Abstract
Introduction
In the randomized placebo-controlled Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) 3:4 trial, tolvaptan slowed kidney growth and renal function decline in subjects with autosomal dominant polycystic kidney disease (ADPKD). Consistent with its primary pharmacologic activity, tolvaptan use was commonly associated with aquaretic adverse events (AAEs) attributable to excess free water clearance.
Methods
A post hoc analysis of tolvaptan-related discontinuations from the pivotal randomized controlled trial TEMPO 3:4 and its open-label extension TEMPO 4:4.
Results
In total, 750 of 961 tolvaptan-treated subjects (78%) in TEMPO 3:4 reported at least one AAE. Of these 750 subjects, 72 (10%) discontinued because of an AAE (aquaretic-discontinued group) and 573 (76%) continued (aquaretic-continued group). The aquaretic-discontinued subjects were younger, had better baseline renal function, and had higher fasting urine osmolality than aquaretic-continued subjects. Of the 750 subjects reporting an AAE, 105 (14%) discontinued for another reason (non-aquaretic-discontinued group). Compared to non-aquaretic-discontinued subjects, aquaretic-discontinued subjects were more commonly male, had better baseline renal function, and discontinued the study drug faster. After 3 years of therapy, 75% of tolvaptan subjects indicated that they could tolerate their current dose for the rest of their lives, compared to 85% of placebo subjects. These findings were corroborated by results in the open-label extension trial TEMPO 4:4.
Discussion
In this study, AAEs were common but well tolerated in ADPKD patients on tolvaptan. ADPKD patients in earlier stages of disease progression may be more sensitive to aquaretic symptoms, which may help in guiding tolvaptan dosing and titration decisions in the future.
Keywords: aquaretic adverse events, autosomal dominant polycystic kidney disease, discontinuation, drug safety, tolerability, tolvaptan
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited disorder characterized by the appearance and slow growth of fluid-filled cysts in the kidneys, liver, and other organs.1, 2 In the kidney, the expanding cysts obstruct renal tubules, blood vessels and lymphatics, resulting in apoptosis, atrophy, and fibrosis.3 As the cysts grow, the kidneys gradually and progressively enlarge, which may ultimately cause end-stage renal disease (ESRD); thus, approximately 50% of patients with ADPKD have ESRD by 60 years of age.4 The disease was responsible for 2.6% of patients on dialysis and 9.9% of patients receiving renal transplants in 2012 in the United States,5 making it the fourth leading cause of ESRD, behind diabetes, hypertension, and glomerulonephritis.6 The prevalence of ADPKD patients on renal replacement therapy (RRT) in the European Union has been estimated at 91.1 per million.7
In the past, therapy for ADPKD was symptomatic, relying primarily on antihypertensives, analgesics, RRT, and transplantation. Recently, however, pharmacotherapy with tolvaptan has proved to have beneficial disease-modifying activity in patients with ADPKD.8, 9 Tolvaptan is an oral selective antagonist of the V2 receptor, the key binding site of the antidiuretic hormone arginine vasopressin (AVP), and acts by lowering the levels of cyclic AMP within the distal renal tubule epithelium, the major site of cyst development in ADPKD.1, 2, 10 Blockade of the V2 receptor has been shown to have an anti-proliferative effect that slows cyst development in orthologous model systems.11, 12, 13, 14, 15 In the pivotal Phase 3 trial Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO 3:4), treatment with tolvaptan halved the rates of total kidney volume growth relative to placebo.8, 9 These effects were associated with other favorable outcomes, including a lower rate of worsening renal function and a lower rate of worsening kidney pain.
By antagonizing the activity of AVP in renal tubule cells, tolvaptan induces aquaresis (i.e., increased free water clearance and decreased urine osmolality) and increased serum sodium concentrations.16 In fact, tolvaptan was initially approved for the treatment of clinically significant hyponatremia, including patients with heart failure and syndrome of inappropriate antidiuretic hormone. Consistent with its mechanism of action, the use of tolvaptan in clinical trials has been associated with aquaretic adverse events (AAEs). Given their high frequency, AAEs might be expected to impose some limitations on tolvaptan use in the clinic, but this has not been directly examined. To dissect this issue in more detail, the current subanalysis of the TEMPO 3:4 trial and its open-label extension TEMPO 4:4 characterized the impact of tolvaptan-related AAEs on a subject’s decision to discontinue from the trial. This study also aimed to identify characteristics of ADPKD patients who were at increased risk for AAEs, which may allow for better tolvaptan titration regimens and encourage compliance.
Methods
Trial Design
This was a post hoc, exploratory subanalysis of the TEMPO 3:4 trial (ClinicalTrials.gov identifier: NCT00428948).8, 9 TEMPO 3:4 was a Phase 3, multicenter, double-blind, placebo-controlled, 3-year trial assessing the efficacy and safety of tolvaptan in subjects with ADPKD. Eligibility requirements included a diagnosis of ADPKD according to the Ravine criteria, a total kidney volume of ≥ 750 ml as measured by magnetic resonance imaging (MRI), an estimated creatinine clearance rate of ≥ 60 ml per minute as calculated by the Cockcroft–Gault formula, and an age of 18 to 50 years. A total of 1445 eligible subjects were randomly assigned in a 2:1 ratio to receive either tolvaptan (n = 961) or matching placebo (n = 484). Following randomization, subjects were to be administered 2 doses of study drug per day for 3 years, initiating with a 3-week titration period. During the first week of the titration period, the active arm received tolvaptan at a dose of 45 mg in the morning and 15 mg in the afternoon. This split dose was increased to 60/30 mg in the second week and to 90/30 mg in the third week according to subject-reported tolerability. Subjects were allowed to down-titrate to 45/15 mg at any time during the trial but were advised to remain on their highest tolerated dose throughout the remaining trial period. The investigators were instructed on the necessity to explain the mechanism of action of the drug in relation to expected AAEs including polyuria and the need to compensate with adequate water intake.
To confirm any conclusions about AAEs in TEMPO 3:4, we further examined prior placebo subjects who crossed over to open-label tolvaptan in TEMPO 4:4 (ClinicalTrials.gov identifier: NCT01214421).17 TEMPO 4:4 was a nonrandomized, open-label extension of TEMPO 3:4 that sought to determine whether tolvaptan modified the progression of ADPKD and whether its effects could be sustained over time. The trial included 871 patients on open-label tolvaptan, 557 who had received tolvaptan in TEMPO 3:4 and 314 who had received placebo. The entry criteria were completion of TEMPO 3:4 and an eGFRMDRD ≥ 30 ml/min per 1.73 m2. Treatment used the same split-dose regimens as the TEMPO 3:4 trial for a minimum of 2 years. Initiation of dosing used the same titration scheme over the first 4 weeks with down-titration to 45/15 mg or lower after discussion with the medical monitor after the first month.
Assessments
In both studies, AAEs were defined as thirst, polyuria (production of large volumes of dilute urine), nocturia (need to wake up to urinate at night), pollakiuria (abnormally frequent urination), and polydipsia (excessive thirst). All adverse events were counted once per subject per specific event.
Three subpopulations of tolvaptan-treated subjects from TEMPO 3:4 and TEMPO 4:4 were examined in the current study: those who reported an AAE and continued on study drug; those who reported an AAE but discontinued for another AE; and those who reported an AAE that led to trial discontinuation. These are referred to in this report as aquaretic-continued (AC), non–aquaretic-discontinued (NAD), and aquaretic-discontinued (AD), respectively. Subjects who experienced an AAE were analyzed separately for those who continued or discontinued the trial and separately for those who discontinued the trial due to polyuria versus all other combined aquaretic AAEs.
Specific assessments conducted on the individual subpopulations included the following: frequency and timing of AAEs and non-AAEs leading to trial discontinuation; dose of tolvaptan at trial discontinuation; highest titrated dose of tolvaptan before discontinuation; baseline demographics, including age, sex, height-adjusted total kidney volume (htTKV), renal function (eGFRCKD-EPI), and fasting baseline urine osmolality (uOsm).
In addition, in a patient-reported outcome analysis in TEMPO 3:4, the percentage of subjects who tolerated their current dose of study drug was determined. The analysis was based on a questionnaire that asked, among other queries, whether the subject could tolerate his or her current dose of study drug for the rest of his or her life (those who answered “no” to this question were either titrated to a lower dose of medication or discontinued from the trial).
Statistical Analyses
Baseline characteristics were calculated for subjects who reported an AAE separately for those who continued or discontinued the trial and separately for those who discontinued the trial due to polyuria versus all other combined aquaretic AAEs. Moreover, those who discontinued the trial for a reason other than an AAE were further assessed by reason for discontinuation: (i) due to an AE other than an AAE (n = 57) or (ii) for another reason (n = 48), which included lost to follow-up (n = 11), subject met withdrawal criteria (n = 3), investigator withdrew subject (n = 2) and subject withdrew consent (n = 32). This analysis reports the full population of individuals who discontinued for reasons other than an AAE (n = 105) and the subpopulation who discontinued because of another AE (n = 57) in Table 1.
Table 1.
Subpopulation characteristics in TEMPO 3:4
| Subgroup | n | Age, mean (SD), yr | Male, % | Baseline eGFR, mean (SD), ml/min per 1.73 m2 | Baseline htTKV, mean (SD), ml/m | Baseline fasting uOsm. mean (SD), mOsm/kg | Time to first AAE report, median (IQR), day | Time to discontinuation, median (IQR), day |
|---|---|---|---|---|---|---|---|---|
| All tolvaptan-treated subjects | 961 | 38.6 (7.1) | 52 | 81.3 (21.0) n = 958 |
979 (515) | 500 (173) n = 700 |
||
| Tolvaptan subjects without AAE | 211 | 38.4 (6.7) | 46 | 80.6 (20.6) | 1033 (563) | 511.7 (170) n = 147 |
||
| Tolvaptan subjects with AAE | 750 | 38.6 (7.2) | 53 | 81.6 (21.1) n = 747 |
963 (500) | 496 (173) n = 553 |
||
| Continued (AC) | 573 | 38.9 (7.1)a | 53 | 80.9 (20.9)f n = 675 |
957 (478) | 492 (176)k n = 504 |
2 (1–8) | |
| Discontinued AAE (AD) | 72 | 36.2 (7.8)a,b | 57c,d | 88.2 (22.2)e,f,g n = 72 |
915 (521)i,j | 544 (134)k n = 49 |
2 (1–6) | 96 (15–209)l,m |
| Polyuria | 40 | 36.4 (8.6) | 50 | 95.6 (18.6)h | 843 (385) | 555 (126) n = 27 |
||
| All other AAEs | 32 | 36.0 (7.0) | 66 | 79.0 (23.0)h | 1005 (648) | 531 (146) n = 22 |
||
| Discontinued other (NAD) | 105 | 38.3 (7.5) | 41d | 78.7 (21.6)g | 1032 (595)i | 490 (158) n = 76 |
1 (1–6) | 372 (121–631)m |
| All other AEs | 57 | 40.0 (7.1)b | 35c | 73.6 (19.2)e | 1046 (518)j | 486 (164) n = 42 |
1 (1–4) | 288 (121–577)l |
AAE, aquaretic adverse event, AC, aquaretic-continued; AD, aquaretic-discontinued; eGFR, estimated glomerular filtration rate, calculated by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation; htTKV, height-adjusted total kidney volume; IQR, interquartile range; NAD, non–aquaretic-discontinued; TEMPO, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes; uOsm, urine osmolality.
Data are presented on subjects allocated to tolvaptan treatment.
Subjects who discontinued for reasons other than AAEs include other AE (n = 57, 54%), lost to follow-up (n = 11, 11%), subject met withdrawal criteria (n = 3, 3%), investigator withdrew subject (n = 2, 2%), subject withdrew consent (n = 32, 31%).
P = 0.006.
P = 0.007.
P = 0.02.
P < 0.05.
P = 0.006.
P = 0.0001.
P = 0.005.
P = 0.001.
P = 0.04.
P = 0.03.
P < 0.05.
P < 0.0001.
P < 0.0001.
Parametric variables were expressed as mean ± SD. Differences in baseline characteristics were calculated with a Fisher exact test for categorical data and a Mann–Whitney–Wilcoxon test for continuous data. Time to first report of an AAE and time to discontinuation are presented as median (interquartile range [IQR]). Difference in time between subjects who reported an AAE and continued or discontinued the trial was calculated with a Mann–Whitney–Wilcoxon test. Time to discontinuation of the trial is expressed as median (IQR). Difference in time between subjects who discontinued the trial due to an AAE or other reason was calculated with a Mann–Whitney–Wilcoxon test. Differences in the proportions of patients maintaining the target dose according to the report of an AAE or not were assessed with a Fisher exact test. All analyses were performed with SAS 9.2 (SAS Institute, Cary, NC) statistical software, and a 2-sided value of P < 0.05 was considered to indicate statistical significance.
Results
Characteristics of Tolvaptan-Treated Subjects Who Reported an AAE
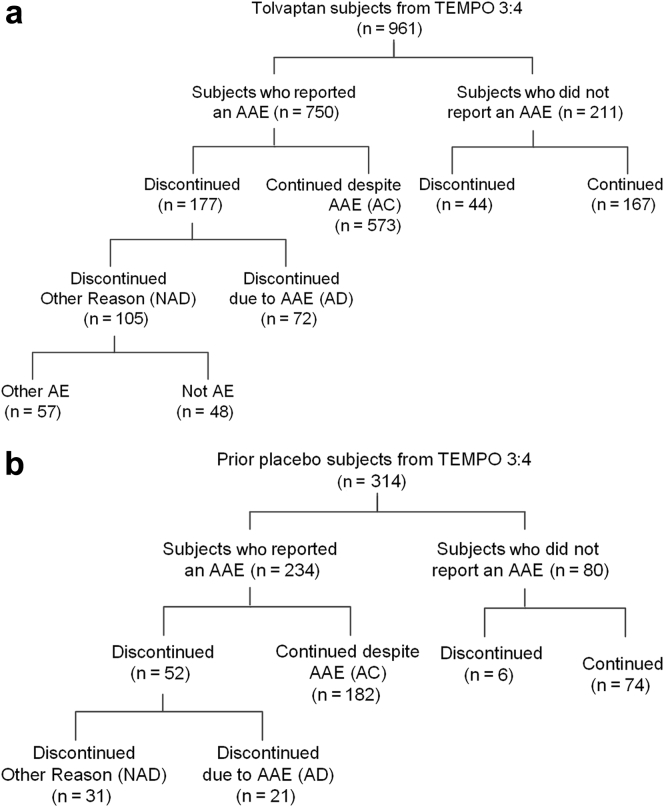
In TEMPO 3:4, 750 of 961 tolvaptan-treated subjects (78%) reported at least 1 AAE (AAE population), whereas 211 (22%) reported no such event (non-AAE population) (Figure 1A). Of the 750 reporting an AAE, 177 (24%) subjects discontinued, including 72 (10%) due to the aquaretic event (aquaretic-discontinued [AD]) and 105 (14%) for another reason (non–aquaretic- discontinued [NAD]). Treatment was not discontinued in 573 (76%) reporting an AAE (aquaretic-continued [AC]).
Figure 1.
Flow chart of tolvaptan subjects into categories of participants who reported or did not report an aquaretic adverse event (AAE). (a) Tolvaptan subjects in the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) 3:4 trial. (b) Prior placebo subjects in TEMPO 4:4. AC, aquaretic-continued; AD, aquaretic-discontinued; AE, adverse event; NAD, non–aquaretic discontinued.
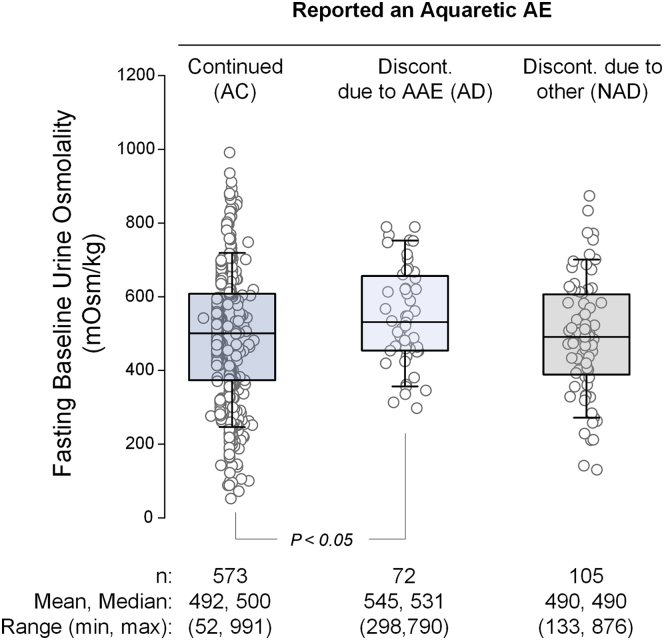
No significant differences were observed between the AAE and non-AAE populations, but differences were detected within the AAE population itself (Table 1). For example, AD subjects were significantly younger (36.2 ± 7.8 vs. 38.9 ± 7.1 years; P = 0.006), had higher baseline renal function (eGFRCKD-EPI, 88.2 ± 22.2 vs. 80.9 ± 20.9 ml/min per 1.73 m2; P = 0.006), and higher fasting baseline uOsm (544 ± 134 vs. 492 ± 176 mOsm/kg; P = 0.045) than AC subjects (Figure 2). Similarly, when compared with NAD subjects, AD subjects were more likely to be male (57 vs. 41%, P < 0.05), had higher baseline renal function (88.2 ± 22.2 vs. 78.7 ± 21.6 ml/min per 1.73 m2; P = 0.005), had lower baseline htTKV (915 ± 521 vs. 1032 ± 595 ml/m, P = 0.03), and were faster to discontinue the trial (median [IQR], 96 [15–209] vs. 372 [121–631] days; P < 0.0001). AD subjects were younger (36.2 ± 7.8 vs. 40.0 ± 7.1 years, respectively, P = 0.007) than subjects in the NAD group who discontinued for a different AE (N = 57).
Figure 2.
Urine osmolality in tolvaptan subjects who reported an aquaretic adverse event (AAE). All patient data points are plotted as circles. Box plot shows mean and interquartile range of fasting baseline urine osmolality. AC, aquaretic-continued; AD, aquaretic-discontinued; AE, adverse event; Discont., discontinued; max, maximum; min, minimum; NAD, non–aquaretic-discontinued.
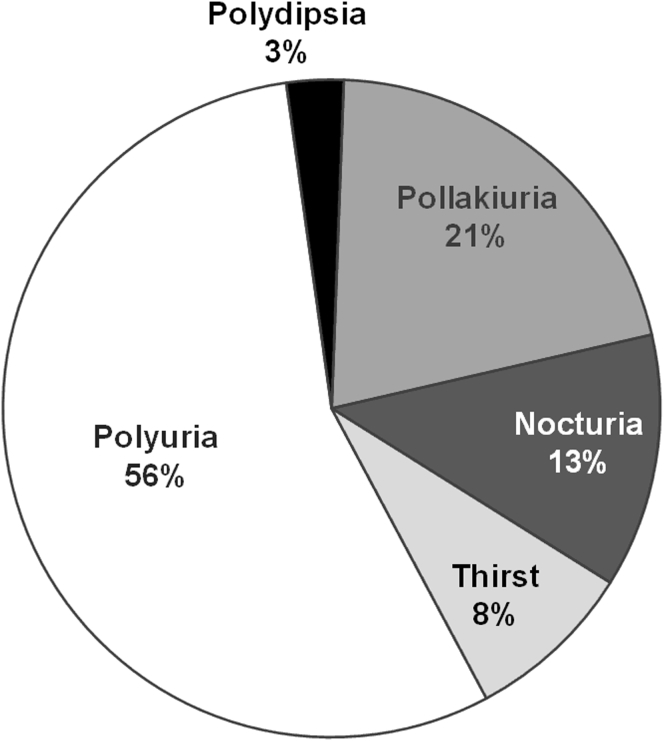
In the AD subgroup, the most common AAE leading to discontinuation was polyuria, accounting for 56% (n = 40) of the total, followed by pollakiuria (15 [21%]), nocturia (9 [13%]), thirst (6 [8%]), and polydipsia (2 [3%]), respectively (Figure 3). Thus, trial discontinuation was caused by polyuria more frequently than all other AAEs combined. Subjects who discontinued because of polyuria had significantly higher baseline renal function than subjects who discontinued for other AAEs (eGFRCKD-EPI, 95.6 ± 18.6 vs. 79.0 ± 23.0 ml/min per 1.73 m2; P = 0.001).
Figure 3.
Frequency of aquaretic adverse events that led to trial discontinuation of tolvaptan subjects in the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) 3:4 trial. Nocturia, need to wake up to urinate at night; pollakiuria, abnormally frequent urination; polydipsia, excessive thirst; polyuria, production of large volumes of dilute urine.
To validate these findings, the cohort of subjects who crossed over from placebo in TEMPO 3:4 to tolvaptan in the open-label extension trial TEMPO 4:4 were also examined (Figure 1B). In total, 234 of 314 (75%) prior placebo subjects reported an AAE following the switch to tolvaptan. Similar to the tolvaptan group in TEMPO 3:4, 21 of 234 subjects (9%) discontinued due to an AAE (Table 2). The AD subjects had significantly higher baseline renal function than the AC subjects (72.4 ± 30.5 vs. 67.5 ± 23.1 ml/min per 1.73 m2; P = 0.048). The AD subjects also discontinued the trial faster than the NAD subjects (median [IQR], 196 [49–910] vs. 658 [216–1218] days, P = 0.02).
Table 2.
Subpopulation characteristics in TEMPO 4:4
| Subgroup | n | Age, mean (SD), yr | Male, % | Baseline eGFR, mean (SD), ml/min per 1.73 m2 | Baseline htTKV, mean (SD), ml/m | Baseline dasting uOsm, mean (SD), mOsm/kg | Time to first AAE report, median (IQR), d | Time to discontinuation, median (IQR), d |
|---|---|---|---|---|---|---|---|---|
| All prior placebo subjects | 314 | 42.5 (7.2) | 50 | 66.6 (25.4) n = 253 |
1196 (676) n = 289 |
442 (165) n = 309 |
||
| Prior placebo without AAE | 80 | 42.1 (8.0) | 58 | 62.4 (24.4) n = 74 |
1286 (773) n = 72 |
429 (149) n = 77 |
||
| Prior placebo with AAE | 234 | 42.6 (6.9) | 48 | 68.4 (25.6) n = 179 |
1167 (640) n = 217 |
446 (171) n = 232 |
||
| Continued (AC) | 182 | 43.3 (6.5) | 47 | 67.5 (23.1)a n = 135 |
1153 (615)c n = 170 |
440 (169) n = 180 |
1 (1–2) | |
| Discontinued (AD) | 21 | 41.2 (8.1) | 62 | 72.4 (30.5)a,b n = 18 |
1145 (604)c n = 18 |
445 (152) | 1 (1–2) | 196d (49–910) |
| Polyuria | 17 | 41.2 (7.8) | 59 | 78.8 (27.2) n = 15 |
981 (474) n = 14 |
443 (148) | ||
| All other AAEs | 4 | 41.0 (10.5) | 75 | 40.4 (29.5) n = 3 |
1719 (729) n = 4 |
453 (191) | ||
| Discontinued Other AE (NAD) | 31 | 44.3 (4.5) | 48 | 51.9 (29.3)b n = 25 |
1499 (910) n = 27 |
425 (132) | 1 (1–2) | 658d (216–1218) |
AAE, aquaretic adverse event, AC, aquaretic-continued; AD, aquaretic-discontinued; eGFR, estimated glomerular filtration rate, calculated by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation; htTKV, height-adjusted total kidney volume; IQR, interquartile range; NAD, non–aquaretic-discontinued; TEMPO, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes; uOsm, urine osmolality.
Data are presented on subjects who were on placebo in TEMPO 3:4 and switched to tolvaptan in TEMPO 4:4.
Subjects who discontinued for other reasons include the following: other AE (n = 17, 55%), completed (n = 11, 36%), investigator withdrew (n = 1, 3%), subject withdrew consent (n = 2, 6%).
P = 0.003.
P = 0.048.
P = 0.02.
P = 0.02.
As was the case in TEMPO 3:4, AD subjects in TEMPO 4:4 were more likely to discontinue because of polyuria than all other AAEs combined (n = 17 vs. n = 4). Subjects who discontinued because of polyuria had lower baseline htTKV and higher baseline renal function than those who discontinued because of other AAEs (Table 2).
Aquaretic Adverse Events in Tolvaptan-Treated Subjects in TEMPO 3:4: Dose, Timing, and Urine Osmolality Relationships
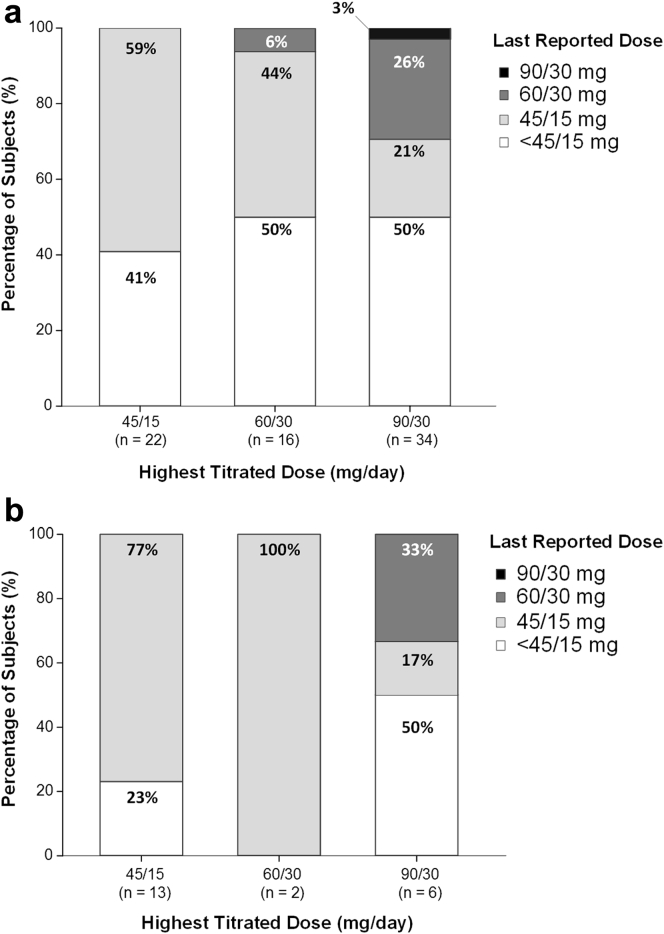
Among the 72 patients comprising the AD population of TEMPO 3:4, 22 (31%), 16 (22%), and 34 (47%) had titrated to a maximum split-daily dosage of 45/15 mg, 60/30 mg, and 90/30 mg, respectively. Analogous numbers for TEMPO 4:4 were 13 (62%), 2 (10%), and 6 (28%), respectively (Figure 4). Down-titration of tolvaptan was permitted in both trials at any time based on tolerability. Before discontinuation, nearly all subjects in the AD populations of both trials had down-titrated to 45/15 mg or had stopped taking study drug before formally withdrawing from the trial.
Figure 4.
Dose of tolvaptan at discontinuation relative to the highest titrated dose in aquaretic-discontinued (AD) subjects. Subjects were titrated to the highest tolerated dose of 45/15, 60/30, or 90/30 mg/d and were permitted to down-titrate as needed during the trial. The highest titrated dose is plotted on the x-axis, and the dose at the time of discontinuation (90/30, 60/30, 45/15, < 45/15 mg/d) is plotted as a box plot based on percentage of patients. (a) Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) 3:4. (b) TEMPO 4:4.
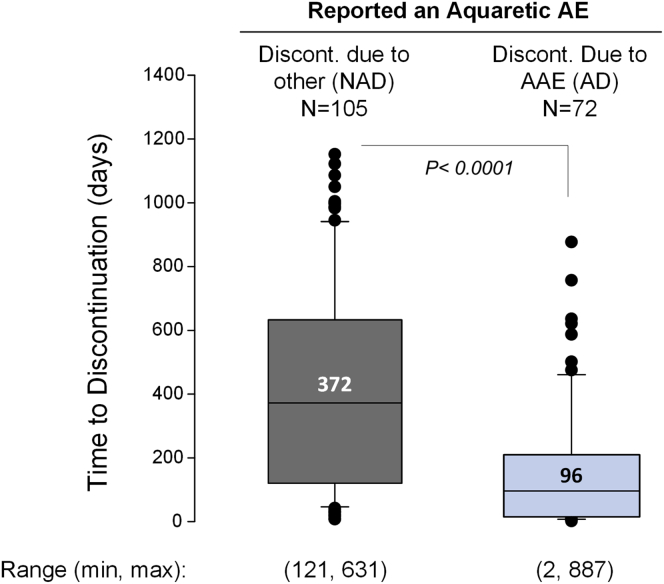
Both AD and AC subjects in TEMPO 3:4 reported AAEs relatively soon after entering the treatment phase, that is, a median of 2 days after the first dose of tolvaptan in both subgroups (Table 1). The median (IQR) time to discontinuation for AD subjects was 96 (15–209) days, which was significantly shorter than the 372 (121–631) days observed among NAD subjects (P < 0.0001) (Figure 5).
Figure 5.
Time to discontinuation due to aquaretic and non-aquaretic adverse events. Box plot of median and interquartile range of time to discontinuation in the aquaretic-discontinued (AD) and non–aquaretic-discontinued (NAD) groups. Data points above and below the 75th and 25th quartiles are depicted in black circles. The overall ranges were 2 to 877 days in the AD group and 8 to 1050 days in the NAD group. Discont., discontinued; max, maximum; min, minimum; TEMPO 3:4, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes.
Tolerance of Tolvaptan Relative to Placebo in TEMPO 3:4
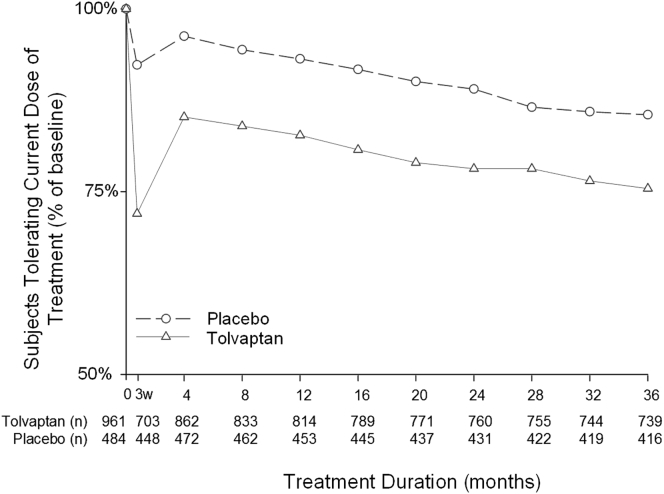
At each visit, subjects were asked if they could tolerate their current dose of medication for the rest of their life. A relatively rapid increase in the percentage of subjects answering “no” to this question occurred during the initial titration period (Figure 6), which resulted in down titration or trial discontinuation. The majority of early discontinuations by week 3 were due to AAEs (30/47 [64%]) (Table 3). The tolerability of tolvaptan appeared to stabilize by the month 4 visit. At the end of the trial (3 years), 75.4% of subjects who were still receiving tolvaptan indicated that they could tolerate their current dose of medication for the rest of their lives, compared with 85.5% of placebo patients. Analysis of the dose regimen at the end of TEMPO 3:4 and 4:4 (Table 4) showed that patients who did not report an AAE were better able to maintain the target dose of 90/30mg than those who reported an AAE in both trials (TEMPO 3:4: 130 of 167 vs. 271 of 573, P < 0.0001; TEMPO 4:4: 46 of 74 vs. 77 of 182, P = 0.0056, respectively).
Figure 6.
Percentage of subjects tolerating their current dose of study drug during the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) 3:4 trial. 3w, 3 weeks (refers to the time point taken at the end of the titration period).
Table 3.
Reasons for early discontinuation during dose titration period (up to week 3) in TEMPO 3:4
| Reason for discontinuation (n = 47) | n (%) |
|---|---|
| Reported an AAE | 41 (87) |
| Discontinued (AD) | 30 (64) |
| Discontinued for other reason (NAD) | 11 (23) |
| Discontinued without reporting AAE | 1 (2) |
| Subject withdrew consent | 4 (9) |
| Protocol deviation | 1 (2) |
AAE, aquaretic adverse event; AD, aquaretic-discontinued; NAD, non–aquaretic-discontinued; TEMPO, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes.
Table 4.
Proportion of patients on various treatment regimens at the end of the TEMPO 3:4 and 4:4 trials: Influence of aquaretic adverse events
| TEMPO 3:4 | n (%) |
|---|---|
| No AAE | 167 (100) |
| 45/15 mg | 19 (11) |
| 60/30 mg | 18 (11) |
| 90/30 mg | 130 (78) |
| AC | 573 (100) |
| 45/15 mg | 157 (27) |
| 60/30 mg | 145 (25) |
| 90/30 mg | 271 (47) |
| TEMPO 4:4 | n (%) |
|---|---|
| No AAE | 74 (100) |
| 45/15 mg | 16 (22) |
| 60/30 mg | 12 (16) |
| 90/30 mg | 46 (62) |
| AC | 182 (100) |
| 45/15 mg | 71 (39) |
| 60/30 mg | 34 (19) |
| 90/30 mg | 77 (42) |
AAE, aquaretic adverse event; AC, aquaretic adverse event continued; No AAE, no aquaretic adverse event; TEMPO, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes.
Proportion of patients maintaining the target dose of 90/30 mg, No AAE versus AC: TEMPO 3:4, 130 of 167 versus 271 of 573, P < 0.0001; TEMPO 4:4, 46 of 74 versus 77 of 182, P = 0.0056, respectively).
Discussion
The most common side effects associated with tolvaptan use in TEMPO 3:4 were related to aquaresis. Thirst (55.3% of tolvaptan-treated subjects vs. 20.5% of placebo-treated subjects; P < 0.001), polyuria (38.3% vs. 17.2%; P < 0.001), and nocturia (29.1% vs. 13.0%; P < 0.001) were the most commonly reported adverse events, whereas pollakiuria (23.2% vs. 5.4%; P < 0.001) and polydipsia (10.4% vs. 3.5%; P < 0.001) were the fifth and tenth most commonly reported adverse events, respectively. These occurred soon after study drug initiation (median, 2 days) and were ranked on subject self-reports as most intolerable during the initial 3-week titration period. Once adjusted to the aquaretic side effects, overall tolerability to ongoing therapy was very favorable in the ADPKD trial population. In fact, 75% of subjects who were still receiving tolvaptan at 3 years indicated that they could tolerate their current dose of medication for the rest of their lives, compared with 85% of placebo patients. Accordingly, only 10% of subjects withdrew because of an AAE over the course of the trial. The tolvaptan-treated subjects who did withdraw because of an AAE were significantly younger, had better baseline renal function, and had higher fasting baseline urine osmolality values than subjects with AAEs who remained in the trial or subjects who discontinued for a non–aquaretic-related AE. These results are consistent with the recent observation that the initial aquaretic response to tolvaptan correlates positively with baseline urine osmolality and baseline eGFR.16 In other words, patients with less advanced disease are more responsive to the pharmacologic action of tolvaptan, and thus experience more pronounced aquaresis.16
Aquaretic-related discontinuations in tolvaptan-naive subjects from TEMPO 3:4 who crossed over to tolvaptan treatment in the open-label extension trial TEMPO 4:4 were largely consistent with the findings from TEMPO 3:4, despite the smaller cohort of subjects (n = 961 vs. n = 314, tolvaptan [TEMPO 3:4] vs. prior placebo [TEMPO 4:4]). In general, the safety profile for tolvaptan in TEMPO 4:4 was similar to that in TEMPO 3:4. Most adverse events were related to the aquaretic effect of tolvaptan. These AAEs were more frequently reported in the delayed-treatment group who had not been previously exposed to tolvaptan. The higher proportion of subjects in TEMPO 4:4 who did not titrate past the starting dose of 45/15 mg could be attributed to a more relaxed outlook on pushing titration to the maximum tolerated dose, as this was an open-label extension and not a pivotal Phase 3 clinical trial.17
Although most tolvaptan-naive subjects from TEMPO 3:4 and TEMPO 4:4 attempted to down-titrate before discontinuation, a large cohort of subjects never titrated past the starting dose of 45/15 mg (TEMPO 3:4, n = 22 [31%]; TEMPO 4:4, n = 13 [62%]). This might indicate that they experienced a higher amount of aquaresis than other subjects. In earlier studies on the short-term effects of tolvaptan, higher 24-hour urine volume excretion was experienced by subjects with higher baseline renal function.18, 19, 20 As discussed above, higher response to tolvaptan in TEMPO3:4 was also observed in patients with better preserved renal function.16 In addition, subjects in earlier stages of disease may be more sensitive to the increase in urine output. Given that urine concentrating ability decreases with disease progression, subjects in later stages of disease may be more accustomed to managing a higher urine volume and, consequently, may perceive the polyuria and nocturia associated with increased aquaresis as being less burdensome.18, 19, 20
From a mechanistic perspective, elevated cAMP levels in renal tubule epithelial cells play a key role in ADPKD pathogenesis.21 Achieving maximal antagonism of cAMP via V2-receptor inhibition, that is, titrating tolvaptan to its highest available dose, is thus expected to have maximal clinical benefit. This therapeutic goal, however, must be balanced by the recognition that excessive aquaresis can be an important factor limiting compliance; thus, 750 of 961 tolvaptan-treated subjects in TEMPO 3:4 (78%) reported at least 1 AAE, of whom 72 (10%) discontinued from the trial because of an aquaretic-related event. The current subanalysis identified 2 important risk factors for tolvaptan discontinuation in ADPKD patients. First, subjects with better renal function, as measured by higher eGFR, and subjects with a greater ability to concentrate their urine were more likely to discontinue tolvaptan. Second, sensitivity to tolvaptan-mediated AAEs appeared most pronounced early after drug initiation, as observed by the significant difference in time to discontinuation between AD and NAD groups (96 vs. 372 days, P < 0.0001, respectively) and those who discontinued for other AEs (96 vs. 288 days, P < 0.0001). This finding is supported by the early-treatment group in TEMPO 4:4, who had completed 3 years of tolvaptan treatment in TEMPO 3:4. Although they continued to report a high frequency of AAEs (350 of 557 [63%]), very few discontinued because of an AAE (3 of 350 [1%]). This was further validated in the recent Replicating Evidence of Preserved Renal Function: An Investigation of Tolvaptan Safety and Efficacy in ADPKD (REPRISE) trial, which used a tolvaptan titration (2 weeks) and run-in period (3 weeks) to enrich the trial for subjects who could tolerate tolvaptan treatment, to minimize the number of early aquaretic-related discontinuations.22 Of the 1491 subjects who entered the tolvaptan titration period, adverse events led to the discontinuation of treatment in 6.8% of subjects, most of them (4.6%) aquaretic-related, consistent with the findings in this report. Practically, this argues that clinicians should take particular care when administering tolvaptan to ADPKD patients who are in early stages of their disease, in particular starting at a low dose (45/15 mg) and delaying subsequent titration until tolerability has been established.
Given that enrollment in the open-label extension trial TEMPO 4:4 occurred continuously upon completion of TEMPO 3:4, the vast majority of subjects were unaware of the positive efficacy data from the pivotal TEMPO 3:4 trial, as the data were not unblinded or published. Indeed, 9% of prior placebo subjects discontinued the TEMPO 4:4 trial because of AAEs associated with tolvaptan treatment, similar to the 10% AAE discontinuations reported in TEMPO 3:4. An intriguing issue is whether tolerability in real life could be higher than in the setting of the trial. Clinical experience suggests that patients newly exposed to tolvaptan may be more tolerant to polyuria, as many were already exposed to high water intake. More generally, patients may be more inclined to accept adverse events resulting from the mode of action of a given drug, once that drug has been approved. The fact that post hoc analyses from the TEMPO 4:4 trial showed positive outcomes for the subgroup of rapid progressors, that is, those patients eligible for tolvaptan prescription, may also be a motivating factor.17
The aim of dose titration is to block activity of vasopressin at the renal V2 receptor as completely and constantly as possible, while maintaining acceptable fluid balance. The adequacy of vasopressin suppression can be monitored through measurement of fasting trough urine osmolality, which could prove to be an effective strategy to optimize dose titration and tolerability to treatment.16 In a recent post hoc analysis of TEMPO 3:4, subjects with the greatest mean change from baseline in urine osmolality were more likely to achieve a better clinical response as measured by slowed renal function decline.16 A target decrease of ≥300 mOsm/kg from baseline may be considered ideal in most cases, although a decrease of ≥200 mOsm/kg from baseline may be appropriate in patients who are relatively early in their disease course and are likely to be more sensitive to the aquaretic side effects of tolvaptan. If possible, an absolute urine osmolality <300 mOsm/kg should be maintained at all times. Thus, measuring changes in urine osmolality over time could help guide dosing and titration decisions.
In conclusion, to support long-term compliance, tolvaptan dosing and titration decisions in ADPKD patients with high baseline renal function and high fasting baseline urine osmolality should be approached conservatively and in partnership with the patient before initiating therapy.
Disclosure
OD and ABC are members of the steering committee of the TEMPO 3:4 trial and have received research funding from Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, NJ). ABC has received consultancy fees from Otsuka Pharmaceutical Development & Commercialization, Inc. JDB, SES, and FSC are employees of Otsuka Pharmaceutical Development & Commercialization, Inc.
Acknowledgments
This trial was funded by Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, New Jersey, USA. David Norris, PhD (Ecosse Medical Communications, Falmouth, Massachusetts, USA) assisted in the medical writing of this manuscript.
References
- 1.Grantham J.J. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–1485. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 2.Torres V.E., Harris P.C., Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 3.Grantham J.J., Mulamalla S., Swenson-Fields K.I. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7:556–566. doi: 10.1038/nrneph.2011.109. [DOI] [PubMed] [Google Scholar]
- 4.Ong A.C., Devuyst O., Knebelmann B. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385:1993–2002. doi: 10.1016/S0140-6736(15)60907-2. [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System: 2014 annual data report: an overview of the epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. [Google Scholar]
- 6.U.S. Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. USRDS 2013 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. [Google Scholar]
- 7.Spithoven E.M., Kramer A., Meijer E. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival—an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014;29(suppl 4):iv15–iv25. doi: 10.1093/ndt/gfu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres V.E., Meijer E., Bae K.T. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3-4 Study. Am J Kidney Dis. 2011;57:692–699. doi: 10.1053/j.ajkd.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Samsca [Package insert] L Otsuka America Pharmaceuticals, Inc.; Rockville, MD: 2014. [Google Scholar]
- 11.Gattone V.H., 2nd, Wang X., Harris P.C. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 12.Hanaoka K., Guggino W.B. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11:1179–1187. doi: 10.1681/ASN.V1171179. [DOI] [PubMed] [Google Scholar]
- 13.Torres V.E., Wang X., Qian Q. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T., Nagao S., Kasahara M. Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am J Kidney Dis. 1997;30:703–709. doi: 10.1016/s0272-6386(97)90496-0. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi T., Nagao S., Wallace D.P. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 16.Devuyst O., Chapman A.B., Gansevoort R.T. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres V.E., Chapman A.B., Devuyst O. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 Trial. Nephrol Dial Transplant. 2017 doi: 10.1093/ndt/gfx079. [DOI] [PubMed] [Google Scholar]
- 18.Boertien W.E., Meijer E., de Jong P.E. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 2013;84:1278–1286. doi: 10.1038/ki.2013.285. [DOI] [PubMed] [Google Scholar]
- 19.Boertien W.E., Meijer E., de Jong P.E. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis. 2015;65:833–841. doi: 10.1053/j.ajkd.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Shoaf S.E., Bricmont P., Mallikaarjun S. Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int. 2014;85:953–961. doi: 10.1038/ki.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres V.E., Harris P.C. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol. 2014;25:18–32. doi: 10.1681/ASN.2013040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres V.E., Devuyst O., Chapman A.B. Rationale and design of a clinical trial investigating tolvaptan safety and efficacy in autosomal dominant polycystic kidney disease. Am J Nephrol. 2017;45:257–266. doi: 10.1159/000456087. [DOI] [PMC free article] [PubMed] [Google Scholar]