Abstract
Candida albicans is a human fungal pathogen that causes millions of mucosal and life-threatening infections annually. C. albicans initially interacts with epithelial cells, resulting in fungal recognition and the formation of hyphae. Hypha formation is critical for host cell damage and immune activation, which are both driven by the secretion of Candidalysin, a recently discovered peptide toxin. Epithelial activation leads to the production of inflammatory mediators that recruit innate immune cells including neutrophils, macrophages and innate Type 17 cells, which together work with epithelial cells to clear the fungal infection. This review will focus on the recent discoveries that have advanced our understanding of C. albicans-epithelial interactions and the induction of mucosal innate immunity.
Keywords: Candida, Candidalysin, pathogenicity, virulence, hyphae, epithelium, mucosal, adhesion, invasion, damage, innate immunity, neutrophil, macrophage, dendritic cell, Type 17 immunity, IL-17
Introduction
Candida albicans is normally a harmless commensal organism within the normal microbiota in approximately half the world’s population. In the commensal phase, C. albicans most likely resides in the mucus layer of mucosal surfaces. However, occasionally and under certain circumstances, C. albicans may encounter host cells directly, which may result in the attachment of the fungus to epithelial cells. Depending on the strain of C. albicans and the physiological and immune status of the host, this interaction event can lead to superficial overgrowth and epithelial invasion, followed by disease and immune activation. C. albicans is the most prevalent Candida species causing infections in humans and is the causative agent of oral and vaginal candidiasis (e.g. thrush), giving rise to severe morbidity in millions of individuals worldwide. Given that potentially fatal systemic infections can arise from breaches of the mucosal barrier (predominantly from the gut) it is of paramount importance to understand how C. albicans interacts with cells of the innate immune system and how this fungus is restricted to the mucosal surface in health. Critical to this is an understanding of how epithelial cells are able to discriminate between harmless (commensal) and dangerous (pathogenic) C. albicans cells, which determines whether a mutually beneficial commensal relationship or immune activation takes place.
C. albicans interaction with epithelial cells: adhesion and invasion
Epithelial cells at mucosal surfaces are the first point of contact with C. albicans and constitute the first line of defence. Although fungal pathogenicity depends on the type of mucosal tissue, there are common virulence mechanisms and principles. C. albicans adhesion to epithelial cells is mediated through the interaction of fungal cell wall moieties and surface proteins with host receptors (Table 1). C. albicans yeast cells are recognized by oral epithelial cells (in the TR146 cell line) and induce three signalling pathways within 15 min; the nuclear factor-kappaB (NF-κB) pathway, the phosphatidylinositol-4,5-bisphosphate 3-kinase (Pi3K), and all three mitogen-activated protein kinase (MAPK) pathways (p38, JNK (c-Jun N-terminal kinase) and ERK1/2 (extracellular signal-regulated protein kinase)). This results in the activation of the p65/p50 transcription factor via NF-κB, the c-Jun transcription factor via JNK and ERK1/2, and AKT (protein kinase B) and mTor (mammalian target of rapamycin) via Pi3K signaling [1,2]. Initial binding may constitute recognition of fungal cell wall mannans and β-glucans but this does not fully activate epithelial cells as proinflammatory cytokines were not induced [2]. Lack of activation by C. albicans cell wall polysaccharides was also found in skin keratinocytes [3], suggesting that fungal polysaccharides play a limited role in inducing epithelial/keratinocyte immune responses. While many other yeast-associated secreted/cell-surface proteins (e.g. Sap1-3/9/10, Als1/3/4/9, Mp65, Phr1, Iff4, Sun41, Pra1, Eap1, Utr2 and Ecm33), cell wall processing proteins (e.g. Big1, Mnt1/2, Mnn9), and protein trafficking/vesicle transport proteins (e.g. Vps11) are thought to promote epithelial adhesion, this is likely to be via indirect mechanisms given that these proteins possess complex, multi-factorial functions that contribute to cell wall integrity and hypha formation [4–7].
Table 1.
C. albicans genes involved during interactions with epithelial cells
| Fungal component/gene | Epithelial function or target receptors | Reference |
|---|---|---|
| Structural polysaccharides | ||
| β-glucan | Induces epithelial signalling. Recognised by Epha2. | [2]. M Swidergall et al (abstract) |
| Mannans | Induces epithelial signalling. Receptors not identified. | [2] |
| Chitin | Induces epithelial signalling. Receptors not identified. | [2] |
| Adhesins | ||
| HWP1 | Adhesion to epithelial cells via transglutaminase activity. Specific host receptors unknown. | [8] |
| ALS1-9 | Adhesin family. Structural studies indicate this family has multiple epithelial targets. | [14,79–82] |
| INT1 | Interaction with epithelial integrins. | [83] |
| Toxins | ||
| ECE1 | Parent protein of Candidalysin. Induces c-Fos and MKP1 signalling. Receptor activation indicated but not identified. | [22••] |
| Endocytosis | ||
| ALS3 | Activation of or interaction with E-cadherin, EGFR/Her2, AhR, NEDD9 and PDGF BB | [13,15,16,18•,20•] |
| SSA1 | HSP70 family member. Activation of or interaction with EGFR/Her2 | [15] |
| Active Penetration/hydrolysis | ||
| SAP1-8 | Secreted aspartic proteases – digestion of epithelial tissues. Sap5 degrades E-cadherin | [84,85] |
| PLB1 | Phospholipase B1 – digestion of epithelial tissues | [86] |
| LIP1-10 | Lipase family – digestion of epithelial tissues | [87] |
Adhesion of C. albicans to an epithelial cell is a strong inducer of hypha formation. The formation of hyphae occurs within 30 – 60 min and this is accompanied by the expression of hypha-associated proteins, which are known to possess critical roles in adhesion, invasion, damage induction and immune activation/evasion. The two key hyphal proteins that promote epithelial adhesion are Hwp1 (hyphal wall protein 1) [8] and Als3 (agglutinin-like sequence 3) [9,10]. Hwp1 is highly expressed in human oral infections [11] and acts as a substrate for epithelial transglutaminases, enabling strong covalent links with other epithelial proteins [12]. Als3 is both an adhesin and an invasin, and together with Ssa1 (heat shock protein) promotes the endocytosis of C. albicans into epithelial cells via E-cadherin [13–15] and the EGFR/Her2 (epidermal growth factor receptor/human epidermal growth factor 2) complex [16]. Endocytosis is an entirely host driven process and does not require viable hyphae [17]. Other pathways that promote C. albicans endocytosis include the PDGF BB (platelet-derived growth factor BB) and NEDD9 (neural precursor-cell-expressed developmentally downregulated protein 9) pathways, which both require hypha formation and Als3 expression [18•]. However, despite possessing adhesion/invasin activities, Als3 does not directly induce epithelial cell damage or cytokine production [19]. The AhR (aryl hydrocarbon receptor) also contributes to the endocytosis of C. albicans via Src family kinase phosphorylation of EGFR, but AhR is not involved in epithelial damage or cytokine induction by C. albicans and it is unknown how AhR is activated [20•]. Currently, the level of redundancy between these different pathways (E-cadherin, EGFR/Her2, AhR, PDGF BB and NEDD9) and how they communicate to promote C. albicans endocytosis is unclear. It is important to note that induced endocytosis is not the only invasion route of C. albicans. Indeed, active penetration, which does not require host activities, seems to be the dominant invasion route depending on the type of epithelial cell [21].
Epithelial damage and immune activation by Candidalysin
While C. albicans adhesion and invasion leads to fungal recognition and signal pathway activation, surprisingly this does not translate into epithelial damage or innate immune activation [2,17]. Recently, it was discovered that C. albicans hyphae induce both epithelial damage and innate immunity through the secretion of a cytolytic peptide toxin called Candidalysin, which is encoded by the hypha-associated ECE1 gene [22••]. Candidalysin is an amphipathic peptide that adopts an α-helical structure and is the first peptide toxin to be identified in any human fungal pathogen. In oral epithelial cells, Candidalysin induces calcium ion influx and lactate dehydrogenase (LDH) release, which are characteristics of cell damage and membrane destabilization (Figure 1). Notably, C. albicans mutants where the entire ECE1 gene or the Candidalysin-encoding region has been deleted, have full invasive potential in vitro but are incapable of inducing tissue damage or cytokine release, and are highly attenuated in a murine model of oropharyngeal candidiasis and a zebrafish swimbladder mucosal model [22••].
Figure 1.
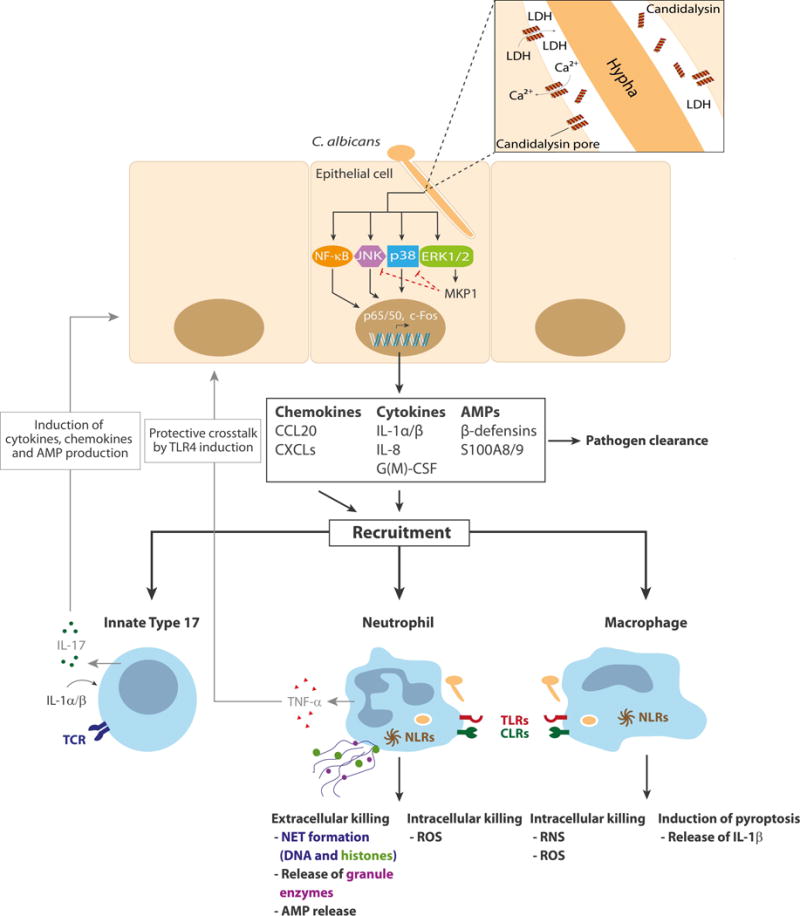
Innate immunity against C. albicans at the oral mucosa. C. albicans hypha formation induces the formation of an invasion pocket and the production of Candidalysin (helical peptide; top right panel). At high concentrations (>15 µM) Candidalysin forms pores that result in membrane damage (LDH release), calcium influx, and the activation of the epithelial cell, predominantly via the MAPK signalling pathways and the transcription factor c-Fos. MKP1 activation (via ERK1/2) contributes to the regulation of the epithelial immune response. Epithelial activation leads to chemokine, cytokine and antimicrobial peptide (AMP) release and the subsequent recruitment of innate immune cells, including macrophages, neutrophils and TCRβ+ type 17 cells. Macrophages and neutrophils recognise and phagocytose the fungus through traditional pattern recognition receptors such as TLRs and CLRs. This results in death of the fungus via oxidative or nitrosative (ROS/RNS) killing or the induction of pyroptosis in macrophages (and the release of IL-1β) or NET formation in neutrophils. Neutrophils also release TNFα, which induces the upregulation of TLR4 in epithelial cells. IL-1α/β released by epithelial cells, macrophages and potentially other cell sources activate TCRβ+ type 17 cells, which in turn release IL-17 that subsequently induces the release of additional chemokines, cytokines and antimicrobial peptides (AMP) from epithelial cells, further promoting fungal clearance and barrier function.
Candidalysin induces epithelial immunity predominantly via MAPK signalling, specifically (i) the p38 pathway, resulting in the activation of the AP-1 transcription factor c-Fos, and (ii) the ERK1/2 pathway, resulting in the activation of MKP1 (MAPK phosphatase 1) that regulates immune responses [22••]. Together, these pathways lead to the production of pro-inflammatory cytokines including IL-1α/β, IL-6, GM-CSF and G-CSF. Importantly, p38/c-Fos and MKP1 is also activated in human vaginal epithelial cells [23] and by other hypha-forming Candida species [24]. Therefore, these signalling pathways may enable different mucosal tissues to detect fungal hyphae, thereby potentially identifying when certain Candida species have become pathogenic. Notably, epithelial activation by Candidalysin is not mediated via C-type lectin receptors (CLRs) or Toll-like receptors (TLRs) [2], suggesting that epithelial cells utilise different sensing mechanisms than myeloid cells; whereby myeloid cells respond to C. albicans cell wall moieties (β-glucan and mannans) (see below) and epithelial cells respond to damage-inducing C. albicans through p38/c-Fos/MKP1 by detecting Candidalysin activity [25–27]. Similar p38 activation has been observed in murine intestinal epithelial cells with bacterial pathogens (Citrobacter rodentium) [28] and in C. elegans (nematode worm) with C. albicans [29], indicating that p38 signalling may be a common epithelial mechanism for the detection of pathogenic microbes.
Innate immunity at mucosal surfaces: neutrophils and macrophages
C. albicans, predominantly through Candidalysin activity, induces proinflammatory cytokines, chemokines and antimicrobial peptides (e.g. IL-1α, IL-1β, IL-8, G-CSF, GM-CSF, β-defensin 3, CCL20 and S100A8/9) from epithelial cells that are required for immune cell recruitment [2,22••,30]. The key myeloid cells that are initially recruited to the site of infection include neutrophils and macrophages (Figure 1). These immune cells recognize C. albicans cell wall mannans and DNA via TLR2, 4 and 9, and fungal β-glucan via CLRs including Dectin-1/−2, DC-SIGN or Mincle [31–33]. Activation of TLRs and CLRs leads to the induction of NF-κB, MAPK and Syk signaling and the production of pro-inflammatory cytokines and further downstream immune effector functions. Nod-like receptors (NLRs) can also be activated by danger signals or internalized fungal compounds and this leads to inflammasome activation and the secretion of IL-1β and IL-18, which help protect against superficial and disseminated C. albicans infection [34].
Upon C. albicans infection, neutrophils are rapidly recruited to the site of entry. Even without physical contact to invading hyphae, neutrophils respond to epithelial derived chemokines and growth factors and release TNFα, which in turn triggers a protective effect in epithelial cells via the upregulation of TLR4 [35]. Neutrophils also inhibit hyphal formation without direct contact [36,37]. Neutrophils can also be recruited by responding directly to C. albicans-derived factors such as the secreted aspartic proteases (Saps) [38•]. Furthermore, neutrophils phagocytose (e.g. via CLRs) and kill C. albicans yeast cells and short hyphae intracellularly predominantly via oxidative burst mechanisms. C. albicans hyphae that are too large to be phagocytosed are either growth-inhibited or killed extracellularly through the formation of neutrophil extracellular traps (NETs or NETosis), via the release of granule enzymes and through secretion of antimicrobial peptides such as calprotectin [39,40,41•]. Indeed, the zinc binding properties of calprotectin inhibits C. albicans growth during NET formation [40]. C. albicans hyphae trigger NETosis more effectively and rapidly than yeast cells, but both morphologies can induce NETs via autophagy and oxidative mechanisms [42•]. Reactive oxygen species [43], fibronectin [44] and Dectin-1 signaling [45] have also been implicated in NET formation. However, the role of Dectin-1 is controversial as other studies indicate that NET release by β-glucan is mediated via complement receptor 3 (CD11b/CD18) and not Dectin-1 [44].
Macrophages are also recruited to the site of infection and ingest non-opsonized C. albicans after recognition by TLRs and CLRs [47]. While macrophages phagocytose and kill C. albicans intracellularly in the phagolysosome through oxidative and nitrosative mechanisms, their activity and efficiency of killing is lower than that of neutrophils. Thus, C. albicans is readily able to survive within and escape from macrophages in vitro [48] and macrophages play a more minor role in vivo during murine disseminated infections [32,34]. Macrophages also recognize C. albicans via intracellular NLRs, which activates the NLRP3 inflammasome, leading to production of pro-inflammatory IL-1β and IL-18 as well as pyroptotic host cell death [49,50]. Although immune cell death was originally thought to be hypha-dependent, hypha-independent triggers of pyroptosis have also been described [51,52•]. Therefore, filamentation alone may not be sufficient to trigger NLRP3 inflammasome-mediated pyroptosis [52•,53]. Intracellular hypha formation is driven by active alkalinization of the phagosome [54] and causes macrophage cell death by at least two different mechanisms: pyroptosis and physical piercing of the macrophage membrane [49,50,54]. Notably, NLRP3 inflammasome activation is not necessarily coupled with pyroptosis and the fungal trigger that activates the inflammasome still remains unknown. Finally, while inflammasome activation can lead to IL-1β and IL-18 production, IL-1β has been implicated with Th17 responses whereas IL-18 appears to promote Th1 activity [55].
Innate immunity at mucosal surfaces: innate Type 17 cells
A key insight into requirements for host defense against mucosal candidiasis came from the recognition that mice lacking the IL-17 receptor or its key downstream signaling adaptor Act1 are highly susceptible to oropharyngeal candidiasis (OPC) [30,56,57]. Even more strikingly, when humans were subsequently identified with loss-of-function mutations in the same genes, their dominant disease susceptibility was chronic mucocutaneous candidiasis (CMC) [58,59,60••]. IL-17 is the eponymous cytokine of the Th17 lineage, and a common misconception is that this cytokine functions mainly in the adaptive immune response. However, a variety of innate cells of lymphoid origin produce IL-17, including γδ-T, natural killer T (NKT), innate lymphoid cell type 3 (ILC3) and TCRβ+ ‘natural’ Th17 cells (nTh17) [61]. In the context of OPC, IL-17 is produced mainly by γδ-T and nTh17 cells, and mice lacking a TCR (e.g., Rag1−/− or IL-7Rα−/− mice) are highly susceptible to infection [62]. Although ILC3s have also been reported in this context [63], Rag1−/− mice have ILC3 cells but still show the same high susceptibility to OPC as IL-17R-deficient mice [62]. The role of neutrophils in producing IL-17 is controversial, but data in the murine OPC model argues against neutrophils as a source of this cytokine [64].
Surprisingly, activation of innate Type 17 cells appears to be quite distinct from activation of adaptive Type 17 immunity. A Dectin-1-Syk-CARD9 pathway was shown to be important for activating immunity to systemic candidiasis [65]. Consistently, CARD9 is essential for the adaptive Th17 recall response in oral candidiasis. However, this adaptor was largely dispensable for induction of the acute innate IL-17 response [66], a finding that was also recently verified for Dectin-1 and TLR2 (A Verma et al., unpublished). This new study finds that Candidalysin production by C. albicans hyphae is the triggering factor for innate IL-17 production in the murine model of OPC (Figure 1). Mice infected with ECE1-deficient strains show only minimal induction of IL-17 or activation of nTh17 cell production of this cytokine. Additionally, IL-1R signaling is required for activation of the innate Type 17 response, with contributions from both hematopoietic and non-hematopoietic compartments (A Verma et al., unpublished). A vital role for IL-1 in defense against OPC was shown previously in studies of the inflammasome in mouse OPC [67] and was recently verified in contributing to neutrophil activation in this setting [68]. Collectively, these data indicate that the early, innate response to C. albicans in the oral mucosa depends on sensing of tissue damage through Candidalysin, and not simply the presence of β-glucan components revealed upon fungal filamentation.
Although the IL-17 receptor is expressed ubiquitously, we found that the essential responder cell in the context of oral candidiasis is the superficial oral epithelial cell [69•]. Mice with a conditional deletion of the IL-17 receptor in Keratin 13+ cells (including oral and buccal epithelial cells, but not skin, gut or other tissues) show a similar fungal susceptibility as mice with a full knockout of this receptor. Moreover, gene pathway signatures induced in the oral mucosa during acute infection were highly conserved with genes induced by C. albicans and IL-17 in human oral keratinocytes [69].
The anti-fungal functions of IL-17 are multi-fold. First, IL-17 is a potent activator of the neutrophil response, which it triggers by inducing expression of neutrophil-recruiting chemokines and cytokines such as G-CSF, CXCL1/2 and 5 in oral tissue [30]. It should be noted that the extent to which IL-17 drives neutrophil signals may be variable [30,64,70]. Second, IL-17 potently induces anti-microbial peptides (AMPs), particularly β-defensins-1 and −3. Mice lacking these defensins show markedly increased susceptibility to OPC [30,69•,71]. IL-17 may also act on salivary gland cells, contributing to the production of antifungal AMPs such as histatins [72,73]. The combined action of IL-17 signaling promotes effective, non-redundant host defense to mucosal candidiasis.
As noted above, several human kindreds were identified with inherited mutations in the IL-17 receptor signaling pathway that cause CMC [58–60]. Additionally, other gene defects that predispose to CMC are associated with defective IL-17 production or function, including mutations in STAT3 (Hyper-IgE Syndrome, HIES), STAT1 and AIRE (APECED) [74]. In the latter case, neutralizing antibodies against Type 17 cytokines are found in affected patients, raising the possibility that disease is associated with reduced IL-17 signaling. Of course, in all these cases, IL-17 is likely produced by both innate cells and conventional (adaptive) Th17 cells. In this regard, HIV patients with low CD4 T cells counts are highly prone to OPC, and this has been particularly associated with Th17 loss [75]. Finally, in 2016 the first biologic drugs (Secukinumab, Ixekizumab) targeting IL-17 (specifically, IL-17A and the IL-17A/F heterodimer) directly came to the market to treat psoriasis, a strongly IL-17-driven autoimmune disease [76]. Surprisingly, the incidence of OPC is quite low, in the range of 4–8% of patients [77]. This may simply mean that blockade is incomplete, due either to dose effects or access of anti-IL-17 antibodies to the oral mucosal tissue. Alternatively, these biologics spare IL-17F, which has been shown to cooperate with IL-17A in promoting resistance to OPC [63,78]. Cumulatively, these findings all support a central role for IL-17 receptor signal transduction in mucosal host defense, at both innate and adaptive levels.
Conclusion
The epithelial cell plays a fundamental role in the host response to C. albicans (Figure 1). Both C. albicans yeast and hyphae are recognized, but only hyphae are able to invade epithelial cells by induced endocytosis and/or active penetration, causing activation of epithelial cells. Endocytosis of C. albicans is mediated via multiple epithelial receptors and the fungal invasin Als3, but epithelial cells are predominantly activated by the hypha-associated peptide toxin Candidalysin. Candidalysin damages epithelial membranes and activates danger response pathways mediated via p38/cFos and ERK/MKP1, which results in immune activation and the secretion of cytokines and chemokines. These effector molecules recruit innate immune cells such as neutrophils, macrophages and innate Type 17 cells. Neutrophils (and macrophages) directly kill or restrict the fungus through phagocytosis mechanisms and/or NET formation, and innate Type 17 cells secrete IL-17 and other inflammatory effectors to further recruit neutrophils and promote mucosal barrier function. These innate immune responses work in conjunction with epithelial cells to control the fungal infection. It is clear that C. albicans hypha formation is critically important for both fungal pathogenicity and the host response. Additional advances into these epithelial-hyphal interaction events will no doubt provide valuable insights into our understanding of C. albicans infections in the future.
Acknowledgments
This work was supported by the Medical Research Council (MR/M011372/1), Biotechnology & Biological Sciences Research Council (BB/N014677/1) and the National Institute for Health Research at Guys and St Thomas’s NHS Foundation Trust and King’s College London Biomedical Research Centre (IS-BRC-1215-20006) to JRN; the Deutsche Forschungsgemeinschaft CRC/TR124 FungiNet Project C1 and SPP 1580 (Hu 528/17-1), Centre for Sepsis Control and Care (CSCC), German Federal Ministry of Education and Health [BMBF] 01EO1002, European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie Grant Agreement number 642095 (OPATHY) to BH; the Leibniz Association InfectoOptics SAS-2015-HKI-LWC to BH and AK; and NIH (DE022550) to SLG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moyes DL, Shen C, Murciano C, Runglall M, Richardson JP, Arno M, Aldecoa-Otalora E, Naglik JR. Protection Against Epithelial Damage During Candida albicans Infection Is Mediated by PI3K/Akt and Mammalian Target of Rapamycin Signaling. J Infect Dis. 2014;209:1816–1826. doi: 10.1093/infdis/jit824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, et al. A Biphasic Innate Immune MAPK Response Discriminates between the Yeast and Hyphal Forms of Candida albicans in Epithelial Cells. Cell Host Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Koning HD, Rodijk-Olthuis D, van Vlijmen-Willems IM, Joosten LA, Netea MG, Schalkwijk J, Zeeuwen PL. A comprehensive analysis of pattern recognition receptors in normal and inflamed human epidermis: upregulation of dectin-1 in psoriasis. Journal of Investigative Dermatology. 2010;130:2611–2620. doi: 10.1038/jid.2010.196. [DOI] [PubMed] [Google Scholar]
- 4.Naglik JR, Moyes DL, Wachtler B, Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect. 2011;13:963–976. doi: 10.1016/j.micinf.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaffin WL. Candida albicans Cell Wall Proteins. Microbiology and Molecular Biology Reviews. 2008;72:495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munro CA, Bates S, Buurman ET, Hughes HB, MacCallum DM, Bertram G, Atrih A, Ferguson MAJ, Bain JM, Brand A, et al. Mnt1p and Mnt2p of Candida albicans Are Partially Redundant {alpha}-1,2-Mannosyltransferases That Participate in O-Linked Mannosylation and Are Required for Adhesion and Virulence. Journal of Biological Chemistry. 2005;280:1051–1060. doi: 10.1074/jbc.M411413200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murciano C, Moyes DL, Runglall M, Islam A, Mille C, Fradin C, Poulain D, Gow NA, Naglik JR. Candida albicans cell wall glycosylation may be indirectly required for activation of epithelial cell proinflammatory responses. Infect Immun. 2011;79:4902–4911. doi: 10.1128/IAI.05591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staab JF, Bradway SD, Fidel P, Jr, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. Candida albicans ALS3 and insights into the nature of the ALS gene family. Current Genetics. 1998;33:451–459. doi: 10.1007/s002940050359. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Oh SH, Cheng G, Green CB, Nuessen JA, Yeater K, Leng RP, Brown AJ, Hoyer LL. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology. 2004;150:2415–2428. doi: 10.1099/mic.0.26943-0. [DOI] [PubMed] [Google Scholar]
- 11.Naglik JR, Fostira F, Ruprai J, Staab JF, Challacombe SJ, Sundstrom P. Candida albicans HWP1 gene expression and host antibody responses in colonization and disease. J Med Microbiol. 2006;55:1323–1327. doi: 10.1099/jmm.0.46737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis. 2002;185:521–530. doi: 10.1086/338836. [DOI] [PubMed] [Google Scholar]
- 13.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE, Filler SG. Als3 Is a Candida albicans Invasin That Binds to Cadherins and Induces Endocytosis by Host Cells. PLoS Biol. 2007;5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cota E, Hoyer LL. The Candida albicans agglutinin-like sequence family of adhesins: functional insights gained from structural analysis. Future Microbiol. 2015;10:1635–1548. doi: 10.2217/fmb.15.79. [DOI] [PubMed] [Google Scholar]
- 15.Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, Liu Y, Dongari-Bagtzoglou A, Edgerton M, Filler SG. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathogens. 2010;6:e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu W, Phan QT, Boontheung P, Solis NV, Loo JA, Filler SG. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci USA. 2012;109:14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wachtler B, Wilson D, Haedicke K, Dalle F, Hube B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS ONE. 2011;6:e17046. doi: 10.1371/journal.pone.0017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Liu Y, Shetty AC, Schwartz JA, Bradford LL, Xu W, Phan QT, Kumari P, Mahurkar A, Mitchell AP, Ravel J, et al. New signaling pathways govern the host response to C. albicans infection in various niches. Genome Res. 2015;25:679–689. doi: 10.1101/gr.187427.114. Identified several new signaling pathways at the interface between C. albicans and host cells in various contexts of infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murciano C, Moyes DL, Runglall M, Tobouti P, Islam A, Hoyer LL, Naglik JR. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS ONE. 2012;7:e33362. doi: 10.1371/journal.pone.0033362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Solis NV, Swidergall M, Bruno VM, Gaffen SL, Filler SG. The Aryl Hydrocarbon Receptor Governs Epithelial Cell Invasion during Oropharyngeal Candidiasis. MBio. 2017;8 doi: 10.1128/mBio.00025-17. Identifies AhR as a new receptor that promotes the endocytosis of C. albicans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wachtler B, Citiulo F, Jablonowski N, Forster S, Dalle F, Schaller M, Wilson D, Hube B. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS ONE. 2012;7:e36952. doi: 10.1371/journal.pone.0036952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J, Runglall M, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. Candidalysin is the first cytolytic peptide toxin idenfied in any human fungal pathogen and is essential for epithelial damage and immune activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyes DL, Murciano C, Runglall M, Islam A, Thavaraj S, Naglik JR. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE. 2011;6:e26580. doi: 10.1371/journal.pone.0026580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyes DL, Murciano C, Runglall M, Kohli A, Islam A, Naglik JR. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med Microbiol Immunol. 2012;201:93–101. doi: 10.1007/s00430-011-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naglik JR, Richardson JP, Moyes DL. Candida albicans Pathogenicity and Epithelial Immunity. PLoS Pathog. 2014;10:e1004257. doi: 10.1371/journal.ppat.1004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang SX, Moyes DL, Richardson JP, Blagojevic M, Naglik JR. Epithelial discrimination of commensal and pathogenic Candida albicans. Oral Diseases. 2016;22:114–119. doi: 10.1111/odi.12395. [DOI] [PubMed] [Google Scholar]
- 27.Wilson D, Naglik JR, Hube B. The Missing Link between Candida albicans Hyphal Morphogenesis and Host Cell Damage. PLoS Pathog. 2016;12:e1005867. doi: 10.1371/journal.ppat.1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff MF, Karin M. Constitutive intestinal NF-{kappa}B does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pukkila-Worley R, Ausubel FM, Mylonakis E. Candida albicans Infection of Caenorhabditis elegans Induces Antifungal Immune Defenses. PLoS Pathogens. 2011;7:e1002074. doi: 10.1371/journal.ppat.1002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naglik JR. Candida Immunity. New Journal of Science. 2014;2014:390227. Article ID 390241. doi:390210.391155/392014/390241. [Google Scholar]
- 32.Duhring S, Germerodt S, Skerka C, Zipfel PF, Dandekar T, Schuster S. Host-pathogen interactions between the human innate immune system and Candida albicans-understanding and modeling defense and evasion strategies. Front Microbiol. 2015;6:625. doi: 10.3389/fmicb.2015.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng SC, Joosten LA, Netea MG. The interplay between central metabolism and innate immune responses. Cytokine Growth Factor Rev. 2014;25:707–713. doi: 10.1016/j.cytogfr.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Cheng SC, Joosten LA, Kullberg BJ, Netea MG. Interplay between Candida albicans and the Mammalian Innate Host Defense. Infect Immun. 2012;80:1304–1313. doi: 10.1128/IAI.06146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J Clin Invest. 2007;117:3664–3672. doi: 10.1172/JCI28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin-Bejerano I, Fraser I, Grisafi P, Fink GR. Phagocytosis by neutrophils induces an amino acid deprivation response in Saccharomyces cerevisiae and Candida albicans. Proc Natl Acad Sci USA. 2003;100:11007–11012. doi: 10.1073/pnas.1834481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fradin C, de GP MacCallum D, Schaller M, Klis F, Odds FC, Hube B. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol. 2005;56:397–415. doi: 10.1111/j.1365-2958.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- 38.Gabrielli E, Sabbatini S, Roselletti E, Kasper L, Perito S, Hube B, Cassone A, Vecchiarelli A, Pericolini E. In vivo induction of neutrophil chemotaxis by secretory aspartyl proteinases of Candida albicans. Virulence. 2016:00–00. doi: 10.1080/21505594.2016.1184385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cellular Microbiology. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 40.Urban CF, Ermert D, Schmid M, bu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. PLoS Pathogens. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, Brinkmann V, Von Bernuth H, Zychlinsky A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6 doi: 10.7554/eLife.24437. Identifies and discusses the different stimuli that activates NETs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenno S, Perito S, Mosci P, Vecchiarelli A, Monari C. Autophagy and Reactive Oxygen Species Are Involved in Neutrophil Extracellular Traps Release Induced by C. albicans Morphotypes. Front Microbiol. 2016;7:879. doi: 10.3389/fmicb.2016.00879. Demonstrates morphology dependent induction of NETS by C. albicans via different dynamics and mechanisms, including stimulation via autophagy and/or ROS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1:181–193. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byrd AS, O’Brien XM, Johnson CM, Lavigne LM, Reichner JS. An Extracellular Matrix-Based Mechanism of Rapid Neutrophil Extracellular Trap Formation in Response to Candida albicans. J Immunol. 2013;190:4136–4148. doi: 10.4049/jimmunol.1202671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nani S, Fumagalli L, Sinha U, Kamen L, Scapini P, Berton G. Src Family Kinases and Syk Are Required for Neutrophil Extracellular Trap Formation in Response to beta-Glucan Particles. J Innate Immun. 2014 doi: 10.1159/000365249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filler SG. Candida-host cell receptor-ligand interactions. Curr Opin Microbiol. 2006;9:333–339. doi: 10.1016/j.mib.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Miramon P, Kasper L, Hube B. Thriving within the host: Candida spp. interactions with phagocytic cells. Med Microbiol Immunol. 2013;202:183–195. doi: 10.1007/s00430-013-0288-z. [DOI] [PubMed] [Google Scholar]
- 49.Wellington M, Koselny K, Sutterwala FS, Krysan DJ. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell. 2014;13:329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, Lu J, Bambery K, Masters SL, Vince JE, Naderer T, et al. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. MBio. 2014;5:e00003–00014. doi: 10.1128/mBio.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKenzie CG, Koser U, Lewis LE, Bain JM, Mora-Montes HM, Barker RN, Gow NA, Erwig LP. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun. 2010;78:1650–1658. doi: 10.1128/IAI.00001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.O’Meara TR, Veri AO, Ketela T, Jiang B, Roemer T, Cowen LE. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat Commun. 2015;6:6741. doi: 10.1038/ncomms7741. First systematic study demonstrating that C. albicans hypha formation is not required for escape from macrophages and that pyroptosis is triggered by cell-wall remodelling and exposure of glycosylated proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wellington M, Koselny K, Krysan DJ. Candida albicans morphogenesis is not required for macrophage interleukin 1beta production. MBio. 2012;4:e00433–00412. doi: 10.1128/mBio.00433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vylkova S, Lorenz MC. Modulation of Phagosomal pH by Candida albicans Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport. PLoS Pathog. 2014;10:e1003995. doi: 10.1371/journal.ppat.1003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van de Veerdonk FL, Joosten LA, Shaw PJ, Smeekens SP, Malireddi RK, Van Der Meer JW, Kullberg BJ, Netea MG, Kanneganti TD. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol. 2011;41:2260–2268. doi: 10.1002/eji.201041226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho AW, Shen F, Conti HR, Patel N, Childs EE, Peterson AC, Hernandez-Santos N, Kolls JK, Kane LP, Ouyang W, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. The Journal of Immunology. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferreira MC, Whibley N, Mamo AJ, Siebenlist U, Chan YR, Gaffen SL. Interleukin-17-induced protein lipocalin 2 is dispensable for immunity to oral candidiasis. Infect Immun. 2014;82:1030–1035. doi: 10.1128/IAI.01389-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, et al. An ACT1 Mutation Selectively Abolishes Interleukin-17 Responses in Humans with Chronic Mucocutaneous Candidiasis. Immunity. 2013;39:676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, Ikinciogullari A, Dogu F, Belkadi A, Levy R, et al. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212:619–631. doi: 10.1084/jem.20141065. Demonstrates that IL-17RC, a specific signaling receptor for IL-17A and IL-17F, causes similar susceptibility to chronic mucocutaneous candidiasis as IL-17RA or Act1 deficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conti HR, Gaffen SL. IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J Immunol. 2015;195:780–788. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ, et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 64.Huppler AR, Verma AH, Conti HR, Gaffen SL. Neutrophils Do Not Express IL-17A in the Context of Acute Oropharyngeal Candidiasis. Pathogens. 2015;4:559–572. doi: 10.3390/pathogens4030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LeibundGut-Landmann S, Grosz O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 66.Bishu S, Hernandez-Santos N, Simpson-Abelson MR, Huppler AR, Conti HR, Ghilardi N, Mamo AJ, Gaffen SL. The adaptor CARD9 is required for adaptive but not innate immunity to oral mucosal Candida albicans infections. Infect Immun. 2014;82:1173–1180. doi: 10.1128/IAI.01335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 Coordinates the Neutrophil Response to C. albicans in the Oral Mucosa. PLoS Pathog. 2016;12:e1005882. doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Conti HR, Bruno VM, Childs EE, Daugherty S, Hunter JP, Mengesha BG, Saevig DL, Hendricks MR, Coleman BM, Brane L, et al. IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell Host Microbe. 2016;20:606–617. doi: 10.1016/j.chom.2016.10.001. Generation of new conditional deletion of IL-17RA in superficial oral and esophageal epithelial cells and showing that oral epithelial cells dominantly control IL-17R-dependent responses to oral candidasis through regulation of human beta-defenisn 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trautwein-Weidner K, Gladiator A, Kirchner FR, Becattini S, Rülicke T, Sallusto F, LeibundGut-Landmann S. Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis. PLoS Pathog. 2015;11:e1005164. doi: 10.1371/journal.ppat.1005164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomalka J, Azodi E, Narra HP, Patel K, O’Neill S, Cardwell C, Hall BA, Wilson JM, Hise AG. beta-Defensin 1 plays a role in acute mucosal defense against Candida albicans. J Immunol. 2015;194:1788–1795. doi: 10.4049/jimmunol.1203239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, Edgerton M, Gaffen SL. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4:448–455. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tati S, Davidow P, McCall A, Hwang-Wong E, Rojas IG, Cormack B, Edgerton M. Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PLoS Pathog. 2016;12:e1005522. doi: 10.1371/journal.ppat.1005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milner JD, Holland SM. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat Rev Immunol. 2013;13:635–648. doi: 10.1038/nri3493. [DOI] [PubMed] [Google Scholar]
- 75.Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5:135–140. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75:329–338. doi: 10.1007/s40265-015-0359-0. [DOI] [PubMed] [Google Scholar]
- 77.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 78.Whibley N, Tritto E, Traggiai E, Kolbinger F, Moulin P, Brees D, Coleman BM, Mamo AJ, Garg AV, Jaycox JR, et al. Antibody blockade of IL-17 family cytokines in immunity to acute murine oral mucosal candidiasis. J Leukoc Biol. 2016;99:1153–1164. doi: 10.1189/jlb.4A0915-428R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu W, Filler SG. Interactions of Candida albicans with epithelial cells. Cell Microbiol. 2010;12:273–282. doi: 10.1111/j.1462-5822.2009.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoyer LL, Cota E. Candida albicans Agglutinin-Like Sequence (Als) Family Vignettes: a Review of Als Protein Structure and function. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan R, Simpson PJ, Matthews SJ, Cota E. Backbone 1H, 15N, 13C and Ile, Leu, Val methyl chemical shift assignments for the 33.5 kDa N-terminal domain of Candida albicans ALS1. Biomol NMR Assign. 2010;4:187–190. doi: 10.1007/s12104-010-9243-8. [DOI] [PubMed] [Google Scholar]
- 82.Lin J, Oh SH, Jones R, Garnett JA, Salgado PS, Rusnakova S, Matthews SJ, Hoyer LL, Cota E. The peptide-binding cavity is essential for Als3-mediated adhesion of Candida albicans to human cells. J Biol Chem. 2014;289:18401–18412. doi: 10.1074/jbc.M114.547877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gale CA, Bendel CM, McClellan M, Hauser M, Becker JM, Berman J, Hostetter MK. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 84.Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun. 2007;75:2126–2135. doi: 10.1128/IAI.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukherjee PK, Seshan KR, Leidich SD, Chandra J, Cole GT, Ghannoum MA. Reintroduction of the PLB1 gene into Candida albicans restores virulence in vivo. Microbiology. 2001;147:2585–2597. doi: 10.1099/00221287-147-9-2585. [DOI] [PubMed] [Google Scholar]
- 87.Schofield DA, Westwater C, Warner T, Balish E. Differential Candida albicans lipase gene expression during alimentary tract colonization and infection. FEMS Microbiology Letters. 2005;244:359–365. doi: 10.1016/j.femsle.2005.02.015. [DOI] [PubMed] [Google Scholar]


