Abstract
Introduction
Membranous glomerulopathy (MG) is a common cause of nephrotic syndrome that results from the formation of immune complexes along the subepithelial aspect of the glomerular basement membranes. Although it is most frequently caused by polytypic deposits, cases with light chain isotype−restricted deposits are rarely seen.
Methods
We conducted a retrospective analysis of 28 cases of MG that showed light chain isotype restriction.
Results
The mean age at diagnosis was 62.2 years and the male-to-female ratio was 1. All patients presented with proteinuria (73.1% nephrotic range), and the mean serum creatinine was 1.5 mg/dl. Six patients had an underlying lymphoproliferative disorder (LPD), 2 had autoimmune disease, and 1 patient was positive for both hepatitis B and syphilis. Only 1 of the patients with an LPD had a detectable monoclonal Ig. Four patients (14.3%) showed focal proliferation or crescents, 3 of whom had an underlying LPD. Kappa (κ) restriction was seen in 26 of 28 patients (85.7%). Staining for IgG subclasses was performed in 19 cases, 14 of which showed positive staining for a single subclass. PLA2R was positive in 7 of 27 cases. 30% of PLA2R-negative patients and 28.6% of those with positive staining for a single IgG subclass had an associated LPD.
Discussion
The majority of MG cases with light chain isotype−restricted deposits lack a recognizable secondary etiology. However, the absence of PLA2R positivity, positive staining for a single IgG subclass, and presence of focal proliferation are worrisome histopathologic features that should prompt a thorough clinical workup to exclude the presence of an underlying LPD.
Keywords: membranous glomerulopathy, MGRS, monoclonal gammopathy of renal significance, PLA2R, proliferative glomerulonephritis with monoclonal IgG deposits
Membranous glomerulopathy (MG) is a common cause of nephrotic syndrome caused by the formation of immune complexes along the subepithelial aspect of the glomerular basement membranes.1 Although these immune complexes, in the vast majority of cases, are composed of polytypic IgG, there are rare cases that show light chain isotype restriction by routine immunofluorescence. Numerous case reports and case series detailing glomerular diseases caused by nonorganized monotypic Ig deposits showing a mesangial proliferative, endocapillary proliferative, or membranoproliferative pattern of glomerular injury have been published, and, depending on the clinical scenario, have been found to be both associated with and independent of underlying lymphoproliferative disorders (LPD).2, 3, 4, 5, 6 However, relatively few case reports and small case series have described a membranous pattern of immune complex deposition with monotypic deposits, and the clinical significance of such deposits remains largely to be explored.2, 3, 7, 8, 9, 10, 11, 12 We report the largest series to date of MG cases with light chain isotype−restricted deposits, with emphasis on morphologic and immunofluorescence characteristics, which may aid in determining the possible risk of an underlying LPD.
Materials and Methods
Patient Selection
A retrospective analysis with clinico-pathological correlation was performed on all renal biopsy samples received at Arkana Laboratories (formerly Nephropath) between January 2010 and February 2016 that met the following inclusion criteria: predominant MG features characterized by glomerular basement membranes with silver-positive “spikes” or silver-negative “pin holes”, with or without mesangial hypercellularity; positive granular capillary wall staining for IgG by routine immunofluorescence; positive staining for a single light chain isotype (kappa [κ] or lambda [λ]) by routine immunofluorescence evaluation; and presence of subepithelial granular electron-dense deposits by electron microscopy. Cases with a predominant membranoproliferative, endocapillary proliferative, or crescentic pattern of injury were excluded from the analysis. The study protocol was approved by Schulman Institutional Review Board and conformed to the principles outlined in the Declaration of Helsinki.
Biopsy Sample Processing Techniques
All cases were processed by light, immunofluorescence, and electron microscopy using standard techniques.13 Kidney biopsy samples were fixed in buffered formalin, dehydrated in graded alcohols, and embedded in paraffin using standard methods. Serial 3-mm-thick sections were cut and treated with hematoxylin and eosin, Jones methenamine silver, Masson trichrome, and periodic acid–Schiff reagent.
Samples submitted for IF studies were transported in Michel’s transport medium, washed in buffer, and frozen in a cryostat. Sections, cut at 4 μm, were rinsed in buffer and reacted with fluorescein-tagged polyclonal rabbit anti-human antibodies to IgG, IgA, IgM, C3, C4, C1q, fibrinogen, and κ-, and λ-light chains (all from Dako, Carpinteria, CA) for 1 hour, rinsed, and a coverslip applied using aqueous mounting media. The staining intensity was graded 0 to 3+ on a semiquantitative scale. If remaining tissue was available, sections were further stained with fluorescein-tagged mouse anti-human antibodies to IgG1, IgG2, IgG3, and IgG4 (Sigma-Aldrich, St. Louis, MO). Phospholipase A2 receptor was detected in paraffin-embedded sections following pronase digestion using rabbit polyclonal antiphospholipase A2 receptor antibodies (Sigma-Aldrich, St. Louis, MO), followed by highly cross-adsorbed Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies, Carlsbad, CA) at a dilution of 1:100, as previously described.14 PLA2R was determined to be positive if there was any degree of positive granular capillary loop staining in the glomeruli and as negative if there was an absence of staining.
Electron microscopy was performed on the formalin fixed tissue. The ends of the renal biopsy specimen were removed as 1-mm cubes, dehydrated using graded alcohols, and embedded in epon/araldite resin. Sections 1 mm thick were cut using an ultramicrotome, stained with toluidine blue, and examined with a light microscope. Thin sections were examined in a Jeol JEM-1011 electron microscope (Jeol, Tokyo, Japan). Photomicrographs were routinely taken at ×5000, ×12,000, and ×20,000 magnifications.
Results
Clinical Features
A total of 30 biopsy samples from 28 patients meeting the inclusion criteria were diagnosed at our institution within the study period (Table 1), representing 0.06% of total biopsy samples (30 of 52,364) and 0.95% of biopsy samples diagnosed as MG (30 of 3,129). Twenty seven patients underwent a single biopsy and one posttransplant patient had a total of three biopsies, all from the allograft. No information was available concerning the native kidney disease in this patient. There were equal numbers of male and female patients, and the average age at diagnosis was 62.2 years (range 37–87 years). Nine patients had renal insufficiency (S Cr > 1.2 mg/dl), and the average creatinine was 1.53 mg/dl (range 0.4–5 mg/dl). All 28 patients presented with proteinuria (mean 5.5 g), 73.1% of them in the nephrotic range (≥ 3.5 g/dl). Results of serum and urine protein electrophoresis with immunofixation (IFE) were available in 23 patients. Only 1 patient with a normal SPEP and UPEP had a detectable free λ light chain by IFE. The remaining patients had no detectable monoclonal Ig in serum or urine. One of 20 patients tested for hepatitis B was positive, and all were negative for hepatitis C. The patient who was positive for hepatitis B also had reactivity for RPR and FTA-ABS. Antinuclear antibodies (ANA) were tested in 23 patients, 2 of them with a positive result. One of these 2 patients had a diagnosis of mixed connective tissue disease, whereas the other did not have a diagnosis of an autoimmune disorder. One additional patient with negative antinuclear antibodies had a history of rheumatoid arthritis. Complement levels were measured in 21 patients. Two patients had mildly decreased C3 (67 and 69 mg/dl), and all patients had normal C4.
Table 1.
Summary of clinical data
| Patient | Age (yr) | Gender | Cr (mg/dl) | Prot (g) | Monoclonal Ig | Underlying disorder |
|---|---|---|---|---|---|---|
| 1 | 64 | F | 1 | 3.7 | – | |
| 2 | 75 | F | 0.6 | 6.9 | – | |
| 3 | 79 | M | 0.8 | 2.3 | – | Carcinoid tumor |
| 4 | 67 | F | 1 | 9.6 | – | CLL/SLL |
| 5 | 59 | F | 0.9 | 6.4 | – | |
| 6 | 87 | F | 1 | 4 | – | |
| 7 | 48 | F | NA | 1.4 | – | |
| 8 | 78 | M | 4.2 | 18.2 | – | |
| 9 | 37 | F | 1.9 | 4.5 | NP | Rheumatoid arthritis |
| 10 | 59 | M | 5 | 6 | NP | Prostate cancer |
| 11 | 79 | F | 0.5 | 5.4 | – | |
| 12 | 40 | F | NA | 4.2 | – | |
| 13 | 67 | F | 2.3 | NA | – | CLL/SLL |
| 14 | 44 | M | NA | 2.7 | NP | |
| 15 | 64 | F | 1.4 | 7.4 | Free λ, urine | CLL/SLL |
| 16 | 64 | M | 1 | 5 | – | CLL/SLL |
| 17 | 57 | M | 2.5 | 0.5 | NP | Transplant |
| 18 | 70 | M | 1 | 2.1 | – | SMZL |
| 19 | 78 | M | 1.3 | 8.4 | – | |
| 20 | 46 | F | 1.63 | 6.4 | NP | |
| 21 | 63 | M | NA | 8 | – | CLL |
| 22 | 51 | M | 1.1 | 0.2 | M-spike, serum | MCTD |
| 23 | 65 | M | 0.63 | 3.1 | – | |
| 24 | 60 | M | 0.6 | 9.8 | – | |
| 25 | 83 | M | 1.1 | 5.6 | – | |
| 26 | 50 | M | 1.2 | 5.9 | – | |
| 27 | 57 | F | 0.4 | 4.2 | – | Hepatitis B, syphilis |
| 28 | 50 | F | 3.7 | NP | – |
CLL/SLL, chronic lymphocytic leukemia/small cell lymphoma; Cr, serum creatinine; F, female; M, male; MCTD, mixed connective tissue disorder; NA, not available; NP, not performed; Prot, 24-hour proteinuria; SMZL, splenic marginal zone lymphoma.
Six of the 28 patients in the series (21.4%) either had a diagnosis of a LPD at the time of the biopsy, or received such a diagnosed subsequently. Four of these patients carried a diagnosis of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) at the time of the biopsy, 1 patient carried a diagnosis of splenic marginal zone lymphoma, and 1 patient was diagnosed with CLL/SLL 2 years after the renal biopsy. In contrast, only 20 of the 3099 patients (0.65%) diagnosed in our institution with MG with polytypic deposits during the study period had a history of an underlying lymphoproliferative disorder. Accordingly, 20 of the 26 patients (76.9%) with MG and an underlying LPD showed polytypic deposits, and 6 (23.1%) showed monotypic deposits. All 6 patients with LPD in our series had a normal serum and urine protein electrophoresis, and only 1 patient had a detectable free λ light chain by urine immunofixation electrophoresis. In addition, 1 patient had a prior history of a carcinoid tumor and 1 patient a prior history of prostate adenocarcinoma.
Pathologic Findings
Light Microscopy
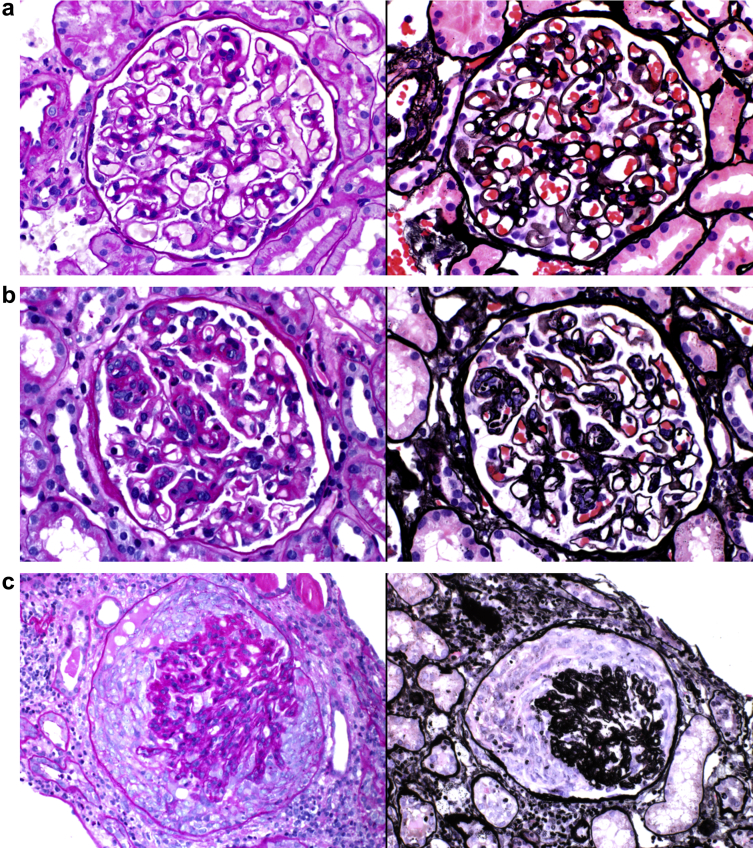
Biopsy samples from 24 of the 28 patients (85.7%) showed exclusively MG features with no endocapillary proliferation or cellular crescent formation (Table 2). These cases had evidence of global or segmental glomerular basement membrane silver-positive “spikes” or silver-negative “pin holes.” Four patients (14.3%) had evidence of focal glomerular active lesions, including 2 (7.1%) with focal and segmental endocapillary proliferation and 2 (7.1%) with focal cellular crescents (Figure 1). Of note, both patients with biopsy samples showing endocapillary proliferation, and 1 of the 2 patients whose biopsy samples showed cellular crescents, had an associated LPD (Table 3). The remaining patient with focal cellular crescents had a history of rheumatoid arthritis with negative anti-neutrophil cytoplasmic antibody (ANCA). There was no significant chronicity in most cases. Minimal-to-mild interstitial fibrosis and tubular atrophy were seen in 20 of the 28 patients, moderate in 7 patients and severe in a single patient.
Table 2.
Summary of morphologic findings
| Patient | LM glomerular pattern | IF | IgG subclass | PLA2R |
|---|---|---|---|---|
| 1 | Normal | IgG, κ | NP | – |
| 2 | Normal | IgG, C3, κ | IgG1 | – |
| 3 | Normal | IgG, IgM, C3, κ | NP | + |
| 4 | Focal proliferation | IgG, C3, κ | NP | – |
| 5 | Normal | IgG, κ | NP | – |
| 6 | Normal | IgG, κ | IgG4 | – |
| 7 | Normal | IgG, C3, C1q, κ | IgG3 | – |
| 8 | Normal | IgG, C3, κ | NP | – |
| 9 | Focal crescents | IgG, C3, κ | NP | – |
| 10 | Normal | IgG, C3, κ | NP | + |
| 11 | Normal | IgG, κ | IgG1 | – |
| 12 | Normal | IgG, C3, κ | IgG2 | – |
| 13 | Focal crescents | IgG, C3, κ | IgG1 | – |
| 14 | Normal | IgG, λ | IgG1 | – |
| 15 | Normal | IgG, C3, λ | NP | – |
| 16 | Normal | IgG, C3, C1q, κ | IgG1 | – |
| 17 | Normal | IgG, C3, κ | IgG1 / IgG4 | + |
| 18 | Normal | IgG, C3, κ | IgG3 | – |
| 19 | Normal | IgG, C3, C1q, κ | IgG3 | – |
| 20 | Normal | IgG, C3, κ | NP | + |
| 21 | Focal proliferation | IgG, C3, C1q, λ | IgG1 | – |
| 22 | Normal | IgG, IgM, C3, κ | IgG1 | – |
| 23 | Normal | IgG, C3, κ | IgG1 / IgG3 | + |
| 24 | Normal | IgG, C3, κ | IgG1 / IgG4 | + |
| 25 | Normal | IgG, C3, κ | IgG1 / IgG2 | NP |
| 26 | Normal | IgG, C3, κ | IgG3 / IgG4 | + |
| 27 | Normal | IgG, C3, C1q, κ | IgG2 | – |
| 28 | Normal | IgG, C3, κ | IgG1 | – |
IF, immunofluorescence; LM, light microscopy; NP, not performed; PLA2R, phospholipase A2 receptor.
Figure 1.
Morphologic spectrum in membranous glomerulopathy with light chain−restricted deposits. (a) Membranous pattern of glomerular injury characterized by thick glomerular basement membranes with subepithelial “spikes” and “pin holes” in patient 14 (periodic acid−Schiff, original magnification ×400 [left]; Jones methenamine silver, original magnification ×400 [right]). (b) Focal and segmental endocapillary hypercellularity in patient 21 (periodic acid−Schiff, original magnification ×400 [left]; Jones methenamine silver, original magnification ×400 [right]). (c) Focal cellular crescents in patient 9 (periodic acid−Schiff, original magnification ×400 [left]; Jones methenamine silver, original magnification ×400 [right]).
Table 3.
Clinical and morphologic characteristics of patients with and without lymphoproliferative disorders
| Characteristic | Patients with history of LPD | Patients with no history of LPD |
|---|---|---|
| No. of patients | 6/28 | 22/28 |
| Age, mean (range) | 65.8 (63–70) | 61.2 (37–87) |
| Gender (M:F) | 3:3 | 11:11 |
| Monoclonal Ig | ||
| Serum (n) | 0/6 | 1/17 |
| Urine (n) | 1/6 | 0/17 |
| Antinuclear antibodies (n) | 0/5 | 2/18 |
| Hepatitis B (n) | 0/5 | 1/15 |
| Hepatitis C (n) | 0/5 | 0/15 |
| Histologic pattern | ||
| No glomerular proliferation, n (%) | 3/6 (50) | 21/22 (95.5) |
| Focal endocapillary proliferation/crescents, n (%) | 3/6 (50) | 1/22 (4.5) |
| IgG subclasses | ||
| Positive for only 1 subclass, n (%) | 4/4 (100) | 10/15 (66.7) |
| Positive for >1 subclass, n (%) | 0/4 (0) | 5/15 (33.3) |
| PLA2R | ||
| Positive, n (%) | 0/6 (0) | 7/21 (33.3) |
| Negative, n (%) | 6/6 (100) | 14/21 (66.7) |
F, female; LPD, lymphoproliferative disorders; M, male; PLA2R, phospholipase A2 receptor.
Immunofluorescence
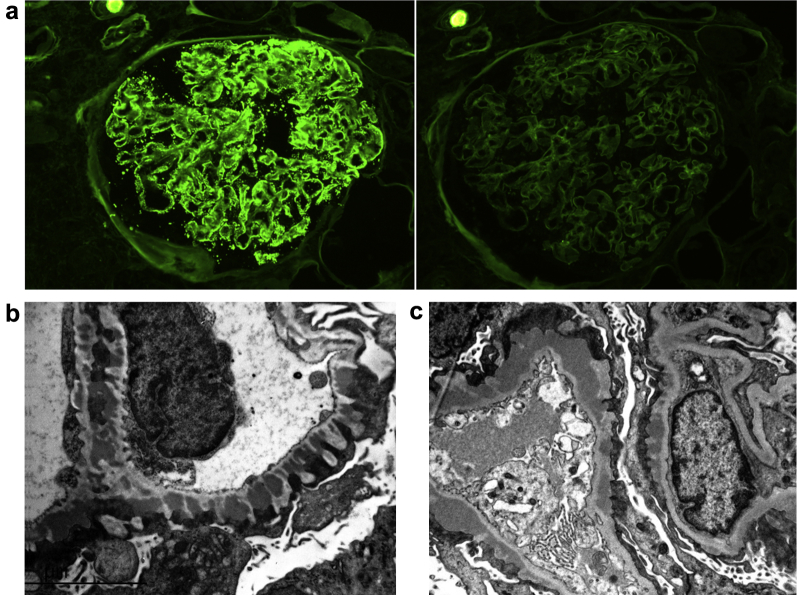
As per the inclusion criteria, the biopsy samples from all 28 patients in our study had positive capillary wall IgG granular staining by routine immunofluorescence, with an average intensity of 2.7+ on a scale of 0 to 3+. IgM was positive in 2 of 30 cases (6.7%), C3 in 23 (76.7%) and C1q in 5 (16.7%), with a mean intensity when positive of 0.5+, 1.5+, and 0.9+, respectively. No IgA staining was present in any of the cases. In all, κ light chain restriction was present in 24 patients (85.7%) and λ restriction in 4 patients (14.3%) (Figure 2). Tissue was available for IgG subclass staining in 19 cases. Fourteen cases (73.7%) had positive staining for a single IgG subclass, and 5 (26.3%) stained positive for 2 subclasses. None of the cases stained positive for more than 2 subclasses. When staining was positive for a single subclass, IgG1 was the most common (8/14, 57.1%), followed by IgG3 (3/14, 21.4%), IgG2 (2/14, 14.3%) and IgG4 (1/14, 7.1%). Similarly, when staining was positive for 2 subclasses, IgG1 staining was present in 4 of the 5 cases (80%).
Figure 2.
Immunofluorescence and electron microscopy findings in membranous glomerulopathy with light chain−restricted deposits. (a) Kappa (κ) light chain−restricted deposits in patient 8. Strongly positive (3+) capillary loop granular staining for κ light chain (left) and negative staining for lambda (λ) light chain (right) (direct immunofluorescence, original magnification ×400). (b,c) Numerous subepithelial electron-dense deposits typical of membranous glomerulopathy are present in patient 25 (b) and patient 16 (c) (unstained, original magnification ×12,000).
Staining for phospholipase A2 receptor (PLA2R) was performed in the biopsy samples from 27 patients, 7 of which (25.9%) were positive. None of the PLA2R positive cases showed endocapillary proliferation or crescent formation by light microscopy, and all were κ light chain restricted by routine immunofluorescence. IgG subclass results were available in 4 of the 7 PLA2R-positive cases, all of which showed positive staining for 2 IgG subclasses. On the contrary, none of the 14 cases with positive staining for a single IgG subclass were positive for PLA2R. Of note, none of the patients whose biopsy samples showed positive PLA2R staining had an associated diagnosis of an LPD.
Electron Microscopy
All patients showed evidence of subepithelial deposits, with Ehrenreich and Churg stage ranging from 1 through 3 (stage 1: 4/30; stage 2: 11/30; and stage 3: 15/30) (Figure 2). A total of 21 cases showed varying degrees of mesangial deposits, and 2 showed occasional subendothelial deposits. The deposits were of the immune complex-type without evidence of substructure. One of the 2 patients with subendothelial deposits had a history of rheumatoid arthritis and showed focal cellular crescents by light microscopy, whereas the other showed no proliferation or crescents and had a diagnosis of splenic marginal zone lymphoma.
Discussion
We present here a large case series of MG cases with light chain−restricted deposits by routine immunofluorescence. This is a rare pattern of glomerular injury, representing only 0.95% of MG cases. A handful of publications have addressed this pattern of glomerular injury, including a descriptions by Komatsuda et al. of 3 cases and by Guiard et al. of 14 cases.2, 7 Nasr et al. included 2 patients with predominant membranous features in the report of the closely related pattern of glomerular injury, proliferative glomerulonephritis with monoclonal IgG deposits.3, 15 The main goal of the present study was to identify any association of this morphologic pattern with underlying disorders, in particular LPD. This is also the first case series to evaluate the PLA2R status and significance in light chain isotype−restricted MG.
Lymphoproliferative disorders can affect the kidney in numerous ways, ranging from direct infiltration of renal parenchyma by neoplastic cells to glomerular and/or tubulointerstitial monoclonal Ig deposition.16, 17 Multiple myeloma and plasma cell dyscrasias are the most common hematologic malignancies that lead to kidney disease; however, other clonal processes can also lead to disease.9, 17, 18, 19 Strati et al.16 reviewed 49 renal biopsy findings from patients with CLL and monoclonal B-cell lymphocytosis, and observed that the most common pattern of glomerular injury was membranoproliferative glomerulonephritis. This cohort also included cases with direct CLL infiltration, minimal change disease, acute interstitial nephritis, and amyloidosis. Only 2 of the 49 cases in the series (4%) showed MG, which is slightly lower than the cumulative reported cases available in the literature, where MG represents 12% of all biopsy results from CLL patients.16 Not all reported cases of MG associated with LPD show light chain restriction by immunofluorescence.20, 21 In our retrospective analysis, we found that 20 of 3099 patients diagnosed with MG with polytypic deposits had an underlying LPD. On the other hand, 5 of the 28 patients with light chain isotype−restricted deposits had an underlying LPD at the time of the biopsy (4 with CLL/SLL and 1 with splenic marginal zone lymphoma), and 1 additional patient was diagnosed with CLL/SLL 2 years after the kidney biopsy. All 6 patients had absence of M-spike on serum and urine protein electrophoresis, and only 1 patient had a detectable free λ light chain by urine immunofixation electrophoresis. Although the absence of a detectable monoclonal Ig in serum/urine is consistent with previous reports of MG with monotypic deposits,2, 19, 22 there are also published cases with positive serum and urine protein electrophoresis, including cases of CLL, multiple myeloma, and follicular lymphoma.2, 9 We speculate that the absence of a detectable monoclonal Ig in serum and/or urine in the majority of these patients is due to a concentration of the monoclonal Ig below the lower limit of detection for the assay.
We were able to identify 3 particular biopsy findings that increase the likelihood of an underlying LPD: negative staining for PLA2R, positive staining for a single IgG subclass, and focal proliferation and/or crescents by light microscopy. Phospholipase A2 receptor (PLA2R), a glycoprotein constituent of normal human podocytes, has been identified as the antigenic target in the majority of cases of primary MG, and staining against this glycoprotein in kidney biopsy samples has been shown to be a useful technique to differentiate primary versus secondary MG.14, 23 We stained 27 cases for PLA2R. Seven of the 27 stained cases were positive, and none of these positive cases had an underlying LPD. On the contrary, up to 30% of PLA2R-negative patients were associated with a hematologic malignancy, which underscores the importance of performing this ancillary test. Of note, all 4 PLA2R-positive patients in our cohort with tissue remaining for subclass analysis had multiple subclasses present. Based on the findings in this series, it appears that the light chain restriction in PLA2R-associated MG is not indicative an underlying clonal process. Debiec et al. reported a case of recurrent membranous glomerulopathy in a renal allograft that showed PLA2R-positive deposits that did show IgG3 κ isotype restriction.24 However, after a thorough clinical investigation, no evidence of an underlying lymphoproliferative disorder was found. Therefore, to date, there are no reported cases of a PLA2R-positive MG representing a monoclonal gammopathy of renal significance.
IgG subclass staining is useful for determining the significance of light chain restriction seen by routine immunofluorescence staining. Similar to PLA2R-negative cases, 28.6% of patients with positive staining for a single IgG subclass had an underlying LPD, compared to none of the cases with positive staining for more than 1 subclass. Overall, the most frequently positive IgG subclass was IgG1. This observation remained true when analyzing only patients with underlying lymphoproliferative disorders. Dominant and co-dominant IgG4 staining has been reported in cases of primary MG25, 26; hence the predominance of IgG1 and the lack of IgG4 positive cases in patients with underlying LPD was not unexpected. These findings are similar to those from the series by Guiard et al., in which 7 of the 11 patients with MG with light chain isotype−restricted deposits and available IgG subclasses, had IgG1 positive deposits, and none were positive for IgG4.2 Furthermore, cases of malignancy-associated MG have been shown to be IgG1 or IgG2 dominant, although none of the associated tumors in the report were of hematologic origin.27 The distribution of IgG subclasses also contrasts with what has been described in proliferative glomerulonephritis with monoclonal IgG deposits, where the majority of cases are IgG3k restricted.3
It is important to highlight that all cases included in this series had light chain isotype−restricted deposits by routine immunofluorescence and did not require antigen retrieval. Although these data are not presented, immunofluorescence staining for IgG, κ, and λ light chains was performed on formalin-fixed, paraffin-embedded tissue after pronase digestion in 5 cases, including 2 cases with positive staining for a single IgG subclass, 1 patient with positive staining for 2 subclasses, and 2 cases with no available tissue for subclass testing. The pattern of staining did not change when compared to routine immunofluorescence, and no deposits were unmasked with this technique. This observation is relevant to avoid confusion with the recently described entity membranous-like glomerulopathy with masked IgG κ deposits, which has somewhat similar morphologic findings but markedly different demographics, clinical presentation, and risk of underlying LPD.6 The patients presented here, in whom the staining for Igs did not require an antigen retrieval step, are older by a mean age of 34.7 years and more often male. The patients with membranous-like glomerulopathy with masked IgG κ deposits had significantly more evidence of autoimmune disease, and none had an associated lymphoproliferative disorder.
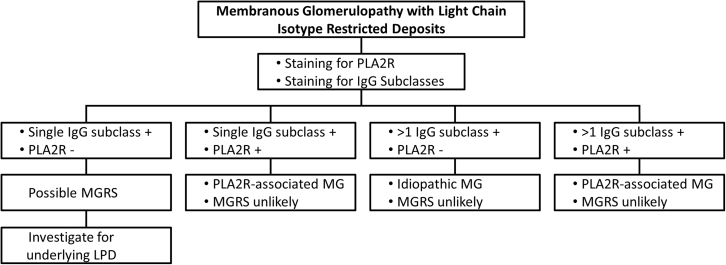
In conclusion, MG with light chain isotype−restricted deposits is a rare pattern of glomerular injury. Although the majority of patients lack a recognizable etiology, there is a considerable risk of an underlying lymphoproliferative disorder, predominantly CLL. We present a proposed algorithm for the approach of these biopsies and the clinical workup of these patients (Figure 3). The absence of PLA2R positivity within glomerular deposits, positive staining for a single IgG subclass, and presence of focal proliferation or crescents by light microscopy are worrisome histopathologic features that should prompt a thorough clinical workup to exclude the presence of an underlying lymphoproliferative disorder, even in the absence of a recognizable monoclonal Ig.
Figure 3.
Proposed algorithm for the clinical and pathologic workup of membranous glomerulopathy cases with light chain isotype−restricted deposits. LPD, lymphoproliferative disorder; MG, membranous glomerulopathy; MGRS, monoclonal gammopathy of renal significance; PLA2R, phospholipase A2 receptor.
Disclosures
All the authors declared no competing interests.
References
- 1.Beck L.H., Jr., Salant D.J. Membranous nephropathy: from models to man. J Clin Invest. 2014;124:2307–2314. doi: 10.1172/JCI72270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guiard E., Karras A., Plaisier E. Patterns of noncryoglobulinemic glomerulonephritis with monoclonal Ig deposits: correlation with IgG subclass and response to rituximab. Clin J Am Soc Nephrol. 2011;6:1609–1616. doi: 10.2215/CJN.10611110. [DOI] [PubMed] [Google Scholar]
- 3.Nasr S.H., Satoskar A., Markowitz G. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055–2064. doi: 10.1681/ASN.2009010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour S.J., Beaulieu M.C., Zalunardo N.Y. Proliferative glomerulonephritis with monoclonal IgG deposits secondary to chronic lymphocytic leukemia. Report of two cases. Nephrol Dial Transplant. 2011;26:2712–2714. doi: 10.1093/ndt/gfr251. [DOI] [PubMed] [Google Scholar]
- 5.Larsen C.P., Messias N.C., Walker P.D. Membranoproliferative glomerulonephritis with masked monotypic immunoglobulin deposits. Kidney Int. 2015;88:867–873. doi: 10.1038/ki.2015.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen C.P., Boils C.L., Cossey L.N. Clinicopathologic features of membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int Rep. 2016;1:299–305. doi: 10.1016/j.ekir.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komatsuda A., Masai R., Ohtani H. Monoclonal immunoglobulin deposition disease associated with membranous features. Nephrol Dial Transplant. 2008;23:3888–3894. doi: 10.1093/ndt/gfn363. [DOI] [PubMed] [Google Scholar]
- 8.de Seigneux S., Bindi P., Debiec H. Immunoglobulin deposition disease with a membranous pattern and a circulating monoclonal immunoglobulin G with charge-dependent aggregation properties. Am J Kidney Dis. 2010;56:117–121. doi: 10.1053/j.ajkd.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Evans D.J., Macanovic M., Dunn M.J. Membranous glomerulonephritis associated with follicular B-cell lymphoma and subepithelial deposition of IgG1-κ paraprotein. Nephron Clin Pract. 2003;93:112–118. doi: 10.1159/000069548. [DOI] [PubMed] [Google Scholar]
- 10.Yamada T., Arakawa Y., Mii A. A case of monoclonal immunoglobulin G1-lambda deposition associated with membranous feature in a patient with hepatitis C viral infection. Clin Exp Nephrol. 2012;16:468–472. doi: 10.1007/s10157-011-0579-x. [DOI] [PubMed] [Google Scholar]
- 11.Omokawa A., Komatsuda A., Hirokawa M. Membranous nephropathy with monoclonal IgG4 deposits and associated IgG4-related lung disease. Clin Kidney J. 2014;7:475–478. doi: 10.1093/ckj/sfu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touchard G., Preud'homme J.L., Aucouturier P. Nephrotic syndrome associated with chronic lymphocytic leukemia: an immunological and pathological study. Clin Nephrol. 1989;31:107–116. [PubMed] [Google Scholar]
- 13.Walker P.D., Cavallo T., Bonsib S.M. Practice guidelines for the renal biopsy. Mod Pathol. 2004;17:1555–1563. doi: 10.1038/modpathol.3800239. [DOI] [PubMed] [Google Scholar]
- 14.Larsen C.P., Messias N.C., Silva F.G. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 15.Nasr S.H., Markowitz G.S., Stokes M.B. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int. 2004;65:85–96. doi: 10.1111/j.1523-1755.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 16.Strati P., Nasr S.H., Leung N. Renal complications in chronic lymphocytic leukemia and monoclonal B-cell lymphocytosis: the Mayo Clinic experience. Haematologica. 2015;100:1180–1188. doi: 10.3324/haematol.2015.128793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doshi M., Lahoti A., Danesh F.R. Paraprotein-related kidney disease: kidney injury from raraproteins—what determines the site of injury? Clin J Am Soc Nephrol. 2016;11:2288–2294. doi: 10.2215/CJN.02560316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heher E.C., Rennke H.G., Laubach J.P. Kidney disease and multiple myeloma. Clin J Am Soc Nephrol. 2013;8:2007–2017. doi: 10.2215/CJN.12231212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulin B., Ronco P.M., Mougenot B. Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992;42:127–135. doi: 10.1038/ki.1992.270. [DOI] [PubMed] [Google Scholar]
- 20.Ziakas P.D., Giannouli S., Psimenou E. Membranous glomerulonephritis in chronic lymphocytic leukemia. Am J Hematol. 2004;76:271–274. doi: 10.1002/ajh.20109. [DOI] [PubMed] [Google Scholar]
- 21.Silva G.E., Costa R.S., Chahud F. Membranous glomerulonephritis associated with splenic marginal zone lymphoma mimicking multiple myeloma. Clin Nephrol. 2013;79:488–493. doi: 10.5414/cn107079. [DOI] [PubMed] [Google Scholar]
- 22.Da'as N., Polliack A., Cohen Y. Kidney involvement and renal manifestations in non-Hodgkin's lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Haematol. 2001;67:158–164. doi: 10.1034/j.1600-0609.2001.5790493.x. [DOI] [PubMed] [Google Scholar]
- 23.Beck L.H., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debiec H., Hanoy M., Francois A. Recurrent membranous nephropathy in an allograft caused by IgG3k targeting the PLA2 receptor. J Am Soc Nephrol. 2012;23:1949–1954. doi: 10.1681/ASN.2012060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin W., Beck L.H., Jr., Zeng C. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kearney N., Podolak J., Matsumura L. Patterns of IgG subclass deposits in membranous glomerulonephritis in renal allografts. Transplant Proc. 2011;43:3743–3746. doi: 10.1016/j.transproceed.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Ohtani H., Wakui H., Komatsuda A. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant. 2004;19:574–579. doi: 10.1093/ndt/gfg616. [DOI] [PubMed] [Google Scholar]