Abstract
Purpose
Patients with severe sepsis who experience rapid, early deterioration and death are of particular concern. Our objective was to identify predictors of early death in Emergency Department (ED) patients with severe sepsis.
Methods
Secondary analysis of two prospective studies of adult ED patients with severe sepsis. The primary outcome was early death, defined as death within 24 hours of triage.
Results
Out of 410 severe sepsis admissions, 20 patients experienced early death. These patients demonstrated significantly higher initial lactate (7.3 versus 3.3 mmol/L, p < 0.001) and modified SOFA (mSOFA) scores (10 vs 6, p<.001), were less likely to normalize their lactate (p<0.001), had lower initial pH (p<0.001), and more frequently had early positive blood cultures (p=0.021). Multivariable logistic regression identified initial serum lactate level (OR 1.19, 95% CI 1.06–1.35) and mSOFA score (OR 1.17, 95% CI 1.00–1.36) as independent predictors of early death. A repeat lactate ≥ 5 mmol/L had a sensitivity of 55% and specificity of 89% for early death. There were no significant treatment differences between groups.
Conclusion
Initial serum lactate and mSOFA score were independent predictors of mortality within 24 hours of ED admission in patients with severe sepsis.
Keywords: Sepsis, shock, organ dysfunction, resuscitation
Introduction
Approximately 570,000 patients with sepsis present to Emergency Departments (ED) in the United States each year.[1] Research suggests outcomes in sepsis are improved with timely recognition and early resuscitation initiated in the ED.[2] However, given the broad spectrum of clinical presentations and variable rates of disease progression, the early identification of septic patients at increased risk for clinical deterioration remains challenging. The need for rapid risk stratification of these patients in the ED has fueled a large body of research on biomarkers and clinical prediction tools.[3,4,5] Several studies have demonstrated the role for these clinical indicators in independently predicting in-hospital mortality in patients with sepsis.[6,7]
Despite this, there remains a paucity of data on predictors of early death, or death within the first 24 hours, in septic patients. Identifying a subset of patients with severe sepsis or septic shock who are at increased risk of early death could aid in the prioritization of care for these patients and assist in predicting which patients are most likely to benefit from higher levels of care. Additionally, this information could help direct future clinical trials investigating novel therapeutic interventions. To our knowledge, no study has specifically evaluated this cohort of septic patients. The objective of this study was to identify independent predictors of mortality within 24 hours of ED arrival in patients with severe sepsis or septic shock. Our secondary objective was to test the hypothesis that early therapeutic interventions decreased the risk of early death.
Methods
Study design
We conducted a secondary analysis of two completed studies of adult ED patients with severe sepsis or septic shock, one single-center study and one multi-center study. The single center study was a prospective, observational cohort study evaluating end-tidal carbon dioxide as a resuscitative end point for sepsis.[8] The study was conducted from 2012 to 2014 in the adult ED and intensive care unit at a tertiary care academic medical center in the United States. The multi-center study was a randomized, clinical trial evaluating the non-inferiority of lactate compared with central venous oxygen saturation during early resuscitation in sepsis which took place from January 2007 to January 2009 in the EDs of 3 large, urban, tertiary care centers.[9] Briefly, both studies included only adult patients (age > 17 years) with severe sepsis (2 of 4 systemic inflammatory response syndrome criteria, infection, and lactate > 4 mmol/L) or septic shock (systolic blood pressure < 90 mm Hg despite at least 20 mL/kg intravenous fluids) within the first 6 hours of sepsis recognition. Patients enrolled in both studies were treated with early, protocolized resuscitation bundles which included early broad-spectrum antibiotics, intravenous fluids, lactate monitoring, and blood cultures within the first six hours. The institutional review boards at each institution approved the enrollment protocols.
Study Protocol & Measures
Prospectively collected data for both original studies included patient demographics, suspected source of infection, patient comorbidities, vital signs, and laboratory values for markers of organ dysfunction, including initial and repeat serum lactate concentrations. Lactate normalization was defined as an initial lactate >2 mmol/L followed by a subsequent measurement <2 mmol/L within 6 hours.[10] Additionally, treatment interventions were available for both cohorts, including the total quantity of intravenous fluids administered during the initial 24 hours and vasopressor usage. For this secondary analysis, two of the authors (AJ and TR) performed a chart review to retrospectively calculate modified Sequential Organ Failure Assessment (mSOFA) scores, an adjustment of the SOFA score that removes the Glasgow Coma Scale (GCS) component as this data was not prospectively collected for all patients. mSOFA has been studied and has been shown to have similar predictive ability as SOFA for predicting organ failure.[11,12] Both ED and inpatient records were reviewed to adjudicate sepsis diagnosis, and systematically collect comorbidities, source of infection, culture results, presence of shock, vasopressor or inotrope use, respiratory failure requiring mechanical ventilation, time to initial antibiotics, time to initial vasopressors, quantity of fluid resuscitation and blood product administration.
Data analysis
The primary outcome for this study was all-cause mortality within 24 hours of ED presentation. Student’s t-test, Wilcoxon rank-sum test, and chi-square or Fisher’s exact tests were used as appropriate to analyze the differences in baseline demographic characteristics, comorbidities, source of infection, treatment data and physiologic parameters between the early death and early survival groups.
Univariate analyses of multiple covariates, which included patient comorbidities, clinical characteristics and interventions were used to derive a multivariate logistic regression model predictive of composite adverse outcome. Candidate variables were chosen from the univariate analyses, and all variables with p-values of 0.1 or less were used to inform the choice of potential predictor variables for the final regression model. To avoid over-fitting, given the relatively low number of primary outcomes (early mortality) in the sample, we limited the number of independent variables to the three most significant, which is based upon a commonly used method to increase the accuracy of regression models in epidemiology.[13] When univariate comparisons revealed significance for collinear variables, the more clinically useful of the two was chosen for inclusion in the regression model. For example, repeat lactate and lactate normalization are collinear, as lactate normalization is derived from repeat lactate. Therefore lactate normalization was included in the model and repeat lactate value was not. All statistical tests were two-tailed and p-values of <0.05 were considered significant. Graphical and statistical analyses were performed using Stata Version 12 (StataCorp LP, College Station, Texas).
Results
A total of 410 patients with severe sepsis or septic shock met the inclusion criteria and were included in the final analysis (Table 1). Of these, 270 (66%) were older than 55 years, 188 (46%) were female. 219 (53%) were white and 158 (39%) were African American. Diabetes mellitus was the most common comorbidity (37%), followed by active cancer (21%), and COPD (20%). The most common sources of infection were pulmonary (49%), followed by urinary tract (28%), and intra-abdominal (15%).
Table 1.
Demographic, source of sepsis and comorbidities for patients meeting the primary outcome (death within 24 hours) versus survival at 24 hours
| Variable | Total (N=410) | Alive at 24 hours (N=390) | Deceased at 24 hours (N= 20) | p-value |
|---|---|---|---|---|
| Age > 55 | 270 (66%) | 254 (64%) | 16 (80%) | 0.172 |
| Sex, female, n (%) | 188 (46%) | 175 (45%) | 13 (65%) | 0.078 |
| African American, n (%) | 160 (39%) | 150 (38%) | 10 (50%) | < 0.001 |
| Source of sepsis, n (%) | ||||
| Pulmonary | 158 (49%) | 147 (38%) | 11 (55%) | 0.121 |
| Urinary | 112 (28%) | 108 (28%) | 5 (25%) | 0.793 |
| Intra-abdominal | 62 (15%) | 59 (15%) | 4 (20%) | 0.556 |
| Bacteremia | 16 (4%) | 16 (4%) | 0 (0%) | 0.356 |
| CNS | 4 (1%) | 4(1%) | 0 (0%) | 0.649 |
| Comorbidities, n (%) | ||||
| Diabetes Mellitus | 152 (37%) | 147 (38%) | 5 (25%) | 0.252 |
| End Stage Renal Disease | 40 (10%) | 38 (12%) | 1 (5%) | 0.481 |
| COPD | 80 (20%) | 73 (19%) | 7 (35%) | 0.074 |
| Human Immunodeficiency Virus | 39 (10%) | 30 (8%) | 1 (5%) | 0.657 |
| Malignancy | 85 (21%) | 77 (20%) | 8 (40%) | 0.030 |
| Transplant | 14 (3%) | 14 (4%) | 0 (0%) | 0.389 |
Comorbidities were defined by presence as documented in the medical record. HIV = Human Immunodeficiency Virus, COPD = Chronic Obstructive Pulmonary Disease, CNS = Central Nervous System.
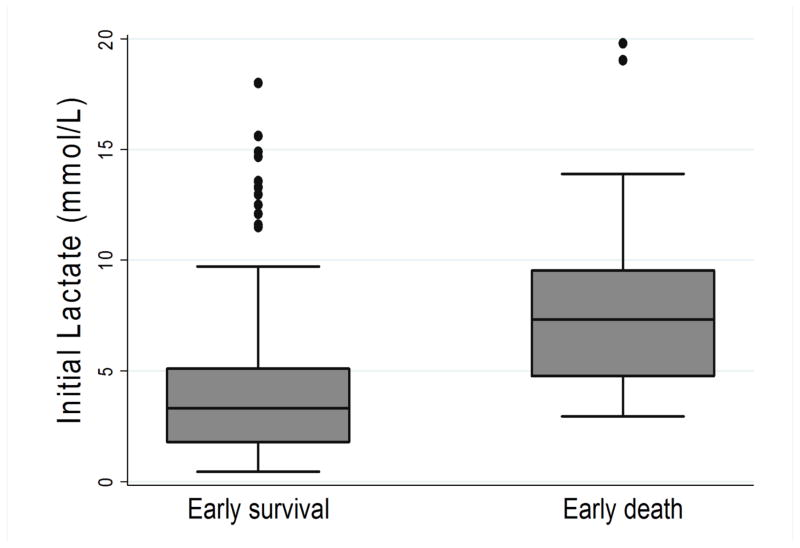
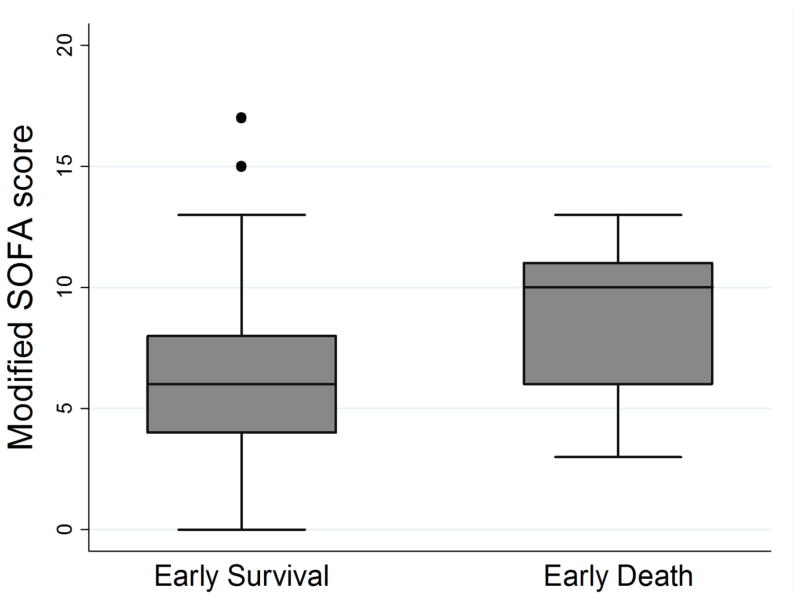
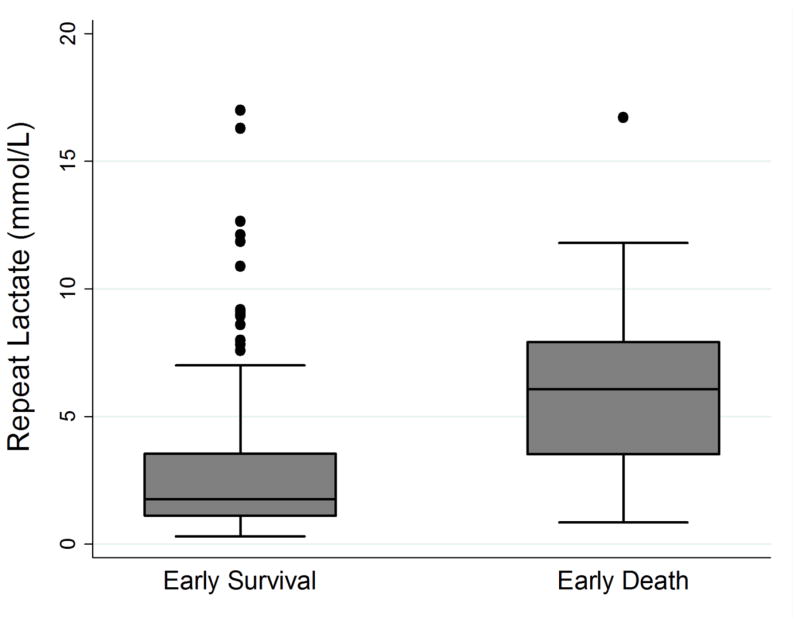
Of the 410 patients in the study, 20 (4.9%) patients experienced the primary outcome of death within 24 hours of ED arrival. Table 2 compares demographics, physiologic parameters, suspected source of infection, patient comorbidities, and treatment data between the early survival and early death groups. Compared to the early survival group, the early death group had statistically significant higher initial lactate values (7.3 vs. 3.3 mmol/L, p <0.001), repeat lactates (6.1 vs 1.8 mmol/L, p <0.001), mSOFA scores (10 (IQR 6,11) vs. 6 (IQR 4,8), p <0.001), lower mean arterial or venous pH (7.20 vs 7.31, p<0.001 N=271) and were more likely to have positive blood cultures (65% vs. 39%, p = 0.021) or active cancer (40% vs 20%, p =0.030). Figures 1–3 contain boxplots illustrating median initial lactate levels, repeat lactate levels, and mSOFA scores across the groups.
Table 2.
Physiologic characteristics and clinical interventions for patients meeting the primary outcome (death within 24 hours) versus survival at 24 hours
| Variable | Total (N=410) | Alive at 24 hours (N=390) | Deceased at 24 hours (N= 20) | p-value |
|---|---|---|---|---|
| Physiologic parameters | ||||
| SBP< 90, n (%) | 283 (68%) | 270 (69%) | 13 (65%) | 0.690 |
| Initial lactate, mmol/L (IQR) | 3.4 (1.8–5.4) | 3.3 (1.8–5.1) | 7.3 (4.8–9.5) | <0.001 |
| Repeat lactate, mmol/L, (IQR) | 2.8 (2.6) | 1.8 (1.1–3.5) | 6.1(3.5–8.0) | <0.001 |
| Delta Lactate (SD) | −1.4 (2.0) | −1.3 (2.0) | −1.8 (2.2) | 0.223 |
| Lactate normalization, n (%) | 190 (46%) | 189 (48%) | 1 (5%) | <0.001 |
| pH (SD) | 7.30 (0.44) | 7.31 (0.45) | 7.20 (0.24) | <0.001 |
| Median mSOFA score (IQR) | 6 (4–8) | 6 (4–8) | 10 (6–11) | <0.001 |
| Blood culture positive within 24h, n (%) | 165 (40%) | 152 (39%) | 13 (65%) | 0.021 |
| Interventions | ||||
| IVF at 6 hours, mL (SD) | 4141 (2258) | 4122 (2248) | 4514 (2484) | 0.450 |
| IVF at 24 hours, mL (SD) | 5048 (3660) | 5028 (3692) | 5419 (3054) | 0.507 |
| Vasopressor use, n (%) | 222 (54%) | 208 (53%) | 14 (70%) | 0.145 |
| Mechanical ventilation, n (%) | 343 (84%) | 327 (84%) | 16 (80%) | 0.651 |
| Time to antibiotics, minutes (SD) | 170 (171) | 171 (173) | 152 (113) | 0.638 |
| Time to vasopressors, minutes (SD) | 303 (335) | 293 (324) | 450 (457) | 0.264 |
| PRBC use, n (%) | 39 (9.5%) | 36 (9.2%) | 3 (15%) | 0.391 |
| PRBCs within 6 hours, units (SD) | 2.6 (1.2) | 2.7 (1.2) | 2.0 (1.0) | 0.373 |
IQR = interquartile range; SD = standard deviation; mSOFA = Modified Sequential Organ Dysfunction Assessment; SBP = systolic blood pressure; IVF = Intravenous Fluids, PRBC = Packed Red Blood Cells;
Figure 1.
Boxplot demonstrating medians and interquartile ranges for initial serum lactate compared between patients with early survival and early death
Figure 3.
Boxplot demonstrating medians and interquartile ranges for modified Sequential Organ Function Assessment (SOFA) score compared between patients with early survival and early death
Lactate normalization differed significantly between groups, with 95% of patients failing to achieve lactate normalization in the early death group, compared to only 48% of patients in the early survivors group (p < .001). However, lactate clearance [(initial lactate– subsequent lactate)/initial lactate] did not differ significantly between the groups (−1.3 vs −1.8, p = 0.223). There were no significant differences in age (age > 55 years) (80% vs 64%, p =0.172), sex (65% vs 45%, p =0.078), comorbidities, or source of infection among patients with early death. (Table 2).
With regards to early interventions for sepsis, patients with early death had no significant differences for rates of vasopressor use (70% vs 53%, p=0.145), time to initiating vasopressors (457 min vs 293 min, p=0.264), time to antibiotics (152 min vs 171 min, p=0.638), need for mechanical ventilation (80% vs 84%, p=0.651), IVF administration at 6 hours (2484 mL vs 2248 mL, p=0.507), IVF administration at 24 hours (5419 vs 5028, p=0.507), need for PRBC infusion (15% vs 9.2%, p=0.391) or quantity of PRBCs administered (2.0 units vs 2.7 units, p=0.373).
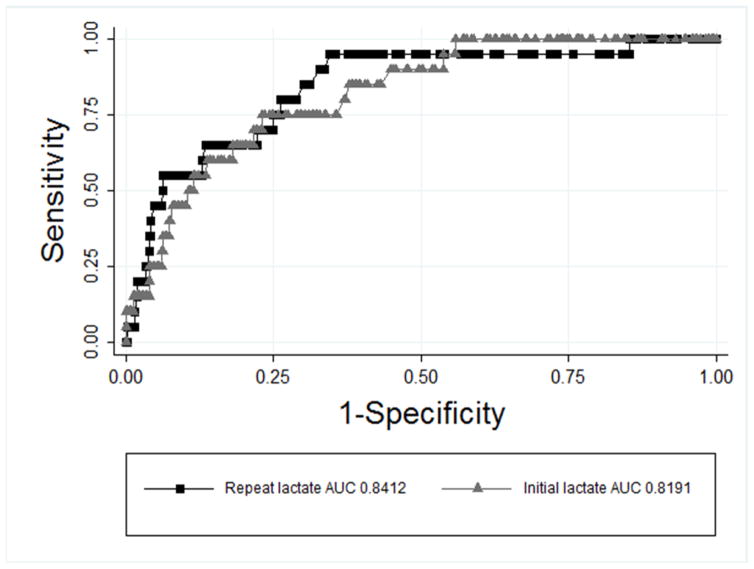
Predictor variables included in the final model were initial serum lactate, lactate normalization, and mSOFA score. The multivariable logistic regression model identified initial serum lactate (OR 1.19, 95% CI 1.05–1.35, p = .004) and initial mSOFA score (OR 1.17, 95% CI 1.00–1.36, p = .046) as clinical predictors of early death (Table 3). Non-parametric ROC analysis identified repeat lactate as the most robust predictor of early death out of all variables in the regression model with an AUC of 0.84, while initial lactate had an AUC of 0.82 (Figure 4). A cut-off repeat lactate ≥ 5 mmol/L had a sensitivity of 55% and specificity of 89% for early death with a positive likelihood ratio of 4.83 for predicting this outcome. Though the percentage of patients achieving lactate normalization in the univariate analysis was significantly different between groups, lactate normalization (OR 0.13, 95% CI 0.02–1.07, p = .056) was not found to be a statistically significant independent predictor of early death.
Table 3.
Predictors of death within 24 hours of emergency department admission
| Predictor | Odds ratio | 95% Confidence interval | p-value |
|---|---|---|---|
| Initial lactate | 1.19 | 1.06 – 1.35 | 0.004 |
| Lactate normalization | 0.13 | 0.02 – 1.07 | 0.058 |
| mSOFA score | 1.17 | 1.00 – 1.36 | 0.046 |
sBP = systolic blood pressure, mSOFA = Modified Sequential Organ Dysfunction Assessment, COPD = Chronic Obstructive Pulmonary Disease
Figure 4.
ROC curve demonstrating predictive value of initial lactate and repeat lactate
To identify the components of the mSOFA that were most predictive of early death, Wilcoxon’s rank sum analysis demonstrated that the respiratory component (3 IQR 1.5,3.5 vs 1, IQR 0,3, p =0.001) and cardiovascular components (3.5, IQR 1,4 vs 2, IQR 1,3, p = 0.018) accounted for most of the difference in mSOFA between those with early death and early survival (Table 4).
Table 4.
Type of organ dysfunction and death within 24 hours
| mSOFA component | Alive at 24 hours* | Deceased at 24 hours* | p-value |
|---|---|---|---|
| Respiratory | 1 (0–3) | 3 (1.5–3.5) | 0.001 |
| Cardiovascular | 2 (1–3) | 3.5 (1–4) | 0.018 |
| Hepatic | 0 (0–0) | 0 (0–2) | 0.211 |
| Renal | 2 (0–2) | 2 (1–2.5) | 0.177 |
| Coagulation | 0 (0–1) | 1 (0–2) | 0.007 |
Abbreviations: mSOFA = modified Sequential Organ Failure Assessment, IQR = interquartile range;
median (IQR).
mSOFA values were derived from laboratory and clinical parameters obtained upon presentation to the ED.
Discussion
In this analysis, we demonstrated that initial serum lactate and mSOFA score were independent predictors of death within 24 hours of ED admission in patients with severe sepsis or septic shock. Univariate testing did not detect significant differences in the rates or rapidity of critical therapeutic interventions among patients experiencing early death; whereas failure to normalize serum lactate (persistent lactate elevation), initial pH, and early blood culture positivity were significant predictors of early death in the univariate analysis. While some of these factors are known to predict in-hospital mortality or increased ICU lengths of stay in sepsis[6,14,15] to our knowledge this is the first study to associate these clinical features with mortality within the first 24 hours of presentation of sepsis. These findings may have implications for risk stratification of patients with severe sepsis or septic shock in addition to early identification of those in whom aggressive management may be needed to decrease risk of death or inform alternative goals of care.
The role of initial serum lactate is well established as a predictor of adverse outcomes in patients with sepsis and our results are consistent with previously published literature. In this analysis, patients with early death had markedly elevated initial serum lactates and repeat serum lactates. We also identified based on our ROC analysis that a threshold for repeat lactate of 5.0 mmol/L was strongly predictive of early death. This may have particular utility in the identification of patients in need of aggressive interventions and novel therapies to improve outcomes. Two previous studies demonstrated that lactate clearance of 10–20% is non-inferior to early quantitative resuscitation.[9,16] However, Puskarich and colleagues have demonstrated that early lactate normalization is the strongest predictor of survival[17] and that lactate clearance is not a substitute for improved microcirculatory flow.[18] In this study, though we were unable to identify a significant difference in delta lactate (initial lactate minus repeat lactate) between the early death and early survivor groups, patients who died within 24 hours had markedly elevated repeat serum lactates in comparison to the early survivors despite adequate clearance. We analyzed delta lactate rather than lactate clearance because a 10% lactate clearance target seemed less informative than the absolute change in lactate given the high initial median lactates in these patients. Comparing median repeat lactates between groups, the early death group had repeat lactates that were significantly and markedly elevated (by 4.3 mmol/L) in comparison to early survivors (Table 2). The starkest individual finding was the difference in lactate normalization between early survivors and non-survivors, with 95% of early death patients failing to achieve lactate normalization compared with 48% of early survivors. It is worth noting that while not reaching statistical significance in our multivariate model, the number of outcomes in the study was relatively low, and a larger study may detect the additional independent value of failure of lactate normalization in this patient population.
SOFA score is a validated measure of organ dysfunction that directly relates to mortality in septic patients[19,20] and is frequently used as an endpoint in clinical trials.[21,22] It has recently gained much attention due to the newest revision of the sepsis definitions (SEP-3) to include SOFA score as the method for quantification of acute organ dysfunction, a requirement for diagnosis of sepsis.[23] Using SOFA as a tool to risk stratify patients and guide early aggressive therapy is especially important in the subset of sepsis patients who do not present with overt signs of shock.[15] As components necessary for the calculation of Glasgow Coma Score (GCS) were not collected prospectively, we utilized a modified SOFA score previously identified to have similar predictive utility.[11,12] In this study, the early death group demonstrated a significantly higher median mSOFA score of 10 compared to 6 in early survivors. Our findings suggest that early SOFA score evaluation may identify patients at risk of death within the first 24 hours of sepsis. Furthermore, specific types of organ dysfunction may be associated with greater risk of early death. In this study, the early mortality group had significantly higher cardiovascular and respiratory SOFA sub-scores which may reflect the current limitations in full organ support in the setting of severe multi-organ dysfunction.
Interestingly, increased early mortality was not associated with differences in ED treatments or interventions. In two of the cornerstone treatments for septic shock, fluid resuscitation and antibiotics, we found no differences in IVF administered at 6 or 24 hours or with time to initial antibiotic administration in the early versus later deaths. The time to administer vasopressors for shock was also similar between the groups. These findings suggest that persistent hyperlactatemia may be an indication for a broader range of therapies including those that are less commonly utilized in the early stages of sepsis, such as inotropes. Therefore, this high-risk subgroup may represent a potential target patient population for novel therapies.
Of note in this study, 6 of 20 patients who died within 24 hours did not initially require vasopressors and therefore would not have met the Sepsis-3 definition of septic shock at presentation despite extremely elevated lactates and high SOFA scores.[23] This has important implications for patients who would not meet the Sepsis-3 criteria for septic shock and may be inappropriately considered low-risk. It also has implications for diagnosis coding and the downstream metrics and quality standards to which hospitals are held accountable. These findings note the importance of using clinical judgment in addition to a consensus definition of septic shock when determining the need for intensive care or patient disposition. Additionally, only seven of 20 patients presented with SBP < 90 mm Hg. All of these patients, however had an initial lactate > 2 mmol/L, while the majority had lactate values > 4. This underscores the value of using lactate in the risk stratification of septic patients in addition to blood pressure in the assessment of patient severity and risk for early death.
Finally, although elevated lactate values and acidosis do not always go hand in hand, particularly in the setting of minor lactate elevations, the finding of lower pH in the early death group was likely a reflection of a reflection of the significant lactate elevations in that group, and thus did not maintain significance in the multivariable analysis. In most settings, point of care lactate testing and blood gases can be performed simultaneously and rapidly. However, in settings where point of care lactate testing is not available, rapid pH testing may be a more widely available early indicator of the septic patients’ physiologic status while awaiting a lactate value.
Limitations
This study had several limitations. Most importantly, a relatively low number of patients experienced the primary outcome which meant that the study was likely underpowered to detect some clinically significant differences between the early death and early survival groups. Nevertheless, we still demonstrated several significant predictors of early death, illustrating the strong prognostic value of these particular factors, specifically mSOFA and lactate. A larger study may be able to detect other important independent clinical predictors. As mentioned previously, we did not find a significant modulatory effect of early interventions on early mortality. There are at least three potential explanations for this; 1) this study may simply be underpowered to detect such differences, 2) relatively homogeneous resuscitation practices between providers may limit the clinical heterogeneity needed to detect treatment effects, or 3) current early therapeutic interventions truly have limited efficacy in preventing early deaths, as the patient’s disease process may simply be too entrenched to truly effect with our current therapies. Finally, we used a modified SOFA score, which removes the GCS component of the originally described SOFA, as GCS was not collected in both parent studies and could not be calculated retrospectively for part of the cohort. However, this modified score has been previously used in studies to assess the degree of organ dysfunction in severe sepsis, so we feel its utilization is still reliable.[24]
Conclusions
This study demonstrates that there is a small group of patients who experience early death from severe sepsis. The initial and repeat serum lactate levels and mSOFA score are significant independent predictors of early death in these patients, even after accounting for treatment interventions. These data may be useful to identify patients at high risk of early death in whom novel therapies and investigational treatments may be warranted.
Figure 2.
Boxplot demonstrating medians and interquartile ranges for repeat serum lactate compared between patients with early survival and early death
Highlights.
Initial serum lactate and SOFA score independently predict death within 24 hours in patients presenting to the ED with sepsis
This outcome was not associated with differences in ED treatments or interventions
These data may help identify patients at high risk of early death and inform their early clinical course
Acknowledgments
Funding Sources: Dr. Guirgis (K23GM115690), Dr. Puskarich (K23GM113041), and Dr. Jones (R01GM103799) have received support from the National Institutes of General Medical Sciences. Dr. Puskarich also receives support from the NIH Loan Repayment Program. The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928–36. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Croft CA, et al. Computer versus paper system for recognition and management of sepsis in surgical intensive care. J Trauma Acute Care Surg. 2014;76:311–319. doi: 10.1097/TA.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, et al. The PIRO concept: P is for predisposition. Crit Care. 2003;7:248–251. doi: 10.1186/cc2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro NI, et al. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31:670–675. doi: 10.1097/01.CCM.0000054867.01688.D1. [DOI] [PubMed] [Google Scholar]
- 6.Thomas-Rueddel DO, et al. Hyperlactatemia is an independent predictor of mortality and denotes distinct subtypes of severe sepsis and septic shock. J Crit Care. 2015;30:439.e1–439.e6. doi: 10.1016/j.jcrc.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Drumheller BC, et al. Risk factors for mortality despite early protocolized resuscitation for severe sepsis and septic shock in the emergency department. J Crit Care. 2015;31:13–20. doi: 10.1016/j.jcrc.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Guirgis FW, et al. End-tidal carbon dioxide as a goal of early sepsis therapy. Am J Emerg Med. 2014;32:1351–6. doi: 10.1016/j.ajem.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Jones AE, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puskarich MA, et al. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest. 2013;143:1548–53. doi: 10.1378/chest.12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent JL, et al. Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med. 2003;31:834–840. doi: 10.1097/01.CCM.0000051515.56179.E1. [DOI] [PubMed] [Google Scholar]
- 12.Arnold RC, et al. Multicenter observational study of the development of progressive organ dysfunction and therapeutic interventions in normotensive sepsis patients in the emergency department. Acad Emerg Med. 2013;20:433–440. doi: 10.1111/acem.12137. [DOI] [PubMed] [Google Scholar]
- 13.FEH, Lee KL, Mark DB. Tutorial in Biostatistics Multivariable Prognostic Models: Issues in Developing Models. Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Whittaker Sa, et al. Epidemiology and outcomes in patients with severe sepsis admitted to the hospital wards. J Crit Care. 2014;30:78–84. doi: 10.1016/j.jcrc.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puskarich MA, et al. Outcomes of patients undergoing early sepsis resuscitation for cryptic shock compared with overt shock. Resuscitation. 2011;82:1289–1293. doi: 10.1016/j.resuscitation.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima A Pa, Willemsen SP, Bakker J. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–61. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 17.Puskarich MA, et al. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest. 2013;143:1548–1553. doi: 10.1378/chest.12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puskarich MA, Shapiro NI, Massey MJ, Kline JA, Jones AE. Lactate clearance in septic shock is not a surrogate for improved microcirculatory flow. Acad Emerg Med. 2016 doi: 10.1111/acem.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro N, et al. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006;48:583–90. 590.e1. doi: 10.1016/j.annemergmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Marshall JC, et al. Outcome measures for clinical research in sepsis: A report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. 2005;33:1708–1716. doi: 10.1097/01.ccm.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL. Endpoints in sepsis trials: more than just 28-day mortality? Crit Care Med. 2004;32:S209–S213. doi: 10.1097/01.ccm.0000126124.41743.86. [DOI] [PubMed] [Google Scholar]
- 23.Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold RC, et al. Multicenter observational study of the development of progressive organ dysfunction and therapeutic interventions in normotensive sepsis patients in the emergency department. Acad Emerg Med. 2013;20:433–440. doi: 10.1111/acem.12137. [DOI] [PubMed] [Google Scholar]