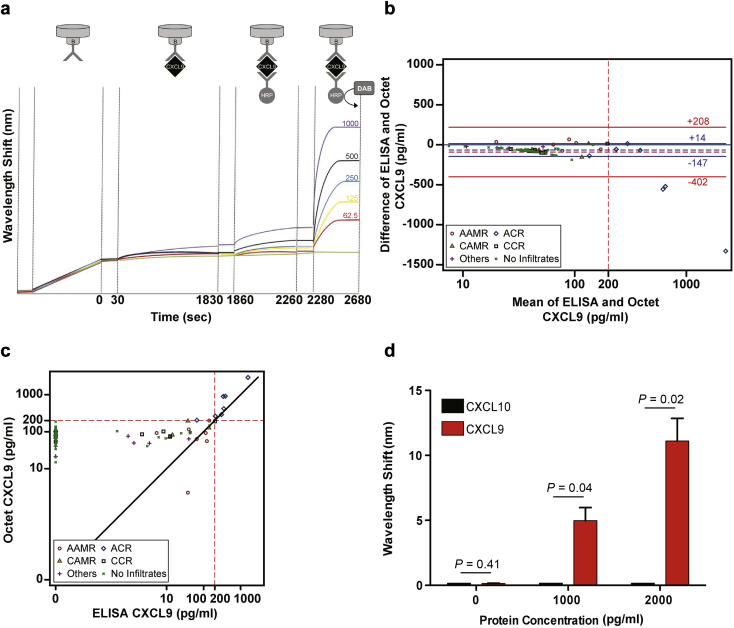
Figure 1.
Biolayer interferometry (BLI) rapidly detects uCXCL9. (a) Schematic representation of assay (top) with primary readout depicting light wavelength shift with each step (y-axis) over time (x-axis) using 62.5 to 1000 pg/ml of recombinant CXCL9 (each colored line is a different concentration) as a standard curve. (b) Bland-Altman plot of CXLC9 as measured by standard enzyme-linked immunosorbent assay (ELISA) versus BLI. The x-axis is on a logarithm scale to allow visualization of the numerical range close to the positivity threshold of the assays (i.e., 200 pg/ml). Symbols represent different diagnoses. Horizontal dashed lines represent the mean difference of ELISA and BLI results, horizontal solid lines represent the 95% limits of agreement, which are defined as the mean difference ±1.96 × the SD of the differences. Red lines include all the data points; blue lines are drawn after exclusion of mean values >500 pg/ml. The numerical values of the limits of agreement are reported above or below the respective lines. (c) Scatterplot of CXCL9 results as performed by ELISA versus BLI (logarithm scale). The solid line passing through the origin with a 45-degree angle represents the line of perfect concordance between ELISA and BLI. Dashed lines passing through 200 pg/ml divide the plot into 4 quadrants. Note that all results lie in the lower left quadrant or in the upper right quadrant, both representing areas of agreement between the 2 methods. Points within the upper left quadrant represent instances in which BLI results are positive (i.e., >200 pg/ml) but standard ELISAs are negative (i.e., <200 pg/l), whereas points within the lower right quadrant would represent instances in which BLI results are negative and ELISA results are positive (note, there are no points in either of these 2 quadrants). (d) BLI-ELISA for CXCL9 does not detect recombinant CXCL10. Each bar is the mean of 3 replicate values; P values show unpaired t-tests. Assay was repeated with similar results. Statistical comparisons performed by t-test. AAMR, acute antibody-mediated rejection; ACR, acute cellular rejection; CAMR, chronic antibody-mediated rejection; CCR, chronic cellular rejection.
We also prospectively collected serial urine samples and clinical data from 46 KTRs with for-cause biopsies who were followed at 4 institutions (Bellvitge University Hospital, IDIBELL, UB, Barcelona, Spain; Mount Sinai Hospital, New York, New York, USA; Parma University Hospital, Parma, Italy; and S. Orsola University Hospital, Bologna, Italy). We also analyzed urine from 10 BKV-negative subjects with stable serum creatinine and no intragraft infiltrates at 6-month surveillance biopsies. Consenting subjects were enrolled at the time of biopsy. The initial urine sample was obtained before the biopsy and before any antirejection treatment.
Inclusion criterion was an acute increase in serum creatinine ≥30%. Patients with pure antibody-mediated rejection,15 as reported by the local pathologist, were excluded. Immunosuppression varied by site but generally included induction with antithymocyte globulin (ATG) or anti-CD25 monoclonal antibody (mAb) and maintenance immunosuppression with a calcineurin inhibitor, and mycophenolic acid with or without corticosteroids. Therapeutic interventions were made at the discretion of the site investigators and were not dictated by study. Therapy of ACR included steroids, ATG, and/or i.v. Ig Serum creatinine values were determined at each hospital laboratory, and the information was collected from hospital records of the subjects. Surveillance studies for viral infections, including the BK virus, were performed according to local practice at each participating site. Bacterial (e.g., urinary) and BK virus infections were routinely tested for in patients with acute renal allograft dysfunction as per local standard of care. Patients included in the study were negative for BK virus infection. The enrollment and sample and/or data collection were performed following institutional review board approval at each site. All patients signed informed consent.
Laboratory Studies
Urine samples for chemokines were centrifuged at 2000g for 30 minutes at 4 °C within 4 hours of collection. The supernatant was divided into aliquots and frozen at −80 °C.
Urine ELISA for CXCL9
Frozen aliquots of urine supernatant were diluted (1:1) in 0.05% Tween 20/0.4% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) (pH 7.2–7.4), and tested by ELISA for CXCL9 (R&D Systems, Minneapolis, Minneapolis) as reported.12, 14
Urine CXCL9 detection by BLI
Samples were run on OctetRED96 using Octet Data Acquisition software (version 8.2, Pall ForteBio LLC, Fremont, CA) and analyzed using Data Analysis software (version 8.2, Pall ForteBio LLC), while continually monitoring wavelength. Assays were performed in black 96-well plates. All steps used a shake speed of 1000 rpm unless otherwise specified. All wells contained 200 μl of fluid. Streptavidin-conjugated sensor tips (Pall ForteBio LLC, cat #18-5019) were incubated in PBS (pH 7.2–7.4) containing 0.025% Tween 20/0.4% BSA for 10 minutes and then incubated with 20 μg/ml (in the previously described buffer) biotinylated mouse antihuman CXCL9 (clone B8-11, BD Bioscience, San Jose, CA) for 10 minutes. Following a 30-second wash, the tips were exposed for 30 minutes to urine supernatants diluted 1:1 with PBS/Tween/BSA or to recombinant CXCL9 (62.5–500 pg/ml, R&D Sys. cat#DY392) diluted in PBS/Tween/BSA to calculate a standard curve. Urine samples obtained from healthy subjects (institutional review board−approved collection of urine from normal volunteers at Mount Sinai Hospital) served as a negative control group. Following an additional 30-second wash, the sensor tips were exposed to horseradish peroxidase (HRP)−conjugated antihuman CXCL9 clone B8-6 (5 μg/ml in PBS/Tween/BSA for 15 min). The tips were washed in stable peroxide buffer (3,3’-diaminobenzidine, DAB substrate kit, Thermo Fischer Scientific, Canoga Park, CA, cat #PI-34065) for 30 seconds on a shaker at 200 rpm and then exposed to metal-enhanced DAB diluted into peroxide buffer for 15 minutes.
Statistical Methods
Data are summarized using descriptive statistics for categorical (counts/percentages) and continuous (mean and SDs) variables. To assess the agreement between BLI and standard ELISA measurements of CXCL9, we used the Lin’s concordance correlation coefficient (the concordance correlation coefficient combines measures of both precision and accuracy to determine how far the observed data deviate from the line of perfect concordance)16 and Bland and Altman's 95% limits-of-agreement17 in urine samples from 86 kidney transplantation patients with various biopsy diagnoses. Bland-Altman’s 95% limits of agreement were additionally computed after exclusion of values of CXCL9 >500 pg/ml because close agreement between BLI and ELISA with respect to such large CXCL9 values was not relevant for the purpose of clinical decision-making. The intraplate assay coefficient of variation (CV) was calculated based on the wavelength shift for samples of recombinant CXCL9 diluted in both PBS/Tween/BSA and urine from healthy subjects, with 4 replicates for each dilution on the same plate. The average of the CVs at each dilution is reported as the intra-assay CV. The interplate CV is calculated based on the mean CV of the different dilutions, with 4 replicates on one plate, repeated 3 times during 1 week. Differences in CXCL9 values were analyzed by Mann-Whitney test (Prism, GraphPad Software, La Jolla, California). A 2-tailed P value <0.05 was considered to be statistically significant.
Results
To shorten the time required to detect uCXCL9, we used BLI, a methodology in which binding of a ligand to a fiber optic sensor tip induces a real-time detectable wavelength shift in the returning beam of light (Δλ) that correlates with the quantity of bound ligand.18 BLI has been used to screen mAb binding affinities among other indications.18 We chose BLI over other available rapid detection systems, including nanoscale and/or microfluidic and multiplexing approaches such as Ella (https://www.proteinsimple.com/ella.html) or Gyros (http://www.bioagilytix.com/gyrolab-for-frequent-automated-sampling), because urine volumes are not limiting (negating the need for nanoscale volumes), high-throughput multiplexing is not required for CXCL9 analysis of clinical samples, the BLI technology is more widely available, and the ability to move BLI technology to a point-of-care clinical setting seems straightforward.
We adapted BLI to detect CXCL9 (Figure 1a) by attaching an anti-CXCL9 mAb to commercially available sensor tips, exposing the tips to CXCL9 and amplifying the signal with a second, HRP-conjugated anti-CXCL9 mAb followed by addition of a metal-enhanced HRP substrate. Using precoated sensor tips, the assay can be completed in <1 hour (Figure 1a). We measured CXCL9 by ELISA and BLI in 86 urinary samples from patients with various biopsy diagnoses.
A Bland-Altman plot17 (Figure 1b) analysis showed good agreement between the 2 assays for values <500 pg/ml; the average difference between ELISA and BLI was −66 ± 41 pg/ml and the 95% limits of agreement were −147 to +14 pg/ml. Depiction of the same data using a scatterplot comparison of ELISA and BLI results (Figure 1c) showed a Lin’s concordance correlation coefficient16 of 0.78 (BLI was more sensitive than ELISA) and a 100% concordance of positive results versus negative results based on the threshold of 200 pg/ml.
Specificity controls using independent samples showed that the BLI CXCL9 assay did not cross-react with CXCL10 (Figure 1d). BLI had the same limit of sensitivity as reported for commercial ELISAs (∼35 pg/ml, see data sheet, R&D catalogue #DY392) with intra- and interplate CVs of <5% and <12%, respectively (data not shown).
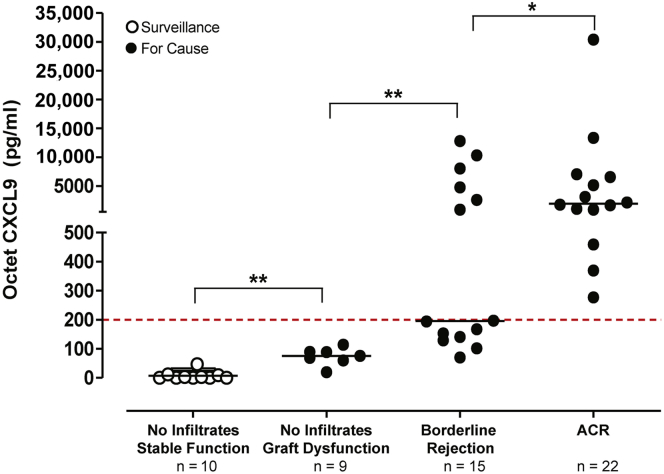
We then measured uCXCL9 by BLI in a different set of samples obtained from 56 BKV-negative KTRs. This new set included 22 samples from subjects with a ≥30% increase serum creatinine and biopsy-proven ACR ≥ Banff 1A (Table 1). Urinary CXCL9 was >200 pg/ml in all of these samples (Figure 2). In contrast, uCXCL9 was <100 pg/ml (P < 0.01 vs. ACR) in stored CTOT urine samples from 10 BKV-negative KTRs with stable serum creatinine and a normal 6-month surveillance biopsy (Table 1 and Figure 2). Urine CXCL9 values in samples from BKV-negative subjects (n = 9) with an acute rise in serum creatinine due to calcineurin inhibitor toxicity and/or volume depletion, but without mononuclear cell infiltrates, were all 100 to 200 pg/ml (P < 0.01 vs. ACR or vs. normal) (Figure 2). CXCL9 measurements in prospectively collected urine from BKV-negative subjects with for-cause biopsies that showed biopsy-proven borderline rejection (n = 15) (Table 1) were higher than those from biopsies without infiltrates (P < 0.01) (Figure 2); 7 of 15 contained ≥200 pg/ml CXCL9 and 8 showed <200 pg/ml (Figure 2).
Table 1.
Baseline demographic and clinical characteristics of study subjects
| Characteristics | Overall (n = 56) |
No infiltratesa (n = 10) |
No infiltrates (n = 9) |
Borderline (n = 15) |
ACR (n = 22) |
|---|---|---|---|---|---|
| Donors | |||||
| Deceased | 30 (53.6) | 2 (20.0) | 5 (55.6) | 11 (73.3) | 12 (54.6) |
| Male | 22 (39.3) | 3 (30.0) | 2 (22.2) | 6 (40.0) | 11 (50.0) |
| Age (yr) | 37.5 ± 15.6 | 34.9 ± 11.5 | 32.9 ± 15.2 | 41.0 ± 14.9 | 38.3 ± 18.5 |
| Race | |||||
| Black or African American | 10 (17.9) | 3 (30.0) | 3 (33.0) | 2 (13.3) | 2 (9.1) |
| Other race | 27 (48.2) | 5 (50.0) | 5 (55.6) | 9 (60.0) | 8 (36.4) |
| Unknown or not reported | 19 (33.9) | 2 (20.0) | 1 (11.1) | 4 (26.7) | 12 (54.5) |
| Recipients | |||||
| Male | 42 (75.0) | 6 (60.0) | 6 (66.7) | 12 (80.0) | 18 (81.8) |
| Age (yr) | 45.4 ± 18.3 | 45.6 ± 9.8 | 45.8 ± 21.7 | 47.1 ± 19.7 | 44.0 ± 19.8 |
| Race | |||||
| Black or African American | 19 (33.9) | 5 (50.0) | 2 (22.2) | 5 (33.3) | 7 (31.8) |
| Other race | 27 (48.2) | 5 (50.0) | 2 (22.2) | 8 (53.3) | 12 (54.5) |
| Unknown or not reported | 10 (17.9) | 0 (0.0) | 5 (55.6) | 2 (13.3) | 3 (13.6) |
| Induction | |||||
| Yes | 52 (92.9) | 8 (80.0) | 8 (88.9) | 15 (100.0) | 21 (95.5) |
| No | 4 (7.1) | 2 (20.0) | 1 (11.1) | 0 (0.0) | 1 (4.5) |
| Time after transplantb | 183 (70–211) | 191 (186–197) | 167 (79–211) | 125 (28–-190) | 120 (76–597) |
ACR, acute cellular rejection.
Variables are expressed as mean ± SD, median (interquartile range), or absolute number (%).
Patients with stable graft function who underwent surveillance biopsies. All the other patients received a biopsy for cause (serum creatinine increase >30%).
Days from transplant to the date of first graft biopsy.
Figure 2.
Biolayer interferometry urine CXCL9 detects intragraft infiltrates in kidney transplant recipients. We analyzed 56 samples from kidney transplant recipients with surveillance (open circles, n = 10) or for-cause (filled circles; ≥30% increase in serum creatinine) biopsies. Subjects were stratified according to the presence of stable graft function and no graft infiltrates (in surveillance biopsies, n = 10), acute graft dysfunction and no graft infiltrates (n = 9), borderline rejection (n = 15), or acute cellular rejection (ACR) (in for-cause biopsies, n = 22). Dotted red line is drawn at the 200 pg/ml threshold for CXCL9 positivity. Horizontal black lines are drawn at the median value for each group. Statistical comparison performed by a Mann-Whitney test. *P < 0.05; **P < 0.01.
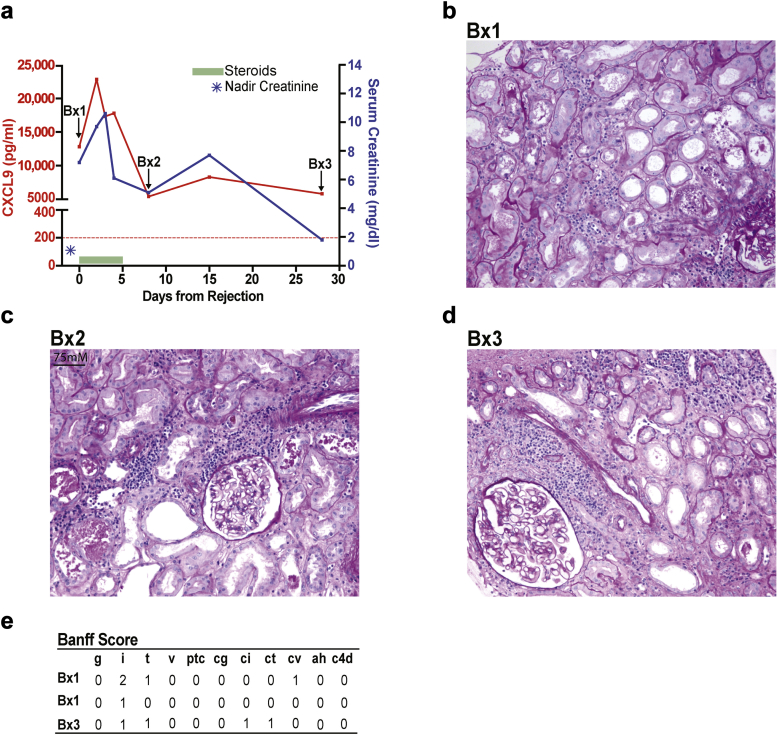
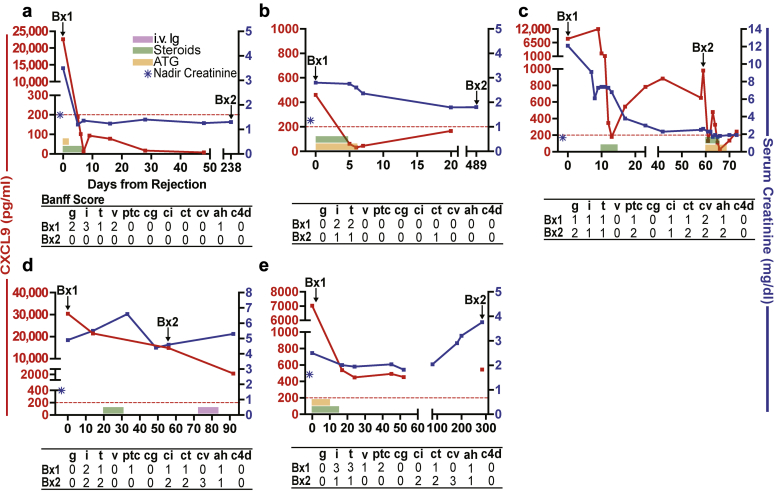
In addition, we collected and analyzed serial urine samples from 6 BKV-negative KTRs with ACR before and after antirejection therapy, and who had follow-up biopsies as part of clinical care. The summarized results from 1 representative subject at 20 days after transplant are depicted in Figure 3. The subject’s baseline serum creatinine was 1.0 mg/dl and increased to 7.2 mg/dl coincident with an episode of biopsy-proven ACR. Steroid pulses reduced serum creatinine levels to <2 mg/dl, but follow-up biopsies on days 7 and 28 after the initial diagnostic biopsy showed intragraft infiltrates associated with persistently elevated levels of uCXCL9. In 2 additional subjects (Figure 4a and b), antirejection therapy resulted in uCXCL9 <200 pg/ml and absence of mononuclear infiltrates in follow-up biopsies. In 3 cases (Figure 4c−e), persistent uCXCL9 >200 pg/ml despite therapy for ACR was associated with intragraft infiltrates, regardless of changes in serum creatinine.
Figure 3.
Persistently elevated uCXCL9 after treatment for acute cellular rejection (ACR) detects subclinical intragraft infiltrates despite a progressive decline in serum creatinine. (a) Clinical course depicting changes in serum creatinine and uCXCL9 (day 0 is the date of the biopsy, 20 days posttransplant). Colored horizontal bars depict time during which each drug was administered (key: upper right panel a). Blue star represents the nadir serum creatinine within the initial 6 months posttransplant. Red dashed line is drawn at the 200 pg/ml threshold for CXCL9 positivity. (b−d) Representative periodic acid–Schiff stained sections of biopsy 1 (Bx1), 2 (Bx2), and 3 (Bx3) depicted in (a) showing areas of mononuclear cell infiltration in each biopsy. (e) Quantitative Banff scores as read by the local pathologist for the 3 biopsies.
Figure 4.
Changes in serum creatinine and urine CXCL9 in 5 BK virus–negative, donor specific antibody–negative subjects (depicted individually in a–e) with acute cellular rejection and follow-up biopsies to monitor treatment efficacy. Tables below each panel depict Banff scores of each of the biopsies. Dotted red line is drawn at the 200 pg/ml threshold for CXCL9 positivity. ATG, antithymocyte globulin.
Discussion
We demonstrated that BLI and ELISA could similarly detect uCXCL9, but BLI was considerably faster and largely automated. These advantages could permit implementing BLI-based uCXLC9 testing in a clinical transplantation practice. Although BLI methods are straightforward, the currently available detection device has a complex and expensive interface designed for broader use. Nonetheless, modifications could simplify the interface and lower costs to accommodate practicing physicians.
We showed that in BKV-negative KTRs with acute graft dysfunction, BLI-measured uCXCL9 ≥200 pg/ml detected Banff grade ≥1A ACR. Approximately 50% of subjects with borderline and/or suspicious rejection also had uCXCL9 >200 pg/ml, with the remainder falling into the normal range. Because (i) histological diagnoses in kidney transplantation are subject to sampling bias and inter-reader variation, and (ii) the significance of borderline rejection remains controversial,19, 20 one testable hypothesis that arose from these observations was that elevated uCXCL9 measurements were better indicators of ongoing pathological inflammation than serum creatinine or histological evidence of suspicious and/or borderline rejection. Although urinary tract infections and BKV increase uCXCL9, routine BKV monitoring and urinalysis together with rapid CXCL9 diagnostics could guide clinical decision-making noninvasively, a hypothesis that is also testable.
We acknowledge that none of the tested subjects in this series had pure antibody-mediated rejection. Other groups showed associations between antibody-mediated rejection and the chemokine CXCL10 (in the absence of CXCL9).10, 21 Pilot studies indicated that we could detect CXCL10 by an analogous BLI-based ELISA (data not shown), which provided feasibility for potentially incorporating both CXCL9 and CXCL10 measurements into clinical care.
We also provided evidence that serial uCXCL9 monitoring after initiating treatment for ACR could be diagnostically informative. In our limited analysis of KTRs with clinically indicated follow-up biopsies, continuous elevation of uCXCL9 detected persistent intragraft cellular infiltrates regardless of serum creatinine. We acknowledge the small numbers of subjects, the inconsistent numbers and timing of biopsies, and the descriptive nature of this case study precluded reaching definitive conclusions regarding the usefulness of BLI uCXCL9 to guide post-treatment decision-making in KTRs with rejection. Nonetheless, these proof-of-concept results provided a foundation to support future controlled studies to test the hypothesis that inclusion of real-time BLI uCXCL9 measurements would lower the need for follow-up biopsies, guide decisions to continue and/or alter antirejection therapy, and consequently, improve patient outcomes.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The study was supported by National Institutes of Health grants U01 AI63594 awarded to PH and U01 AI084146 awarded to MA. PC was supported by T32 AI078892. JR-A was supported by grant UL1TR000067 from the National Center for Advancing Translational Sciences, National Institutes of Health.
References
- 1.Kramer B.K., Klinger M., Vitko S. Tacrolimus-based, steroid-free regimens in renal transplantation: 3-year follow-up of the ATLAS trial. Transplantation. 2012;94:492–498. doi: 10.1097/TP.0b013e31825c1d6c. [DOI] [PubMed] [Google Scholar]
- 2.Tanriover B., Jaikaransingh V., MacConmara M.P. Acute rejection rates and graft outcomes according to induction regimen among recipients of kidneys from deceased donors treated with tacrolimus and mycophenolate. Clin J Am Soc Nephrol. 2016;11:1650–1661. doi: 10.2215/CJN.13171215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald S., Russ G., Campbell S. Kidney transplant rejection in Australia and New Zealand: relationships between rejection and graft outcome. Am J Transplant. 2007;7:1201–1208. doi: 10.1111/j.1600-6143.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- 4.Nankivell B.J., Alexander S.I. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 5.Anglicheau D., Naesens M., Essig M. Establishing biomarkers in transplant medicine: a critical review of current approaches. Transplantation. 2016;100:2024–2038. doi: 10.1097/TP.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 6.Menon M.C., Murphy B., Heeger P.S. Moving biomarkers toward clinical implementation in kidney transplantation. J Am Soc Nephrol. 2017;28:735–747. doi: 10.1681/ASN.2016080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazzeri E., Romagnani P. CXCR3-binding chemokines: novel multifunctional therapeutic targets. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:109–118. doi: 10.2174/1568008053174723. [DOI] [PubMed] [Google Scholar]
- 8.Hu H., Aizenstein B.D., Puchalski A. Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am J Transplant. 2004;4:432–437. doi: 10.1111/j.1600-6143.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 9.Hauser I.A., Spiegler S., Kiss E. Prediction of acute renal allograft rejection by urinary monokine induced by IFN-gamma (MIG) J Am Soc Nephrol. 2005;16:1849–1858. doi: 10.1681/ASN.2004100836. [DOI] [PubMed] [Google Scholar]
- 10.Schaub S., Nickerson P., Rush D. Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am J Transplant. 2009;9:1347–1353. doi: 10.1111/j.1600-6143.2009.02645.x. [DOI] [PubMed] [Google Scholar]
- 11.Jackson J.A., Kim E.J., Begley B. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am J Transplant. 2011;11:2228–2234. doi: 10.1111/j.1600-6143.2011.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hricik D.E., Nickerson P., Formica R.N. Multicenter validation of urinary CXCL9 as a risk-stratifying biomarker for kidney transplant injury. Am J Transplant. 2013;13:2634–2644. doi: 10.1111/ajt.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.C., Page E.K., Knechtle S.J. Urine proteomics in kidney transplantation. Transplant Rev (Orlando) 2014;28:15–20. doi: 10.1016/j.trre.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Hricik D.E., Formica R.N., Nickerson P. Adverse outcomes of tacrolimus withdrawal in immune-quiescent kidney transplant recipients. J Am Soc Nephrol. 2015;26:3114–3122. doi: 10.1681/ASN.2014121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas M., Sis B., Racusen L.C. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 16.Liao J.J., Lewis J.W. A note on concordance correlation coefficient. PDA J Pharm Sci Technol. 2000;54:23–26. [PubMed] [Google Scholar]
- 17.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 18.Dysinger M., King L.E. Practical quantitative and kinetic applications of bio-layer interferometry for toxicokinetic analysis of a monoclonal antibody therapeutic. J Immunol Methods. 2012;379:30–41. doi: 10.1016/j.jim.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Becker J.U., Chang A., Nickeleit V. Banff borderline changes suspicious for acute T cell-mediated rejection: where do we stand? Am J Transplant. 2016;16:2654–2660. doi: 10.1111/ajt.13784. [DOI] [PubMed] [Google Scholar]
- 20.Ozluk Y., Blanco P.L., Mengel M. Superiority of virtual microscopy versus light microscopy in transplantation pathology. Clin Transplant. 2012;26:336–344. doi: 10.1111/j.1399-0012.2011.01506.x. [DOI] [PubMed] [Google Scholar]
- 21.Ho J., Rush D.N., Karpinski M. Validation of urinary CXCL10 as a marker of borderline, subclinical, and clinical tubulitis. Transplantation. 2011;92:878–882. doi: 10.1097/TP.0b013e31822d4de1. [DOI] [PubMed] [Google Scholar]